Mathematical Modelling of Bioethanol Production from Raw Sugar Beet Cossettes in a Horizontal Rotating Tubular Bioreactor
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bioethanol Production in the HRTB
2.2. Mathematical Modelling of Bioethanol Production in the HRTB
2.2.1. Yeast Growth during Bioethanol Production
2.2.2. Products Formation during Bioethanol Production
2.2.3. Substrate Utilization during Bioethanol Production
2.2.4. Mathematical Model and Bioprocess Evaluation Criteria
3. Results
3.1. Bioethanol Production in the HRTB
3.2. Mathematical Model
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhaoa, J.; Xua, Y.; Wang, W.; Griffin, J.; Roozeboomd, K.; Wang, D. Bioconversion of industrial hemp biomass for bioethanol production: A review. Fuel 2020, 281, 118725. [Google Scholar] [CrossRef]
- Kumar Prasad, R.; Chatterjee, S.; Mazumder Pranab, B.; Kumar Gupta, S.; Sharma, S.; Gunvant Vairale, M.; Datta, S.; Kumar Dwivedi, S.; Kumar Gupta, D. Bioethanol production from waste lignocelluloses: A review on microbial degradation potential. Chemosphere 2019, 231, 588–606. [Google Scholar] [CrossRef]
- Dave, N.; Selvaraj, R.; Varadavenkatesan, T.; Vinayagam, R. A critical review on production of bioethanol from macroalgal biomass. Algal Res. 2019, 42, 101606. [Google Scholar] [CrossRef]
- Saadiah Hafid, H.; Abdul Rahman, N.A.; Md Shah, U.K.; Samsu Baharuddin, A.; Ariff, A.B. Feasibility of using kitchen waste as future substrate for bioethanol production: A review. Renew. Sustain. Energy Rev. 2017, 74, 671–686. [Google Scholar] [CrossRef]
- Torroba, A. Liquid Biofuels Atlas. 2021, pp. 1–35. Available online: http://www.iica.int (accessed on 24 December 2021).
- Bai, F.W.; Chen, L.J.; Anderson, W.A.; Moo-Young, M. Parameter oscillations in very high gravity medium continuous ethanol fermentation and their attenuation on multi-stage packed column bioreactor system. Biotechnol. Bioeng. 2004, 88, 558–566. [Google Scholar] [CrossRef]
- Bai, F.W.; Anderson, W.A.; Moo-Young, M. Ethanol fermentation technologies from sugar and starch feedstocks. Biotechnol. Adv. 2008, 26, 89–105. [Google Scholar] [CrossRef]
- Cardona, C.A.; Sánchez, Ó.J. Fuel ethanol production: Process design trends and integration opportunities. Bioresour. Technol. 2007, 98, 2415–2457. [Google Scholar] [CrossRef]
- Giubbina, F.F.; Scaramboni, C.; De Martinis, B.S.; Godoy-Silva, D.; Mello, I.N.P.D.; Nogueira, R.F.P.; Campos, M.L.A.M. Temporal variation of ethanol in rainwater from the sugar cane belt of SãoPaulo State (Brazil). Atmos. Environ. 2019, 216, 116926. [Google Scholar] [CrossRef]
- Kawa-Rygielska, J.; Pietrzak, W.; Regiec, P.; Stencel, P. Utilization of concentrate after membrane filtration of sugar beet thin juice for ethanol production. Bioresour. Technol. 2013, 133, 134–141. [Google Scholar] [CrossRef]
- Vučurović, D.G.; Dodić, S.N.; Popov, S.D.; Dodić, J.M.; Grahovac, J.A. Process model and economic analysis of ethanol production from sugar beet raw juice as part of the cleaner production concept. Bioresour. Technol. 2012, 104, 367–372. [Google Scholar] [CrossRef]
- Tan, L.; Sun, Z.Y.; Okamoto, S.; Takaki, M.; Tang, Y.Q.; Morimura, S.; Kida, K. Production of ethanol from raw juice and thick juice of sugar beet by continuous ethanol fermentation with flocculating yeast strain KF-7. Biomass Bioenergy 2015, 81, 265–272. [Google Scholar] [CrossRef]
- Dodić, S.; Popov, S.; Dodić, J.; Ranković, J.; Zavargo, Z.; Jevtić Mučibabić, R. Bioethanol production from thick juice as intermediate of sugar beet processing. Biomass Bioenergy 2009, 33, 822–827. [Google Scholar] [CrossRef]
- Šantek, B.; Gwehenberger, G.; Ivančić Šantek, M.; Narodoslawsky, M.; Horvat, P. Evaluation of energy demand and the sustainability of different bioethanol production processes from sugar beet. Resour. Conserv. Recycl. 2010, 54, 872–877. [Google Scholar] [CrossRef]
- Dziugan, P.; Balcerek, M.; Pielech-Przybylska, K.; Patelski, P. Evaluation of the fermentation of high gravity thick sugar beet juice worts for efficient bioethanol production. Biotechnol. Biofuels 2013, 6, 158. [Google Scholar] [CrossRef] [Green Version]
- Pavlečić, M.; Rezić, T.; Ivančić Šantek, M.; Horvat, P.; Šantek, B. Bioethanol production from raw sugar beet cossettes in horizontal rotating tubular bioreactor. Bioprocess Biosyst. Eng. 2017, 40, 1679–1688. [Google Scholar] [CrossRef] [PubMed]
- Lilley, K.A.; Fry, C.J.; Bailey, J.M.; Day, J.M. Comparison of aerobic heterotrophic taxa isolatedfrom four root domains of mature sugar beet (Beta vulgaris). FEMS Microbiol. Ecol. 1996, 21, 23l–242. [Google Scholar] [CrossRef]
- Li, M.; Yang, F.; Wu, X.; Yan, H.; Liu, Y. Effects of continuous cropping of sugar beet (Beta vulgaris L.) on its endophytic andsoil bacterial community by high throughput sequencing. Ann. Microbiol. 2020, 70, 39. [Google Scholar] [CrossRef]
- Sereš, Z.; Maravic, N.; Rakic, D.; Dokic, L.J.; Nikolic, I.; Soronja-Simovic, D.; Ðorđevic, M. Application of biocides in the process of sucrose extraction from sugar beet: Effect on sucrose content, number of Leuconostoc colonies and wet pulp characteristics. Food Sci. Technol. 2017, 75, 17–24. [Google Scholar] [CrossRef]
- Kohout, K.C.; Ukowitz, C.; Reiter, D.; Zitz, U.; Moder, K.; Emerstorfer., F.; Hein, W.; Kneifel, W.; Domig, J.K. Bacterial growth dynamics and corresponding metabolite levels in the extraction area of an Austrian sugar beet factory using antimicrobial treatment. J. Sci. Food Agric. 2020, 100, 2713–2721. [Google Scholar] [CrossRef] [PubMed]
- Kłosowski, G.; Mikulski, D.; Czupryński, B.; Kotarska, K. Characterisation of fermentation of high-gravity maize mashes with the application of pullulanase, proteolytic enzymes and enzymes degrading non-starch polysaccharides. J. Biosci. Bioeng. 2010, 109, 466–471. [Google Scholar] [CrossRef]
- Grahovac, J.; Jokic, A.; Dodic, J.; Vucurovic, D.; Dodic, S. Modelling and prediction of bioethanol production from intermediates and byproduct of sugar beet processing using neural networks. Renew. Energy 2016, 85, 953–958. [Google Scholar] [CrossRef]
- Dodic, M.J.; Vucurovic, G.D.; Dodic, S.N.; Grahovac, J.A.; Popov, S.D.; Nedeljkovic, N.M. Kinetic modelling of batch ethanol production from sugar beet raw juice. Appl. Energy 2012, 99, 192–197. [Google Scholar] [CrossRef]
- Andrews, J.F. A mathematical model for the continuous culture of microorganisms utilizing inhibitory substrates. Biotechnol. Bioeng. 1968, 10, 707–723. [Google Scholar] [CrossRef]
- Joannis-Cassan, C.; Riess, J.; Jolibert, F.; Taillandier, P. Optimization of very high gravity fermentationn process for ethanol production from industrial sugar beet syrup. Biomass Bioenergy 2014, 70, 165–173. [Google Scholar] [CrossRef] [Green Version]
- Attfield, P.V.; Kletsas, S. Hyperosmotic stress response by strains of bakers’ yeasts in high sugar concentration medium. Lett. Appl. Microbiol. 2001, 31, 323–327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gomar-Alba, M.; Morcillo-Parra, M.A.; del Olmo, M.L. Response of yeast cells to high glucose involves molecular and physiological differences when compared to other osmostress conditions. FEMS Yeast Res. 2015, 15, fov039. [Google Scholar] [CrossRef]
- Andre, L.; Hemming, A.; Adler, L. Osmoregulation in Saccharomyces cerevisiae studies on the osmotic induction of glycerol production and glycerol 3-phosphate dehydrogenase (NAD+). FEBS Lett. 1991, 286, 13–17. [Google Scholar] [CrossRef] [Green Version]
- Hohmann, S. Osmotic adaptation in yeast-control of the yeast osmolyte system. Int. Rev. Cytol. 2002, 215, 149–187. [Google Scholar] [CrossRef]
- Paraggio, M.; Fiore, C. Screening of Saccharomyces cerevisiae wine strains for the production of acetic acid. World J. Microbiol. Biotechnol. 2004, 20, 743–747. [Google Scholar] [CrossRef]
- Moruno, E.G.; Delfini, C.; Pessione, E.; Giunta, C. Factors affecting acetic acid production by yeasts in strongly clarified grape musts. Microbios 1993, 74, 249–256. [Google Scholar] [PubMed]
- Eglinton, J.M.; Heinrich, A.J.; Pollnitz, A.P.; Langridge, P.; Henschke, P.A.; de Barros Lopes, M. Decreasing acetic acid accumulation by a glycerol overproducing strain of Saccharomyces cerevisiae by deleting the ALD6 aldehyde dehydrogenase gene. Yeasts 2002, 19, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Marques, W.L.; Raghavendran, V.; Ugarte Stambuk, B.; Karoly Gombert, A. Sucrose and Saccharomyces cerevisiae: A relationship most sweet. FEMS Yeast Res. 2016, 16, fov107. [Google Scholar] [CrossRef] [Green Version]
- Vucurovic, V.M.; Razmovski, R.N. Sugar beet pulp as support for Saccharomyces cerivisiae immobilization in bioethanol production. Ind. Crops Prod. 2012, 39, 128–134. [Google Scholar] [CrossRef]
- Pavlečić, M.; Vrana, I.; Vibovec, K.; Ivančić Šantek, M.; Horvat, P.; Šantek, B. Ethanol production from different intermediates of sugar beet processing. Food Technol. Biotechnol. 2010, 48, 362–367. [Google Scholar]
- Terebiznik, M.R.; Pilosof, A.M.R. Biomass estimation in solid state fermentation by modeling dry matter weight loss. Biotechnol. Tech. 1999, 13, 215–219. [Google Scholar] [CrossRef]
- Bhargav, S.; Panda, B.P.M.; Ali, M.; Javed, S. Solid-state fermentation: An overview. Chem. Biochem. Eng. Q. 2008, 22, 49–70. [Google Scholar]
- Steudler, S.; Böhmer, U.; Weber, J.; Bley, T. Biomass measurement by flow cytometry during solid-state fermentation of basidiomycetes. Cytom. Part A 2015, 87, 176–188. [Google Scholar] [CrossRef] [PubMed]
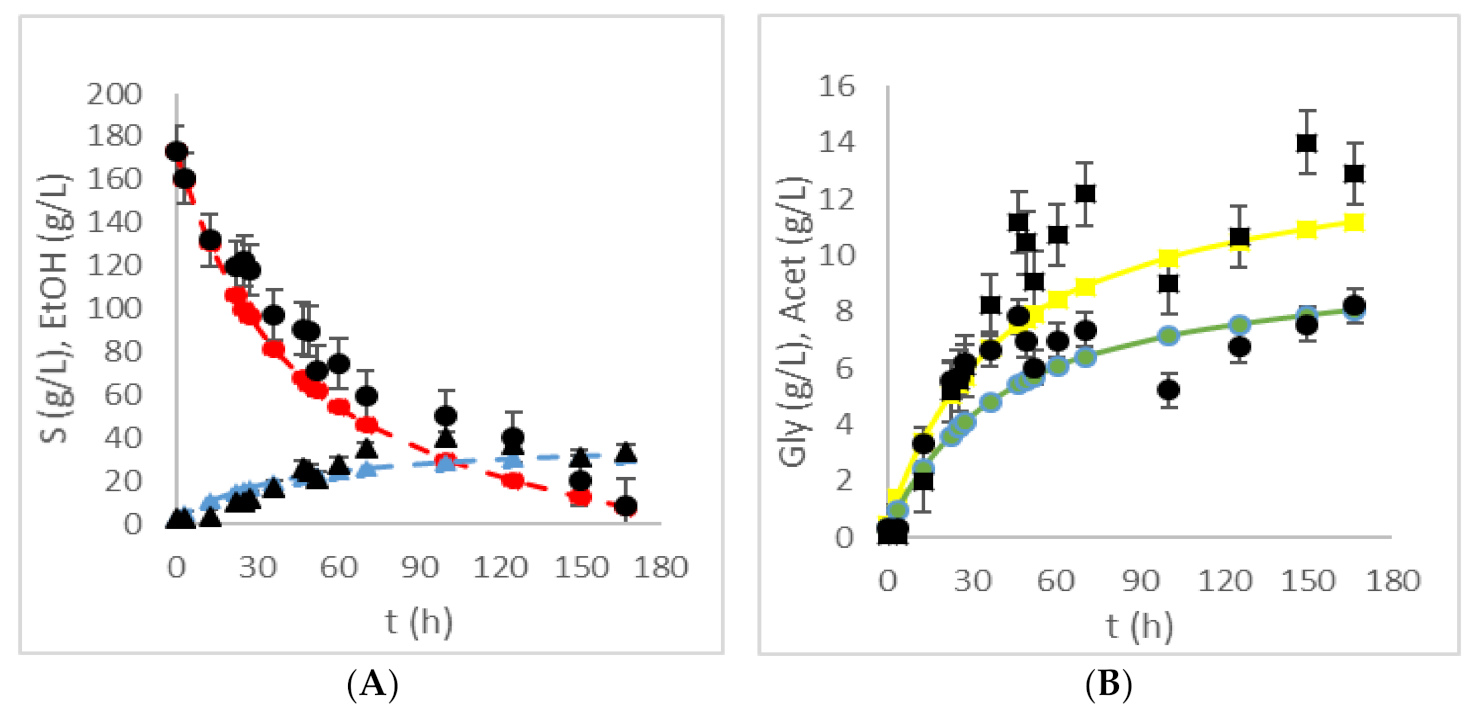
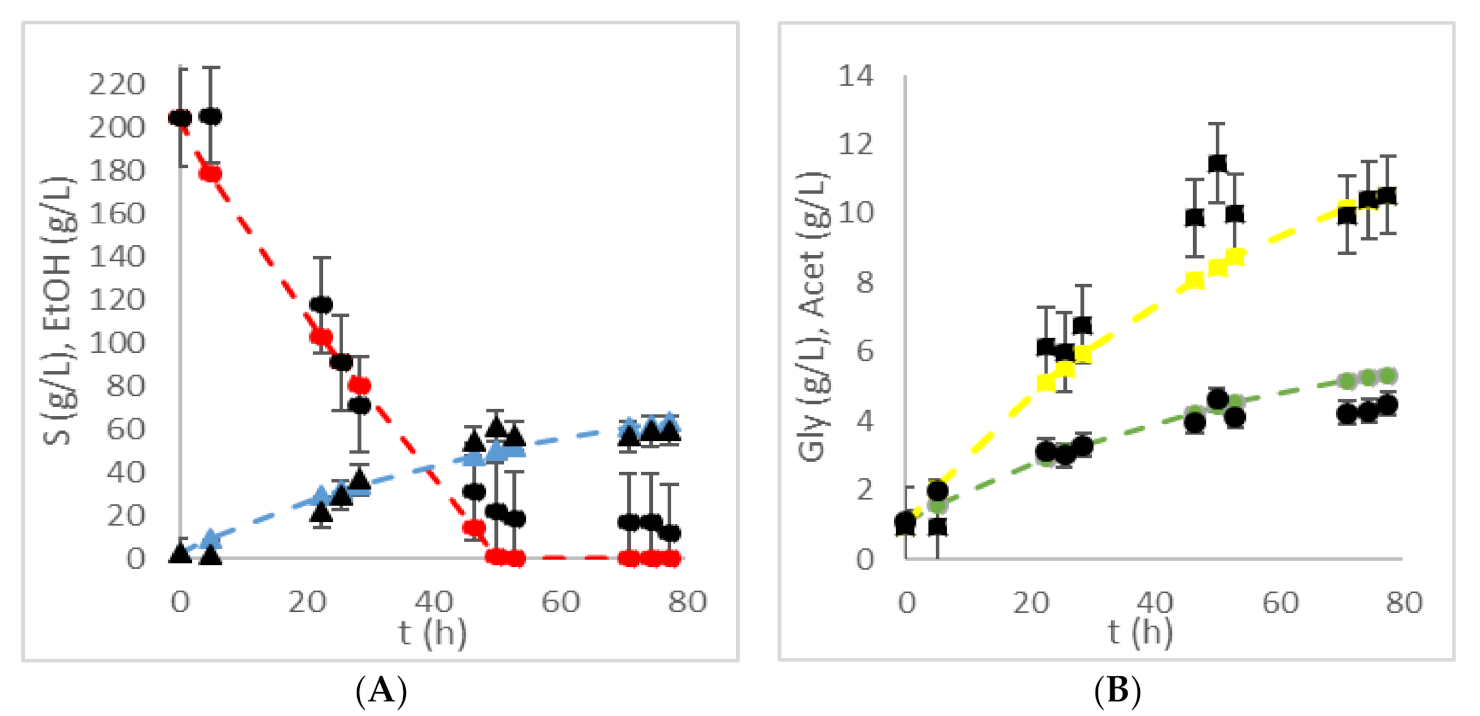
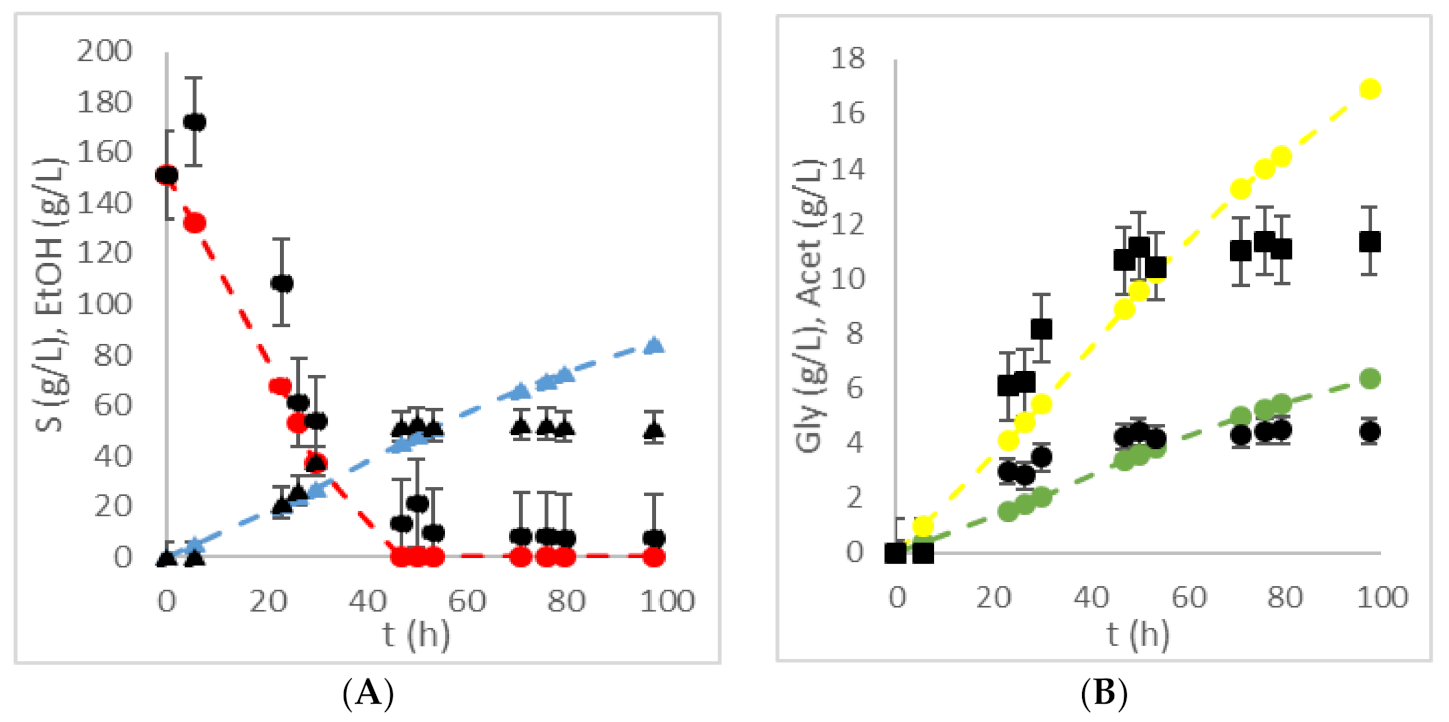
| HRTB Operational Conditions | Bioprocess Efficiency Parameters | ||
|---|---|---|---|
| YEtOH/S (g g−1) | E (%) | Pr (g L−1 h−1) | |
| Constant HRTB rotation mode | |||
| 5 rpm | 0.27 | 50.20 | 0.247 |
| 10 rpm | 0.19 | 35.20 | 0.185 |
| 15 rpm | 0.26 | 49.30 | 0.196 |
| Interval HRTB rotation mode | |||
| 3 min; 5 rpm | 0.36 | 66.90 | 0.832 |
| 6 min; 5 rpm | 0.44 | 81.80 | 0.682 |
| 9 min; 5 rpm | 0.35 | 65.00 | 0.613 |
| 12 min; 5 rpm | 0.43 | 79.90 | 0.769 |
| 15 min; 5 rpm | 0.26 | 48.30 | 0.497 |
| 3 min; 10 rpm | 0.40 | 74.30 | 0.552 |
| 6 min; 10 rpm | 0.23 | 42.70 | 0.357 |
| 9 min; 10 rpm | 0.35 | 65.00 | 0.812 |
| 12 min; 10 rpm | 0.25 | 46.50 | 0.665 |
| 15 min; 10 rpm | 0.33 | 60.70 | 0.583 |
| 3 min;15 rpm | 0.35 | 64.30 | 0.934 |
| 6 min;15 rpm | 0.31 | 57.60 | 0.327 |
| 9 min;15 rpm | 0.32 | 58.70 | 0.606 |
| 12 min; 15 rpm | 0.47 | 87.36 | 0.618 |
| 15 min; 15 rpm | 0.22 | 40.90 | 0.332 |
| Interval HRTB rotation mode (12 min; 15 rpm)—different initial mass of sugar beet cossettes | |||
| 10.0 kg | 0.45 | 83.64 | 0.560 |
| 12.5 kg | 0.38 | 70.63 | 0.312 |
| 15.0 kg | 0.37 | 68.77 | 0.548 |
| 17.5 kg | 0.37 | 68.77 | 0.552 |
| Parameter | YX/S (g g−1) | YEtOH/S (g g−1) | YGLY/S (g g−1) | YACET/S (g g−1) | Ks (g L−1) | Ki (g L−1) |
|---|---|---|---|---|---|---|
| Value | 0.02 | 0.50 | 0.18 | 0.28 | 6.07 | 20.00 |
| HRTB Operational Conditions | Adjustable Model Parameters | |||||
|---|---|---|---|---|---|---|
| Xk (g L−1) | µmax (h−1) | qEtOH (g gx−1 h−1) | qGly (g gx−1 h−1) | qAcet (g gx−1 h−1) | kd (h−1) | |
| Constant HRTB rotation mode | ||||||
| 5 rpm | 1.36 | 0.05 | 0.79 | 0.10 | 0.16 | 0.04 |
| 10 rpm | 0.71 | 0.07 | 1.10 | 0.40 | 0.29 | 0.04 |
| 15 rpm | 1.03 | 0.01 | 0.27 | 0.63 | 0.10 | 0.04 |
| Interval HRTB rotation mode | ||||||
| 3 min; 5 rpm | 2.82 | 0.03 | 0.88 | 0.10 | 0.03 | 0.04 |
| 6 min; 5 rpm | 1.49 | 0.05 | 0.80 | 0.01 | 0.08 | 0.04 |
| 9 min; 5 rpm | 1.60 | 0.11 | 0.80 | 0.05 | 0.01 | 0.04 |
| 12 min; 5 rpm | 1.66 | 0.06 | 0.90 | 0.14 | 0.06 | 0.04 |
| 15 min; 5 rpm | 0.70 | 0.04 | 0.78 | 0.20 | 0.15 | 0.01 |
| 3 min; 10 rpm | 1.13 | 0.08 | 0.65 | 0.08 | 0.08 | 0.01 |
| 6 min; 10 rpm | 1.00 | 0.09 | 0.80 | 0.27 | 0.15 | 0.04 |
| 9 min; 10 rpm | 1.70 | 0.10 | 0.68 | 0.06 | 0.003 | 0.02 |
| 12 min; 10 rpm | 2.63 | 0.07 | 0.57 | 0.09 | 0.04 | 0.03 |
| 15 min; 10 rpm | 1.35 | 0.02 | 0.61 | 0.13 | 0.07 | 0.01 |
| 3 min;15 rpm | 2.10 | 0.07 | 0.75 | 0.07 | 0.05 | 0.02 |
| 6 min;15 rpm | 1.00 | 0.07 | 0.30 | 0.10 | 0.06 | 0.01 |
| 9 min;15 rpm | 1.60 | 0.09 | 0.48 | 0.09 | 0.02 | 0.02 |
| 12 min; 15 rpm | 0.75 | 0.07 | 0.80 | 0.03 | 0.02 | 0.005 |
| 15 min; 15 rpm | 0.80 | 0.07 | 0.82 | 0.26 | 0.17 | 0.03 |
| Interval HRTB rotation mode (12 min; 15 rpm)—different initial mass of sugar beet cossettes | ||||||
| 10.0 kg | 0.58 | 0.07 | 0.80 | 0.18 | 0.07 | 0.007 |
| 12.5 kg | 0.50 | 0.07 | 0.25 | 0.05 | 0.05 | 0.010 |
| 15.0 kg | 2.00 | 0.07 | 0.13 | 0.04 | 0.01 | 0.001 |
| 17.5 kg | 1.12 | 0.07 | 0.80 | 0.16 | 0.06 | 0.010 |
| HRTB Operational Conditions | MSE | |||
|---|---|---|---|---|
| Substrate | Ethanol | Glycerol | Acetic Acid | |
| Constant HRTB rotation mode | ||||
| 5 rpm | 480.60 | 53.05 | 42.70 | 0.26 |
| 10 rpm | 273.79 | 30.38 | 3.84 | 2.02 |
| 15 rpm | 629.86 | 18.83 | 15.79 | 11.83 |
| Interval HRTB rotation mode | ||||
| 3 min; 5 rpm | 265.64 | 29.35 | 3.56 | 1.22 |
| 6 min; 5 rpm | 334.17 | 278.58 | 59.70 | 0.14 |
| 9 min; 5 rpm | 75.38 | 173.65 | 14.24 | 0.54 |
| 12 min; 5 rpm | 1468.88 | 265.26 | 3.18 | 0.49 |
| 15 min; 5 rpm | 524.14 | 20.09 | 3.24 | 0.80 |
| 3 min; 10 rpm | 144.53 | 1276.16 | 17.11 | 4.67 |
| 6 min; 10 rpm | 649.08 | 505.06 | 8667.72 | 345.15 |
| 9 min; 10 rpm | 690.02 | 4.52 | 3.15 | 0.04 |
| 12 min; 10 rpm | 282.46 | 36.75 | 1.72 | 0.30 |
| 15 min; 10 rpm | 171.00 | 40.83 | 2.97 | 1.94 |
| 3 min;15 rpm | 336.39 | 53.30 | 1.04 | 2.09 |
| 6 min;15 rpm | 365.11 | 27.05 | 7.42 | 4.70 |
| 9 min;15 rpm | 601.61 | 250.88 | 8.05 | 0.59 |
| 12 min; 15 rpm | 562.54 | 299.83 | 27.01 | 0.30 |
| 15 min; 15 rpm | 279.14 | 57.95 | 4.14 | 1.92 |
| Interval HRTB rotation mode (12 min; 15 rpm)—different initial mass of sugar beet cossettes | ||||
| 10.0 kg | 377.57 | 81.06 | 8.51 | 1.69 |
| 12.5 kg | 460.67 | 18.69 | 27.54 | 3.60 |
| 15.0 kg | 401.92 | 528.26 | 14.28 | 3.79 |
| 17.5 kg | 383.48 | 374.22 | 6.20 | 1.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pavlečić, M.; Novak, M.; Trontel, A.; Marđetko, N.; Grubišić, M.; Ljubas, B.D.; Tominac, V.P.; Rakovac, R.Č.; Šantek, B. Mathematical Modelling of Bioethanol Production from Raw Sugar Beet Cossettes in a Horizontal Rotating Tubular Bioreactor. Fermentation 2022, 8, 13. https://doi.org/10.3390/fermentation8010013
Pavlečić M, Novak M, Trontel A, Marđetko N, Grubišić M, Ljubas BD, Tominac VP, Rakovac RČ, Šantek B. Mathematical Modelling of Bioethanol Production from Raw Sugar Beet Cossettes in a Horizontal Rotating Tubular Bioreactor. Fermentation. 2022; 8(1):13. https://doi.org/10.3390/fermentation8010013
Chicago/Turabian StylePavlečić, Mladen, Mario Novak, Antonija Trontel, Nenad Marđetko, Marina Grubišić, Blanka Didak Ljubas, Vlatka Petravić Tominac, Rozelindra Čož Rakovac, and Božidar Šantek. 2022. "Mathematical Modelling of Bioethanol Production from Raw Sugar Beet Cossettes in a Horizontal Rotating Tubular Bioreactor" Fermentation 8, no. 1: 13. https://doi.org/10.3390/fermentation8010013
APA StylePavlečić, M., Novak, M., Trontel, A., Marđetko, N., Grubišić, M., Ljubas, B. D., Tominac, V. P., Rakovac, R. Č., & Šantek, B. (2022). Mathematical Modelling of Bioethanol Production from Raw Sugar Beet Cossettes in a Horizontal Rotating Tubular Bioreactor. Fermentation, 8(1), 13. https://doi.org/10.3390/fermentation8010013









