1. Introduction
Tibetan pigs are a rare plateau-type pig breed that have the capacity to adapt to the low oxygen environment of a plateau region with strong resistance to adversity. They are mainly found in high altitude areas (altitudes = 2500–3500 m) such as Yunnan and Tibet in China and are the pig breed with the highest altitude distribution in the world. At present, the research on the Diqing Tibetan pig mainly focuses on growth and development [
1,
2], meat quality [
3], and reproductive performance [
4,
5], while relatively little research has been done on the mechanisms of their herbivory. It has been reported that forage could account for 90% of the dietary composition of Diqing Tibetan pigs under the condition of house feeding [
6]. However, previous reports have mostly focused on the roughage tolerance of Diqing Tibetan pigs, while little has been reported on their ability to utilize fiber efficiently. Some researchers are attempting to screen microbial strains with fiber-degrading ability directly from gut microorganisms of Diqing Tibetan pigs [
6,
7]. The lack of basic data poses great difficulties for subsequent investigations and many scientific hypotheses need to be reconfirmed.
Fibers, as the main component of cell walls, are the most widely distributed and abundant renewable resources on the Earth. Non-starch polysaccharides (NSPs), as the most important component of fiber besides lignin, play a vital role in the functional regulation of fiber. Pigs are unable to synthesize cellulase, hemicellulase, and pectinase, so the degradation of dietary fiber is largely dependent on fermentation and non-enzymatic hydrolysis by microorganisms [
8]. Microorganisms in the pig’s gut use dietary fiber as a primary carbon source and eventually convert it to SCFAs, which can provide 5–20% of the total energy requirement of the host [
9,
10,
11]. However, fiber has long been defined as an anti-nutritional factor and high fiber levels in the diet will affect the digestibility of energy and protein and other nutrients and reduce feed conversion efficiency [
12,
13].
An appropriate level of fiber in the diet has a positive effect on promoting development of the animal intestine and its microecological balance [
14,
15,
16,
17]. Fiber can provide a good substrate for microorganisms in the hindgut of pigs [
18,
19], while the short chain fat acid (SCFA) produced by fermentation can defend against infections and pathogenic bacteria by lowering intestinal pH [
20,
21,
22]. Fiber can play a role in metabolism, supply of energy, etc. and that role could be influenced by the degree of degradation by intestinal microbes. Therefore, the screening of strains with efficient fiber degradation is particularly important.
2. Materials and Methods
Management and design of the experiment followed animal care rules approved by the Department of China Agricultural University Animal Care and Use Ethics Committee.
2.1. Sample Collection of Original Diet
In order to carry out a preliminary investigation of the composition and nutritional level of the original diet of Diqing Tibetan pigs, we entered Diqing Tibetan Autonomous Prefecture (altitude = 3220 m) twice in April and October of the same year to collect samples of the original diet of Diqing Tibetan pigs and local feed ingredients. Samples were prepared as air-dried samples and brought back to the laboratory for chemical analysis.
2.2. Animals and Dietary Treatments
A total of 60 healthy adult Diqing Tibetan pigs of similar body condition (male, 10 months old) were randomly divided into 2 groups with 6 replicates each and 5 pigs in each replicate. Diqing Tibetan pig rations were formulated with reference to NY (2004) nutritional requirements, and the NDF levels of treatment 1 and 2 were adjusted to 20% and 40%, respectively (
Table 1). The experimental period lasted for 6 days after the pre-experimental period of 10 days. Water and rations were provided ad libitum for piglets.
The external indicator method (Cr2O3) was used to evaluate the total tract apparent digestibility of nutrients in Diqing Tibetan pigs. Pigs were kept in a pig sty (10 pigs in each pig sty) and fed three times in the morning, noon, and evening every day. Due to the high level of NDF in our experimental diet, Diqing Tibetan pigs usually defecated quickly, within one hour after feeding. We assigned special personnel to be responsible for fecal collection in each pig sty to avoid microbial loss caused by prolonged exposure to the air.
The total quantity of feces excreted by each pig was collected at 7 a.m. every morning. Feces were weighed and mixed. Representative samples were sampled and stored at −20 °C. For crude protein analysis, feces excreted were preserved in 1:3 diluted sulfuric acid. Subsamples of feeds and feces were dried in a 65 °C oven until constant weight, then let the samples regain moisture for 72 hours under natural conditions. After that, the samples were finely ground by mortar and pestle to pass through a 1 mm screen and then stored in sealed containers for the analysis of digestibility.
2.3. Chemical Analysis
According to AOAC international methods, crude protein (CP), dry matter (DM), ether extract (EE), ash, calcium (Ca), phosphorus (P), acid detergent fiber (ADF), and neutral detergent fiber (NDF) were determined using a fiber analyzer (Ankom-220, Ankom Products, New York, NY, USA). The chromium content was determined by wet digestion (nitric acid + perchloric acid, GB/T 13088-2006) using an atomic absorption spectrometer (Hitachi-Z-5000, Hitachi Group, Tokyo, Japan).
2.4. Analysis of Non-Starch Polysaccharides and SCFAs
Total non-starch polysaccharide (NSP), insoluble non-starch polysaccharide (INSP), soluble non-starch polysaccharide (SNSP), and their constituent sugars were determined by gas-liquid chromatography (GLC) for neutral sugars. Colorimetry was used to determine glucuronic acid (Bach, 1997). The GLC of constituent sugars was performed on an Agilent GC 6890 with a flow of 20 mL/min and split 40:1. A 30 m × 0.25 mm × 0.25 μm column (Agilent DB-225, film thickness 0.25 μm) was used. The temperature of column and detector were 220 °C and 250 °C, respectively.
The concentration of SCFA and lactate in the feces were analyzed using the method described in a previous report [
23] and with slight modifications. About 0.5 g of fecal sample was put into a 10 mL centrifuge tube. Then, 8.0 mL of ultrapure water was added, mixed, and centrifuged at 4000 rpm to obtain the supernate where 160 μL supernatant was placed into a 10 mL centrifuge tube and fixed to 8 mL. The supernate was filtered using a 0.20 mm nylon membrane filter (Millipore, Bedford, OH, USA) and poured into a liquid chromatography system (Agilent Technologies 1200, Agilent Technologies, Santa Clara, CA, USA).
2.5. Preparation of Fermentation
In order to avoid the short residence of digesta in the digestive tract caused by high fiber content and eliminate the influence of the foregut on the fiber digestibility, the in vitro fermentation tests of fecal microorganisms were carried out. Then, the role of microorganisms in fiber degradation was further verified by comparing the changes of NDF content in substrates before and after fermentation.
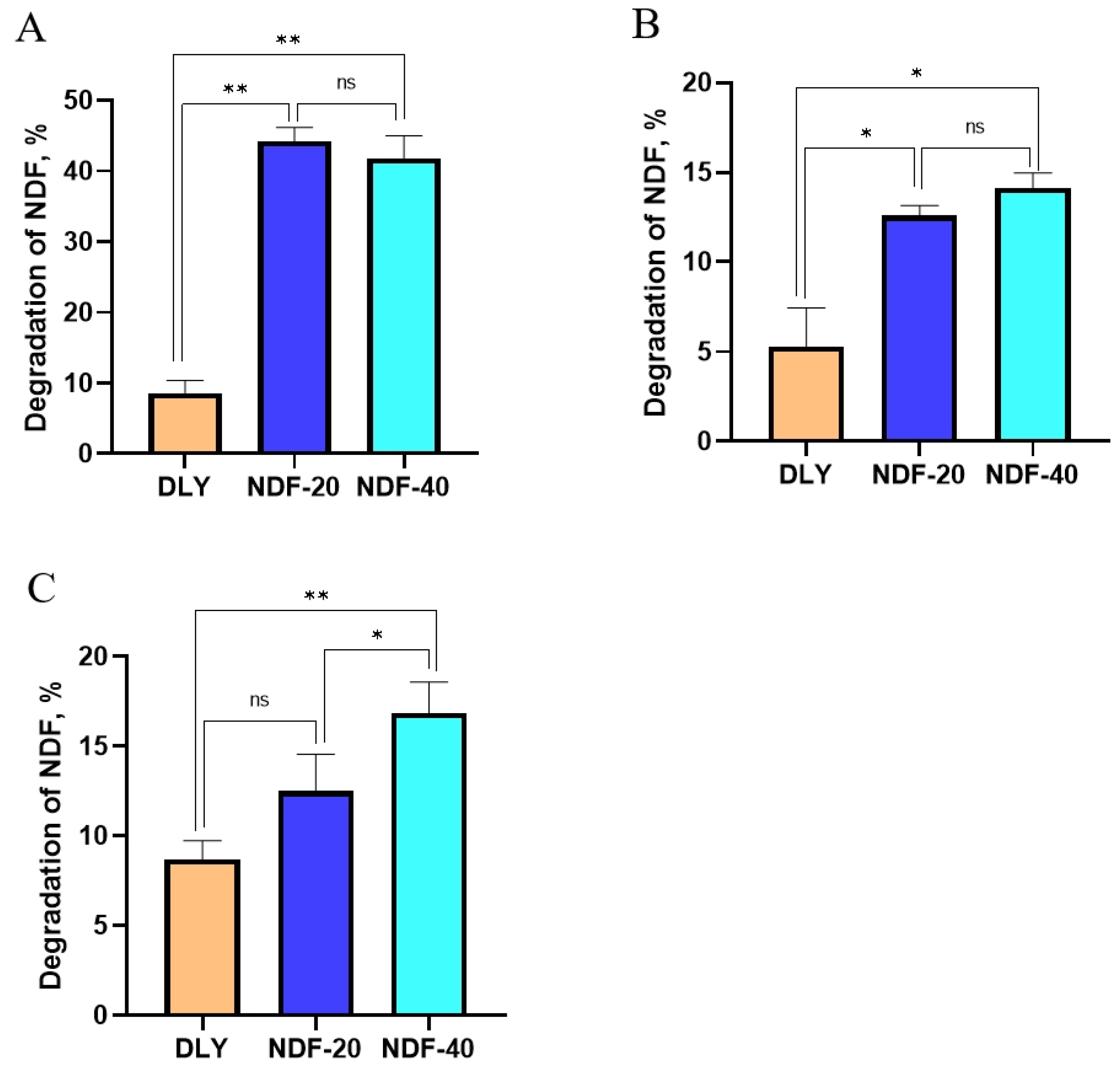
Exp.1. Feces from Diqing Tibetan pigs (the content of NDF in the feed are 20% and 40%, respectively) and Duroc × Landrace × Yorkshire pigs (DLY, BW = 55 kg, feed with commercial complete formula feed) were selected as starter culture. The total quantity of feces excreted by each pig was collected at 7 a.m. every morning. The samples needed to be quickly mixed under sterile conditions and stored in liquid nitrogen. In each treatment, the fecal samples of every 3 pigs were mixed into one for fermentation experiments. After treatment, fecal samples were mixed with PBS and glycerol and sealed in sterile sampling bags to form a sample system. Then, we filtered the system under anaerobic conditions to obtain bacterial liquid, which was used as the starter culture of fermentation test. The feed of Diqing Tibetan pigs and DLY pig were used as fermentation substrates and fermented anaerobically at 37 °C for 5 d. The system of sampling and fermentation are shown in
Table 2 and
Table 3.
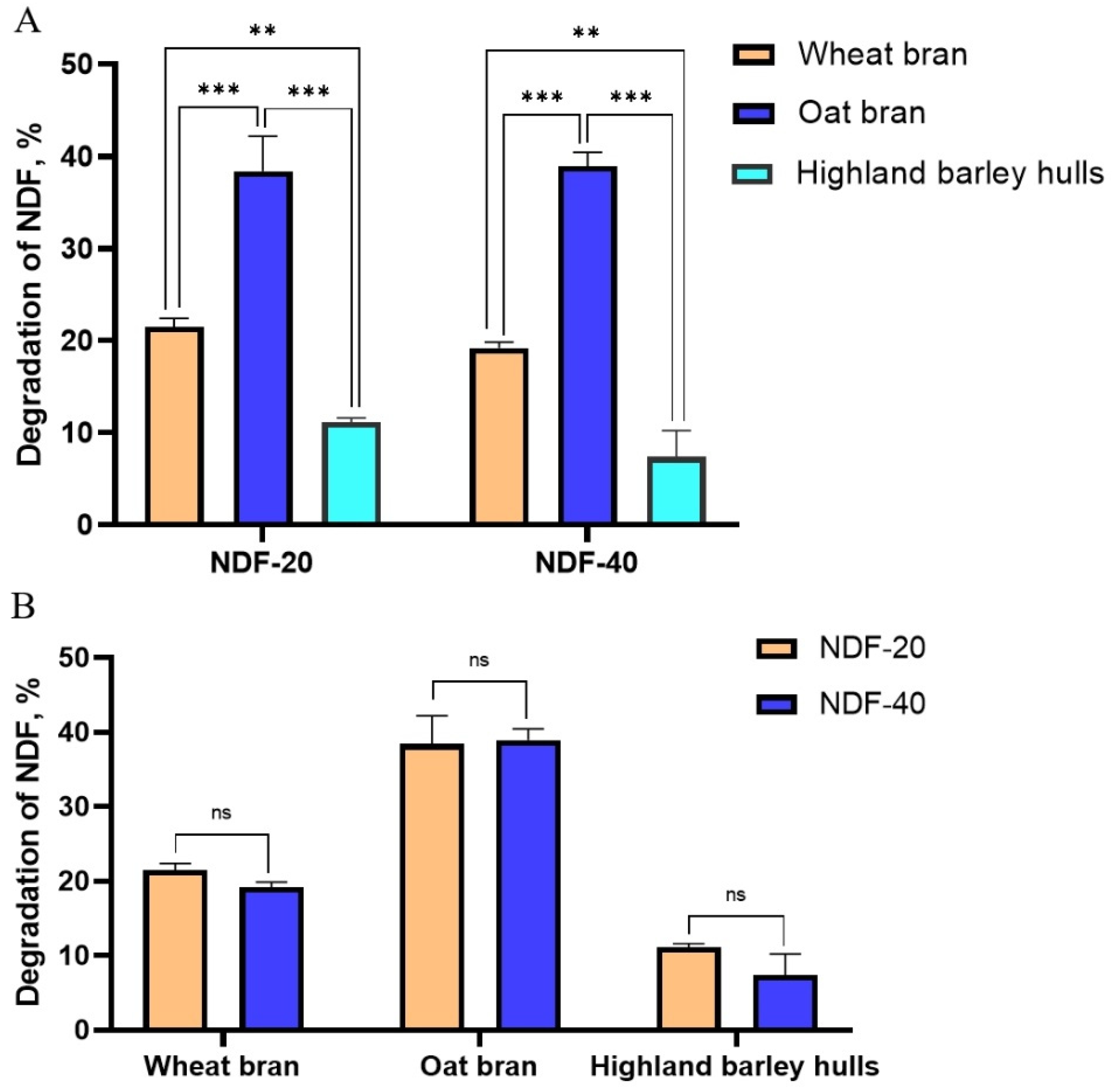
Exp.2. Feces from Diqing Tibetan pigs (the content of NDF in the feed are 20% and 40%, respectively) were selected as starter culture. Wheat bran, oat bran, and highland barley hulls were used as fermentation substrates and fermented anaerobically at 37 °C for 5 d.
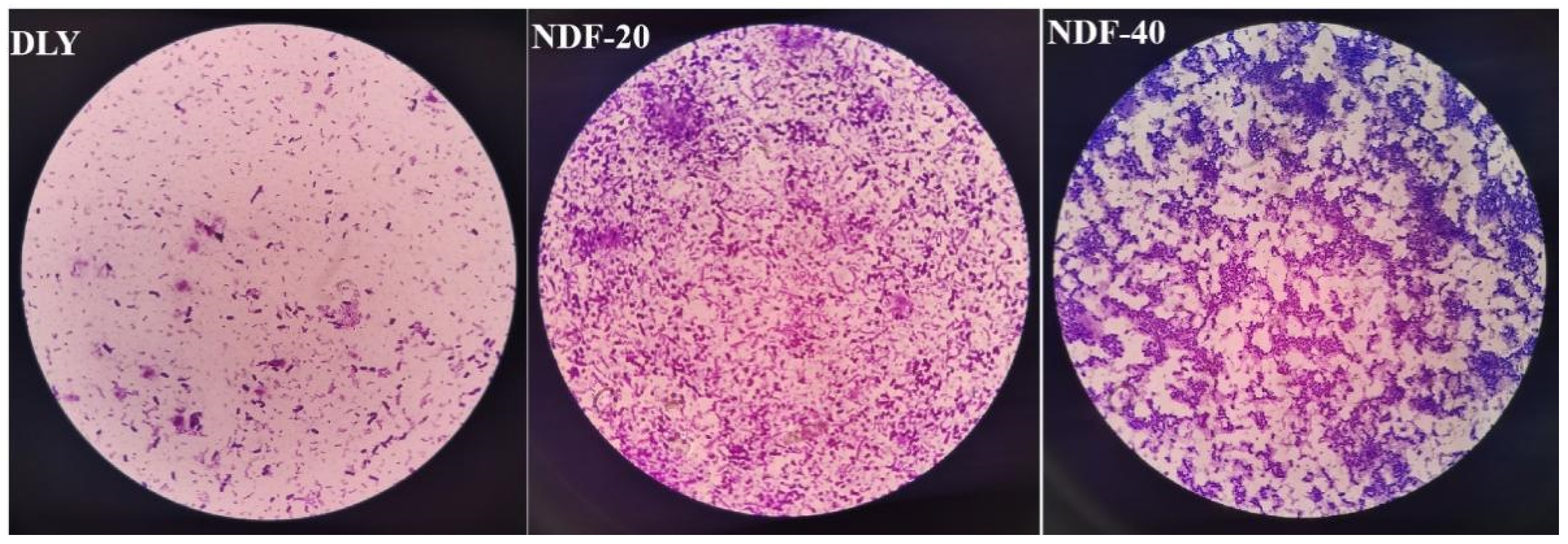
2.6. Detection of Bacteria by Crystal Violet Staining
Firstly, 10 uL bacterial liquid was dropped into the center of the clean slide and applied into a bacterial film with a sterile inoculation ring. Then, the side with bacterial film was placed upward and the water evaporated dry with a low fire. After the slide was cooled, an appropriate amount of ammonium oxalate crystal violet dye was added and incubated for 1 min. The dye solution was poured out and the slide washed with distilled water until there was no color of the dye solution in the flowing water. Finally, the excess water was removed with absorbent paper, observed, and recorded under the microscope. Sections of bacterial strains were observed at a magnification of 400× (Nikon ECLIPSE 80i, Tokyo, Japan). Bacterial strains were counted and classified as cocci, brevibacterium, and longbacterium.
2.7. Statistical Analysis
To determine differences among treatments, the data of digestive trial and fermentation trial were analyzed using t-tests procedures and one-way ANOVA procedures of GraphPad Prism 8.0.2, respectively. The data were initially analyzed using a completely randomized design. All statements of significance were based on the probability of p < 0.05.
5. Conclusions
Our results strongly suggested that Diqing Tibetan pigs have the potential to efficiently utilize fiber, and their unique intestinal microbial composition is the main reason for their efficient utilization of dietary fiber. The results of the study provide a theoretical basis for an in-depth understanding of the biological characteristics of Diqing Tibetan pigs and fully exploit the resource advantages of its intestinal microorganisms. Furthermore, our results are of great significance to improve the utilization efficiency of fiber feed, save renewable resources, and alleviate the situation of human-animal food competition. However, the composition of key gut microorganisms for efficient fiber utilization in Diqing Tibetan pigs and the contribution and mechanism of these microorganisms in the degradation of fiber fraction need further study.