Abstract
In this study, four different kinds of table olive fermentations belonging to Olea europaea L. Itrana cultivar were evaluated: A, spontaneous fermentation; B, fermentation with a single inoculum (Lactiplantibacillus plantarum B1); C, fermentation with multiple inoculum (L. plantarum B1 + L. plantarum B51 + L. plantarum B124, 1:1:1); and D, fermentation with mixed (bacterium + yeast) inoculum (L. plantarum B1 + Candida boidinii). This research focuses on the correlation between the different mixes of inoculations and their effect under the chemical, sensorial, and textural profiles in the final products (olives) for potential applications on table olive fermentation. During the fermentation, some specific parameters were monitored: chemical characterization of oil fraction (pigments, tocopherols, fatty acids, alkyl esters, and sterol composition), Texture Profile Analysis (TPA), determination of olive color, and sensory evaluation of the final products. The use of LAB starters (single and multiple inocula) compared to spontaneous process revealed a greater performance in preventing the spoilage process and in developing favorable physico-chemical conditions during the fermentation. In fact, the highest values of fatty acid alkyl esters were reached in spontaneous fermentation (~480 mg/kg in jar A). The presence of C. boidinii as inoculum in jar D was involved in table olive softening: the fermented olives showed the lowest values of the parameters related to consistence of fruit as hardness (~2300 g) and gumminess (~990 g) and high value of fatty acid methyl esters (~110 mg/kg).
1. Introduction
Fermentation is an ancient method always used to preserve food. A food can be considered fermented when its components are used by microorganisms to produce a biologically processed final product, acceptable for human consumption. Natural fermentations are triggered with no addition of any starter microorganisms and their control is limited to maintaining the external environmental conditions [1]. The whole fermentation process and the sensory and rheological characteristics of the final product are influenced by the composition of the microflora [2]. Table olives are the most widespread fermented plant food product of the Mediterranean area and have a high nutritional value due to the content in fibers, amino acids, unsaturated fatty acids, vitamins, and antioxidant compounds, so they can be considered an important functional food. The microbial pool involved in the fermentation of table olives mainly includes lactic acid bacteria (LAB) and yeasts [3]. Metabolic activity of these microorganisms causes changes in food: they help to preserve the foodstuff, to extend its shelf-life, and to influence the sensory profile of the final product [3,4]. The fermentation process can also be guided by starter cultures. These selected microorganisms can reduce the effects of spoilage, inhibiting the growth of unwanted microorganisms or pathogens, reducing the debittering times, and improving the quality of the final product [5]. The selected starter cultures are generally represented by a single strain (single starter) or by a mixture of strains (mixed starters). Among the species of lactic acid bacteria (LAB), the most used as starters are Lactiplantibacillus plantarum and Lactiplantibacillus pentosus, either as single or mixed starters with other bacteria or yeasts [3,6]. The main activity carried out by the LAB in the fermentation of table olives is to produce lactic acid (acidifying the fermentation brine), thus pH is lowered and consequently a microbiological stability is obtained in the final product. Properly selected yeasts contribute to the determination of aroma and taste thanks to the production of desired metabolites and volatile compounds, while at the same time, they promote the growth of LAB and the degradation of phenolic compounds [7]. Among the species of yeasts, the most used as starters are Wickerhamomyces anomalus and Candida boidinii [8]. The role played by yeasts in olive fermentation is twofold: they can be either beneficial or unfavorable causing potential microorganism deterioration. Since low pH and high salt concentrations are usually obtained in the final products, they can cause the deterioration of the fruits leading to the production of bad smells and tastes; they can also determine the accumulation of CO2 resulting in a swelling of the containers, a darkening of the brines, or a softening of the fruit, which is particularly harmful in the packaging or in the storage of olives.
In this study, four different fermentations of table olives (Olea europaea L. Itrana cultivar) were evaluated: A, spontaneous fermentation; B, fermentation with a single inoculum (Lactiplantibacillus plantarum B1); C, fermentation with multiple inoculum (L. plantarum B1 + L. plantarum B51 + L. plantarum B124, 1:1:1); and D, fermentation with mixed (bacterium + yeast) inoculum (L. plantarum B1 + Candida boidinii). This research focuses on the correlation between the different mixes of inoculations and their effect under the chemical, sensorial, and textural profiles in the final products (olives) for possible applications on table olive fermentation. The following parameters were monitored during the fermentation: chemical characterization of oil fraction (pigments, tocopherols, fatty acids, alkyl esters, and sterol composition), Texture Profile Analysis (TPA), determination of olive color, and sensory evaluation of the final products. This work follows previous research [9] involving some parameters (pH, titratable acidity, salinity, and biophenol compounds) that were monitored during the fermentation in the same jars.
2. Materials and Methods
2.1. Fermentation
Fruits of Olea europaea L. Itrana cv. were hand-harvested at their mature-green stage of ripening during December 2019 and supplied by the farm Frantoio Oleario F.lli Feudi located in Sonnino (LT), Italy. The olives were divided in four lots of 2700 g of olives (about 700 drupes) and placed in glass jars, then submerged in 8% brine, prepared with 80 g/L (w/v) of sterilized Trapani PGI sea salt, according to the Greek-style method [10]. Four different fermentations were evaluated: A, spontaneous fermentation; B, fermentation with a single inoculum (Lactiplantibacillus plantarum B1); C, fermentation with multiple inoculum (L. plantarum B1 + L. plantarum B51 + L. plantarum B124, 1:1:1); and D, fermentation with mixed (bacterium + yeast) inoculum (L. plantarum B1 + Candida boidinii). L. plantarum B1 comes from unknown olives brine, L. plantarum B51 derives from Olea europaea L. Cucco cv. olive brine, L. plantarum B124 is from Olea europaea L. Sant’Agostino cv. olive brine as described in Lanza et al. [9], while C. boidinii was isolated from Leucocarpa cv. olives brine as reported in Lanza et al. [11]. After 72 h of immersion of the olives in brine, each starter culture mixture (inoculum level: 6 log/mL) was distributed in glass jars and then incubated at 20–25 °C. In order to obtain the different inocula to be used as starter cultures in jar B and C, bacterial strains were revitalized in MRS broth (De Man, Rogosa, and Sharpe medium; Thermo Scientific™ Oxoid™, Waltham, MA, USA) at 30 °C for 24 h and inoculated as described in Lanza et al. [9]. Then, 2.5 mL of strain L. plantarum B1 and 2.5 mL of C. boidinii were respectively reactivated in 50 mL of MRS broth and 50 mL of MEB (Malt Extract broth medium; Thermo Scientific™ Oxoid™, Waltham, MA, USA) and finally inoculated in jar D following the procedure described for jar B and C [9]. The olives’ fermentation process was considered complete in 8 months.
2.2. Characterization of Fresh Fruits and Final Products
2.2.1. Jaen Maturity Index
The ripening level of the olive samples was assessed by a maturity index which is derived from the average color of the olives at that time; this method is also called “the Jaen Maturity Index” (JMI) and it is currently the most common method to evaluate the ripening level [12]. A sample of 100 olives was taken and divided into the following categories:
Category 0: skin color deep green; Category 1: skin color yellow-green; Category 2: skin color green with reddish spots on <half the fruit surface. Start of color change; Category 3: skin color with >half the fruit surface turning reddish or purple. End of color change; Category 4: skin color black with white flesh; Category 5: skin color black with <half the flesh turning purple; Category 6: skin color black with not all the flesh purple to the stone; and Category 7: skin color black with all the flesh purple to the stone.
The maturity index was obtained by applying the following formula where A, B, C, D, E, F, G, and H are the number of fruits in each of the color categories 0, 1, 2, 3, 4, 5, 6, and 7 respectively:
JMI = [(A*0) + (B*1) + (C*2) + (D*3) + (E*4) + (F*5) + (G*6) + (H*7)]/100
2.2.2. Pulp Weight and Pulp to Stone Ratio (P/S)
A representative sample of 20 olives was weighed and stoned. Each stone was well cleaned and weighed, and then the weight of pulp was obtained by subtracting the stone weight from the whole fruit. Finally, the relationships between them (pulp to stone ratio (P/S), percentage of pulp, weight of a single olive) were calculated.
2.2.3. Moisture, Ash, and Oil Content
Moisture was determined by a drying process. The test protocol consists of placing precisely weighed (precision ± 0.01 g) olive pulp samples in an oven at a temperature of 105 °C for 24 h, calculating the weights obtained before and after the drying process. Ash content was determined by submitting precisely weighed (precision ±0.001 g) olive pulp samples in a preheated and thermostatically controlled muffle at 550 °C oven, until constant weight. Oil content was determined by extracting the dried samples re-weighted (precision ±0.01 g) with 40–70 °C petroleum ether, for 6 h in a Soxhlet apparatus.
2.2.4. Determination of Water Activity (aw)
Water activity was determined by AquaLab Pre Water Activity Meter (Decagon). An initial calibration with 2 standard solutions (aw 0.760 and aw 0.920) was carried out in duplicate. The analysis was performed taking three different olives from each jar, destoning, and then cutting them into small pieces (about 3 mm). These chopped pieces were put in a plastic flat capsule arranged in such a way to completely fill the surface of the sample support, so to be inserted into the equipment’s analytical chamber. When the loading slot was closed, the measurement started automatically. The assay was repeated twice on the same sample from every jar to gather repeated acquisitions in duplicate. The analysis was carried out at room temperature always recorded by the instrument because of the sensitivity of the aw parameter to temperature changes.
2.2.5. Chemical Characterization of Oil Fraction
Oil Extraction
A total of 30 olive fruits were manually de-pitted and triturated with a grinder. The olive paste was warmed up in a water bath at 28 ± 2 °C for 30 min and then centrifuged at 3500 rpm for 30 min in a refrigerated centrifuge (ALC PK 120R; Thermo Electron Corporation, Waltham, MA, USA) at 10 °C. The resulting supernatant oil was collected with a Pasteur pipette and filtered in the presence of anhydrous sodium sulphate, and then stored in 50 mL plastic tubes (Falcon) wrapped with aluminum foil, which was kept at 4 °C until analysis. This procedure simulates the extraction of olive oil in olive mills (i.e., crushing, mixing, and centrifugation) and it was used with the aim of preventing changes in the oil quality as far as possible.
Pigments
The chlorophyll content of the olive oil was determined by measuring the absorbance at 630, 670, and 710 nm by using a Lambda 2 UV/VIS spectrophotometer (Perkin Elmer, Waltham, MA, USA), according to the AOCS Cc 13c-50 method [13]. The chlorophyll content was given as milligram per kilogram oil, calculated according to the following formula:
where L is the thickness of the cuvette (cm).
Chlorophyll (mg kg−1) = [A670 − (A630 + A710) × 0.5]/0.1086L
Tocopherols
For the determination of the tocopherols, a solution of 1 g olive oil in 10 mL acetone was used directly for HPLC. α-Tocopherol (0.32 μg/10 μL) was used as the external standard. The HPLC analysis was conducted using a high-resolution LC 200 liquid chromatograph, equipped with a Series 200 UV/Vis detector (Perkin Elmer, Waltham, MA, USA), a 7725 Rheodyne injector, a 20 μL sample loop, and a TotalChrom workstation for data acquisition (Perkin Elmer, Waltham, MA, USA). Separation on a Spherisorb ODS2 column (250 × 4.6 mm I.D., 5 μm; Waters, Milford, MA, USA) was performed at 25 °C at a constant flow rate of 1 mL min−1 with the mobile phase of 0.2% H3PO4 in water (v/v)/methanol/acetonitrile (2/49/49; v/v/v). The eluted compounds were detected at 292 nm [14].
Fatty-Acid Composition
The fatty-acid composition of the oil was determined according to the method described in European Union Commission Regulation EEC/2568/91 and its subsequent modifications (Annex X.B) [15]. The procedure employs a gas chromatography system (HRGC Mega 2 series 8560; Carlo Erba, Milan, Italy) equipped with an SPTM-2380 (Supelco, Bellefonte, PA, USA) fused silica capillary column (60 m × 0.32 mm ID × 0.2 μm film thickness). The oven temperature program was from 70 °C to 165 °C at 20 °C min−1 and held at 165 °C for 23 min; then from 165 °C to 200 °C at 1.5 °C min−1 and held at 200 °C for 5 min; and then from 200 °C to 220 °C at 2 °C min−1 and held at 220 °C for 5 min. The detector temperature was 230 °C. Hydrogen was used as the carrier gas at a column head pressure of 60 kPa. The samples (0.4 μL) were applied by on-column injection.
Alkyl Esters
Fatty acid methyl esters (FAMEs) and fatty acid ethyl esters (FAEEs) were determined according to the method described in the European Union Commission Regulation EEC/2568/91 and its subsequent modifications (Annex XX) [15]. This procedure involves the use of a Thermo Scientific Trace GC ultra multi-channel gas chromatograph equipped with a low polarity fused silica capillary column (15 m length × 0.32 mm ID × 0.10 μm film thickness). The oven temperature program was 80 °C for 1 min, then from 80° to 140 °C at 20 °C min−1, then from 140 °C to 195 at 5.0 °C min−1, then from 195 to 340 °C at 30.0 °C min−1, then held at 340 °C for 12 min. The detector temperature was 350 °C. Hydrogen was used as the carrier gas at a column head pressure of 70 kPa. The samples (0.6 μL) were applied by on-column injection. The analysis was carried out in duplicate for each sample.
Sterol Composition
The sterol profile and contents were determined according to the European Union Commission Regulation EEC/2568/91 and its subsequent modifications (Annex V) [15]. The olive oil, with added α-cholestanol as internal standards, was saponified with 2 N potassium hydroxide in ethanolic solution, then the unsaponifiable was extracted with ethyl ether. The sterol fraction was separated from the extract by thin-layer chromatography on a basic gel plate, then recovered from the plate and transformed into trimethylsilyl ethers and analyzed by an HRGC 5160 Mega series (Perkin Elmer) equipped with a Zebron Phenomenex ZB-5 capillary column (30 m × 0.32 mm ID × 0.25 µm film thickness). The gas chromatographic conditions were column temperature 265 °C; hydrogen was used as the carrier gas at a column head pressure of 50 kPa; split ratio 1:50 and substance amount injected into the split system 1 µL; injector and detector temperatures were 280 and 290 °C, respectively.
2.3. Texture Profile Analysis (TPA)
TPA is a method that allows to quantify multiple textural parameters in just one experiment. TPA was performed by using the TA.XTPlus texture analyzer (Stable Micro Systems Ltd., Godalming, UK) and data acquisition and integration by using Exponent Connect version 7.0.7.0 software from the same manufacturer. Each sample was composed by a batch of 10 olives from each jar, chosen taking care to avoid physical deformities and/or mechanical damages, also checking uniformity in size. Each batch sample was taken directly from the jar and placed horizontally, centered under the probe before measurement. A calibration of the instrument was performed before analyzing each batch. The fruit was then compressed twice by means of a cylindrical flat probe (P/25, diameter 25 mm). The trigger force was 5 g. The test speed was set at 1.0 mm s−1 with an acquisition rate of 250 data points per second. All analyses were done at room temperature. TPA parameters measured for our samples were hardness, fracturability, adhesiveness, springiness, cohesiveness, gumminess, and chewiness.
A representative TPA plot is shown in Figure 1. Hardness (h) is the force required to achieve a given deformation or penetration of the product. It is the maximum peak force that occurs during the first compression (expressed as Force in grams). Fracturability (f) is represented by the first significant peak where the force decreases during the first compression of the product (expressed as Force in grams). Adhesiveness is the force required to remove material that adheres to the mouth. It is the work required to overcome the sticky forces between the sample and the probe (expressed as Area 3 in mm2).
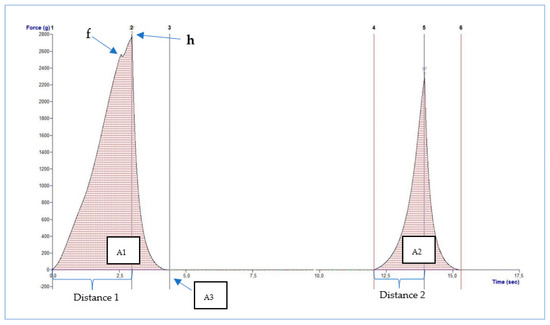
Figure 1.
Representative plot of TPA of Itrana cv. olive. A1, Area 1; A2, Area 2; A3, Area 3; f, fracturability; h, hardness.
Springiness is the rate at which a deformed sample returns to its original size and shape. It is measured by the distance of the detected height during the second compression divided by the original compression distance (expressed as Distance 2/Distance 1 in mm). Cohesiveness is the degree to which a product can be deformed before it breaks. It is measured as the area of work during the second compression divided by the area of work during the first compression (expressed as Area 2/Area 1 in mm2). Gumminess is the energy required to disintegrate the product to the state ready for swallowing (expressed as hardness × cohesiveness). Chewiness is the number of chews needed to masticate the product until it is ready for swallowing (expressed as gumminess × springiness).
2.4. Determination of Color
The epicarp color of the fruits was measured using a color-view spectrophotometer (Konica Minolta Optics, 2970 Ishikawa-machi, Hachioji, Tokyo, Japan; Model CM-2600D). The analysis of color was made on 10 uniformly sized olive fruits. Color was expressed in terms of CIE (Commission Internationale de l’Eclairage) L* (lightness), a* (redness/greenness), b* (yellowness/blueness), and their derivative Chroma ().
2.5. Sensory Evaluation
The sensory characteristics of table olives were evaluated by professional tasters of the CREA-IT Panel, as expected from the COI/OT/MO No 1/Rev.2 [16]. The profile sheet used was the one required by IOC with a few additions to better assess kinesthetic properties. The evaluated attributes were negative sensations (abnormal fermentation, cooking effect, rancid, musty, or other defects), gustatory sensations (salty, bitter, acid), and kinesthetic sensations (flesh hardness, skin hardness, skin persistence, fibrousness, detachment of the flesh from the pit, and crunchiness). It can be noticed that the “hardness” already reported in the original IOC sheet has been split into “skin hardness” and “flesh hardness”. The first was assessed by initially placing half an olive between the incisors to detect resistance in cutting and penetration; the second was then detected by chewing with the molar teeth, perceiving the resistance to deformation. Samples were submitted to the panel composed of 10 tasters, inside the appropriate glasses as described by the official table olive tasting method. The table olive profile sheet uses a 10 cm intensity scale ranging from 1 (no perception) to 11 (extreme). The results obtained were statistically processed using the specific software provided by the IOC Method COI/OT/MO/Doc.1/Rev.2 Annex 3: Sensory analysis of table olives-computer program [16], so to obtain the necessary values for the definition of sensory profiles.
2.6. Statistical Analyses
All data significance was evaluated by one-way ANOVA using the F-test (p ≤ 0.05) and processed in the Past PAleontological STatistics software (Version 2.12, Øyvind Hammer, Natural History Museum, University of Oslo, Oslo, Norway).
3. Results
Figure 2 shows a portion of the olives used for the experiments (Itrana cv.). The first assay to be done on the fresh product was the determination of the Jaen Maturity Index. According to paragraph 2.2.1, the JMI calculated (1.04) confirms that the assessed sample is between “Category 1” (Skin color yellow-green) and “Category 2” (skin color green with reddish spots on <half the fruit surface. Start of color change).

Figure 2.
Aspect and maturation degree of fresh fruits of Itrana cv.
Due to the nature of the olives cultivar and to the different veraison degrees, fresh olives were further divided in three different groups: green, changing color, and black, and to better characterize the relationship between color and ripening, a color assessment was subsequently performed (Table 1). Once assessed, the sample was again gathered into one before proceeding in further chemical analysis.

Table 1.
Color assessment of fresh fruits. The data are expressed as mean of two replicates and standard deviation between parentheses.
Figure 3 shows the aspect of final products (jars A, B, C and D). Since the color attribute of food products is another crucial factor in the acceptance of a food, it was also analyzed on processed products: all the results are displayed in Table 2. In general, significant differences were observed among all the color parameters at the end of fermentation process. Jar D, inoculated with L. plantarum B51 and C. boidinii, was significantly different from the other jars in the lightness parameter (L*). The lightness parameter is mainly variety-dependent. The jars inoculated with different bacteria (B, C) had similar results to the spontaneous jar (A). The chromaticity coordinate a* value (redness) of the jar B was significantly different from the others, while similar values between jar A, C, and D were found. Last parameters such as b* and CHROMA, or yellowness and chromaticity, respectively, showed the same difference: spontaneous fermentation into jar A was significantly different from the others, as indicated by different letters. In conclusion, the evolution of the color was different under non-inoculated and inoculated conditions.

Figure 3.
Aspect of final products.

Table 2.
Color characterization of final products. The data are expressed as mean of two replicates followed by ANOVA test. S, significant. Significant differences are indicated by different letters (p < 0.05).
In Table 3, the chemico-physical characteristics of Itrana fresh olives and final products are reported. The size of the fresh fruit is medium (3.71 g). Both pulp percentage (>83%) and pulp/stone ratio (4.94) are good and compliant with the characteristic features of Itrana cultivar. Moisture is 67.28% while the amount of chemically no-bound water measured as water activity (aw) has an average of 0.977. The values obtained by these preliminary analyses certify the optimal conditions of the olives sample, making them ready to be transformed into table olives. However, the Itrana cv. is already well-known to be a dual-purpose variety (both for olive oil and table olives production) and the oil content in the pulp is high (22.17%). At the end of fermentation, all chemico-physical values of final product in each jar were lower than fresh fruit, except for the ash (1.63 g/100 g of pulp in fresh fruit). A weight reduction by 20–23% could be observed at the end of fermentation process, such as all the other parameters that showed a decline as expected, due to the loss of both bound and not-bound water. It is easily explained by the hypertonic external brine in which olives were submerged for months, that caused the leakage of water from the olives’ pulp cells. Jar D showed lower value of moisture and AW than other jars, while all the other chemico-physical values were higher than other jars. ANOVA statistical analysis highlights significant differences among the jars regarding all the chemico-physical parameters that were tested.

Table 3.
Chemico-physical characteristics of fresh and final products. The data are expressed as mean of two replicates followed by ANOVA test. S, significant. Significant differences are indicated by different letters (p < 0.05).
Fats can be classified as saturated fatty acids (SFA), monounsaturated fatty acids (MUFA), and polyunsaturated fatty acids (PUFA), corresponding to the different chemical fractions of fatty acids. Many authors highlight the importance of PUFAs for human nutrition, enough to be considered essential fatty acids because their intake can be supplied just from the diet. Moreover, MUFAs are also deemed significant fatty acids for humans since they can contribute to decreasing the concentration of Low-Density Lipoprotein (LDL) cholesterol in the blood and at the same time, they can help in maintaining or raising the concentration of High-Density Lipoprotein (HDL) cholesterol [17]. Among the fatty acids present in olive oils, MUFAs and particularly oleic acid, are responsible for the beneficial effects on human health [17]. The nutritional value of table olives depends mostly on a balanced profile of polyunsaturated and monounsaturated fatty acids.
In the present study, as shown in Table 4, a large amount of MUFAs was found. Moreover, the fatty acid profile of fermented table olives revealed significant qualitative and quantitative differences among the assayed jars, except for stearic acid (C 18:0), SFA, linoleic/palmitic, and PUFA/SFA. As expected, oleic acid (C18:1) was the most abundant fatty acid among all the jars, ranging from 74.90% in jar D to 75.51% in jar B of total fatty acids, followed by palmitic and linoleic acids, ranging from 12.87% in jar B to 13.13% in jar A and from 6.72% in jar A to 7.25% in jar D, respectively. PUFA/SFA is an important ratio to evaluate the nutritional quality of the lipid fraction of foods [18]. The World Health Organization (WHO) has recommended a PUFA/SFA ratio > 0.4–0.5 as an optimal value in a balanced diet. In the present study, the ratio PUFA/SFA ranged from 0.48 in jar A to 0.52 in jar D, even if they were not significantly different.

Table 4.
Fatty acid composition of fresh and final products. The data are expressed as mean of two replicates followed by ANOVA test. S, significant. Significant differences are indicated by different letters (p < 0.05). NS, not significant.
Table 5 shows the fatty acid alkyl esters profile of fresh olives and final products. In fresh samples, FAEEs and FAMEs were very low (16.37 and 8.74 mg/kg, respectively) and did not surpass the limits for extra virgin olive oil (FAEEs ≤ 30 mg/kg or FAMEs + FAEEs ≤ 75 mg/kg) [15]. As regards fatty acid ethyl esters (FAEEs) derived from the esterification of fatty acids and ethanol, there was a significant increase during the fermentation process in all the jars up to 370 mg/kg in jar A at the eighth month of fermentation. In jars B and C, the levels of FAEEs reached ~230–250 mg/kg at the end of fermentation, while they remained lower in jar D (116.89 mg/kg). In jar D (inoculated with L. plantarum B51 + C. boidinii), there was the lowest level of FAEE-oleate and FAEE-linoleate after 8 months of fermentation between the inoculate ones (94.09 mg/kg). The over-production of FAEEs is generally considered the most reliable indicator of abnormal fermentation processes. In previous work, Lanza et al. [19] found very high level of FAEEs (>5100 mg/kg) in defected olives spontaneously fermented. Fatty acid methyl esters (FAMEs) also increased but they achieved lower quantities at the end of fermentation. Methyl alcohol is formed when the olives were overripe and enzymes such as lipases hydrolyzed the pectins. The highest values were reached in jar A and jar D (~110 mg/kg) but they did not reach the values found in Lanza et al. [19] in defected olives spontaneously fermented (>550 mg/kg).

Table 5.
Fatty acid alkyl esters of fresh and final products. The data are expressed as mean of two replicates followed by ANOVA test. S, significant. Significant differences are indicated by different letters (p < 0.05).
The sterol composition of the Itrana cultivar was analyzed in all jars (Table 6) and significant differences were found between fresh olives, spontaneous fermentation (jar A), and inoculated jars (jar B, C, D).

Table 6.
Total sterol content and composition of fresh and final products. The data are expressed as mean of two replicates followed by ANOVA test. S, significant. Significant differences are indicated by different letters (p < 0.05).
The main sterols were β-Sitosterol with the values between 92.02% (jar A) and 92.82% (jar B); Campesterol in quantities between 2.48% (fresh olives) and 2.93% (jar B) and Δ-5-Avenasterol between 1.78% (jar B) and 2.18 (jar D). The β-Sitosterol apparent (including Δ-5,23-Stigmastadienol, Clerosterol, β-Sitosterol, Sitostanol, Δ-5-Avenasterol, Δ-5,24-Sigmastadienol) was ≥ 93%. Total sterol content, in all the products, was higher than 1000 mg/kg. Both these parameters exceed the minimum limits fixed by European Union Commission Regulation EEC/2568/91 and its subsequent modifications [15] for extra virgin olive oil (EVOO). An oil rich in phytosterols helps to reduce LDL-cholesterol, and thus, foods with a high content of these compounds are considered as functional foods [20].
Figure 4 shows the content of tocopherols and chlorophylls in the oil extracted both from the fresh Itrana cultivar olives and from the processed olives. Tocopherols are important in daily nutrition and disease prevention and their content is mainly influenced by the variety but also by other factors [21,22]. The total tocopherols content in fresh Itrana cultivar olives was 248.7 mg/kg, while it decreased after fermentation in all jars at a rate of about 30% less: jar A 171.8, jar B 171.3, jar C 171.5, and jar D 170.8 mg/kg. The chlorophyll content of fresh olives was found to be 25.20 mg/kg. After the fermentation process, there was a degradation of the chlorophyll in all jars. However, no significant differences were found between spontaneous fermentation (jar A) with 22.60 mg/kg and fermentation with single, multiple inocula, and mixed of bacterium and yeast (jar B, jar C, and jar D) with values of 21.70, 22.97, and 22.96 mg/kg, respectively.
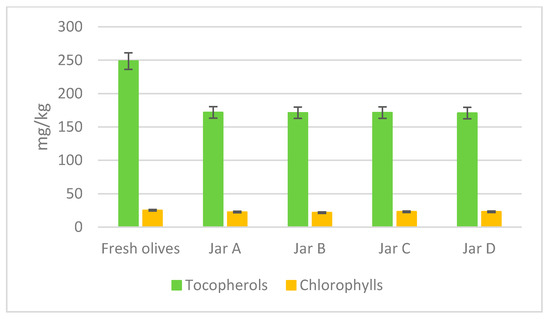
Figure 4.
Tocopherols and chlorophylls of fresh and final products.
TPA tests, carried out at the end of the fermentation, showed differences (p < 0.05) among olives from the four jars in all the textural parameters (Table 7). The olives analyzed in this study showed a significantly higher resistance to deformation, as shown by gumminess and chewiness. Texture loss is strongly influenced by the enzymatic activity of dominant microflora and, in some cases, it can lead to a softening of the fruit due to the degradation of pectic substances on the cell wall. However, significant differences were later observed, indicating that inoculation affected the texture profile of the final product. In fact, as shown in Table 7, the lowest value of the parameters relating to consistency (hardness and gumminess) belonged to jar D, inoculated with L. plantarum B51 and C. boidinii. This is due to the presence of C. boidinii enzymatic action on the fruit. As for hardness and gumminess, all other textural parameters relating to jar D were also lower than the others, except for springiness. All olives showed absence of fracturability and adhesiveness.

Table 7.
Texture Profile Analysis (TPA) of final products. The data are expressed as mean of two replicates followed by ANOVA test. S, significant. Significant differences are indicated by different letters (p < 0.05). NS, not significant.
The organoleptic profile of olives after 8 months of fermentation is presented in Figure 5. Regarding the sensory profiles of table olives, the panel did not find any anomalous fermentation or other defects in jars A, B, or C. During fermentation in jar D, molds developed on the brine surface and produced a thick layer on the top. This can be explained by the high pH value (6.2) reached in jar D at the end of fermentation compared to pH values of other jars (5.1 for jar A, 4.7 for jar B, and 4.5 for jar C) (data not shown in table). These spoilage microorganisms are responsible for product alterations such as flesh softening, development of off-odor compounds, and mycotoxin production [23]. The most representative identified mold genera are Aspergillus and Penicillium [24]. The CREA-IT Panel leader chose not to taste the final product of jar D.
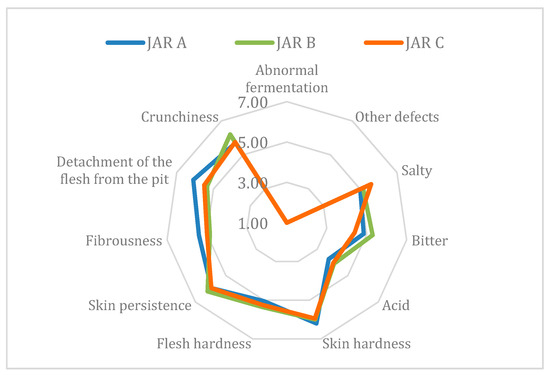
Figure 5.
Sensory profile of final products.
The samples were characterized by low residual bitterness, good texture, and satisfying acid taste. Jar A (without inoculum) and jar B (with only one L. plantarum) were more bitter and fibrous than jar C (with three strains of L. plantarum) at the end of fermentation. In jar A, the olives were less acid than those in the other two jars, probably due to the absence of Lactobacillus strains which produce an acid environment in the brine. From a kinesthetic point of view, jar B and jar C seem to be very close to each other, except for the crunchiness parameter: the olives in jar B were the crunchiest (6.20 median value).
4. Discussion
The use of microbial starters as yeasts or LAB spp. has replaced the “wild” natural fermentation in order to enhance fermentation performances, speeding up the acidification of brines, preventing the spoilage of bacteria, or giving probiotic characteristics to the product.
L. plantarum B1 was chosen to inoculate jar B as a single starter because it has a β-glucosidase gene [25], a marked oleuropeinolytic activity, and grows well at high concentrations of NaCl. Strain B1 is also sensitive to different phages found in fermentation brines, so we thought of inserting it in a multiple starter, together with two other strains of L. plantarum (B51 and B124) already tested for different pro-technological activities in previous works [25,26]. This multiple starter was chosen to inoculate jar C. The choice of a multiple starter was suggested by the synergic activity of the selected strains in carrying out the debittering process, together with their resistance to phage attacks. L. plantarum B51 is a good producer of D/L lactate and resists to acid pH because it produces lactic acid, reduces the pH of the medium, and grows in a strongly acid environment. There was evidence of B51 (as well as B124) being resistant to various phages [26]. This strain demonstrated the ability to hydrolyze both sodium glycodeoxycholate (GDCA) and sodium taurodeoxycholate (TDCA). It proved to be resistant to kanamycin, gentamicin, and ampicillin. L. plantarum B124 has pro-technological characteristics, like B51, but it does not possess probiotic characteristics.
The fermentation process aims to stabilize table olives, reducing pH and positively changing their sensory properties. The use of starter cultures made up of LAB, yeasts, or their mix may help in preventing the spoilage problems and producing high quality products. Technologically, yeasts should resist low pH and high salt concentrations, produce desirable aromas, improve lactic acid bacteria growth, and inhibit spoilage microorganisms [3,27].
The results shown above reveal that treated mature-green olives may have an important nutritional profile, regarding the adequate FA content and the appropriate PUFA/SFA ratio. Many researchers noted that the fatty acid compositions of table olives were very complicated to determine because of their variability, and that they depend on many factors, such as olive cultivars, ripening index, agricultural practices, growing and climatic conditions, and processing methods.
According to Luckett [28], texture and color are relevant descriptors in consumer acceptance. Differences among treatments, especially between spontaneous and guided fermentation, were detected on color parameters. The same differences were also noted for most fatty acids’ compositions among all the jars, so different treatments corresponded to a different content of fatty acids. Sensory characteristics are process-dependent: a relevant contribution is given by the starters, not only in decreasing the bitterness of fruits, but also bringing new and typical flavor to table olives [29]. The inoculated LAB samples were more appreciated than non-inoculated ones, as they were found to have a less bitter taste. The assessors found that the samples obtained with the three mixed LAB were debittered (Jar C) at the end of the process so the use of these starters may be suggested to quickly reduce fermentation times. In the present study, since significant differences were found in texture and color parameters between non-inoculated and inoculated treatments, we can conclude that inoculation was also useful in final products to improve their functional properties, as well as their organoleptic characteristics, according to sensory analysis data results.
Some yeasts have strain-specific metabolic properties, such as esterase and lipolytic activities. The former has frequently been detected, while the isolation of strains with lipolytic activities has been reported to a lesser extent. Esterase positive yeasts are appreciated because they are able to improve the flavor of olives from the formation of esters coming from free fatty acids. Both strong and weak lipase activities have been detected in vitro in some yeasts’ species. The former has been reported in Candida boidinii. Those authors have described the change of free fatty acids’ composition of olives that occurred with the yeast inoculum in contrast with sterile conditions, indicating that lipases produced by these microorganisms modify the characteristics of fruit lipids and therefore its organoleptic characteristics [27,30].
Results showed that the activity of the yeast population could play a key role in the final characteristics of table olives: in fact, yeasts can significantly contribute to the improvement of table olives’ quality and safety. Moreover, they could be useful for: (a) favoring a bacterial growth able to enhance a lactic acid production that allows to preserve the product from spoilage by microorganisms; (b) acting as biocontrol agents against fungi, and other undesirable yeast and bacteria species; (c) producing compounds that positively affect both flavor and texture (i.e., free fatty acids); and (e) preventing unsaturated fatty acids oxidation and peroxides formation.
Lactobacilli are the dominant microorganisms in the fermentation of olives; they can produce compounds as organic acids and H2O2 that, characterizing the ecosystem and developing a precise environment, limit the growth of pathogens [5]. Their important role in the rheology and texture properties of fermented food products through the production of aromatic compounds and organic acids should also be emphasized [3]. Jars A, B, and C were analyzed in a previous work by Lanza et al. [9]. The data reported in that study showed that the multiple starters inoculated into jar C were the only ones able to drive the fermentation until the end of the process, probably due to the alternation of the strains during the technological process. The jars inoculated only with L. plantarum showed lower values of pH in their brine than jar A, reaching values between 4.5 and 4.7, which are necessary to prevent an excessive growth of Gram-negative bacteria; instead, a higher pH value (6.2) was found in jar D (data not shown in the article). Therefore, at the end of the process, the sour taste of both non-inoculated and inoculated treatments reflects the pH values: on sensory analysis, the olives in jar C were the most acid and their brine had the lowest pH value. The titratable acidity of the olive juice was higher in jar C after eight months of fermentation.
Furthermore, as described previously, a textural loss was evident in jar D, where the lowest values of “hardness” and “gumminess” were found. Texture loss is strongly influenced by the enzymatic activity of dominant microflora and it may cause softening due to the degradation of the pectic substances in the cell walls: softening occurred during fermentation and during storage due to higher pH values (greater than 4.8) and/or very low salt concentration [31]. The oleuropein degradation during the fermentation process is a fundamental prerequisite for olives to lose their bitterness and thus become edible. According to Lanza et al. [9], we observed a much faster degradation of secoiridoids in the inoculated jars than in the spontaneously fermented jar and sensory results obtained in the present study confirmed that the multiple inocula ensured a complete olive debittering. The presence of L. plantarum B1 and B124 as a fermentation starter guarantees an optimal trend of de-bittering and fermentation variables, thus ensuring the production of a better final product. Sensory analysis showed us that the olives in jars A and B were the most bitter according to the tasters; the highest levels of hydroxtyrosol, which is the major compound derived from oleuropein degradation and the most responsible for the bitter taste, were found in the analyzed pulp of olives in jar C. An increase in the bitterness of the fruit would be expected due to phenolic compounds, as shown in this study. Hydroxytyrosol has been associated with oleuropein degradation and with the diffusion of phenols from olive fruit to brine. In our study, multiple inoculations of three bacteria could have produced the hydrolysis of other phenols; in fact, hydroxytyrosol and tyrosol concentrations increased in brines during the fermentation. As reported in the previous work by Lanza et al. [9], at the end of fermentation, jar A had the lowest level of hydroxytyrosol and the olives were therefore more bitter when tasted because a lower degradation of oleuropein occurred. The starters allowed a more rapid debittering process and permitted an increase in hydroxytyrosol, tyrosol, and verbascoside on the olives compared to the control sample, so to improve the nutritional value.
5. Conclusions
The use of starter cultures changes the microbial dominance and leads to a faster acidification of brines and to a faster degradation of oleuropein, thus accelerating the completion of the fermentation process. Moreover, the use of starters (single and multiple inocula) reveals a greater performance in preventing the spoilage process and in the development of favorable chemico-physical conditions during the fermentation compared with spontaneous process (jar A). Yeasts are involved in milder taste defects and in olives cell walls degradation, but they can also improve the final product by the production of desirable volatile compounds and by the enhancement of LAB growth. Olives at the same stage of ripening and processed with the same technology, although they were inoculated with different starter microorganisms, show significant differences in textural and sensorial features. The presence of C. boidinii as inoculum in jar D is involved in table olive softening. The results obtained from sensorial evaluation suggest that technological procedures of debittering are involved in both gustatory and kinesthetic sensations. It is very important to note that the use of starter microorganisms allows to significantly reduce the time of the fermentation process, as demonstrated by the results obtained from jar C. Inoculation of table olives with a C. boidinii strain combined with LAB negatively affects table olives’ texture.
Author Contributions
Conceptualization, B.L., S.D.M., M.B., G.D.L., M.C. and N.S.; methodology, B.L., S.D.M., M.B., M.G.D.S., G.D.L., M.C. and N.S.; formal analysis, S.D.M., M.B., M.G.D.S., G.D.L., M.C. and N.S.; data curation, B.L., S.D.M., M.B., M.G.D.S., G.D.L., M.C. and N.S.; writing—original draft preparation, B.L., S.D.M., M.B., M.G.D.S., G.D.L., M.C. and N.S.; writing—review and editing, B.L., S.D.M., M.B., M.G.D.S., G.D.L., M.C. and N.S.; project administration, B.L.; funding acquisition, B.L. and N.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the projects INFOLIVA (D.M. 12479/2018) and DEAOLIVA (D.M. 93882/2017 and D.M. 35902/2019) funded by the Italian Ministry of Agricultural, Food, and Forestry Policies (MiPAAF).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ross, P.R.; Morgan, S.; Hill, C. Preservation and fermentation: Past, present and future. Int. J. Food Microbiol. 2002, 79, 3–16. [Google Scholar] [CrossRef]
- Sulieman, A.M. Microbial Starter Cultures; LAP Lambert Academic Publishing: Beau Bassin, Mauritius, 2017. [Google Scholar]
- Chytiri, A.; Tasioula-Margari, M.; Bleve, G.; Kontogianni, V.G.; Kallimanis, A.; Kontominas, M.G. Effect of different inocu-lation strategies of selected yeast and LAB cultures on Conservolea and Kalamata table olives considering phenol content, texture, and sensory attributes. J. Sci. Food Agric. 2020, 100, 926–935. [Google Scholar] [CrossRef]
- Bonatsou, S.; Tassou, C.C.; Panagou, E.Z.; Nychas, G.-J.E. Table Olive Fermentation Using Starter Cultures with Multifunctional Potential. Microorganisms 2017, 5, 30. [Google Scholar] [CrossRef] [PubMed]
- Perpetuini, G.; Prete, R.; Garcia-Gonzalez, N.; Alam, M.K.; Corsetti, A. Table Olives More than a Fermented Food. Foods 2020, 9, 178. [Google Scholar] [CrossRef] [PubMed]
- De Angelis, M.; Campanella, D.; Cosmai, L.; Summo, C.; Rizzello, C.G.; Caponio, F. Microbiota and metabolome of un-started and started Greek-type fermentation of Bella di Cerignola table olives. Food Microbiol. 2015, 52, 18–30. [Google Scholar] [CrossRef]
- Corsetti, A.; Perpetuini, G.; Schirone, M.; Tofalo, R.; Suzzi, G. Application of starter cultures to table olive fermentation: An overview on the experimental studies. Front. Microbiol. 2012, 3, 248. [Google Scholar] [CrossRef]
- Rodriguez-Gomez, F.; Romero-Gil, V.; Bautista-Gallego, J.; Fernández, A.G.; Arroyo-López, F.N. Multivariate analysis to discriminate yeast strains with technological applications in table olive processing. World J. Microbiol. Biotechnol. 2011, 28, 1761–1770. [Google Scholar] [CrossRef]
- Lanza, B.; Zago, M.; Di Marco, S.; Di Loreto, G.; Cellini, M.; Tidona, F.; Bonvini, B.; Bacceli, M.; Simone, N. Single and Multiple Inoculum of Lactiplantibacillus plantarum Strains in Table Olive Lab-Scale Fermentations. Fermentation 2020, 6, 126. [Google Scholar] [CrossRef]
- CODEX STAN 66-1981 (rev. 2013). Standard for Table Olives. Available online: http://www.fao.org/fao-who-codexalimentarius/sh-proxy/en/?lnk=1&url=https%253A%252F%252Fworkspace.fao.org%252Fsites%252Fcodex%252FStandards%252FCXS%2B66-1981%252FCXS_066e.pdf (accessed on 25 January 2020).
- Lanza, B.; Di Marco, S.; Simone, N.; Di Marco, C.; Gabriele, F. Table olives fermented in iodized sea salt brines: Nutraceuti-cal/sensory properties and microbial biodiversity. Foods 2020, 9, 301. [Google Scholar] [CrossRef] [PubMed]
- IOC (International Olive Council). Guide for the Determination of the Characteristics of Oil-Olives, COI/OH/Doc. No 1 November 2011. Available online: http://www.internationaloliveoil.org (accessed on 16 December 2020).
- American Oil Chemists' Society (AOCS). Method Cc13c-50. In Official Methods and Recommended Practices, 5th ed.; Firestone, D., Ed.; American Oil Chemists' Society: Champaign, IL, USA, 1999. [Google Scholar]
- Rovellini, P.; Azzolini, M.; Cortesi, N. Tocoferoli e tocotrienoli in oli e grassi vegetali mediante HPLC. Riv. Ital. Delle Sostanze Grasse 1997, 74, 1–5. [Google Scholar]
- REG EEC/2568 (1991). Consolidated Version of the Commission Regulation EEC No 2568/91 of 11 July 1991 on the Charac-teristics of Olive Oil and Olive-Residue Oil and on the Relevant Methods of Analysis. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:01991R2568-20161204&from=IT (accessed on 12 December 2020).
- IOC. Method for the Sensory Analysis of Table Olives COI/OT/MO/Doc. No 1/Rev. 2; International Olive Oil Council: Madrid, Spain, 2011. [Google Scholar]
- Sokoła-Wysoczańska, E.; Wysoczański, T.; Wagner, J.; Czyż, K.; Bodkowski, R.; Lochyński, S.; Patkowska-Sokoła, B. Poly-unsaturated fatty acids and their potential therapeutic role in cardiovascular system disorders—A Review. Nutrients 2018, 10, 1561. [Google Scholar] [CrossRef]
- Durante, M.; Tufariello, M.; Tommasi, L.; Lenucci, M.S.; Bleve, G.; Mita, G. Evaluation of bioactive compounds in black table olives fermented with selected microbial starters. J. Sci. Food Agric. 2017, 98, 96–103. [Google Scholar] [CrossRef]
- Lanza, B.; Di Serio, M.G.; Di Giacinto, L. Fatty-Acid alkyl esters in table olives in relation to abnormal fermentation and poorly conducted technological treatments. Grasas Y Aceites 2016, 67, e130. [Google Scholar] [CrossRef]
- Ostlund, R.E., Jr. Phytosterols in human nutrition. Ann. Rev. Nutr. 2002, 22, 533–549. [Google Scholar] [CrossRef] [PubMed]
- Beltrán, G.; Jiménez, A.; del Rio, C.; Sánchez, S.; Martínez, L.; Uceda, M.; Aguilera, M.P. Variability of vitamin E in virgin olive oil by agronomical and genetic factor. J. Food Compos. Anal. 2010, 23, 633–639. [Google Scholar] [CrossRef]
- Pika, M.J.; Kraljić, K.; Škevin, D. Tocopherols: Chemical structure, bioactivity, and variability in Croatian virgin olive oils. In Products from Olive Tree; Boskou, D., Clodoveo, M.L., Eds.; IntechOpen Limited: London, UK, 2016. [Google Scholar]
- Lanza, B.; Amoruso, F. Sensory analysis of natural table olives: Relationship between appearance of defect and gustatory-kinaesthetic sensation changes. LWT Food Sci. Technol. 2016, 68, 365–372. [Google Scholar] [CrossRef]
- Angerosa, F.; Lanza, B.; D'Alessandro, N.; Marsilio, V.; Cumitini, S. Olive oil off-odour compounds produced by aspergillus and penicillium. Acta Hortic. 1999, 695–700. [Google Scholar] [CrossRef]
- Zago, M.; Lanza, B.; Rossetti, L.; Muzzalupo, I.; Carminati, D.; Giraffa, G. Selection of Lactobacillus plantarum strains to use as starters in fermented table olives: Oleuropeinase activity and phage sensitivity. Food Microbiol. 2012, 34, 81–87. [Google Scholar] [CrossRef]
- Lanza, B.; Zago, M.; Carminati, D.; Rossetti, L.; Meucci, A.; Marfisi, P.; Russi, F.; Iannucci, E.; Di Serio, M.G.; Giraffa, G. Isolation and preliminary characterization of Lactobacillus plantarum bacteriophages from table olive fermentation. Ann. Microbiol. 2011, 62, 1467–1472. [Google Scholar] [CrossRef]
- López, F.N.A.; Romero-Gil, V.; Bautista-Gallego, J.; Rodríguez-Gómez, F.; Jiménez-Díaz, R.; García-García, P.; Querol, A.; Garrido-Fernández, A. Potential benefits of the application of yeast starters in table olive processing. Front. Microbiol. 2012, 3, 34. [Google Scholar] [CrossRef]
- Luckett, C.R. The Influences of Texture and Mastication Pattern on Flavor Perception across the Lifespan. Ph.D. Thesis, Degree of Doctor of Philosophy in Food Science. University of Arkansas, Fayetteville, AR, USA, 2016. [Google Scholar]
- Lanza, B.; Di Serio, M.G.; Iannucci, E. Effects of maturation and processing technologies on nutritional and sensory qualities of Itrana table olives. Grasas Y Aceites 2013, 64, 272–284. [Google Scholar] [CrossRef][Green Version]
- Anagnostopoulos, D.; Bozoudi, D.; Tsaltas, D. Yeast ecology of fermented table olives: A tool for biotechnological applications. In Yeast Industrial Application; Morata, A., Loira, I., Eds.; IntechOpen Limited: London, UK, 2017. [Google Scholar]
- Lanza, B. Abnormal fermentations in table-olive processing: Microbial origin and sensory evaluation. Front. Microbiol. 2013, 4, 91. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).