1. Introduction
In the 1980s, the mean life span of the Japanese general population became the world’s longest [
1]. While Japan is still known as a country with a healthy and long-living populace, the proportion of obese people has recently increased due to changes in lifestyle such as Westernized diets [
2,
3]. Japan’s prevalence of obesity is now a social issue [
4].
Obesity results from an accumulation of excess triglycerides stored in adipocyte cells due to energy intake exceeding energy expenditure. Adipocytes usually secrete hundreds of adipocytokines that have proinflammatory and anti-inflammatory properties. When adipocytes increase in size due to excessive energy intake, they produce more proinflammatory adipocytokines. Malignant adipocytokines are closely related to lifestyle-related diseases; therefore, adipocyte proliferation is linked to the occurrence of lifestyle-related diseases.
Interest has grown in the use of fermented foods as a technique to solve obesity-related health problems [
5,
6]. Sake lees, a byproduct of sake brewing, represent a fermented food with health benefits [
7,
8]. Sake lees consist of components of rice, yeast, and koji (a fungus used in the brewing process, the metabolites of which are highly nutritious and rich in amino acids, peptides, and dietary fiber). Animal studies have revealed that sake lees have functional properties, such as inhibiting cholesterol increase, lowering blood pressure, and inhibiting liver damage in mice. Moreover, clinical studies have reported improved environmental conditions in human intestines [
9,
10]. However, the mechanisms of action of sake lees and the roles of their specific components are unknown.
On the other hand, although IL6 is a pluripotent cytokine secreted by many different types of cells and tissues, overexpression by adipose tissue is a strong predictor of abnormalities in adipocytes and systemic metabolism [
11,
12]. In obese people, IL6 expression is increased in adipose tissue [
13]. Therefore, we hypothesized that, by analyzing the expression of IL6 in adipocytes, we might be able to clarify the mechanism underlying the effect of sake lees on IL6 and their effect on lipolysis.
In this study, we investigated the effects of the indigestible components of sake lees on lipid accumulation in adipocytes. In order to investigate the mechanism underlying the inhibition of adipocyte fat accumulation, we analyzed the expression level of IL6 in cells cultured in a medium containing indigestible sake lees components.
2. Materials and Methods
2.1. Materials
Sake lees were refermented at 25 °C for 3 days. The precipitate from the re-fermented mixture was collected by filtration and incubated at 60 °C for 2 h to remove the fat. The residue was collected by filtration, lyophilized, and powderized to obtain ISLCs. The composition of ISLCs is shown in
Table 1.
The ISLCs were diluted in distilled water at a ratio of 9 mL of water per 1 g of ISLCs and heated at 100 °C for 20 min. The solution was then centrifuged, and the supernatant was used for the experiment.
2.2. Cell Culture
Mouse 3T3-L1 cells (Japanese Collection of Research Bioresources Cell Bank, Osaka, Japan) were grown in Dulbecco’s modified Eagle’s medium (DMEM) (with a high glucose content (4500 mg/L), 1% antibiotics, and 10% fetal bovine serum (FBS)) at a temperature of 37 °C in 5% CO2 until confluence was achieved. Cell differentiation was induced by placing the cells in DMEM supplemented with 0.5 mM 3-isobutyl-1-methylxanthine (IBMX), 0.25 µM dexamethasone (DEX), 10% FBS, and 10 μg/mL insulin. Furthermore, 1.0 mg/mL ISLCs was added to the differentiation medium of the cells to create a group of treated cells, while the remainder of the cells acted as non-ISCLs-treated controls. The cells were incubated for 48 h; the differentiation medium was replaced with DMEM containing 10% FBS and 5 μg/mL of insulin, and this was changed every 48 h for 8 days.
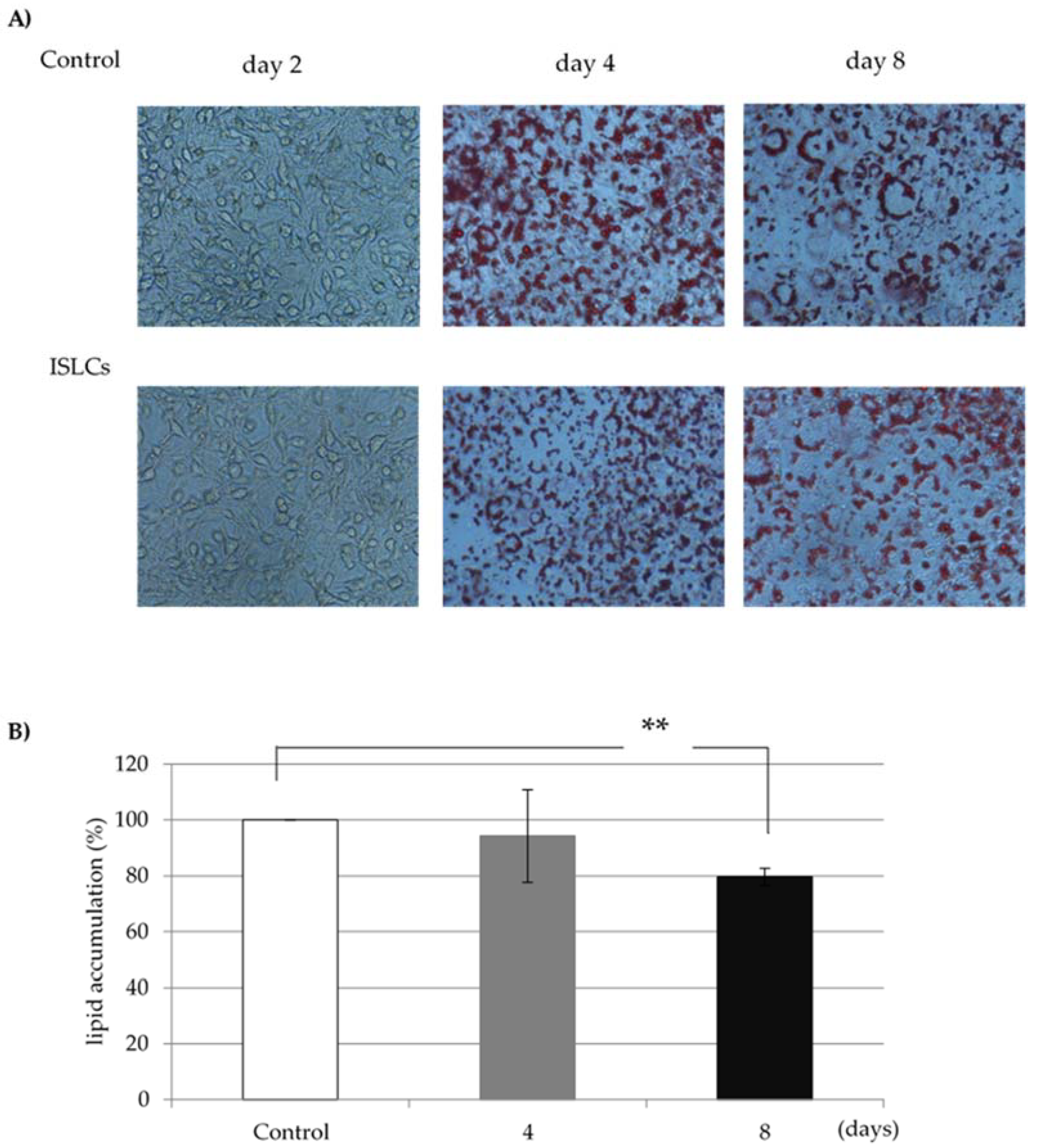
2.3. Oil Red O Stain
Oil Red O staining was used to assess lipid accumulation in fully differentiated adipocyte cells collected 8 days after confluence. Adipocytes were fixed with 10% formalin for 1 h, after which the fixing solution was removed. The cells were subsequently stained with filtered 0.3% Oil Red O solution in 60% isopropanol for 1 h before the staining solution was removed by washing with water. Oil Red O was then eluted with isopropanol, and the cells were dried. Absorbance in the eluate was measured at 560 nm using a microplate reader.
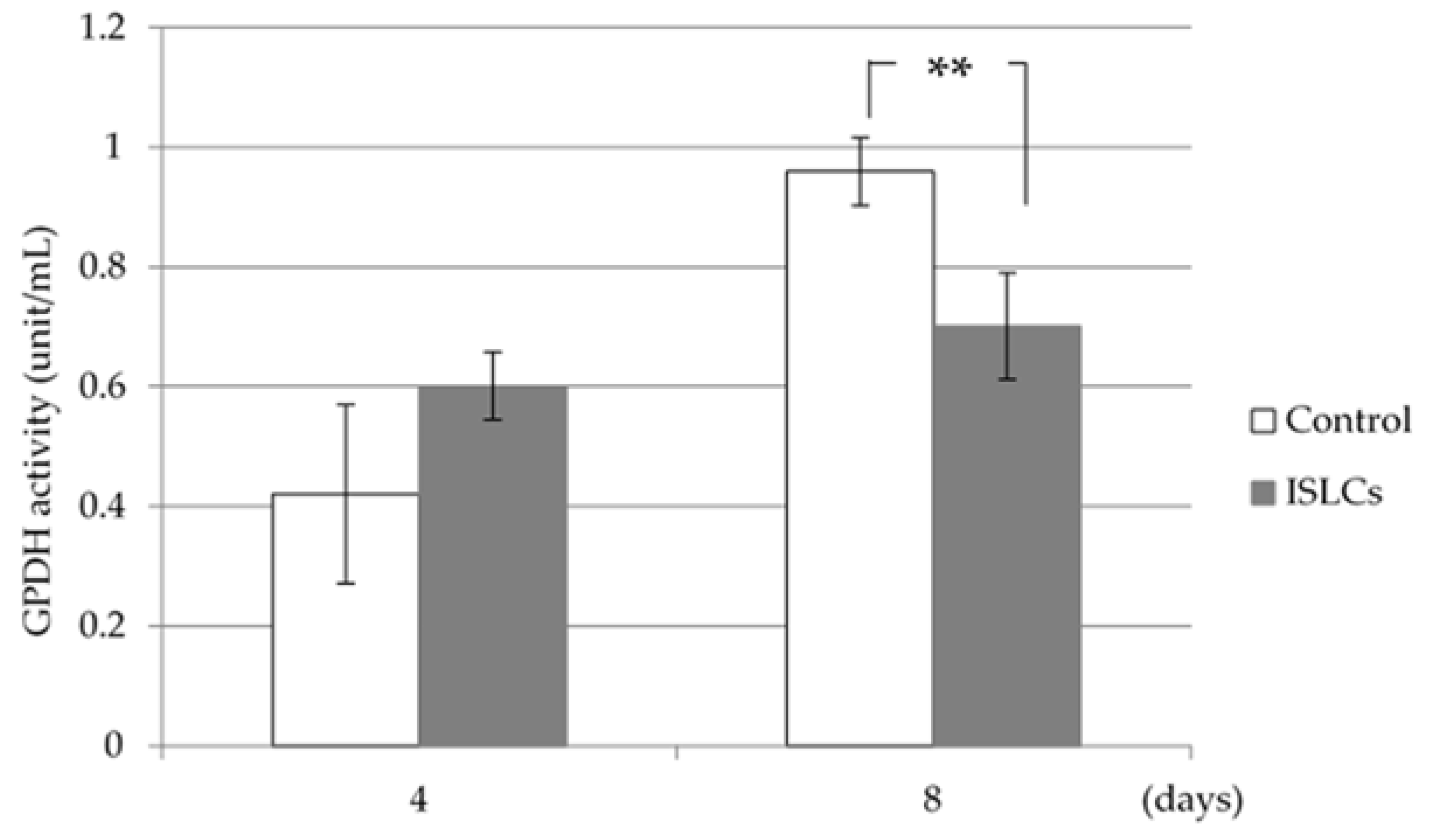
2.4. GPDH Activity
GPDH activity was measured using a GPDH activity assay kit (TAKARA BIO INC., Shiga, Japan) in 3T3-L1 cells collected 4 and 8 days after differentiation was initiated. The cells were washed twice with PBS and scraped into the enzyme extract buffer provided with a GPDH activity assay kit. The collected cells were centrifuged at 10,000× g for 5 min at 4 °C to remove the fat fraction, and the liquid component was used as the experimental sample. Following the removal of nicotinamide adenine di-nucleotide via an enzyme-catalyzed dihydroxyacetone phosphate reduction, the GPDH activity was spectrophotometrically determined at 340 nm.
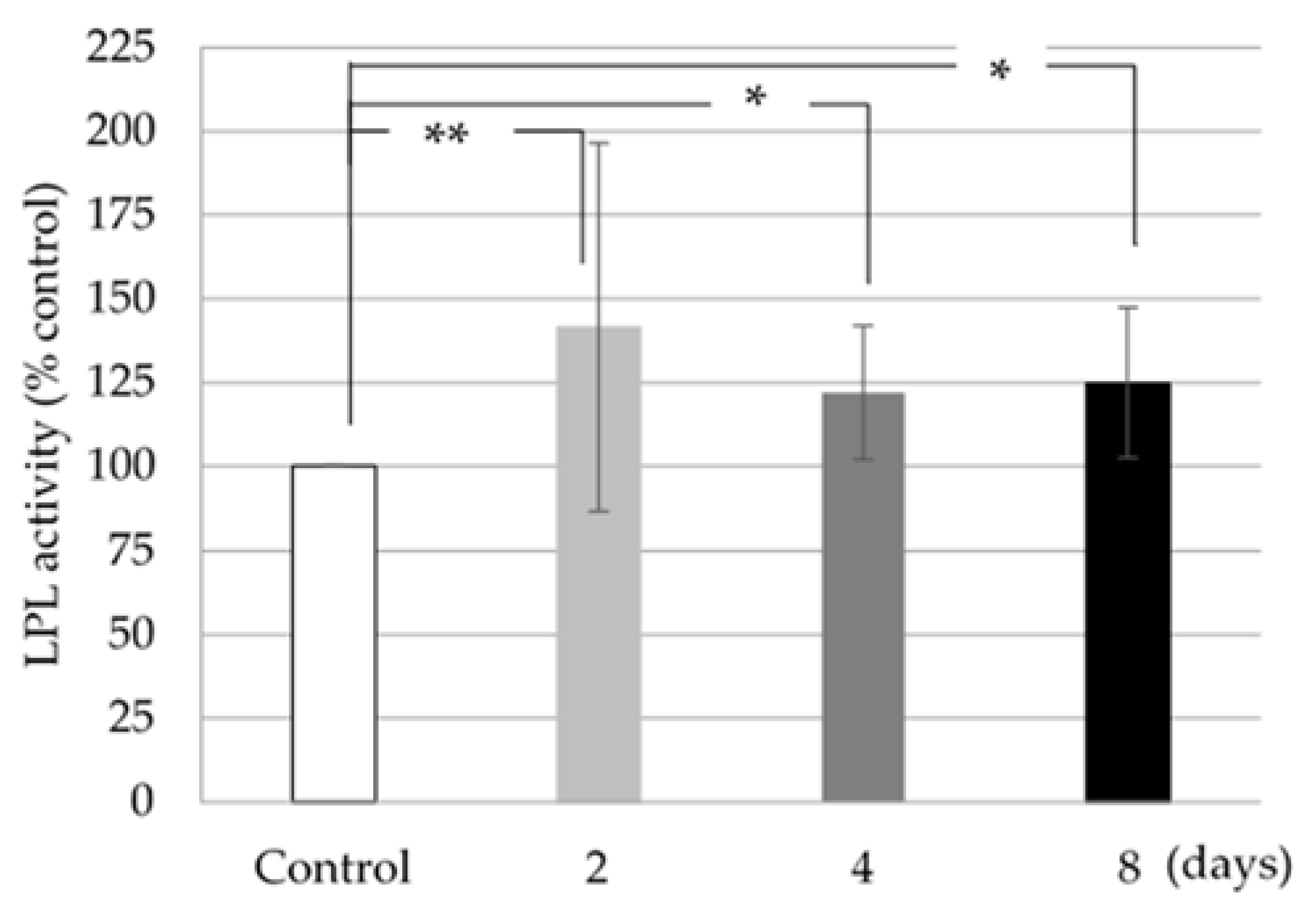
2.5. LPL Activity Assay
LPL is an enzyme that regulates the uptake of circulating triglycerides into adipocytes. LPL activity was measured using a lipase activity assay kit (Cayman Chemical, Ann Arbor, Michigan, USA) in 3T3-L1 cells collected 2, 4, and 8 days after cell differentiation. The activity was read as the cell fluorescence at 37 °C every 30 s for 15 min using an excitation wavelength of 385 nm and an emission wavelength of 515 nm. One unit of LPL activity was defined as the amount of enzyme that led to the formation of 1 nmol of triglycerol per min.
2.6. Real-Time PCR Analysis
We used real-time PCR to analyze IL6 gene expression in adipocytes treated with sake lees. The mRNA was extracted from 3T3-L1 cells collected 2, 4, and 8 days after cell differentiation commenced, using a Nucleo Spin RNA
® Kit (TAKARA BIO INC., Shiga, Japan). The recovered mRNA was used to synthesize first-strand cDNA using a PrimeScript RT reagent kit with IL6 primers (Cosmo Bio Co., Ltd., Tokyo, Japan). The primer sequences used for real-time PCR analysis are shown in
Table 2. Adipocyte IL6 gene expression was analyzed using 39 cycles of real-time PCR at 95 °C for 5 s and 60 °C for 30 s. The change in expression was analyzed using the ΔΔCt method and normalized to an internal control of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA.
2.7. Statistical Analysis
All data are expressed as the mean ± standard deviation. Dunnett’s method was used to assess the lipid accumulation rate and GPDH activity, whereas Student’s t-test was used to describe the LPL activity and IL6 gene expression. Accordingly, p-values <0.05 and <0.01 were considered significant. Excel Tokei (Version 2.12: Social Survey Research Information Co., Ltd., Tokyo, Japan.) was used for data analysis.
3. Results
3.1. Lipid Accumulation
The lipid accumulation rate of each sample of indigestible ingredients was calculated using the fat content of cells in the no-addition group as a control. In the cells treated with 1.0 mg/mL ISLCs, the lipid accumulation was 80.9% ± 6.1% of that in non-ISLC-treated cells (
Figure 1).
3.2. GPDH Activity
The GPDH activity 4 days after differentiation did not differ between the ISCL-treated and control cells; however, the GPDH activity was significantly decreased in the treated cells collected on day 8 (
Figure 2).
3.3. LPL Activity
The LPL activity was increased in 3T3-L1 cells treated with 1.0 mg/mL ISLCs compared to control cells; this increase was statistically significant (
Figure 3).
3.4. Adipocyte IL6 Gene Expression
Adipocyte IL6 gene expression was decreased in 3T3-L1 cells treated with 1.0 mg/mL ISLCs, collected on days 2, 4, and 8 after differentiation when compared to that in non-ISLC-treated cells; this decrease was statistically significant (
Figure 4).
4. Discussion
In this study, we investigated the effects of ISLCs on lipid accumulation in 3T3-L1 adipocytes. The results reveal that the addition of 1.0 mg/mL ISLCs to the culture medium was associated with significantly less lipid accumulation compared to untreated controls; thus, ISLCs may contain components that inhibit lipid accumulation in adipocytes (
Figure 1). Furthermore, the GDPH activity in fully differentiated cells was decreased compared to that in treated cells (
Figure 2). GPDH is known to be an enzyme involved in adipogenesis in fat cells. In this study, the GPDH activity was decreased in ISLC-treated cells, which suggests that ISLCs might have an inhibitory effect on lipogenesis in adipocytes.
IL6 has multiple effects on adipose tissue. Chronically high levels of IL6 may alter gene or protein expression, thus influencing basal lipolysis [
14]. Moreover, high local concentrations of IL6 can modulate leptin production and lipid metabolism in human adipose tissue [
15,
16]. IL6 may also directly influence human adipocyte metabolism by decreasing LPL activity, according to studies on the 3T3-L1 adipocyte model system [
17,
18].
In this study, the IL6 expression of 3T3-L1 cells was decreased and the adipocyte LPL activity was increased following ISLCs treatment compared with untreated controls (
Figure 3 and
Figure 4). Thus, ISLC-related factors might suppress IL6 expression and indirectly affect LPL activity. IL6 is thought to be a paracrine modulator of adipose tissue in obese human beings because LPL activity is inhibited by an increase in leptin production [
19]. Although this study did not assess leptin levels, we postulate that the decreased IL6 expression was associated with reduced leptin levels, thereby increasing LPL activity.
In conclusion, ISLCs were associated with reduced lipid accumulation in adipocytes, with effects on GPDH activity, IL6 expression, and LPL activity, as observed throughout the differentiation period. IL6 is believed to decrease LPL activity and control adipocyte size in humans; however, it is unclear how ISLCs and which specific ISLC components affect IL6 expression. We fractionated the ISLCs extracted at various temperatures into functional components and found that only the ISLCs extracted at 100 °C showed a significant difference. We found insoluble fractions with a molecular weight under 10,000 that were thought to be involved in lipid accumulation in adipocytes. However, the functional components have not yet been concretely determined. In this study, we could not clarify the detailed relationship between IL6 expression and the suppression of lipid accumulation in cells treated with ISLCs. Therefore, future research should be undertaken to elucidate this mechanism and detail the functional components in ISLCs.
Author Contributions
All authors contributed to writing and editing this paper (Y.M., T.I. (Takeshi Imai), T.E., T.I. (Takafumi Iguchi) and M.T.). All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported in part by Kobe College and Kobe College Megumi Association.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ministry of Health, Labor and Welfare. Available online: https://www.mhlw.go.jp/toukei/saikin/hw/life/life19/dl/life19-15.pdf (accessed on 6 June 2021).
- Ministry of Agriculture, Forestry and Fisheries. Available online: http://www.maff.go.jp/j/wpaper/w_maff/h26/h26_h/trend/part1/chap1/c1_3_01.html (accessed on 20 May 2021).
- Ministry of Agriculture, Forestry and Fisheries. Available online: https://www.e-stat.go.jp/stat-search/files?page=1&layout=datalist&toukei=00500300&tstat=000001017950&cycle=8&year=20191&month=0&tclass1=000001032890&tclass2=000001151387 (accessed on 20 May 2021).
- Rodgers, R.J.; Tschöp, M.H.; Wilding, J.P. Anti-obesity drugs: Past, present and future. Dis. Models Mech. 2012, 5, 621–626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barengolts, E.; Smith, E.D.; Reutrakul, S.; Tonucci, L.; Anothaisintawee, T. The Effect of Probiotic Yogurt on Glycemic Control in Type 2 Diabetes or Obesity: A Meta-Analysis of Nine Randomized Controlled Trials. Nutrients 2019, 11, 671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- González, S.; Fernández-Navarro, T.; Arboleya, S.; de Los Reyes-Gavilán, C.G.; Salazar, N.; Gueimonde, M. Fermented Dairy Foods: Impact on Intestinal Microbiota and Health-Linked Biomarkers. Front. Microbiol. 2019, 10, 1046. [Google Scholar] [CrossRef] [PubMed]
- Saito, Y.; Wanezaki, K.; Kawato, A.; Imayasu, S. Structure and activity of angiotensin I converting enzyme inhibitory peptides from sake and sake lees. Biosci. Biotechnol. Biochem. 1994, 58, 1767–1771. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Izu, H.; Shibata, S.; Fujii, T.; Matsubara, K. Sake cake (sake-kasu) ingestion increases branched-chain amino acids in the plasma, muscles, and brains of senescence-accelerated mice prone 8. Biosci. Biotechnol. Biochem. 2019, 83, 1490–1497. [Google Scholar] [CrossRef] [PubMed]
- Kubo, H.; Hoshi, M.; Matsumoto, T.; Irie, M.; Oura, S.; Tsutsumi, H.; Hata, Y.; Yamamoto, Y.; Saito, K. Sake lees extract improves hepatic lipid accumulation in high fat diet-fed mice. Lipids Health Dis. 2017, 16, 106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawakami, K.; Moritani, C.; Uraji, M.; Fujita, A.; Kawakami, K.; Hatanaka, T.; Suzaki, E.; Tsuboi, S. Sake lees hydrolysate protects against acetaminophen-induced hepatotoxicity via activation of the Nrf2 antioxidant pathway. J. Clin. Biochem. Nutr. 2017, 61, 203–209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vicennati, V.; Vottero, A.; Friedman, C.; Papanicolaou, D.A. Hormonal regulation of interleukin-6 production in human adipocyte. Int. J. Obes. 2002, 26, 905–911. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roytblat, L.; Rachinsky, M.; Fisher, A.; Greemberg, L.; Shapira, Y.; Douvdevani, A.; Gelman, S. Raised Interleukin-6 Levels in Obese Patients. Obes. Res. 2000, 8, 673–675. [Google Scholar] [CrossRef]
- Kern, P.A.; Ranganathan, S.; Li, C.; Wood, L.; Ranganathan, G. Adipose tissue tumor necrosis factor and interleukin-6 expression in human obesity and insulin resistance. Am. J. Physiol. Endocrinol. Metab. 2001, 280, 745–751. [Google Scholar] [CrossRef]
- Rotter, V.; Nagaev, I.; Smith, U. Interleukin-6(IL-6)Induces Insulin Resistance in 3T3-L1 Adipocytes and Is, Like IL-8 and Tumor Necrosis Factor-,Overexpressed in Human Fat Cells from Insulin-resistant Subjects. J. Biol. Chem. 2003, 278, 45777–45784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Granowitz, E.V. Transforming Growth Factor-β Enhances and Pro-inflammatory Cytokines Inhibit OB Gene Expression in 3T3-L1 Adipocytes. Biochem. Biophys. Res. Commun. 1997, 240, 382–385. [Google Scholar] [CrossRef] [PubMed]
- Bruun, J.M.; Pedersen, S.B.; Kristensen, K.; Richelsen, B. Effects of pro-inflammatory cytokines and chemokines on leptin production in human adipose tissue in vitro. Mol. Cell. Endocrinol. 2002, 190, 91–99. [Google Scholar] [CrossRef]
- Trujillo, M.E.; Sullivan, S.; Harten, I.; Schneider, S.H.; Greenberg, A.S.; Fried, S.K. Interleukin-6 Regulates Human Adipose Tissue Lipid Metabolism and Leptin Production in Vitro. J. Clin. Endocrinol. Metab. 2004, 89, 5577–5582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greenberg, A.S.; Nordan, R.P.; McIntosh, J.; Calvo, J.C.; Scow, R.O.; Jablons, D. Interleukin 6 Reduces Lipoprotein Lipase Activity in Adipose Tissue of Mice in Vivo and in 3T3-L1 Adipocytes: A Possible Role for Interleukin 6 in Cancer Cachexia. Cancer Res. 1992, 52, 4113–4116. [Google Scholar] [PubMed]
- Fried, S.K.; Bunkin, D.A.; Greenberg, A.S. Omental and Subcutaneous Adipose Tissues of Obese Subjects Release Interleukin-6: Depot Difference and Regulation by Glucocorticoid. J. Clin. Endocrinol. Metab. 1998, 83, 847–850. [Google Scholar] [CrossRef] [PubMed]
| Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).