Evaluating the Effect of Lignocellulose-Derived Microbial Inhibitors on the Growth and Lactic Acid Production by Bacillus coagulans Azu-10
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Isolation and Fermentative Media
2.2. Characterization and Identification of Bacterial Strain
2.3. Inoculum Preparation and Batch Fermentations
2.4. Analytical Methods
3. Results
3.1. Isolation and Identification of Isolate Azu-10
3.2. Effect of pH Values on Lactic Acid Fermentation from Xylose
3.3. Effect of Temperature on Lactic Acid Fermentation from Xylose
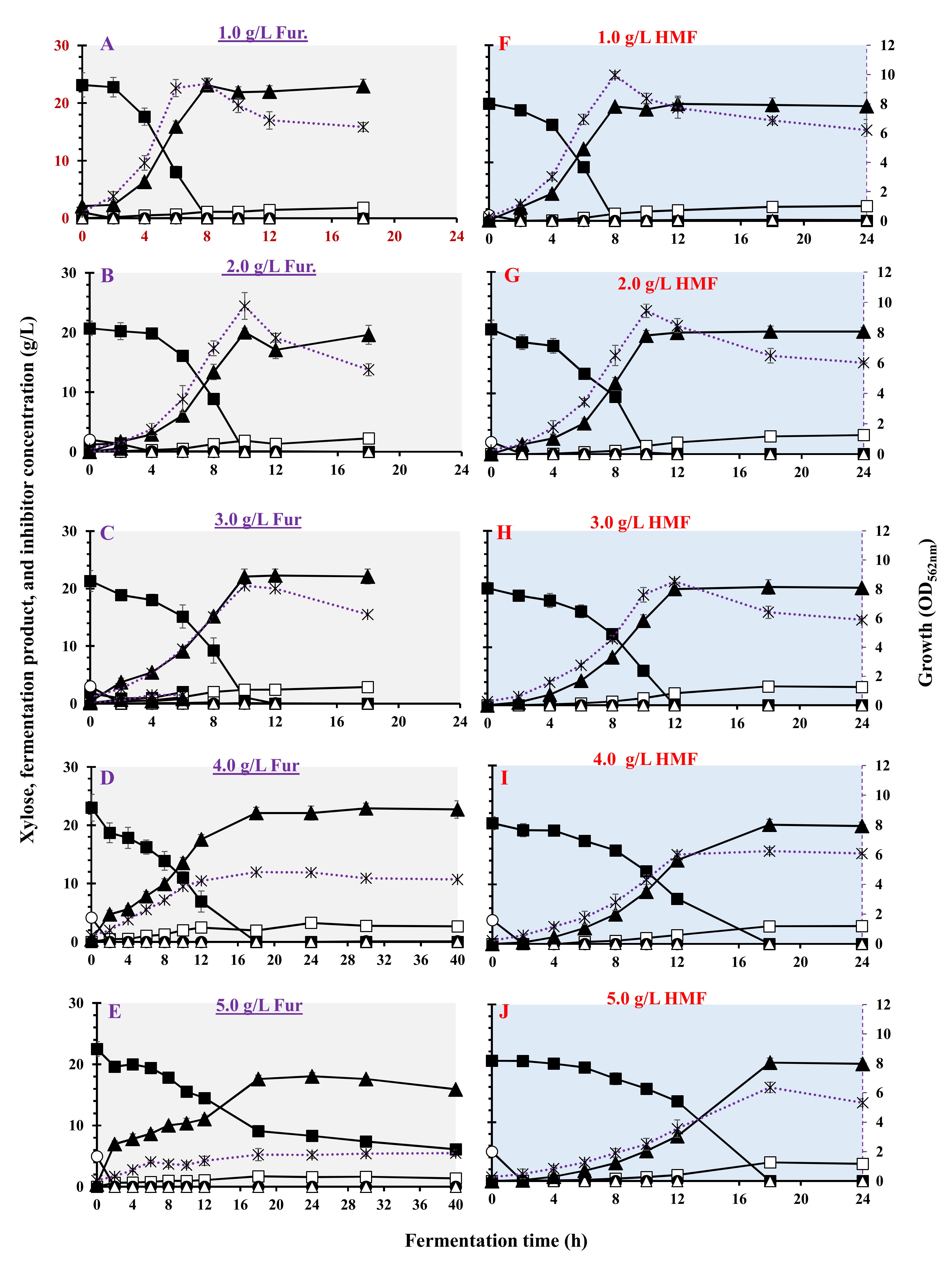
3.4. Effect of Furan on Bacterial Growth and LA Fermentation
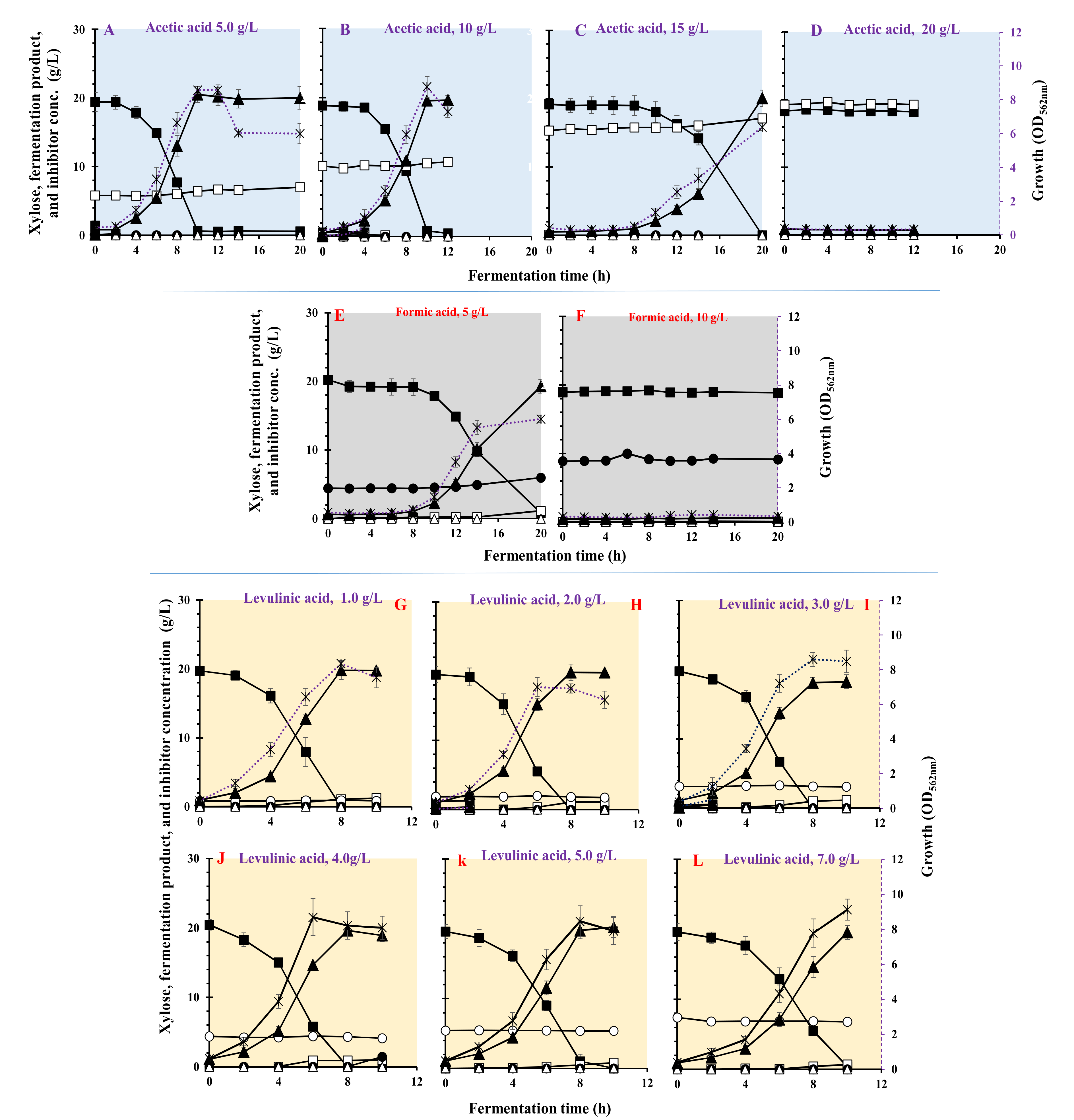
3.5. Effect of Carboxylic Acids on Growth and LA Fermentation
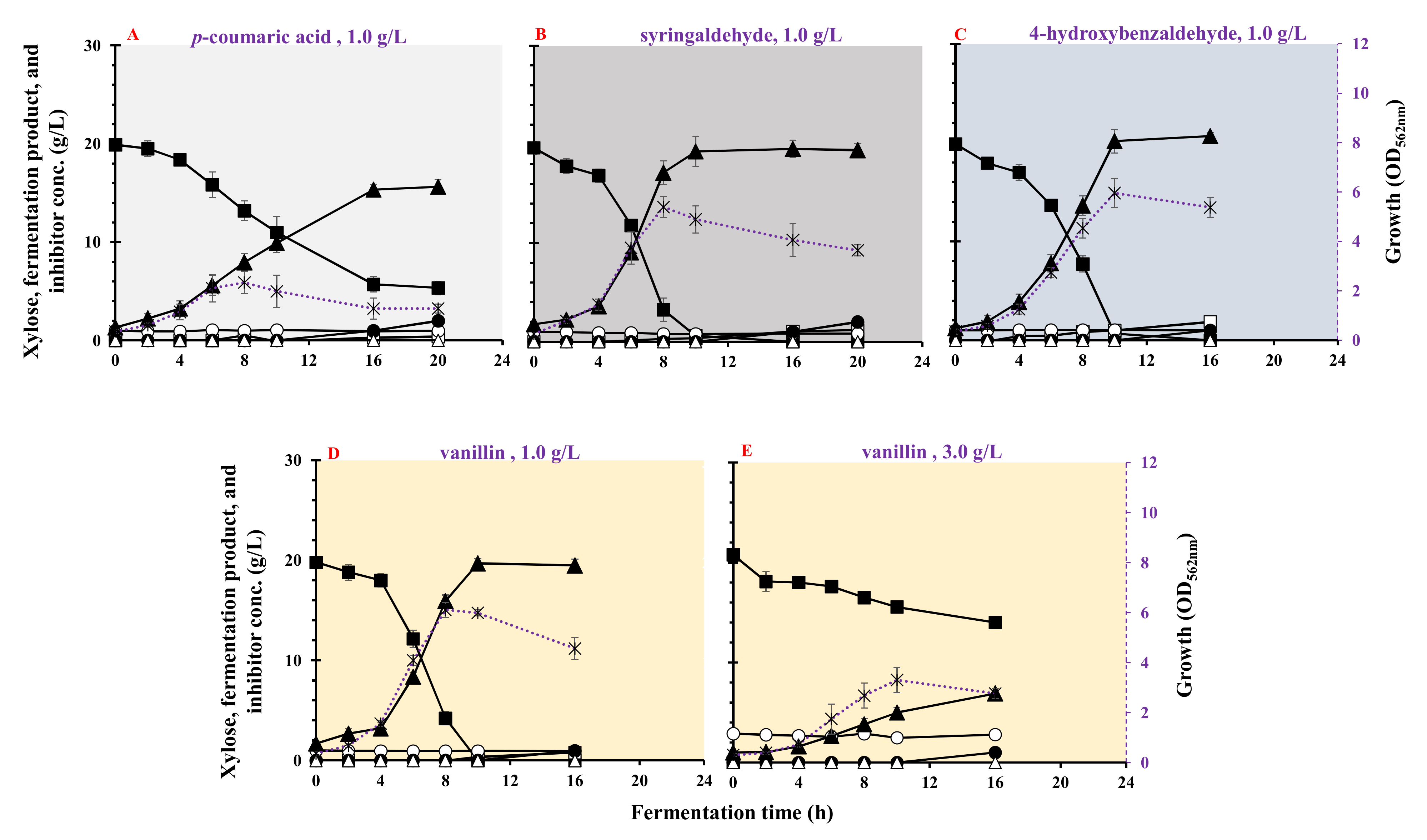
3.6. Effect of Phenolic Compounds on Growth and LA Fermentation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abedi, E.; Hashemi, S.M.B. Lactic acid production—Producing microorganisms and substrates sources-state of art. Heliyon 2020, 6, 04974. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Rahman, M.A.; Tashiro, Y.; Sonomoto, K. Lactic acid production from lignocellulose- derived sugars using lactic acid bacteria: Overview and limits. J. Biotechnol. 2011, 156, 286–301. [Google Scholar] [CrossRef] [PubMed]
- Cubas-Cano, E.; González-Fernández, C.; Ballesteros, M.; Tomás-Pejó, E. Biotechnological advances in lactic acid production by lactic acid bacteria: Lignocellulose as novel substrate. Biofuel Bioprod. Biorefin. 2018, 12, 290–303. [Google Scholar] [CrossRef]
- Abdel-Rahman, M.A.; Sonomoto, K. Opportunities to overcome the current limitations and challenges for efficient microbial production of optically pure lactic acid. J. Biotechnol. 2016, 236, 176–192. [Google Scholar] [CrossRef] [PubMed]
- Saini, R.; Osorio-Gonzalez, C.S.; Hegde, K.; Brar, S.K.; Magdouli, S.; Vezina, P.; Avalos-Ramirez, A. Lignocellulosic Biomass-Based Biorefinery: An Insight into Commercialization and Economic Standout. Curr. Sustain. Energy Rep. 2020, 7, 122–136. [Google Scholar] [CrossRef]
- Sara, M.; Brar, S.; Blais, J. Production of Drop-In and Novel Bio-Based Platform Chemicals. Platf. Chem. Biorefinery 2016, 249–283. [Google Scholar] [CrossRef]
- Sara, M.; Rouissi, T.; Brar, S.K.; Blais, J.F. Life cycle analysis of potential substrates of sustainable biorefinery. In Platform Chemical Biorefinery; Elsevier: Amsterdam, The Netherlands, 2016; pp. 55–76. [Google Scholar]
- Van Der Pol, E.; Vaessen, E.; Weusthuis, R.A.; Eggink, G. Identifying inhibitory effects of lignocellulosic by-products on growth of lactic acid producing micro-organisms using a rapid small-scale screening method. Bioresour. Technol. 2016, 209, 297–304. [Google Scholar] [CrossRef]
- Zhang, L.; Li, X.; Yong, Q.; Yang, S.-T.; Ouyang, J.; Yu, S. Impacts of lignocellulose-derived inhibitors on l-lactic acid fermentation by Rhizopus oryzae. Bioresour. Technol. 2016, 203, 173–180. [Google Scholar] [CrossRef]
- Kim, D. Physico-Chemical Conversion of Lignocellulose: Inhibitor Effects and Detoxification Strategies: A Mini Review. Molecules 2018, 23, 309. [Google Scholar] [CrossRef]
- Abdel-Rahman, M.A.; Tashiro, Y.; Zendo, T.; Sakai, K.; Sonomoto, K. Highly efficient l-lactic acid production from xylose in cell recycle continuous fermentation using Enterococcus mundtii QU 25. RSC Adv. 2016, 6, 17659–17668. [Google Scholar] [CrossRef]
- Abdel-Rahman, M.A.; Tashiro, Y.; Sonomoto, K. Recent advances in lactic acid production by microbial fermentation processes. Biotechnol. Adv. 2013, 31, 877–902. [Google Scholar] [CrossRef] [PubMed]
- Hassan, S.E.-D.; Abdel-Rahman, M.A.; Roushdy, M.M.; Azab, M.S.; Gaber, M.A. Effective biorefinery approach for lactic acid production based on co-fermentation of mixed organic wastes by Enterococcus durans BP130. Biocatal. Agric. Biotechnol. 2019, 20, 101203. [Google Scholar] [CrossRef]
- Abdel-Rahman, M.A.; Tashiro, Y.; Zendo, T.; Sakai, K.; Sonomoto, K. Enterococcus faecium QU 50: A novel thermophilic lactic acid bacterium for high-yield l-lactic acid production from xylose. FEMS Microbiol. Lett. 2015, 362, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.N.; Bryant, J.E.; Madsen, E.L.; Ghiorse, W.C. Evaluation and optimization of DNA extraction and purification procedures for soil and sediment samples. Appl. Environ. Microbiol. 1999, 65, 4715–4724. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Komiyama, A.; Sonomoto, K.; Ishizaki, A.; Hall, S.; Stanbury, P. Two different pathways for D-xylose metabolism and the effect of xylose concentration on the yield coefficient of l-lactate in mixed-acid fermentation by the lactic acid bacterium Lactococcus lactis IO-1. Appl. Microbiol. Biotechnol. 2002, 60, 160–167. [Google Scholar] [PubMed]
- Patel, M.A.; Ou, M.S.; Harbrucker, R.; Aldrich, H.C.; Buszko, M.L.; Ingram, L.O.; Shanmugam, K.T. Isolation and Characterization of Acid-Tolerant, Thermophilic Bacteria for Effective Fermentation of Biomass-Derived Sugars to Lactic Acid. Appl. Environ. Microbiol. 2006, 72, 3228–3235. [Google Scholar] [CrossRef]
- Poudel, P.; Tashiro, Y.; Sakai, K. New application of Bacillus strains for optically pure l-lactic acid production: General overview and future prospects. Biosci. Biotechnol. Biochem. 2016, 80, 642–654. [Google Scholar] [CrossRef]
- Zhou, X.; Ye, L.; Wu, J.C. Efficient production of l-lactic acid by newly isolated thermophilic Bacillus coagulans WCP10-4 with high glucose tolerance. Appl. Microbiol. Biotechnol. 2013, 97, 4309–4314. [Google Scholar] [CrossRef]
- Meng, Y.; Xue, Y.; Yu, B.; Gao, C.; Ma, Y. Efficient production of l-lactic acid with high optical purity by alkaliphilic Bacillus sp. WL-S20. Bioresour. Technol. 2012, 116, 334–339. [Google Scholar] [CrossRef]
- Albert, R.A.; Archambault, J.; Rossello-Mora, R.; Tindall, B.J.; Matheny, M. Bacillus acidicola sp. nov., a novel mesophilic, acidophilic species isolated from acidic Sphagnum peat bogs in Wisconsin. Int. J. Syst. Evol. Microbiol. 2005, 55, 2125–2130. [Google Scholar] [CrossRef]
- Othman, M.; Ariff, A.B.; Rios-Solis, L.; Halim, M. Extractive fermentation of lactic acid in lactic acid bacteria cultivation: A review. Front. Microbiol. 2017, 8, 2285. [Google Scholar] [CrossRef] [PubMed]
- Trcek, J.; Mira, N.P.; Jarboe, L.R. Adaptation and tolerance of bacteria against acetic acid. Appl. Microbiol. Biotechnol. 2015, 99, 6215–6229. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Zhou, X.; Bin Hudari, M.S.; Li, Z.; Wu, J.C. Highly efficient production of l-lactic acid from xylose by newly isolated Bacillus coagulans C106. Bioresour. Technol. 2013, 132, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Ou, M.S.; Mohammed, N.; Ingram, L.O.; Shanmugam, K.T. Thermophilic Bacillus coagulans requires less cellulases for simul-taneous saccharification and fermentation of cellulose to products than mesophilic microbial biocatalysts. Appl. Biochem. Biotechnol. 2009, 155, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Rahman, M.A.; Hassan, S.E.D.; Roushdy, M.M.; Azab, M.S.; Gaber, M.A. Free-nutrient supply and thermo-alkaline conditions for direct lactic acid production from mixed lignocellulosic and food waste materials. Bioresour. Technol. Rep. 2019, 7, 100256. [Google Scholar] [CrossRef]
- Chundawat, S.P.S.; Balan, V.; Da Costa Sousa, L.; Dale, B.E. Thermochemical pretreatment of lignocellulosic biomass. In Bioalcohol Production; Woodhead Publishing: Cambridge, UK, 2010; pp. 24–72. [Google Scholar]
- Jönsson, L.J.; Martín, C. Pretreatment of lignocellulose: Formation of inhibitory by-products and strategies for minimizing their effects. Bioresour. Technol. 2016, 199, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Ximenes, E.; Kim, Y.; Mosier, N.; Dien, B.; Ladisch, M.R. Deactivation of cellulases by phenols. Enzym. Microb. Technol. 2011, 48, 54–60. [Google Scholar] [CrossRef]
- Xiros, C.; Olsson, L. Comparison of strategies to overcome the inhibitory effects in high-gravity fermentation of lignocellulosic hydrolysates. Biomass Bioenergy 2014, 65, 79–90. [Google Scholar] [CrossRef]
- Van Der Pol, E.; Bakker, R.R.; Baets, P.; Eggink, G. By-products resulting from lignocellulose pretreatment and their inhibitory effect on fermentations for (bio)chemicals and fuels. Appl. Microbiol. Biotechnol. 2014, 98, 9579–9593. [Google Scholar] [CrossRef]
- Yu, X.; Zeng, J.; Zheng, Y.; Chen, S. Effect of lignocellulose degradation products on microbial biomass and lipid production by the oleaginous yeast Cryptococcus curvatus. Process. Biochem. 2014, 49, 457–465. [Google Scholar] [CrossRef]
- Kabel, M.A.; Bos, G.; Zeevalking, J.; Voragen, A.G.J.; Schols, H.A. Effect of pretreatment severity on xylan solubility and enzymatic breakdown of the remaining cellulose from wheat straw. Bioresour. Technol. 2007, 98, 2034–2042. [Google Scholar] [CrossRef] [PubMed]
- Klinke, H.B.; Olsson, L.; Thomsen, A.B.; Ahring, B.K. Potential inhibitors from wet oxidation of wheat straw and their effect on ethanol production of Saccharomyces cerevisiae: Wet oxidation and fermentation by yeast. Biotechnol. Bioeng. 2003, 81, 738–747. [Google Scholar] [CrossRef] [PubMed]
- Mills, T.Y.; Sandoval, N.R.; Gill, R.T. Cellulosic hydrolysate toxicity and tolerance mechanisms in Escherichia coli. Biotechnol. Biofuels 2009, 2, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Cortez, D.V.; Roberto, I.C. Individual and interaction effects of vanillin and syringaldehyde on the xylitol formation by Candida guilliermondii. Bioresour. Technol. 2010, 101, 1858–1865. [Google Scholar] [CrossRef] [PubMed]
- Allen, S.A.; Clark, W.; McCaffery, J.M.; Cai, Z.; Lanctot, A.; Slininger, P.J.; Liu, Z.L.; Gorsich, S.W. Furfural induces reactive oxygen species accumulation and cellular damage in Saccharomyces cerevisiae. Biotechnol. Biofuels 2010, 3, 2. [Google Scholar] [CrossRef]
- Larsson, S.; Quintana-Sáinz, A.; Reimann, A.; Nilvebrant, N.-O.; Jönsson, L.J. Influence of Lignocellulose-Derived Aromatic Compounds on Oxygen-Limited Growth and Ethanolic Fermentation by Saccharomyces cerevisiae. Appl. Biochem. Biotechnol. 2000, 84, 617–632. [Google Scholar] [CrossRef]
- Adeboye, P.T.; Bettiga, M.; Olsson, L. The chemical nature of phenolic compounds determines their toxicity and induces distinct physiological responses in Saccharomyces cerevisiae in lignocellulose hydrolysates. AMB Express 2014, 4, 46. [Google Scholar] [CrossRef]
- Almeida, J.R.M.; Modig, T.; Petersson, A.; Hähn-Hägerdal, B.; Lidén, G.; Gorwa- Grauslund, M.F. Increased tolerance and conversion of inhibitors in lignocellulosic hydrolysates by Saccharomyces cerevisiae. J. Chem. Technol. Biotechnol. 2007, 82, 340–349. [Google Scholar] [CrossRef]
- Walton, S.L.; Bischoff, K.M.; Van Heiningen, A.R.P.; Van Walsum, G.P. Production of lactic acid from hemicellulose extracts by Bacillus coagulans MXL-9. J. Ind. Microbiol. Biotechnol. 2010, 37, 823–830. [Google Scholar] [CrossRef]
- Van der Pol, E.C.; Bakker, R.R.; van Zeeland, A.N.T.; Sanchez Garcia, D.; Punt, A. Eggink, G. Analysis of by-product formation and sugar monomerization in sugarcane bagasse pretreated at pilot plant scale: Differences between autohydrolysis, alkaline and acid pretreatment. Bioresour. Technol. 2015, 181, 114–123. [Google Scholar] [CrossRef]
| Temp. (°C) | Max. Biomass (OD562nm) | Residual Xylose (g/L) | LA (g/L) | Acetic Acid (g/L) | Ethanol (g/L) | LA Yield (g/g) | LA Productivity (g/(L·h)) | Max. LA Productivity (g/(L·h)) |
|---|---|---|---|---|---|---|---|---|
| 30 | 5.06 ± 0.15 | <0.1 | 20.5 ± 1.05 | 4.57 ± 0.18 | <0.1 | 0.900 ± 0.025 | 0.890 ± 0.013 | 1.70 ± 0.12 |
| 35 | 1.78 ± 0.01 | <0.1 | 20.4 ± 1.01 | 3.82 ± 0.34 | <0.1 | 0.900 ± 0.013 | 1.13 ± 0.025 | 0.796 ± 0.10 |
| 40 | 2.68 ± 0.32 | <0.1 | 21.9 ± 0.85 | 2.30 ± 0.65 | 0.450 ± 0.045 | 0.910 ± 0.024 | 1.83 ± 0.022 | 2.13 ± 0.34 |
| 45 | 2.90 ± 0.21 | <0.1 | 21.4 ± 0.47 | 1.82 ± 0.13 | 0.420 ± 0.008 | 0.970 ± 0.018 | 1.70 ± 0.145 | 2.62 ± 0.51 |
| 50 | 9.42 ± 0.39 | <0.1 | 22.0 ± 1.07 | 1.03 ± 0.16 | 0.0 | 1.02 ± 0.005 | 3.00 ± 0.023 | 5.70 ± 0.23 |
| 55 | 6.38 ± 0.17 | <0.1 | 21.5 ± 0.83 | 0.314 ± 0.05 | 0.388 ± 0.007 | 0.970 ± 0.013 | 2.69±0.245 | 5.12 ± 0.62 |
| 60 | 4.26 ± 0.22 | 7.04 ± 0.35 | 17.5 ± 0.64 | 0.430 ± 0.04 | 0.469 ± 0.013 | 1.00 ± 0.023 | 0.580 ± 0.041 | 1.66 ± 0.22 |
| 63 | 0.480 ± 0.09 | 18.75 ± 0.27 | 0.680 ± 0.15 | <0.06 | <0.1 | 0.22 ± 0.013 | 0.030 ± 0.005 | 0.030 ± 0.02 |
| Furans | Inhibitor Conc. (g/L) | Max. Biomass (OD562nm) | µmax (h−1) | Residual Xylose (g/L) | LA (g/L) | Acetic Acid (g/L) | Ethanol (g/L) | LA Yield (g/g) | LA Productivity (g/(L·h)) | Max. LA Productivity (g/(L·h)) | Residual Inhibitor (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Furfural | 0 | 9.58 ± 0.456 | 0.804 ± 0.022 | <0.1 | 19.0 ± 1.76 | 0.70 ± 0.01 | <0.1 | 0.850 ± 0.013 | 2.38 ± 0.120 | 4.02 ± 0.013 | 0.0 |
| 1 | 9.34 ± 0.501 | 0.649 ± 0.012 | <0.1 | 23.1 ± 1.08 | 1.15 ± 0.08 | <0.1 | 0.998 ± 0.011 | 2.88 ± 0.213 | 4.78 ± 0.125 | 0.0 | |
| 2 | 9.78 ± 0.903 | 0.565 ± 0.021 | <0.1 | 20.0 ± 0.67 | 1.85 ± 0.12 | <0.1 | 0.968 ± 0.005 | 2.04 ± 0.188 | 3.64 ± 0.065 | 0.0 | |
| 3 | 8.2 ± 0.312 | 0.430 ± 0.010 | <0.1 | 22.3 ± 1.11 | 2.45 ± 0.12 | <0.1 | 1.04 ± 0.008 | 1.85 ± 0.356 | 3.44 ± 0.096 | 0.0 | |
| 4 | 4.78 ± 0.785 | 0.333 ± 0.013 | <0.1 | 22.1 ± 0.85 | 1.94 ± 0.88 | <0.1 | 0.956 ± 0.005 | 1.46 ± 0.256 | 2.16 ± 0.153 | 0.0 | |
| 5 | 2.1 ± 0.411 | 0.240 ± 0.016 | 6.14 ± 0.88 | 15.9 ± 0.34 | 1.38 ± 0.03 | <0.1 | 0.973 ± 0.003 | 0.398 ± 0.365 | 3.31 ± 0.245 | 0.0 | |
| 6 | 0.42 ± 0.106 | 0.158 ± 0.018 | 20.2 ± 1.63 | 0.0 | 0.060 ± 0.002 | <0.1 | 0.0 | 0.0 | 0.0 | 2.16 ± 0.130 | |
| HydroxyMethyl Furfural (HMF) | 1 | 9.96 ± 0.188 | 0.739 ± 0.091 | <0.1 | 19.5 ± 0.29 | 1.23 ± 0.009 | <0.1 | 0.975 ± 0.012 | 2.44 ± 0.122 | 3.81 ± 0.210 | 0.0 |
| 2 | 9.44 ± 0.442 | 0.521 ± 0.072 | <0.1 | 19.5 ± 0.83 | 1.35 ± 0.11 | <0.1 | 0.962 ± 0.011 | 1.95 ± 0.061 | 3.91 ± 0.102 | 0.0 | |
| 3 | 8.52 ± 0.226 | 0.467 ± 0.012 | <0.1 | 20.0 ± 1.18 | 2.06 ± 0.15 | <0.1 | 0.992 ± 0.016 | 1.66 ± 0.091 | 3.13 ± 0.131 | 0.0 | |
| 4 | 6.24 ± 0.243 | 0.484 ± 0.017 | <0.1 | 20.0 ± 0.89 | 1.48 ± 0.08 | <0.1 | 0.987 ± 0.006 | 1.17 ± 0.026 | 2.65 ± 0.214 | 0.0 | |
| 5 | 6.36 ± 0.358 | 0.306 ± 0.022 | <0.1 | 20.1 ± 0.79 | 3.15 ± 0.03 | <0.1 | 0.984 ± 0.007 | 1.11 ± 0.110 | 2.08 ± 0.256 | 0.0 |
| Carboxylic Acids | Inhibition (g/L) | Max. Biomass (OD562nm) | µmax (h−1) | Residual Xylose (g/L) | LA (g/L) | Acetic Acid (g/L) | Ethanol (g/L) | LA Yield (g/g) | LA Productivity (g/(L·h)) | Max. LA Productivity (g/(L·h)) | Residual Inhibitor (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetic acid | 5 | 8.46 ± 0.302 | 0.401 ± 0.021 | <0.1 | 20.5 ± 1.12 | 0.60 ± 0.16 | <0.1 | 1.05 ± 0.041 | 2.05 ± 0.065 | 3.81 ± 0.141 | 100 |
| 10 | 8.64 ± 0.611 | 0.446 ± 0.036 | <0.1 | 18.4 ± 1.33 | 0.42 ± 0.08 | <0.1 | 1.0 ± 0.063 | 1.96 ± 0.120 | 4.32 ± 0.215 | 100 | |
| 15 | 6.34 ± 0.223 | 0.454 ± 0.006 | <0.1 | 20.0 ± 0.99 | 1.76 ± 0.11 | <0.1 | 1.01 ± 0.032 | 1.04 ± 0.012 | 1.40 ± 0.161 | 100 | |
| 20 | 0.32 ± 0.005 | 0.030 ± 0.009 | <0.1 | - | - | <0.1 | - | - | - | 100 | |
| Formic acid | 5 | 5.3 ± 0.405 | 0.481 ± 0.008 | <0.1 | 19.2 ± 0.85 | 1.13 ± 0.04 | <0.1 | 0.991 ± 0.021 | 0.963 ± 0.032 | 2.47 ± 0.099 | 100 |
| 10 | 0.42 ± 0.012 | 0.189 ± 0.012 | 18.85 ± 1.34 | 0.616 ± 0.15 | 0.08 ± 0.01 | <0.1 | 0 | 0 | 0 | 100 | |
| Levulinic acid | 1 | 8.3 ± 0.215 | 0.693 ± 0.025 | <0.1 | 19.7 ± 1.32 | 1.05 ± 0.23 | <0.1 | 1.01 ± 0.012 | 2.47 ± 0.025 | 4.17 ± 0.102 | 98.8 |
| 2 | 7.08 ± 0.561 | 0.508 ± 0.035 | <0.1 | 19.5 ± 1.17 | 1.87 ± 0.44 | <0.1 | 1.00 ± 0.021 | 2.47 ± 0.017 | 4.79 ± 0.135 | 94.1 | |
| 3 | 8.6 ± 0.421 | 0.626 ± 0.017 | <0.1 | 18.1 ± 1.55 | 0.99 ± 0.15 | <0.1 | 0.915 ± 0.005 | 2.26 ± 0.020 | 4.33 ± 0.120 | 98.7 | |
| 4 | 7.18 ± 0.892 | 0.575 ± 0.032 | <0.1 | 19.6 ± 1.19 | 0.90 ± 0.12 | <0.1 | 0.955 ± 0.004 | 2.45 ± 0.023 | 4.76 ± 0.142 | 94.3 | |
| 5 | 8.44 ± 0.883 | 0.541 ± 0.043 | <0.1 | 20.2 ± 1.14 | 0.438 ± 0.07 | <0.1 | 1.01 ± 0.052 | 2.47 ± 0.024 | 4.12 ± 0.097 | 98.8 | |
| 7 | 9.12 ± 0.604 | 0.466 ± 0.035 | <0.1 | 19.5 ± 1.31 | 0.69 ± 0.02 | <0.1 | 1.00 ± 0.019 | 1.95 ± 0.036 | 3.76 ± 0.081 | 91.6 |
| Phenols | Inhibitors (g/L) | Max. Biomass (OD562nm) | µmax (h−1) | Residual Xylose (g/L) | LA (g/L) | Acetic Acid (g/L) | Ethanol (g/L) | LA Yield (g/g) | LA Productivity (g/(L·h)) | Max. LA Productivity (g/(L·h)) | Residual Inhibitor (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| p-coumaric acid | 1.0 | 2.36 ± 0.442 | 0.313 ± 0.012 | 5.33 ± 0.67 | 15.6 ± 0.94 | 0.40 ± 0.02 | <0.1 | 1.01 ± 0.021 | 0.782 ± 0.111 | 1.18 ± 0.105 | 98.0 |
| Syringaldehyde | 1.0 | 4.96 ± 0.554 | 0.482 ± 0.056 | <0.1 | 19.2 ± 0.88 | 0.36 ± 0.01 | <0.1 | 0.991 ± 0.032 | 1.92 ± 0.069 | 4.04 ± 0.026 | 82.0 |
| p-hydroxybenzaldhyde | 1.0 | 5.96 ± 0.602 | 0.388 ± 0.053 | <0.1 | 20.1 ± 0.49 | 1.02 ± 0.35 | <0.1 | 1.00 ± 0.010 | 2.01 ± 0.06 | 3.26 ± 0.183 | 100 |
| Vanillin | 1.0 | 6.02 ± 0.321 | 0.497 ± 0.011 | <0.1 | 19.6 | 0.36 ± 0.01 | <0.1 | 0.98 ± 0.016 | 1.96 ± 0.035 | 3.79 ± 0.187 | 94.0 |
| 3.0 | 3.3 ± 0.531 | 0.441 ± 0.021 | 13.9 ± 0.59 | 7.1 | <0.06 | <0.1 | 1.01 ± 0.020 | 0.432 ± 0.015 | 0.524 ± 0.009 | 97.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdel-Rahman, M.A.; Hassan, S.E.-D.; Fouda, A.; Radwan, A.A.; Barghoth, M.G.; Desouky, S.G. Evaluating the Effect of Lignocellulose-Derived Microbial Inhibitors on the Growth and Lactic Acid Production by Bacillus coagulans Azu-10. Fermentation 2021, 7, 17. https://doi.org/10.3390/fermentation7010017
Abdel-Rahman MA, Hassan SE-D, Fouda A, Radwan AA, Barghoth MG, Desouky SG. Evaluating the Effect of Lignocellulose-Derived Microbial Inhibitors on the Growth and Lactic Acid Production by Bacillus coagulans Azu-10. Fermentation. 2021; 7(1):17. https://doi.org/10.3390/fermentation7010017
Chicago/Turabian StyleAbdel-Rahman, Mohamed Ali, Saad El-Din Hassan, Amr Fouda, Ahmed A. Radwan, Mohammed G. Barghoth, and Salha G. Desouky. 2021. "Evaluating the Effect of Lignocellulose-Derived Microbial Inhibitors on the Growth and Lactic Acid Production by Bacillus coagulans Azu-10" Fermentation 7, no. 1: 17. https://doi.org/10.3390/fermentation7010017
APA StyleAbdel-Rahman, M. A., Hassan, S. E.-D., Fouda, A., Radwan, A. A., Barghoth, M. G., & Desouky, S. G. (2021). Evaluating the Effect of Lignocellulose-Derived Microbial Inhibitors on the Growth and Lactic Acid Production by Bacillus coagulans Azu-10. Fermentation, 7(1), 17. https://doi.org/10.3390/fermentation7010017






