Abstract
The urgent need to replace fossil fuels has seen macroalgae advancing as a potential feedstock for anaerobic digestion. The natural methane productivity (dry weight per hectare) of seaweeds is greater than in many terrestrial plant systems. As part of their defence systems, seaweeds, unlike terrestrial plants, produce a range of halogenated secondary metabolites, especially chlorinated and brominated compounds. Some orders of brown seaweeds also accumulate iodine, up to 1.2% of their dry weight. Fluorine remains rather unusual within the chemical structure. Halogenated hydrocarbons have moderate to high toxicities. In addition, halogenated organic compounds constitute a large group of environmental chemicals due to their extensive use in industry and agriculture. In recent years, concerns over the environmental fate and release of these halogenated organic compounds have resulted in research into their biodegradation and the evidence emerging shows that many of these compounds are more easily degraded under strictly anaerobic conditions compared to aerobic biodegradation. Biosorption via seaweed has become an alternative to the existing technologies in removing these pollutants. Halogenated compounds are known inhibitors of methane production from ruminants and humanmade anaerobic digesters. The focus of this paper is reviewing the available information on the effects of halogenated organic compounds on anaerobic digestion.
1. Introduction
Seaweed-derived biogas has received much interest over the past 50 years due to the high biomass production (2–20 times greater than terrestrial plants), with the additional global benefits of not competing against conventional crops for land or freshwater [1,2]. Estimates indicate that the energy potential from methane production of marine biomass is more than 100 EJ yr−1, significantly higher than the terrestrial biomass (22 EJ yr−1) or municipal solid waste (7 EJ yr−1) [3]. Besides in Solrod (Denmark), where beach-cast seaweed is co-digested with manure, seaweeds are currently not collected (naturally occurring seaweed, mainly Europe) or cultivated (predominantly Asia and Pacific) solely for biofuel production. The vast majority is cultivated or naturally harvested for either human consumption or pharmaceutical (mainly hydrocolloids) applications [4].
Marine algal biomethane production processes are currently unprofitable and unsustainable unless considered as adjuncts to processes that aim for a circular economy, such as wastewater treatment. Milledge et al., [4] concluded that wastewater treatment ponds are currently the most economical approach to the production of microalgal biofuel. This may very well also apply to macroalgae biofuels [5]. Ulva lactuca has been cultivated successfully for biomass production and bioremediation of wastewater [6]. Another strategy would be to use parts of the seaweeds, for example, the stems which are treated as waste and not used for human consumption, for biofuel production [7]. Encouragingly, Tabassum and co-workers [8] reported that the highest specific methane yield was obtained at 286 L CH4 kg VS−1 from the stipe (stem) of Laminaria digitata, when comparing methane potential from different parts (holdfast, stem, frond and bladder) of seaweeds.
Anaerobic digestion (AD) consists of a series of actions by various syntrophic groups of bacteria and archaea that convert organic materials into methane, carbon dioxide and bacterial biomass [9]. The roles of fungi and protozoa during AD are less clear, and such research is not abundant in the literature. Fungi were found in thermophilic digesters and suggested to degrade complex organic compounds [10]. A high population of protozoa correlated with higher methane production, with potential roles in different stages of AD [11].
During the four stages (Figure 1), hydrolysis, acidogenesis, acetogenesis and methanogenesis, insoluble complex polymers comprising carbohydrates, proteins, lipids and other organics are initially digested into simpler sugars, amino acids and fatty acids. These are further converted into volatile fatty acids (mainly acetic acid, propionic acid, butyric acid and valeric acid); alcohols; and different kinds of gases (CO2, H2 and NH3), which are converted into acetate, H2 and CO2 [12,13]. Methanogenesis, the final step in the anaerobic digestion, produces methane gas via two main groups of methanogenic archaea. The first group of methanogens, the acetoclastic methanogens, cleave acetate to generate CO2 and CH4, while the second group, the hydrogenotrophic methanogens, produce CH4 from the reduction of CO2 using H2 gas as the electron donor [14].
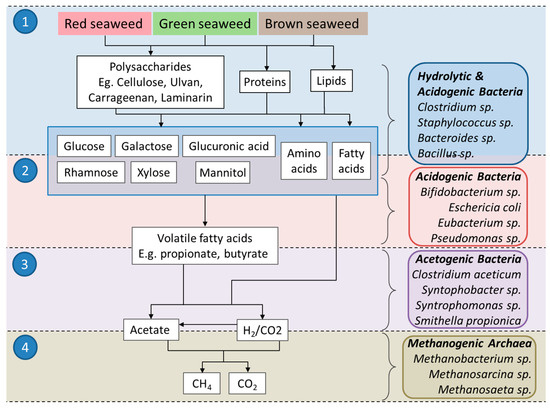
Figure 1.
A simplified schematic of the processes and microorganisms involved during AD. Numbered circles represent the stages of AD: (1) hydrolysis, (2) acidogenesis, (3) acetogenesis, (4) methanogenesis. Examples of bacteria and archaea involved in these stages [9] are shown on the right-hand side of the diagram. Schematic adapted from [17] Maneein et al. (2019).
Biogas produced from seaweed typically contains 50–70% methane, 30–45% carbon dioxide, <2% hydrogen and <3.5% hydrogen sulphide [15,16]. AD is considered a good method of choice for biomass with high water content, such as seaweed, and though hurdles still exist, the suitability of seaweed for AD is generally accepted [4,17,18].
2. Inhibitors of AD by Halogenated Organic Compounds
The differences between practical and potential biogas yields are often ascribed to the presence of inhibitors [19]. In AD, an inhibitor is a compound that will inhibit the growth of a microbe or disrupt its metabolic activity leading to lower or no methane production, and potentially reactor failure. As feedstocks for AD digesters are organic wastes from industry or agriculture, many common pollutants (chemical, biological and physical impurities) are often removed or reduced. Nevertheless, a variety of compounds, such as heavy metals, salts, ammonia, sulphides, nitrates and phenolic compounds, are known to inhibit AD and have been reviewed elsewhere [17,20,21].
These compounds can have varying effects on different stages of AD (Figure 1). For example, acetogenic bacteria can be dominated by sulphate-reducing bacteria at high sulphate concentrations. Another review showed the inhibitory effects of bromochloromethane and other chlorinated aliphatic hydrocarbons on the methanogenic population [20]. Methanogens are considered to be more sensitive to inhibitors than the other microbial groups involved in the anaerobic digestion of contaminated biomass [22]. In the following, the effects of halogenated organic compounds on the AD process are reviewed.
2.1. Chlorinated Organic Inhibitors of Anaerobic Digestion
Halogenated aliphatics are organic chemicals which are widely used in many industrial processes and persist in the environment. Of the chloro-aliphatic compounds, chloroform (CHCl3) is the most widely used and its methanogenic toxicity is well described [23,24,25,26,27]. Complete inhibition of methanogenesis was reported at concentrations ranging from 0.09 to 6 mg L−1 chloroform [23,28]. Van Beelen and van Vlaardingen [24] suggested that the extreme toxicity of chloroform might be attributed to the formation of very toxic and reactive intermediates formed during the slow anaerobic degradation. Direct inhibition of the methanogens occurs as the halogenated aliphatic compounds bind to intercellular proteins, blocking essential binding sites in the methanogenic pathway [20].
Substrate structure and concentration have an important influence on methanogenic inhibition. However, for chloro-aliphatic compounds, unlike chloro-aromatics, no relationship exists between the number of chloro-substituents and toxicity. Lian et al. [29] showed that the potential for hexachlorocyclohexane (γ, α and β) (HCH) degradation in the AD system was restricted to axial Cl atoms of HCH, and saturated chloro-aliphatics compounds were more toxic compared to unsaturated [30].
The effects of chlorophenols (mono, di, tri, tetra and penta-chlorophenol) on methanogenesis have been widely studied; the indications are that penta-chlorophenol (PCP) is the most toxic chlorophenol (50% inhibition of methane production at 8 mg L−1), which has been related to its hydrophobicity [31]. High hydrophobicity eases the adhesion onto the bacterial membranes, disrupts the gradient of protons along the membranes and interferes with cellular energy transduction, decreasing the cell growth due to the uncoupling of catabolic and anabolic cycles. Compounds of greater hydrophobicity accumulate more efficiently in membranes, causing a larger disturbance to the membrane structure [32,33,34,35].
In addition, Uberoi and Bhattacharya [36] suggested that between the individual isomers, the toxicities of di-chlorophenol and tri-chlorophenol to both propionate and acetate degradation were dependent on the substitution positions of chlorine atoms on the benzene ring. However, it is also well known that though the toxicities of chlorophenols have been investigated by many researchers, specific toxicities of these compounds are somewhat contradictory. Conflicting results could be explained by acclimation and the adaptive nature of some bacteria [34], and that the specific growth rates or the physiological state under the experimental conditions determine the sensitivity [37]. The construction of novel microbial consortia with enhanced abilities for degradation and detoxification of hazardous pollutants are now being reported [38,39,40].
2.2. Brominated Organic Inhibitors of Anaerobic Digestion
Brominated organic compounds are used as agrochemicals, pharmaceuticals and flame-retardants. Among halogenated aliphatics, it has been suggested that brominated aliphatics, in general, are more inhibitory to methanogens than their chlorinated analogues [41]. Bromochloromethane (BCM) reduced methane production between 29% and 32% in BCM treated goats (1.4 l kg−1 DW and 12% BCM by weight) with a three-fold reduction in cell count of the methanogenic archaeal population [42,43]. Brominated halogens such as 2-bromoethanesulfonic acid (BESA) have been shown to competitively inhibit the methyl transfer reaction at the terminal reductive step during methane formation using H2 and CO2 [44]. 1 μmol mL−1 BESA completely inhibited methanogenesis from 14CH3COO−, whereas 50 μmol mL−1 was required for complete inhibition of 14CO2 reduction [45]. However, a study by Bouwer and McCarty [46] found that BESA was not a potent inhibitor of methanogens. They concluded that either previous exposure of the anaerobes to halogenated organic compounds might have conferred resistance to BESA and/or degradation of the halogens occurred.
Environmental polybrominated diphenylethers pollutants undergo reductive debromination in anaerobic conditions. Formation of two nonabromodiphenyl ether and six octabromodiphenyl ether congeners proved that decabromodiphenyl ether underwent reductive debromination during anaerobic conditions [47,48], with thermophilic conditions significantly more beneficial for degradation than mesophilic conditions [49] and with enhancements of degradation efficiencies by addition of halogenated electron acceptors [50,51]. Generally, AD under thermophilic (55 °C) conditions exhibits a greater removal rate and allows a higher organic loading rate than digestion under mesophilic (35 °C) conditions. However, biological dehalogenases are unlikely to be active at these high temperatures. Indeed, temperature may cause important changes in the microbial cultures and their conversion rates of substrates. A reductive dechlorination pattern of polychlorinated biphenyl (PCB) was observed to be temperature-dependent; relatively small changes in the temperature caused changes in the rate, extent and pathway of dechlorination, with no or restricted dechlorination at higher temperatures (>37–60 °C) [52,53].
2.3. Iodinated and Fluorinated Organic Inhibitors of Anaerobic Digestion
Little information is available on dehalogenation of iodinated and fluorinated compounds by anaerobic microbial processes. A study by Harmeson and Dietz [54] tested the effects of iodine on the AD of sewage and synthetic sludges. Microscopic examinations indicated that the number of organisms in the sludge decreased as the concentration of potassium iodide (KI) increased. Additionally, gas production decreased when KI or sodium iodide (NaI) concentrations exceeded 0.2%. After 90 h of digestion, there was a 91% and almost 96% reduction in gas production with the addition of 2.5% and 5% iodine, respectively, compared to the blank control. This was also the case when NaI was added as the source of iodine.
A study assessing the mobility of iodine (from disposal facilities into the surroundings) found little interaction between anaerobic microorganisms and iodine [55]. Nevertheless, the fate of iodine in an anaerobic digestor warrants further investigation. Methanogens contain corrinoids which function as methyl carriers during acetate synthesis via reductive carboxylation. Under dark reducing conditions, iodopropane could bind to corrinoid enzymes, thereby inactivating them [56,57]. The effects of iodopropane concentrations on H2-CO2 metabolism of Methanobacterium thermoautotrophicum, Methanobacterium formicicum and Methanosarcina barkeri were concentration-dependent in all four species with the quantitative effect of iodopropane inhibition of methanogen metabolism being reduced by acetate [58].
Methyl fluoride (CH3F) supplementation (1.3%) was found to be a rather specific inhibitor of acetoclastic methanogenesis, whereas hydrogenotrophic methanogenesis was unaffected [59,60,61]. Fluorophenols are used as phenol analogues to investigate the transformation of phenol to benzoate by anaerobic, phenol-degrading consortia; the pathways of five fluoronitrobenzenes (FNBs) biotransformation were different from those of the chloronitrobenzenes under methanogenic conditions [62]. Similarly, 2-fluorobenzoate-utilising and 4-fluorobenzoate-utilising cultures were specific for fluorobenzoates and did not utilise other halogenated (chloro, bromo, iodo) benzoic acids [63]. Likewise, the aromatic compounds (0.1–0.82 mmol L−1) 2,3- or 4-iodobenzoate in addition to 2,3- or 4-bromobenzoate but not 3- or 4-fluorobenzoate underwent reductive dehalogenation by anaerobic lake sediments, and by fresh and acclimated sludge, to CH4 and CO2 indicating that the bromo and iodo substituents seem to be more readily removed [64]. Three fluorinated surfactants were degraded under both aerobic and anaerobic conditions. Surfactant 1 (aqueous solution of a fluorinated surfactant) was readily degradable under both aerobic and anaerobic conditions (28 and 60 days respectively). Surfactant 2 (a nonionic fluorinated surfactant) was degraded under aerobic but not anaerobic conditions. Surfactant 3 (an anionic fluorinated surfactant) was neither degraded under aerobic nor anaerobic conditions. Under anaerobic conditions, surfactant 3 inhibited the methane production rate of sludge from a digester with an EC50 of 160 mg L−1 [65]. Removal of perfluorosulfonate (PFOS) and subsequently of perfluorooctanoic acid (PFOA) was observed in an anaerobic reactor containing sludge from wastewater treatment plants. Neither of the two compounds could be found in the reactor after 26 days of degradation. No metabolites or increases in fluoride concentration were detected [66]. Though reports on microbially catalysed C–F cleavage reactions exist [67,68], there are no reports on anaerobic reductive defluorination, as most studies on the degradation of fluorinated chemicals have focused on aerobic conditions.
3. Anaerobic Dehalogenation
Microorganisms are now known to be able to remove halogens from aliphatic compounds by the activity of enzymes known as dehalogenases [69]. Reductive dehalogenation is mainly known to occur under anaerobic conditions and is the initial step in the anaerobic biodegradation of most aryl halides [29]. Reductive dehalogenation reactions catalysed by anaerobic bacteria are either co-metabolic processes or linked to respiration—a process termed dehalorespiration. Lian et al. [29] reported the presence of Dehalococcoidia, a taxon containing organohalide-respiring bacteria, in AD reactors responding to hexachlorocyclohexane (HCH) addition, highlighting the intrinsic potential of the AD microbiota to deal with halogenated compounds. Indeed, Lian and co-workers [70] reported that in continuous stirred tank reactor setups, HCH contaminated plants showed no negative influence on methane production, suggesting HCHs as electron acceptors can be co-metabolised by microorganisms. Concentrations of HCH higher than 150 mg L−1, however, caused a temporary inhibition on acetoclastic methanogenesis [70].
Chloroform has been shown to completely inhibit the production of methane even at very low concentrations (0.09 mg L−1), whereas other chlorinated compounds such as perchloroethylene did not inhibit methanogenesis [23]. The authors suggested that inhibition of methanogenesis and dechlorination is determined by both the extent of chlorination and the molecular structure of polychlorinated hydrocarbons. It is now well recognised that poly-halogenated substrates are more easily dehalogenated under anaerobic conditions, consistent with a more negative free energy change during reductive dehalogenation, and poly-chlorinated phenols have a more lipophilic nature and are more strongly sorbed by cells [69,71]. Dehalogenases have also been shown to be isomer selective and reductive dehalogenation to be isomer specific [72,73,74].
The dehalogenases make use of fundamentally different strategies with a common mechanism to cleave carbon-halogen bonds. The reductive dehalogenation mechanism consists of the carbon-halogen bond being cleaved catalysed by the dehalogenases replacing the halogen by hydrogen (hydrogenolysis). An active-site carboxylate group attacks the substrate C atom bound to the halogen atom to form an ester intermediate and a halide ion with subsequent hydrolysis of the intermediate. During dihaloelimination (vicinal reduction), two halogens from adjacent carbon atoms are removed, and an additional bond between the carbon atoms is created. Other dehalogenation reactions exist and are described elsewhere [69].
Hydrogenolysis can transform alkyl or aryl halides, whereas vicinal reduction can transform only alkyl halides. Both processes require an electron donor. In a one-step reaction, the transfer of electrons and one proton is carried out by H2, or dehalogenation might occur in a two-step reduction by an electron donor (reduced organic substrate) and with proton abstraction from the solvent [69]. Methanogens are hydrogenotrophs, and methane production is mainly produced by the conversion of CO2 and H2 into CH4; however, acetoclastic methanogenesis where CH3COOH is converted into CH4 and CO2 is another possible pathway. If H2 is diverted from hydrogenotrophic methanogens to the dehalogenation processes, methane production could be adversely affected. However, even though methane production consumes a large amount of H2, Yang and McCarty [75] reported that it does not compete for dechlorination as the threshold of hydrogen required for dechlorination is very low.
On halogenated degradations, most studies have focused on chlorinated compounds. Information on iodinated and brominated compounds is mainly a result of studies including these as analogues to the chlorinated compounds. Further, little literature exists on the biodegradation of naturally occurring brominated compounds and even less for iodinated or fluorinated. Though many studies have examined the relation between dechlorinating bacteria and methanogens, the exact mechanisms by which methanogens affect dechlorinating communities are still somewhat unclear [76], and even more so for communities metabolising brominated and iodinated compounds, albeit possibly being carried out by the same bacterial groups. For example, anaerobic dehalogenation by a strain of Desulfomonile tiedjei (obligate anaerobe) was shown to dehalogenate 3-chlorobenzoate, 3-bromobenzoate and 3-iodobenzoate, all producing benzoate though at different rates with 3-iodobenzoate more readily degraded of the three analogues [72]. In contrast, several strains of Anaeromyxobacter dehalogenans can dehalogenate a number of ortho-halogenated phenols including 2-chloro-and 2-bromophenol (by oxidising acetate) but neither 2-fluoro-and 2-iodophenols [77]. However, in another study, 2-chlorophenol but not 2-bromophenol was dehalogenated by Desulfovibrio dechloracetivorans also using acetate as the electron donor [78]. Though data are somewhat sparse, Allard and Neilson [71] concluded that pathways of degradation and transformation of organoiodine, organochlorine and organobromine are broadly similar, with the degradation of organoiodine and organobromine compounds generally proceeding more easily compared to their chlorinated and fluorinated analogues [71,79].
4. Halogenated Compounds in Macroalgae and Their Effect on AD
Hundreds of secondary halogenated metabolites have been isolated from macroalgae; many of them have been shown to possess strong antimicrobial properties, and they can inhibit a wide range of microorganisms, including Escherichia coli, Pseudomonas aeruginosa and Staphylococcus spp. strains [80,81]. Though one might expect that the broad antimicrobial activity might extend to anaerobes, this remains to be determined. Halogens are incorporated into various components of macroalgae, such as into peptides, polyketides, indoles, terpenes, acetogenins, phenols, volatile hydrocarbons and fatty acids [82,83]. Macroalgal metabolites are either iodinated, chlorinated or brominated, with fluorinated metabolites being rare [82]. Brominated and chlorinated secondary metabolites are predominant in red (90%) and green (7%) macroalgae, while iodinated secondary metabolites are more dominant in brown macroalgae [82]. Chlorinated and brominated secondary metabolites make up less than 1% of the secondary metabolites in brown macroalgae [82]. The formation of halogenated metabolites is catalysed by haloperoxidases (bromoperoxidases, chloroperoxidases and iodoperoxidases) via oxidation of halides in the presence of hydrogen peroxide [84]. Haloperoxidases, and iodo- and bromoperoxidases, have been isolated from red and brown seaweeds (many vanadium dependent enzymes) [85,86,87,88]. However, biohalogenation is a complex process and might not only be mediated by conventional haloperoxidases. Mechanisms have been reviewed in [71].
Halogenation of macroalgal components is associated with cell wall strengthening and chemical defence mechanisms as halogenated metabolites were discovered with antibacterial, antifungal and antioxidant properties [82,89,90]. The antimicrobial properties of halogenated compounds in macroalgae may have inhibitive effects on the microbial community in anaerobic digesters. Methanogenesis was indicated to be inhibited by halogenated aliphatics [91], which can be produced by macroalgae [92]. Saccharina latissima can produce a total of 205 mg of aliphatic organobromine and organochlorine per kg of seaweed [92].
Macroalgae not only accumulate but also emit concentrated bromomethanes and are well recognised as a globally significant source of organic bromine to the atmosphere [93,94,95]. Evolution rates of bromoform, dibromomethane and dibromochloromethane correlate with the bromoperoxidase activities in the algae, and with the bromomethanes released in the air during the growth phase of the algae (winter and early spring) corresponding to the high bromoperoxidase activity in the algae [95]. Release of CHCl3 and CH3I has also been reported [96]. The inhibitory potential of these aliphatic halogenated compounds on AD is yet to be explored. The following section discusses halogenated compounds discovered in seaweed and their potential inhibitory effects on AD (where data exist).
4.1. Iodinated Compounds
Iodine is essential for many biotas, including humans, where iodinated tyrosine plays a vital role in thyroid function. Compared to terrestrial plants where iodine content is <1 mg kg−1, many marine species are rich in iodine. In brown seaweed, iodine content can be up to 1.2% (dry weight) and almost 5% in young macroalgae [97]. Laminaria japonica has been used in China for centuries as a dietary iodine supplement [98]. Only small amounts of seaweeds in a portion of food are needed for it to become a “good source” of iodine and to allow its associated health benefits to be noted on packaging under EU (1924/2006) Approved Health Claims regulations. However, the risks of excess iodine intake from dietary sources such as seaweeds are starting to emerge [99,100].
In seaweed, iodine occurs as inorganic, I− and IO3− and as organic iodine; the proportions, bioavailabilities and toxicities of the chemical species widely differ depending on the seaweed species but also the area of origin, age and condition of the plant [101,102]. However, the total iodine content is usually only investigated and reported for marine algae in the literature (Table 1). Most iodine is water-soluble (I− and IO3−) and can be completely removed after leaching thrice [101]. Non-water soluble iodine is mainly bound to different proteins, cellulosic materials, fucoidan, alginic acid and polyphenols [103,104]. Hou et al. [101] reported that the major portion of iodine in seaweed is iodide (I−) accounting for 60% of total soluble iodine and more than 94% of inorganic iodine, while the content of IO3− is low. 51% of total organic iodine compounds in Laminaria japonica were determined to be iodo-amino acids (mainly 3,5-diiodotyrosine); 49% of iodo-amino acids existed in free state amino acids and another 2% in the combined state [105].

Table 1.
Iodine content in marine algae.
Dominguez-Gonzalez et al. [106] estimated the in vitro bioavailability of iodine from different commercialised seaweeds and reported that iodine is available after gastrointestinal digestion for absorption (bioaccessibility: 49–82%), kombu (brown algae) being the seaweed with the highest bioaccessibility.
Although the effect of iodine on AD is not fully known, Sheppard and Hawkins [115] demonstrated that anaerobes seem to be more sensitive to iodine than aerobes; in agar plates with a broad spectrum of cultures, severe toxicity of iodine to microorganisms did not occur under aerobic conditions at concentrations ≤2000 mg I L−1. However, toxic effects were evident under anaerobic conditions with significantly fewer anaerobes in bog groundwater (110–980 mg I L−1). Aqueous iodine is generally unstable and exists in a complex equilibrium with several species of iodine (I−, I2, I3−, I5−, I62, HOI, OI−, HI2O−, IO2− and H2OI+), with molecular (I2) and HOI being primarily responsible for the biological action [116]. Iodine rapidly penetrates the cell membrane of microorganisms and attacks the key amino acids, such as methionine and cystine, fatty acids and nucleotides leading to cell death [117].
The biomethane potential of seaweeds has been demonstrated in various works [17]. For example, Chynoweth [118] reported biomethane yields between 260 and 280 L kg−1 vs. for AD of macrophyte biomass from the Laminaria genera. The biomethane yield for Saccharina latissima was 342 L kg−1 vs. [119], which is ~81% of the theoretical yield even though this seaweed can accumulate up to 2.6 mg I g−1 seaweed. Other seaweeds found to accumulate less iodine, such as S. muticum (Table 1), produced methane yields as low as 17% of the theoretical yield [120]. The reason for lower experimental methane yields compared to theoretical yields observed in most studies is likely due to the presence of other AD organic inhibitors [4,17].
As most iodine is water-soluble, it is easily removed by a washing pre-treatment before AD. Mixed-effects of washing seaweed on methane production have been found for different species [17] with washed and cut L. digitata also found to have higher methane yields compared to unwashed and cut, and hot water washing (40 °C) of L. digitata increased vs. content by up to 31%, due to the removal of ash and nitrogenous compounds, compared to cold water washed (15 °C) and unwashed samples. The lower ash to vs. ratio for hot water washing, proposed to contribute to higher methane yields, do not seem to correlate with the changes in methane content for other washing experiments [121].
4.2. Brominated Compounds
Washed brown seaweed investigated by [92] contained up to 360 mg organobromine kg−1 seaweed, the majority being aromatic compounds. The degree of bromination appears to be a factor influencing the potency of brominated compounds as antimicrobials [122]. The exudation of antifungal halogenated metabolites, such as bromophycolides, were found on specific areas of the macroalgal surfaces of Callophycus serratus [123]. Bromophenols isolated from Rhodomela confervoides (bis (2,3-dibromo-4,5-dihydroxybenzyl) ether) and Polysiphonia morrowii (3-bromo-4,5-dihydroxybenzaldehyde) also showed antibacterial and antiviral properties [122,124]. It is likely that these compounds also identified in the brown seaweed, Leathesia nana, could also elicit these antimicrobial responses [81,125]. L. digitata was indicated to release hypobromous acid that prevented biofouling by interfering with bacterial communication [126], in a similar manner to brominated furanones from red macroalgae [127].
Certain compounds, such as the fungicide 2,4,6-tribromophenol, found in red macroalgae (Delisea fimbriata), were also found in green (Ulva lactuca) and brown macroalgae (Eisenia bicyclis and Ecklonia kurome) [93,128,129]. The inhibitory effects of bromophenols on anaerobic digestion have been less explored compared to chlorophenols. The degradation of phenols by a methanogenic consortium was revealed to be slower and more difficult with the substitution of halogenated compounds in phenol groups [130]. Nevertheless, anaerobic microorganisms could overcome initial inhibition by 2, 4-dibromophenol and this inhibition could be eliminated when microbes were pre-incubated with the bromophenol [131]. Similarly, anaerobic microorganisms could adapt to highly toxic chlorophenols and subsequently degrade these compounds [132]. The difficulty in degrading halogenated phenols, however, increases with an increase in the concentration and diversity of the types of phenols present [133]. This highlights the importance of adaption to halogenated compounds by the microorganisms in anaerobic digesters.
The antimethanogenic properties of halogenated metabolites with antimicrobial properties were discovered in red seaweed. The release of bromoform and dibromoacetic acid macroalgal surfaces of the red macroalgae Asparagopsis armata, were associated with antibacterial properties against epiphytic bacteria [80]. The concentration of bromoform produced by Asparagopsis taxiformis (1.72 mg of bromoform per gram dry weight) was sufficient to cause antimicrobial effects against anaerobic microorganisms, namely, the archaea population in ruminal fluid [134]. It is, therefore, likely that the archaeal population in an anaerobic digester will also be inhibited.
The ability of algae to reduce microbial methanogenesis has recently received favourable press, and there has been considerable research and commercial interest in the use of brominated compounds, and bromoform (tribromomethane) in particular, on reducing methane emissions from ruminants [134,135,136]. Livestock dietary inclusion (0.5% to 2%) of algal biomass from the red macroalgae Asparagopsis sp. have been shown to reduce up to 99% CH4, attributed to the CH4 inhibitor bromoform, thereby reducing greenhouse gas emissions (GHG), in which, CH4 is the most significant single source of GHG emissions and 25 times more potent than CO2 [137,138,139]. The effects of other brominated metabolites with antimicrobial properties on anaerobic microorganisms will need to be investigated. The use of seaweeds on the management of methanogenesis in ruminants has recently been extensively reviewed by McCauley et al. [139]. The review highlighted that the inhibition concentration, characteristics, adverse effects of halogenated compounds and their application still require investigation due to their inconsistent efficacy. Nevertheless, the results suggest that the use of seaweed as a feedstock for AD could be limited by the availability of anti-methanogenic and antimicrobial properties of halogenated metabolites in seaweed.
Further studies are needed to confirm the inhibitory effects on the highly adaptable anaerobic consortium of microorganisms during AD over a longer period. Bromoform, with concentrations as low as 1 µM, was shown to inhibit methanogenesis in the first 24 h [134]. The longest assay period of the halogenated halocarbons in ruminal fluid was 72 h. Low initial methane production has been correlated with low overall methane potential [140].
4.3. Chlorinated Compounds
Total chloride contents measured in seaweeds are generally higher than bromide content. The inorganic chloride content was found to be highest in brown seaweed (13.5% DW in Himanthalia elongata) compared to the red algae (3.4% in Gigartina pistillata) [141]. In contrast, the highest inorganic bromide content was 0.03% in Bifurcaria bifurcata and Mastocarpus stellatus [141]. The total aliphatic and aromatic chloride content (630 mg kg−1 DW) was found to be higher than the total aliphatic and aromatic bromide content (295 mg kg−1 DW) in the brown seaweed, S. latissima [92]. The total chloride content can reach up to 238 g kg−1 DW in Codium tomentosum, a green macroalga [142]. The chloride content of seaweeds was suggested to be dependent on the salinity of seawater and the depth at which the seaweed grows [141].
Despite the higher chloride content, halogenated compounds which are exclusively chlorinated with antimicrobial activity are less reported in the literature compared to brominated compounds. An acyclic chlorinated monoterpene isolated from the red macroalgae, Portieria hornemannii, showed antifungal activity against Penicillium oxalixum at 0.1 µg compared to Miconazole at 0.8 µg [143]. Another exclusively chlorinated compound was a chlorinated C15 acetogenin which showed potent antibacterial activity against some Staphylococcus aureus strains [129].
Chlorinated compounds reported showing antimicrobial activity are often also brominated. This includes a range of compounds isolated from the red macroalgae genus, Laurencia spp., such as puertitols and pannosanol, with antibacterial activity; 9-deoxyelatol, with antibacterial and antifungal properties [144]; elatol and obtusol, with antiprotozoal activity [145]. Other red macroalgal genera such as Plocamium, Ochtodes and Beckerella can also produce compounds with some of these properties. Hence, there is potential for the disruption of the microbial community within AD by these halogenated compounds produced by macroalgae.
The inhibitory properties of these compounds on AD are lacking in the literature. However, their potential inhibitory effects on AD may be inferred from studies that compare the inhibitory potential of the compounds to antibiotics. For example, fractionated compounds containing 0.09% chlorobenzene from A. taxiformis showed antimicrobial properties comparable to those of doxycycline [146], an antibiotic that can inhibit acetogenic bacteria during AD [147]. The potential inhibitory effects of the compounds isolated from A. taxiformis on AD, therefore, warrant further investigation.
Furthermore, compounds isolated from macroalgae show antimicrobial properties against some genera of bacteria also present in AD (Figure 1). These include a range of compounds (examples in Table 2) from Laurencia spp. and Rhodomela confervoides such as allolaurinterol acetate and bis (2,3-dibromo-4,5-dihydroxybenzyl) ether (a bromophenol also found in L. nana) which have inhibitory effects on Pseudomonas spp., E. coli and Staphylococcus spp. [124,148]. Elucidating the antibacterial activity of the halogenated compounds against these microorganisms under anaerobic conditions may, therefore, implicate the macroalgae’s potential as a novel feedstock for biogas production.

Table 2.
Antibacterial activities of compounds isolated from macroalgae.
4.4. Fluorinated Compounds
Unlike naturally occurring organobromine, and to a lesser extent organoiodine metabolites, produced mostly by marine biota, naturally occurring organo-fluorines are structurally limited and confined to higher plants [71]. Additionally, organofluorines exist only in the monovalent state whereas other halogens may exist at oxidation levels up to 7. El-Said and El-Sikaily [157] reported fluoride concentration in seaweed species of different classes from 40.97 to 177.88 mg g−1 in red algae (Jania rubens, Gracilaria compressa, Gracilaria verrucosa, Pterocladia capillacea and Hypnea musciformis), from 82.03 to 128.23 mg g−1 in green algae (Ulva lactuca, Codium tomentosum and Enteromorpha intestinalis) and from 144.74 to 166.66 mg g−1 in brown algae (Colpomenia sinuosa and Sargassum linifolium). These values were all higher than previously reported concentrations by Masoud et al. [158] who reported a fluoride concentration ranging between 19.17 and 53.70 mg g−1 in five different macroalgae (Codium bursa, Ulva lactuca, Amphiroa sp., Asparagoposis sp. and Sargassum sp.). Though all harvested from similar geographical locations, seasonal, environmental, physiological and species variation will affect the reported ranges.
The C-F bond is one of the strongest and hard to break. The order of C-Halogen bond strength is C-F > C-Cl > C-Br > C-I. In addition, fluorine has the highest electronegativity of all elements, conversely, polarisability increases in the order F < Cl < Br < I; fluorine atoms exhibit very low polarisability because their electrons are tightly held, unlike iodine atoms which are easily polarised. Additionally, fluorine is the strongest known oxidising agent and iodine the least reactive halogen, though still reactive enough to form compounds with most element [159]. The strong C-F bond and higher electronegativity of fluorine could make organofluorines more persistent compared to structurally similar chloro, -bromo and iodinated compounds.
Organo-halogenated compounds are moderate to extremely hydrophobic which in many cells causes toxicological consequences as the permeability barrier of biological membranes are highly hydrophobic, allowing the organohalogens to diffuse through and readily be taken up by any cells. This reduced water solubility, however, could cause increased bioavailability to cells during anaerobic degradation. Degradation and effect on AD remain to be determined.
5. Bioremediation as Part of Refining Macroalgae for Biofuel Production and Its Challenges
The presence of halogen organic compounds has been confirmed in municipal and industrial wastewater. Chlorinated hydrocarbons are frequently cited as important contributors to the inhibition of AD, and aliphatic organochlorides include many of the most toxic and environmentally persistent pollutants [160]. In addition, though biodegradable, chlorophenols which are widely used in pesticides, herbicides, antiseptics and fungicides, are highly persistent in aquatic environments and listed as a priority pollutant by the U.S. Environmental Protection Agency [161]. Almost 50% of all compounds on the priority pollutant list under the clean water act, are chlorinated.
Seaweeds are to this date not cultivated solely for energy purposes due to the high costs of harvesting, concentrating and drying. AD of algal biomass could theoretically reduce costs associated with drying wet biomass before processing. Currently, the vast majority of seaweed is cultivated or naturally harvested for either human consumption or pharmaceutical (hydrocolloids) applications but none for the sole purpose of energy [162].
If macroalgae are to be harvested or cultivated for biofuel production, an economic approach to the production could involve bioremediation. Biosorption via seaweed has become an alternative to the existing technologies in effectively removing these pollutants from wastewater as the majority of macroalgae grow submerged in water and can take up dissolved nutrients across their entire surface area which make them ideal for extraction of nutrients from nutrient-rich wastewaters [163]. For example, the green seaweed Ulva is presently successfully used in several bioremediation processes [6,164,165] and has been shown to grow on seawater and effluent from a sewage treatment plant [166] or on reject/drainage wastewater from dewatered sludge [6]. One of the main restrictive factors of growing marine species on wastewater effluents is low salinity. However, Tsagkamilis [166] suggested cultivation on a mixture using a ratio of 60% sewage effluent to 40% seawater to overcome this issue. Additionally, the authors suggested that Ulva lactuca has high survivability in low salinity waters.
A by-product of the anaerobic digestion process is a nutrient-rich digestate which can be used as a fertiliser for agriculture and thus decrease the use of energy-intensive mineral fertilisers [167]. For example, Han et al., [168] grew microalgae in iodine-rich groundwater (2.0 mg iodide L−1). The algae were stabilised in an anaerobic environment. Of the initial algae iodine concentration (0.35 ± 0.05 mg g−1 VS), 0.19 ± 0.01 mg g−1 VS remained in the solid phase in the anaerobic environment. The authors concluded that the stabilised material had potential to be used as an iodine-rich fertiliser but also noted that the iodine content of the stabilised material was five times higher than what has been reported to be an effective I-fertilisation dose [168]. Biomass for AD and the resulting digestate as part of a bioremediation process must, therefore, be carefully characterised prior to use.
Piringer and Bhattacharya [169] examined the fate of PCP in anaerobic acidogenic systems. Extraction of PCP in serum bottles showed sorption to biomass was the dominant mechanism for PCP removal in acidogenic cultures. The authors concluded that, contrary to expectations, the usefulness of anaerobic acidogenic cultures for degradation of PCP could not be substantiated [169]. As the effectiveness of the AD digestion process and therefore biogas production is limited by pollutants inhibiting the microorganisms, in particular, the methanogens. Acclimation can develop the ability or capacity of the microbes to degrade pollutants faster. Acclimation of an anaerobic sludge to continuous exposure of a toxicant was reported to be dependent on the nature of the toxicant [170]. Acclimation to PCP over 6 months improved the ability of the methanogens to remove chlorine from ortho, meta and para positions whereas the unacclimated methanogens only degraded PCP from the ortho position [171]. Acclimation was found to increase the PCP threshold level of the methanogens from 200 µg L−1 to 600 µg L−1 [172]. Likewise, Boucquey et al. [173] developed a microbial consortium acclimated to a mixture of about 30 chlorinated aliphatics in a fixed-film stationary-bed bioreactor. They observed a complete disappearance of hexachloroethane and its lower homologues, penta, tetra and trichloroethane, present in the toxic mixture. Additionally, octachlorocyclopentene, carbon tetrachloride, trichloroethylene, tetrachloroethylene and hexachloro-1,3-butadiene also completely disappeared, demonstrating the possibility of concurrent degradation of highly toxic chlorinated compounds [173]. The IC50 concentration for chloroform was also reported to increase up to 50 mg L−1 in acclimated methanogenic consortia [174].
6. Conclusions
The shift towards a bio-based economy has put the focus on the development of a seaweed-based biofuel industry. AD is a well-established technology which generates biogas from the degradation of organic matter through a cascade of bacterial and archaeal metabolic pathways. It is also the preferred method for the generation of biofuel from biomass with a high-water content, such as seaweed. Seaweed biomass production as part of bioremediation of wastewater could be one way forward for economic seaweed cultivation for biofuel.
Although microorganisms can remove halogens from aliphatic compounds by the activity of dehalogenases, many halogenated compounds are nevertheless reported as strong inhibitors of methanogenesis, and microorganisms have limited catabolic pathways for the complete mineralisation of these compounds. This, of course, has received much positive attention from scientists taking advantage of seaweed’s ability to inhibit the ruminal methanogenic population of livestock, thereby reducing CH4 greenhouse gasses. However, to achieve higher rates and better yields during AD of halogenated organic compounds, it is necessary to gather more information on the organisms involved and to learn more about their nutritional requirements and metabolic pathways. In addition, the ability of microorganisms to adapt to inhibitory substances, using their inducible enzyme system to produce dehalogenases, has been proven to improve the waste treatment efficiency and methane yields significantly.
Overall, a sustainable route to biofuel production using macroalgae as the AD feedstock may be possible when the biosorption ability of seaweed is combined with the use of anaerobic microbial processes in the removal and recovery of halogenated toxic pollutants.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kerrison, P.D.; Stanley, M.S.; Edwards, M.D.; Black, K.D.; Hughes, A.D. The cultivation of European kelp for bioenergy: Site and species selection. Biomass Bioenergy 2015, 80, 229–242. [Google Scholar] [CrossRef]
- Kraan, S. Mass-cultivation of carbohydrate rich macroalgae, a possible solution for sustainable biofuel production. Mitig. Adapt. Strateg. Glob. Chang. 2013, 18, 27–46. [Google Scholar] [CrossRef]
- Chynoweth, D.P.; Owens, J.M.; Legrand, R. Renewable methane from anaerobic digestion of biomass. Renew. Energy 2001, 22, 1–8. [Google Scholar] [CrossRef]
- Milledge, J.; Nielsen, B.; Maneein, S.; Harvey, P. A brief review of anaerobic digestion of algae for bioenergy. Energies 2019, 12, 1166. [Google Scholar] [CrossRef]
- Gao, K.; McKinley, K.R. Use of macroalgae for marine biomass production and CO2 remediation: A review. J. Appl. Phycol. 1994, 6, 45–60. [Google Scholar] [CrossRef]
- Sode, S.; Bruhn, A.; Balsby, T.J.S.; Larsen, M.M.; Gotfredsen, A.; Rasmussen, M.B. Bioremediation of reject water from anaerobically digested waste water sludge with macroalgae (Ulva lactuca, Chlorophyta). Bioresour. Technol. 2013, 146, 426–435. [Google Scholar] [CrossRef]
- Kuroda, K.; Fujii, K.; Akase, M. Experimental Study of Hydrogen Fermentation of Seaweeds for Efficient Operation. In Proceedings of the 2018 OCEANS—MTS/IEEE Kobe Techno-Oceans (OTO), Kobe, Japan, 28–31 May 2018; pp. 1–4. [Google Scholar]
- Tabassum, M.R.; Xia, A.; Murphy, J.D. Biomethane production from various segments of brown seaweed. Energy Convers. Manag. 2018, 174, 855–862. [Google Scholar] [CrossRef]
- Amani, T.; Nosrati, M.; Sreekrishnan, T.R. Anaerobic digestion from the viewpoint of microbiological, chemical, and operational aspects—A review. Environ. Rev. 2010, 18, 255–278. [Google Scholar] [CrossRef]
- Sun, W.; Yu, G.; Louie, T.; Liu, T.; Zhu, C.; Xue, G.; Gao, P. From mesophilic to thermophilic digestion: The transitions of anaerobic bacterial, archaeal, and fungal community structures in sludge and manure samples. Appl. Microbiol. Biotechnol. 2015, 99, 10271–10282. [Google Scholar] [CrossRef]
- Prabhakaran, P.; Bhasi, A.; Ali, S.; Narayanan, N.; Balakrishnan, M.V.; Bhaskaran, K. Community dynamics and significance of anaerobic protozoa during biomethanation of lignocellulosic waste. Renew. Energy 2016, 98, 148–152. [Google Scholar] [CrossRef]
- Zhang, C.; Su, H.; Baeyens, J.; Tan, T. Reviewing the anaerobic digestion of food waste for biogas production. Renew. Sustain. Energy Rev. 2014, 38, 383–392. [Google Scholar] [CrossRef]
- Appels, L.; Van Assche, A.; Willems, K.; Degrève, J.; Van Impe, J.; Dewil, R. Peracetic acid oxidation as an alternative pre-treatment for the anaerobic digestion of waste activated sludge. Bioresour. Technol. 2011, 102, 4124–4130. [Google Scholar] [CrossRef] [PubMed]
- Parkin, G.F.; Owen, W.F. Fundamentals of Anaerobic Digestion of Wastewater Sludges. J. Environ. Eng. 1986, 112, 867–920. [Google Scholar] [CrossRef]
- Vanegas, C.H.; Bartlett, J. Green energy from marine algae: Biogas production and composition from the anaerobic digestion of Irish seaweed species. Environ. Technol. 2013, 34, 2277–2283. [Google Scholar] [CrossRef] [PubMed]
- Peu, P.; Sassi, J.-F.; Girault, R.; Picard, S.; Saint-Cast, P.; Béline, F.; Dabert, P. Sulphur fate and anaerobic biodegradation potential during co-digestion of seaweed biomass (Ulva sp.) with pig slurry. Bioresour. Technol. 2011, 102, 10794–10802. [Google Scholar] [CrossRef]
- Maneein, S.; Milledge, J.J.; Nielsen, V.B.; Harvey, J.P. A review of seaweed pre-treatment methods for enhanced biofuel production by anaerobic digestion or fermentation. Fermentation 2018, 4, 100. [Google Scholar] [CrossRef]
- Sutherland, A.D.; Varela, J.C. Comparison of various microbial inocula for the efficient anaerobic digestion of Laminaria hyperborea. BMC Biotechnol. 2014, 14, 7. [Google Scholar] [CrossRef]
- Milledge, J.; Nielsen, B.; Harvey, P. The inhibition of anaerobic digestion by model phenolic compounds representative of those from Sargassum muticum. J. Appl. Phycol. 2018. [Google Scholar] [CrossRef]
- Jha, P.; Schmidt, S. Reappraisal of chemical interference in anaerobic digestion processes. Renew. Sustain. Energy Rev. 2017, 75, 954–971. [Google Scholar] [CrossRef]
- Chen, J.L.; Ortiz, R.; Steele, T.W.J.; Stuckey, D.C. Toxicants inhibiting anaerobic digestion: A review. Biotechnol. Adv. 2014, 32, 1523–1534. [Google Scholar] [CrossRef]
- Wang, H.; Fotidis, I.A.; Angelidaki, I. Ammonia effect on hydrogenotrophic methanogens and syntrophic acetate-oxidizing bacteria. FEMS Microbiol. Ecol. 2015, 91, 5512–5520. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Smith, G.B. Inhibition of methanogenesis by C1- and C2-polychlorinated aliphatic hydrocarbons. Environ. Toxicol. Chem. 2000, 19, 2212–2217. [Google Scholar] [CrossRef]
- Van Beelen, P.; van Vlaardingen, P. Toxic effects of pollutants on the mineralization of 4-chlorophenol and benzoate in methanogenic river sediment. Environ. Toxicol. Chem. 1994, 13, 1051–1060. [Google Scholar] [CrossRef]
- Weathers, L.J.; Parkin, G.F. Toxicity of chloroform biotransformation to methanogenic bacteria. Environ. Sci. Technol. 2000, 34, 2764–2767. [Google Scholar] [CrossRef]
- Bagley, D.M.; Lalonde, M.; Kaseros, V.; Stasiuk, K.E.; Sleep, B.E. Acclimation of anaerobic systems to biodegrade tetrachloroethene in the presence of carbon tetrachloride and chloroform. Water Res. 2000, 34, 171–178. [Google Scholar] [CrossRef]
- Maymó-Gatell, X.; Nijenhuis, I.; Zinder, S.H. Reductive dechlorination of cis-1,2-dichloroethene and vinyl chloride by “Dehalococcoides ethenogenes”. Environ. Sci. Technol. 2001, 35, 516–521. [Google Scholar] [CrossRef]
- Gunsalus, R.P.; Wolfe, R.S. ATP activation and properties of the methyl coenzyme M reductase system in Methanobacterium thermoautotrophicum. J. Bacteriol. 1978, 135, 851–857. [Google Scholar] [CrossRef]
- Lian, S.; Nikolausz, M.; Nijenhuis, I.; da Rocha, U.N.; Liu, B.; Corrêa, F.B.; Saraiva, J.P.; Richnow, H.H. Biotransformation of hexachlorocyclohexanes contaminated biomass for energetic utilization demonstrated in continuous anaerobic digestion system. J. Hazard. Mater. 2020, 384, 121448. [Google Scholar] [CrossRef]
- Sanz, J.L.; Rodríguez, N.; Amils, R. Effect of chlorinated aliphatic hydrocarbons on the acetoclastic methanogenic activity of granular sludge. Appl. Microbiol. Biotechnol. 1997, 47, 324–328. [Google Scholar] [CrossRef]
- Patel, G.B.; Agnew, B.J.; Dicaire, C.J. Inhibition of pure cultures of methanogens by benzene ring compounds. Appl. Environ. Microbiol. 1991, 57, 2969–2974. [Google Scholar] [CrossRef]
- Liu, Y.; Tay, J.-H. Strategy for minimization of excess sludge production from the activated sludge process. Biotechnol. Adv. 2001, 19, 97–107. [Google Scholar] [CrossRef]
- Sikkema, J.; de Bont, J.A.; Poolman, B. Mechanisms of membrane toxicity of hydrocarbons. Microbiol. Rev. 1995, 59, 201–222. [Google Scholar] [CrossRef]
- Heipieper, H.J.; Weber, F.J.; Sikkema, J.; Keweloh, H.; de Bont, J.A.M. Mechanisms of resistance of whole cells to toxic organic solvents. Trends Biotechnol. 1994, 12, 409–415. [Google Scholar] [CrossRef]
- Sierra-Alvarez, R.; Lettinga, G. The effect of aromatic structure on the inhibition of acetoclastic methanogenesis in granular sludge. Appl. Microbiol. Biotechnol. 1991, 34, 544–550. [Google Scholar] [CrossRef]
- Uberoi, V.; Bhattacharya, S.K. Effects of chlorophenols and nitrophenols on the kinetics of propionate degradation in sulfate-reducing anaerobic systems. Environ. Sci. Technol. 1997, 31, 1607–1614. [Google Scholar] [CrossRef]
- Ennik-Maarsen, K.A.; Louwerse, A.; Roelofsen, W.; Stams, A.J.M. Influence of monochlorophenols on methanogenic activity in granular sludge. Water Res. 1998, 32, 2977–2982. [Google Scholar] [CrossRef]
- Ali, S.S.; Kornaros, M.; Manni, A.; Sun, J.; El-Shanshoury, A.E.-R.R.; Kenawy, E.-R.; Khalil, M.A. Enhanced anaerobic digestion performance by two artificially constructed microbial consortia capable of woody biomass degradation and chlorophenols detoxification. J. Hazard. Mater. 2020, 389, 122076. [Google Scholar] [CrossRef]
- Ali, S.S.; Abomohra, A.E.-F.; Sun, J. Effective bio-pretreatment of sawdust waste with a novel microbial consortium for enhanced biomethanation. Bioresour. Technol. 2017, 238, 425–432. [Google Scholar] [CrossRef]
- Liang, J.; Fang, X.; Lin, Y.; Wang, D. A new screened microbial consortium OEM2 for lignocellulosic biomass deconstruction and chlorophenols detoxification. J. Hazard. Mater. 2018, 347, 341–348. [Google Scholar] [CrossRef]
- Belay, N.; Daniels, L. Production of Ethane, Ethylene, and Acetylene from Halogenated Hydrocarbons by Methanogenic Bacteria. Appl. Environ. Microbiol. 1987, 53, 1604–1610. [Google Scholar] [CrossRef]
- Denman, S.E.; Tomkins, N.W.; McSweeney, C.S. Quantitation and diversity analysis of ruminal methanogenic populations in response to the antimethanogenic compound bromochloromethane. FEMS Microbiol. Ecol. 2007, 62, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Abecia, L.; Toral, P.G.; Martín-García, A.I.; Martínez, G.; Tomkins, N.W.; Molina-Alcaide, E.; Newbold, C.J.; Yáñez-Ruiz, D.R. Effect of bromochloromethane on methane emission, rumen fermentation pattern, milk yield, and fatty acid profile in lactating dairy goats. J. Dairy Sci. 2012, 95, 2027–2036. [Google Scholar] [CrossRef]
- Healy, J.B.; Young, L.Y.; Reinhard, M. Methanogenic decomposition of ferulic Acid, a model lignin derivative. Appl. Environ. Microbiol. 1980, 39, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Zinder, S.H.; Anguish, T.; Cardwell, S.C. Selective Inhibition by 2-Bromoethanesulfonate of Methanogenesis from Acetate in a Thermophilic Anaerobic Digestor. Appl. Environ. Microbiol. 1984, 47, 1343–1345. [Google Scholar] [CrossRef]
- Bouwer, E.J.; McCarty, P.L. Effects of 2-bromoethanesulfonic Acid and 2- chloroethanesulfonic Acid on acetate utilization in a continuous-flow methanogenic fixed-film column. Appl. Environ. Microbiol. 1983, 45, 1408–1410. [Google Scholar] [CrossRef] [PubMed]
- Gerecke, A.C.; Hartmann, P.C.; Heeb, N.V.; Kohler, H.-P.E.; Giger, W.; Schmid, P.; Zennegg, M.; Kohler, M. Anaerobic degradation of decabromodiphenyl ether. Environ. Sci. Technol. 2005, 39, 1078–1083. [Google Scholar] [CrossRef]
- Shin, M.; Duncan, B.; Seto, P.; Falletta, P.; Lee, D.-Y. Dynamics of selected pre-existing polybrominated diphenylethers (PBDEs) in municipal wastewater sludge under anaerobic conditions. Chemosphere 2010, 78, 1220–1224. [Google Scholar] [CrossRef]
- Shi, C.; Hu, Y.; Kobayashi, T.; Zhang, N.; Kuramochi, H.; Zhang, Z.; Xu, K.-Q. Anaerobic degradation of deca-brominated diphenyl ether contaminated in products: Effect of temperature on degradation characteristics. Bioresour. Technol. 2019, 283, 28–35. [Google Scholar] [CrossRef]
- Lee, L.K.; He, J. Reductive debromination of polybrominated diphenyl ethers by anaerobic bacteria from soils and sediments. Appl. Environ. Microbiol. 2010, 76, 794–802. [Google Scholar] [CrossRef]
- Song, M.; Luo, C.; Li, F.; Jiang, L.; Wang, Y.; Zhang, D.; Zhang, G. Anaerobic degradation of polychlorinated biphenyls (PCBs) and Polychlorinated Biphenyls Ethers (PBDEs), and microbial community dynamics of electronic waste-contaminated soil. Sci. Total Environ. 2015, 502, 426–433. [Google Scholar] [CrossRef]
- Wu, Q.; Bedard, D.L.; Wiegel, J. Temperature determines the pattern of anaerobic microbial dechlorination of Aroclor 1260 primed by 2,3,4,6-tetrachlorobiphenyl in woods pond sediment. Appl. Environ. Microbiol. 1997, 63, 4818–4825. [Google Scholar] [CrossRef]
- Wu, Q.; Bedard, D.L.; Wiegel, J. Effect of incubation temperature on the route of microbial reductive dechlorination of 2,3,4,6-tetrachlorobiphenyl in polychlorinated biphenyl (PCB)-contaminated and PCB-free freshwater sediments. Appl. Environ. Microbiol. 1997, 63, 2836–2843. [Google Scholar] [CrossRef]
- Harmeson, R.H.; Dietz, J. The effect of radioactive substances on sludge digestionNo Title. Eng. Exp. Stn. Bull. 1957, 441. [Google Scholar]
- Amachi, S.; Minami, K.; Miyasaka, I.; Fukunaga, S. Ability of anaerobic microorganisms to associate with iodine: 125I tracer experiments using laboratory strains and enriched microbial communities from subsurface formation water. Chemosphere 2010, 79, 349–354. [Google Scholar] [CrossRef] [PubMed]
- Barker, H.A. Corrinoid-dependent enzymic reactions. Annu. Rev. Biochem. 1972, 41, 55–90. [Google Scholar] [CrossRef] [PubMed]
- Londry, K.L.; Fedorak, P.M. Fluorophenols and 3-fluorobenzoate in phenol-degrading methanogenic cultures. Arch. Microbiol. 1993, 160, 137–143. [Google Scholar] [CrossRef]
- Kenealy, W.; Zeikus, J.G. Influence of corrinoid antagonists on methanogen metabolism. J. Bacteriol. 1981, 146, 133–140. [Google Scholar] [CrossRef]
- Penning, H.; Conrad, R. Effect of inhibition of acetoclastic methanogenesis on growth of archaeal populations in an anoxic model environment. Appl. Environ. Microbiol. 2006, 72, 178–184. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Janssen, P.H.; Frenzel, P. Inhibition of methanogenesis by methyl fluoride: Studies of pure and defined mixed cultures of anaerobic bacteria and archaea. Appl. Environ. Microbiol. 1997, 63, 4552–4557. [Google Scholar] [CrossRef] [PubMed]
- Hao, L.-P.; Lü, F.; He, P.-J.; Li, L.; Shao, L.-M. Quantification of the inhibitory effect of methyl fluoride on methanogenesis in mesophilic anaerobic granular systems. Chemosphere 2011, 84, 1194–1199. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Feng, Y.; Feng, H.; Ghulam, A.; Su, Y.; Shen, D. Anaerobic biotransformation of fluoronitrobenzenes and microbial communities in methanogenic systems. J. Environ. Sci. Health Part A 2014, 49, 1187–1197. [Google Scholar] [CrossRef] [PubMed]
- Vargas, C.; Song, B.; Camps, M.; Häggblom, M.M. Anaerobic degradation of fluorinated aromatic compounds. Appl. Microbiol. Biotechnol. 2000, 53, 342–347. [Google Scholar] [CrossRef] [PubMed]
- Schoberth, S. A new strain of Desulfovibrio gigas isolated from a sewage plant. Arch. Mikrobiol. 1973, 92, 365–368. [Google Scholar] [CrossRef] [PubMed]
- Remde, A.; Debus, R. Biodegradability of fluorinated surfactants under aerobic and anaerobic conditions. Chemosphere 1996, 32, 1563–1574. [Google Scholar] [CrossRef]
- Meesters, R.J.W.; Schröder, H.F. Perfluorooctane sulfonate—A quite mobile anionic anthropogenic surfactant, ubiquitously found in the environment. Water Sci. Technol. J. Int. Assoc. Water Pollut. Res. 2004, 50, 235–242. [Google Scholar] [CrossRef]
- Wang, N.; Szostek, B.; Folsom, P.W.; Sulecki, L.M.; Capka, V.; Buck, R.C.; Berti, W.R.; Gannon, J.T. Aerobic biotransformation of 14C-labeled 8-2 telomer B alcohol by activated sludge from a domestic sewage treatment plant. Environ. Sci. Technol. 2005, 39, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Szostek, B.; Buck, R.C.; Folsom, P.W.; Sulecki, L.M.; Capka, V.; Berti, W.R.; Gannon, J.T. Fluorotelomer alcohol biodegradation-direct evidence that perfluorinated carbon chains breakdown. Environ. Sci. Technol. 2005, 39, 7516–7528. [Google Scholar] [CrossRef]
- Fetzner, S.; Lingens, F. Bacterial dehalogenases: Biochemistry, genetics, and biotechnological applications. Microbiol. Rev. 1994, 58, 641–685. [Google Scholar] [CrossRef]
- Lian, S.; Nikolausz, M.; Nijenhuis, I.; Francisco Leite, A.; Richnow, H.H. Biotransformation and inhibition effects of hexachlorocyclohexanes during biogas production from contaminated biomass characterized by isotope fractionation concepts. Bioresour. Technol. 2018, 250, 683–690. [Google Scholar] [CrossRef]
- Allard, A.-S.; Neilson, A.H. Degradation and Transformation of Organic Bromine and Iodine Compounds: Comparison with Their Chlorinated Analogues BT—Organic Bromine and Iodine Compounds; Neilson, A.H., Ed.; Springer: Berlin/Heidelberg, Germany, 2003; pp. 1–74. [Google Scholar]
- DeWeerd, K.A.; Suflita, J.M. Anaerobic Aryl Reductive Dehalogenation of Halobenzoates by Cell Extracts of “Desulfomonile tiedjei”. Appl. Environ. Microbiol. 1990, 56, 2999–3005. [Google Scholar] [CrossRef]
- Tsang, J.S.H.; Sallis, P.J.; Bull, A.T.; Hardman, D.J. A monobromoacetate dehalogenase from Pseudomonas cepacia MBA4. Arch. Microbiol. 1988, 150, 441–446. [Google Scholar] [CrossRef]
- Barrio-Lage, G.; Parsons, F.Z.; Nassar, R.S.; Lorenzo, P.A. Sequential dehalogenation of chlorinated ethenes. Environ. Sci. Technol. 1986, 20, 96–99. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; McCarty, P.L. Competition for Hydrogen within a Chlorinated Solvent Dehalogenating Anaerobic Mixed Culture. Environ. Sci. Technol. 1998, 32, 3591–3597. [Google Scholar] [CrossRef]
- Teng, Y.; Xu, Y.; Wang, X.; Christie, P. Function of Biohydrogen Metabolism and Related Microbial Communities in Environmental Bioremediation. Front. Microbiol. 2019, 10, 106. [Google Scholar] [CrossRef]
- Sanford, R.A.; Cole, J.R.; Tiedje, J.M. Characterization and Description of Anaeromyxobacter dehalogenans gen. nov., sp. nov., an Aryl-Halorespiring Facultative Anaerobic Myxobacterium. Appl. Environ. Microbiol. 2002, 68, 893–900. [Google Scholar] [CrossRef]
- Sun, B.; Cole, J.R.; Sanford, R.A.; Tiedje, J.M. Isolation and characterization of desulfovibrio dechloracetivorans sp. nov., a marine dechlorinating bacterium growing by coupling the oxidation of acetate to the reductive dechlorination of 2-chlorophenol. Appl. Environ. Microbiol. 2000, 66, 2408–2413. [Google Scholar] [CrossRef]
- Susarla, S.; Masunaga, S.; Yonezawa, Y. Biotransformation of halogenated benzenes in anaerobic sediments. Environ. Sci. Pollut. Res. 1996, 3, 71–74. [Google Scholar] [CrossRef]
- Paul, N.A.; de Nys, R.; Steinberg, P.D. Chemical defence against bacteria in the red alga Asparagopsis armata: Linking structure with function. Mar. Ecol. Prog. Ser. 2006, 306, 87–101. [Google Scholar] [CrossRef]
- Jesus, A.; Correia-da-silva, M.; Afonso, C.; Pinto, M.; Cidade, H. Isolation and potential biological applications of haloaryl secondary metabolites from macroalgae. Mar. Drugs 2019, 17, 73. [Google Scholar] [CrossRef]
- Cabrita, M.T.; Vale, C.; Rauter, A.P. Halogenated compounds from marine algae. Mar. Drugs 2010, 8, 2301–2317. [Google Scholar] [CrossRef]
- Dembitsky, V.M.; Srebnik, M. Natural halogenated fatty acids: Their analogues and derivatives. Prog. Lipid Res. 2002, 41, 315–367. [Google Scholar] [CrossRef]
- Mehrtens, G.; Laturnus, F. Halogenating activity in an arctic population of brown macroalga Laminaria saccharina (L.) Lamour. Polar Res. 1997, 16, 19–26. [Google Scholar] [CrossRef]
- Leblanc, C.; Colin, C.; Cosse, A.; Delage, L.; La Barre, S.; Morin, P.; Fiévet, B.; Voiseux, C.; Ambroise, Y.; Verhaeghe, E.; et al. Iodine transfers in the coastal marine environment: The key role of brown algae and of their vanadium-dependent haloperoxidases. Biochimie 2006, 88, 1773–1785. [Google Scholar] [CrossRef] [PubMed]
- Wever, R.; Plat, H.; de Boer, E. Isolation procedure and some properties of the bromoperoxidase from the seaweed Ascophyllum nodosum. Biochim. Biophys. Acta Protein Struct. Mol. Enzymol. 1985, 830, 181–186. [Google Scholar] [CrossRef]
- Krenn, B.E.; Plat, H.; Wever, R. The bromoperoxidase from the red alga Ceramium rubrum also contains vanadium as a prosthetic group. Biochim. Biophys. Acta Protein Struct. Mol. Enzymol. 1987, 912, 287–291. [Google Scholar] [CrossRef]
- Almeida, M.G.; Humanes, M.; Melo, R.; Silva, J.A.; da Silva, J.J.; Wever, R. Purification and characterisation of vanadium haloperoxidases from the brown alga Pelvetia canaliculata. Phytochemistry 2000, 54, 5–11. [Google Scholar] [CrossRef]
- Pérez, M.J.; Falqué, E.; Domínguez, H. Antimicrobial action of compounds from marine seaweed. Mar. Drugs 2016, 14, 52. [Google Scholar] [CrossRef]
- Küpper, F.C.; Miller, E.P.; Andrews, S.J.; Hughes, C.; Carpenter, L.J.; Meyer-Klaucke, W.; Toyama, C.; Muramatsu, Y.; Feiters, M.C.; Carrano, C.J. Emission of volatile halogenated compounds, speciation and localization of bromine and iodine in the brown algal genome model Ectocarpus siliculosus. J. Biol. Inorg. Chem. 2018, 23, 1119–1128. [Google Scholar] [CrossRef]
- Czatzkowska, M.; Harnisz, M. Inhibitors of the methane fermentation process with particular emphasis on the microbiological aspect: A review. Energy Sci. Eng. 2020, 8, 1880–1897. [Google Scholar] [CrossRef]
- Leri, A.C.; Dunigan, M.R.; Wenrich, R.L.; Ravel, B. Particulate organohalogens in edible brown seaweeds. Food Chem. 2019, 272, 126–132. [Google Scholar] [CrossRef]
- La Barre, S.; Potin, P.; Leblanc, C.; Delage, L. The halogenated metabolism of brown algae (phaeophyta), its biological importance and its environmental significance. Mar. Drugs 2010, 8, 988–1010. [Google Scholar] [CrossRef] [PubMed]
- Wever, R.; van der Horst, M.A. The role of vanadium haloperoxidases in the formation of volatile brominated compounds and their impact on the environment. Dalton Trans. 2013, 42, 11778–11786. [Google Scholar] [CrossRef] [PubMed]
- Itoh, N.; Shinya, M. Seasonal evolution of bromomethanes from coralline algae (Corallinaceae) and its effect on atmospheric ozone. Mar. Chem. 1994, 45, 95–103. [Google Scholar] [CrossRef]
- Nightingale, P.D.; Malin, G.; Liss, P.S. Production of chloroform and other low molecular-weight halocarbons by some species of macroalgae. Limnol. Oceanogr. 1995, 40, 680–689. [Google Scholar] [CrossRef]
- Gall, E.; Küpper, F.; Kloareg, B. A survey of iodine content in Laminaria digitata. Bot. Mar. 2004, 47, 30–37. [Google Scholar] [CrossRef]
- Holdt, S.L.; Kraan, S. Bioactive compounds in seaweed: Functional food applications and legislation. J. Appl. Phycol. 2011, 23, 543–597. [Google Scholar] [CrossRef]
- Crawford, B.A.; Cowell, C.T.; Emder, P.J.; Learoyd, D.L.; Chua, E.L.; Sinn, J.; Jack, M.M. Iodine toxicity from soy milk and seaweed ingestion is associated with serious thyroid dysfunction. Med. J. Aust. 2010, 193, 413–415. [Google Scholar] [CrossRef]
- Zava, T.T.; Zava, D.T. Assessment of Japanese iodine intake based on seaweed consumption in Japan: A literature-based analysis. Thyroid Res. 2011, 4, 14. [Google Scholar] [CrossRef]
- Hou, X.; Chai, C.; Qian, Q.; Yan, X.; Fan, X. Determination of chemical species of iodine in some seaweeds (I). Sci. Total Environ. 1997, 204, 215–221. [Google Scholar] [CrossRef]
- Teas, J.; Pino, S.; Critchley, A.; Braverman, L.E. Variability of iodine content in common commercially available edible seaweeds. Thyroid 2004, 14, 836–841. [Google Scholar] [CrossRef]
- Scott, R. Observations on the Iodo-Amino-Acids of Marine Algae using Iodine-131. Nature 1954, 173, 1098–1099. [Google Scholar] [CrossRef]
- Rosell, K.-G.; Srivastava, L.M. Binding of inorganic elements to kelp residues. Hydrobiology 1984, 116–117, 505–509. [Google Scholar] [CrossRef]
- Han, L.; Fan, X.; Xiancui, L. Studies on organic iodine in seaweed II. The states and content of organic iodine in seaneed. Stud. Mar. Sin. 2001, 43, 129–135. [Google Scholar]
- Domínguez-González, M.R.; Chiocchetti, G.M.; Herbello-Hermelo, P.; Vélez, D.; Devesa, V.; Bermejo-Barrera, P. Evaluation of iodine bioavailability in seaweed using in vitro methods. J. Agric. Food Chem. 2017, 65, 8435–8442. [Google Scholar] [CrossRef]
- Wong, M.H.; Cheung, Y.H. Gas production and digestion efficiency of sewage sludge containing elevated toxic metals. Bioresour. Technol. 1995, 54, 261–268. [Google Scholar] [CrossRef]
- Meguro, H.; Abe, T.; Ogsawara, T.; Tuzimura, K. Analytical studies of iodine in food substances. Agric. Biol. Chem. 1967, 31, 999–1002. [Google Scholar]
- Van Netten, C.; Hoption Cann, S.A.; Morley, D.R.; van Netten, J.P. Elemental and radioactive analysis of commercially available seaweed. Sci. Total Environ. 2000, 255, 169–175. [Google Scholar] [CrossRef]
- Lee, S.M.; Lewis, J.; Buss, D.H.; Holcombe, G.D.; Lawrance, P.R. Iodine in British foods and diets. Br. J. Nutr. 1994, 72, 435–446. [Google Scholar] [CrossRef]
- Romarís-hortas, V.; Moreda-pi, A.; Bermejo-barrera, P. Microwave assisted extraction of iodine and bromine from edible seaweed for inductively coupled plasma-mass spectrometry determination. Talanta 2009, 79, 947–952. [Google Scholar] [CrossRef]
- Jensen, A.; Haug, A. Geographical and Seasonal Variation in the Chemical Composition of Laminaria hyperborea and Laminaria digitata from the Norwegian Coast; Akademisk Trykningssentral: Oslo, Norway, 1956; Volume 14, pp. 1–8. [Google Scholar]
- Bruhn, A.; Brynning, G.; Johansen, A.; Lindegaard, M.S.; Sveigaard, H.H.; Aarup, B.; Fonager, L.; Andersen, L.L.; Rasmussen, M.B.; Larsen, M.M.; et al. Fermentation of sugar kelp (Saccharina latissima)—Effects on sensory properties, and content of minerals and metals. J. Appl. Phycol. 2019, 31, 3175–3187. [Google Scholar] [CrossRef]
- Matanjun, P.; Mohamed, S.; Mustapha, N.M.; Muhammad, K. Nutrient content of tropical edible seaweeds, Eucheuma cottonii, Caulerpa lentillifera and Sargassum polycystum. J. Appl. Phycol. 2009, 21, 75–80. [Google Scholar] [CrossRef]
- Sheppard, M.I.; Hawkins, J.L. Iodine and microbial interactions in an organic soil. J. Environ. Radioact. 1995, 29, 91–109. [Google Scholar] [CrossRef]
- Gottardi, W. Iodine and Disinfection: Theoretical Study on Mode of Action, Efficiency, Stability, and Analytical Aspects in the Aqueous System. Arch. Pharm. (Weinheim) 1999, 332, 151–157. [Google Scholar] [CrossRef]
- McDonnell, G.; Russell, A.D. Antiseptics and disinfectants: Activity, action, and resistance. Clin. Microbiol. Rev. 1999, 12, 147–179. [Google Scholar] [CrossRef] [PubMed]
- Chynoweth, D.P. Renewable biomethane from land and ocean energy crops and organic wastes. HortScience 2005, 40, 283–286. [Google Scholar] [CrossRef]
- Allen, E.; Wall, D.M.; Herrmann, C.; Xia, A.; Murphy, J.D. What is the gross energy yield of third generation gaseous biofuel sourced from seaweed? Energy 2015, 81, 352–360. [Google Scholar] [CrossRef]
- Milledge, J.J.; Harvey, P.J. Ensilage and anaerobic digestion of Sargassum muticum. J. Appl. Phycol. 2016, 28, 3021–3030. [Google Scholar] [CrossRef]
- Tabassum, M.R.; Xia, A.; Murphy, J.D. Comparison of pre-treatments to reduce salinity and enhance biomethane yields of Laminaria digitata harvested in different seasons. Energy 2017, 140, 546–551. [Google Scholar] [CrossRef]
- Liu, M.; Hansen, P.E.; Lin, X. Bromophenols in marine algae and their bioactivities. Mar. Drugs 2011, 9, 1273–1292. [Google Scholar] [CrossRef]
- Lane, A.L.; Nyadong, L.; Galhena, A.S.; Shearer, T.L.; Stout, E.P.; Parry, R.M.; Kwasnik, M.; Wang, M.D.; Hay, M.E.; Fernandez, F.M.; et al. Desorption electrospray ionization mass spectrometry reveals surface-mediated antifungal chemical defense of a tropical seaweed. Proc. Natl. Acad. Sci. USA 2009, 106, 7314–7319. [Google Scholar] [CrossRef]
- Xu, N.; Fan, X.; Yan, X.; Li, X.; Niu, R.; Tseng, C.K. Antibacterial bromophenols from the marine red alga Rhodomela confervoides. Phytochemistry 2003, 62, 1221–1224. [Google Scholar] [CrossRef]
- Xu, X.; Song, F.; Wang, S.; Li, S.; Xiao, F.; Zhao, J.; Yang, Y.; Shang, S. Dibenzyl Bromophenols with Diverse Dimerization Patterns from the Brown Alga Leathesia nana. J. Nat. Prod. 2004, 1, 1661–1666. [Google Scholar] [CrossRef] [PubMed]
- Borchardt, S.A.; Allain, E.J.; Michels, J.J.; Stearns, G.W.; Kelly, F.; Mccoy, W.F. Reaction of acylated homoserine lactone bacterial signaling molecules with oxidized halogen antimicrobials reaction of acylated homoserine lactone bacterial signaling molecules with oxidized halogen antimicrobials. Appl. Environ. Microbiol. 2001, 67, 3174–3179. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, A.P.; Batista, D.; Dobretsov, S.; Coutinho, R. Extracts of seaweeds as potential inhibitors of quorum sensing and bacterial growth. J. Appl. Phycol. 2017, 29, 789–797. [Google Scholar] [CrossRef]
- Flodin, C.; Whitfield, F.B. 4-hydroxybenzoic acid: A likely precursor of 2,4,6-tribromophenol in Ulva lactuca. Phytochemistry 1999, 51, 249–255. [Google Scholar] [CrossRef]
- Kladi, M.; Vagias, C.; Roussis, V. Volatile halogenated metabolites from marine red algae. Phytochem. Rev. 2004, 3, 337–366. [Google Scholar] [CrossRef]
- Bisaillon, J.G.; Lepine, F.; Beaudet, R.; Sylvestre, M. Potential for carboxylation-dehydroxylation of phenolic compounds by a methanogenic consortium. Can. J. Microbiol. 1993, 39, 642–648. [Google Scholar] [CrossRef]
- King, G. Inhibition of microbial activity in marine sediments by a bromophenol from a hemichordate. Nature 1986, 323, 257–259. [Google Scholar] [CrossRef]
- Vallecillo, A.; Garcia-Encina, P.A.; Peña, M. Anaerobic biodegradability and toxicity of chlorophenols. Water Sci. Technol. 1999, 40, 161–168. [Google Scholar] [CrossRef]
- Sahoo, N.K.; Pakshirajan, K.; Ghosh, P.K. Evaluation of 4-bromophenol biodegradation in mixed pollutants system by Arthrobacter chlorophenolicus A6 in an upflow packed bed reactor. Biodegradation 2014, 25, 705–718. [Google Scholar] [CrossRef]
- Machado, L.; Magnusson, M.; Paul, N.A.; Kinley, R.; De Nys, R.; Tomkins, N. Identification of bioactives from the red seaweed Asparagopsis taxiformis that promote antimethanogenic activity in vitro. J. Appl. Phycol. 2016, 28, 3117–3126. [Google Scholar] [CrossRef]
- Machado, L.; Magnusson, M.; Paul, N.A.; de Nys, R.; Tomkins, N. Effects of marine and freshwater macroalgae on in vitro total gas and methane production. PLoS ONE 2014, 9, e85289. [Google Scholar] [CrossRef]
- Nelson, D. Research Led by Ermias Kebreab Asks If Seaweed Can Cut Methane Emissions on Dairy Farms. Available online: https://animalscience.ucdavis.edu/news/research-led-ermias-kebreab-tests-if-seaweed-cuts-methane-emissions-dairy-farms (accessed on 26 March 2020).
- Cottle, D.; Nolan, J.; Wiedemann, S. Ruminant enteric methane mitigation: A review. Anim. Prod. Sci. 2011, 51, 491–514. [Google Scholar] [CrossRef]
- Chagas, J.C.; Ramin, M.; Krizsan, S.J. In vitro evaluation of different dietary methane mitigation strategies. Animals 2019, 9, 1120. [Google Scholar] [CrossRef] [PubMed]
- McCauley, J.I.; Labeeuw, L.; Jaramillo-Madrid, A.C.; Nguyen, L.N.; Nghiem, L.D.; Chaves, A.V.; Ralph, P.J. Management of enteric methanogenesis in ruminants by algal-derived feed additives. Curr. Pollut. Rep. 2020. [Google Scholar] [CrossRef]
- Hessami, M.J.; Phang, S.M.; Sohrabipoor, J.; Zafar, F.F.; Aslanzadeh, S. The bio-methane potential of whole plant and solid residues of two species of red seaweeds: Gracilaria manilaensis and Gracilariopsis persica. Algal Res. 2019, 42, 101581. [Google Scholar] [CrossRef]
- Gómez-Ordóñez, E.; Alonso, E.; Rupérez, P. A simple ion chromatography method for inorganic anion analysis in edible seaweeds. Talanta 2010, 82, 1313–1317. [Google Scholar] [CrossRef]
- Jard, G.; Marfaing, H.; Carrère, H.; Delgenes, J.P.; Steyer, J.P.; Dumas, C. French Brittany macroalgae screening: Composition and methane potential for potential alternative sources of energy and products. Bioresour. Technol. 2013, 144, 492–498. [Google Scholar] [CrossRef]
- Konig, G.M.; Wright, A.D.; Sticher, O.; Angerhofer, C.K.; Pezzuto, J.M. Biological activities of selected marine natural products. Planta Med. 1994, 60, 532–537. [Google Scholar] [CrossRef]
- Rao Kundeti, L.S.; Ambati, S.; Srividya, G.S.; Yadav, J.S.; Kommu, N. A review on chloro substituted marine natural product, chemical examination and biological activity. Curr. Trends Biotechnol. Pharm. 2019, 13, 72–82. [Google Scholar]
- Torres, F.A.E.; Passalacqua, T.G.; Velásquez, A.M.A.; de Souza, R.A.; Colepicolo, P.; Graminha, M.A.S. New drugs with antiprotozoal activity from marine Algae: A review. Braz. J. Pharmacogn. 2014, 24, 265–276. [Google Scholar] [CrossRef]
- Vedhagiri, K.; Manilal, A.; Valliyammai, T.; Shanmughapriya, S.; Sujith, S.; Selvin, J.; Natarajaseenivasan, K. Antimicrobial potential of a marine seaweed Asparagopsis taxiformis against Leptospira javanica isolates of rodent reservoirs. Ann. Microbial. 2009, 59, 431–437. [Google Scholar] [CrossRef]
- Sanz, J.L.; Rodríguez, N.; Amils, R. The action of antibiotics on the anaerobic digestion process. Appl. Microbiol. Biotechnol. 1996, 46, 587–592. [Google Scholar] [CrossRef] [PubMed]
- Li, X.D.; Miao, F.P.; Li, K.; Ji, N.Y. Sesquiterpenes and acetogenins from the marine red alga Laurencia okamurai. Fitoterapia 2012, 83, 518–522. [Google Scholar] [CrossRef] [PubMed]
- Smyrniotopoulos, V.; Vagias, C.; Rahman, M.M.; Gibbons, S.; Roussis, V. Structure and antibacterial activity of brominated diterpenes from the red alga Sphaerococcus coronopifolius. Chem. Biodivers. 2010, 7, 186–195. [Google Scholar] [CrossRef]
- Smyrniotopoulos, V.; Vagias, C.; Rahman, M.M.; Gibbons, S.; Roussis, V. Ioniols I and II, tetracyclic diterpenes with antibacterial activity, from Sphaerococcus coronopifolius. Chem. Biodivers. 2010, 7, 666–676. [Google Scholar] [CrossRef]
- Lane, A.L.; Mular, L.; Drenkard, E.J.; Shearer, T.L.; Engel, S.; Fredericq, S.; Fairchild, C.R.; Prudhomme, J.; Le Roch, K.; Hay, M.E.; et al. Ecological leads for natural product discovery: Novel sesquiterpene hydroquinones from the red macroalga Peyssonnelia sp. Tetrahedron 2011, 66, 455–461. [Google Scholar] [CrossRef]
- Vairappan, C.S.; Suzuki, M.; Abe, T.; Masuda, M. Halogenated metabolites with antibacterial activity from the Okinawan Laurencia species. Phytochemistry 2001, 58, 517–523. [Google Scholar] [CrossRef]
- Vairappan, C.S.; Kawamoto, T.; Miwa, H.; Suzuki, M. Potent antibacterial activity of halogenated compounds against antibiotic-resistant bacteria. Planta Med. 2004, 70, 1087–1090. [Google Scholar] [CrossRef]
- Sims, J.J.; Donnell, M.S.; Leary, J.V.; Lacy, G.H. Antimicrobial agents from marine algae. Antimicrob. Agents Chemother. 1975, 7, 320–321. [Google Scholar] [CrossRef]
- Dias, D.A.; White, J.M.; Urban, S. Laurencia filiformis: Phytochemical Profiling by Conventional and HPLC-NMR Approaches. Nat. Prod. Commun. 2009, 4, 157–172. [Google Scholar] [CrossRef] [PubMed]
- König, G.M.; Wright, A.D.; Franzblau, S.G. Assessment of antimycobacterial activity of a series of mainly marine derived natural products. Planta Med. 2000, 66, 337–342. [Google Scholar] [CrossRef] [PubMed]
- El-Said, G.F.; El-Sikaily, A. Chemical composition of some seaweed from Mediterranean Sea coast, Egypt. Environ. Monit. Assess. 2013, 185, 6089–6099. [Google Scholar] [CrossRef] [PubMed]
- Masoud, M. The effect of fluoride and other ions on algae and fish of coastal water of mediterranean sea, egypt. Am. J. Environ. Sci. 2006, 2, 53–63. [Google Scholar] [CrossRef]
- Cousins, I.T.; Palm, A. Physical-Chemical Properties and Estimated Environmental Fate of Brominated and Iodinated Organic Compounds BT—Organic Bromine and Iodine Compounds; Neilson, A.H., Ed.; Springer: Berlin/Heidelberg, Germany, 2003; pp. 301–334. ISBN 978-3-540-37055-0. [Google Scholar]
- Mohn, W.; Tiedje, J.; Mohn, W.W.; Tiedje, J.M. Microbial reductive dehalogenation. Microbiol. Rev. 1992, 56, 482–507. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency. Priority Pollutant List EPA. Available online: https://www.epa.gov/sites/production/files/2015-09/documents/priority-pollutant-list-epa.pdf (accessed on 18 April 2020).
- Milledge, J.J.; Harvey, P.J. Potential process ‘hurdles’ in the use of macroalgae as feedstock for biofuel production in the British Isles. J. Chem. Technol. Biotechnol. 2016, 91, 2221–2234. [Google Scholar] [CrossRef]
- Milledge, J.J.; Thompson, E.P.; Sauvêtre, A.; Schroeder, P.; Harvey, P.J. Chapter 8—Novel developments in biological technologies for wastewater processing. In Sustainable Water and Wastewater Processing; Galanakis, C.M., Agrafioti, E., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 239–278. [Google Scholar]
- Da Copertino, M.S.; Tormena, T.; Seeliger, U. Biofiltering efficiency, uptake and assimilation rates of Ulva clathrata (Roth) J. Agardh (Clorophyceae) cultivated in shrimp aquaculture waste water. J. Appl. Phycol. 2009, 21, 31–45. [Google Scholar] [CrossRef]
- Robertson-Andersson, D.V.; Potgieter, M.; Hansen, J.; Bolton, J.J.; Troell, M.; Anderson, R.J.; Halling, C.; Probyn, T. Integrated seaweed cultivation on an abalone farm in South Africa. J. Appl. Phycol. 2008, 20, 579–595. [Google Scholar] [CrossRef]
- Tsagkamilis, P.; Danielidis, D.; Dring, M.J.; Katsaros, C. Removal of phosphate by the green seaweed Ulva lactuca in a small-scale sewage treatment plant (Ios Island, Aegean Sea, Greece). J. Appl. Phycol. 2010, 22, 331–339. [Google Scholar] [CrossRef]
- Tampio, E.; Marttinen, S.; Rintala, J. Liquid fertilizer products from anaerobic digestion of food waste: Mass, nutrient and energy balance of four digestate liquid treatment systems. J. Clean. Prod. 2016, 125, 22–32. [Google Scholar] [CrossRef]
- Han, W.; Clarke, W.; Pratt, S. Stabilisation of microalgae: Iodine mobilisation under aerobic and anaerobic conditions. Bioresour. Technol. 2015, 193, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Piringer, G.; Bhattacharya, S.K. Toxicity and fate of pentachlorophenol in anaerobic acidogenic systems. Water Res. 1999, 33, 2674–2682. [Google Scholar] [CrossRef]
- Rodríguez, N.; Sanz, J. Response of an anaerobic granular sludge to chlorinated aliphatic hydrocarbons in different conditions. J. Ferment. Bioeng. 1998, 86, 226–232. [Google Scholar] [CrossRef]
- Nicholson, D.K.; Woods, S.L.; Istok, J.D.; Peek, D.C. Reductive dechlorination of chlorophenols by a pentachlorophenol- acclimated methanogenic consortium. Appl. Environ. Microbiol. 1992, 58, 2280–2286. [Google Scholar] [CrossRef]
- Guthrie, M.A.; Kirsch, E.J.; Wukasch, R.F.; Grady, C.P.L. Pentachlorophenol biodegradation—II: Anaerobic. Water Res. 1984, 18, 451–461. [Google Scholar] [CrossRef]
- Boucquey, J.-B.; Renard, P.; Amerlynck, P.; Filho, P.M.; Agathos, S.N.; Naveau, H.; Nyns, E.-J. High-rate continuous biodegradation of concentrated chlorinated aliphatics by a durable enrichment of methanogenic origin under carrier-dependent conditions. Biotechnol. Bioeng. 1995, 47, 298–307. [Google Scholar] [CrossRef]
- Chen, Y.; Cheng, J.J.; Creamer, K.S. Inhibition of anaerobic digestion process: A review. Bioresour. Technol. 2008, 99, 4044–4064. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).