Effect of Substrate Characteristics on the Growth and Sporulation of Two Biocontrol Microorganisms during Solid State Cultivation
Abstract
1. Introduction
2. Material and Methods
2.1. Microbial Strains
2.2. Substrate Preparation
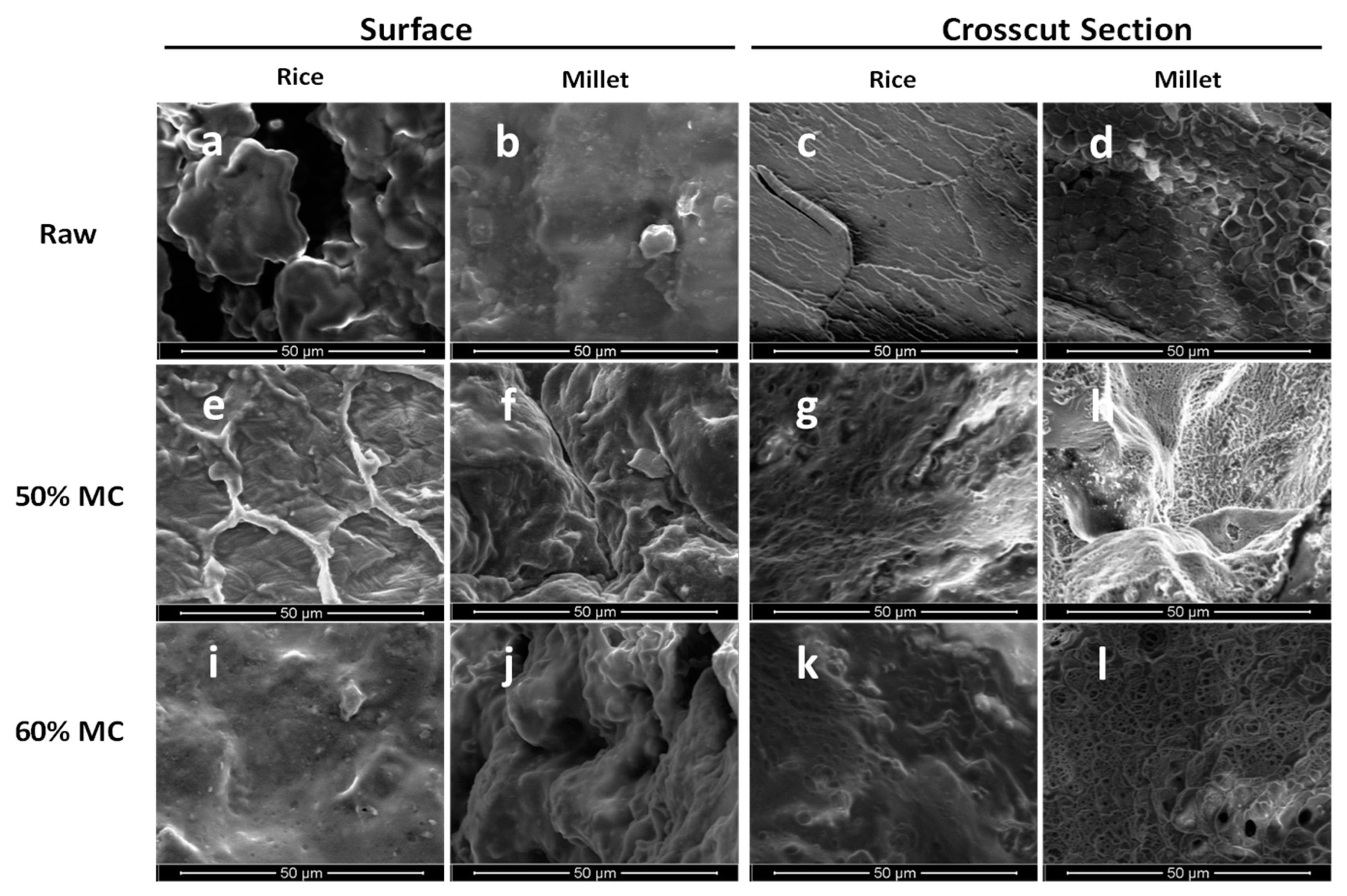
2.3. Texture Analysis and Microscopic Imaging
2.4. Solid-State Fermentation and Spore Production
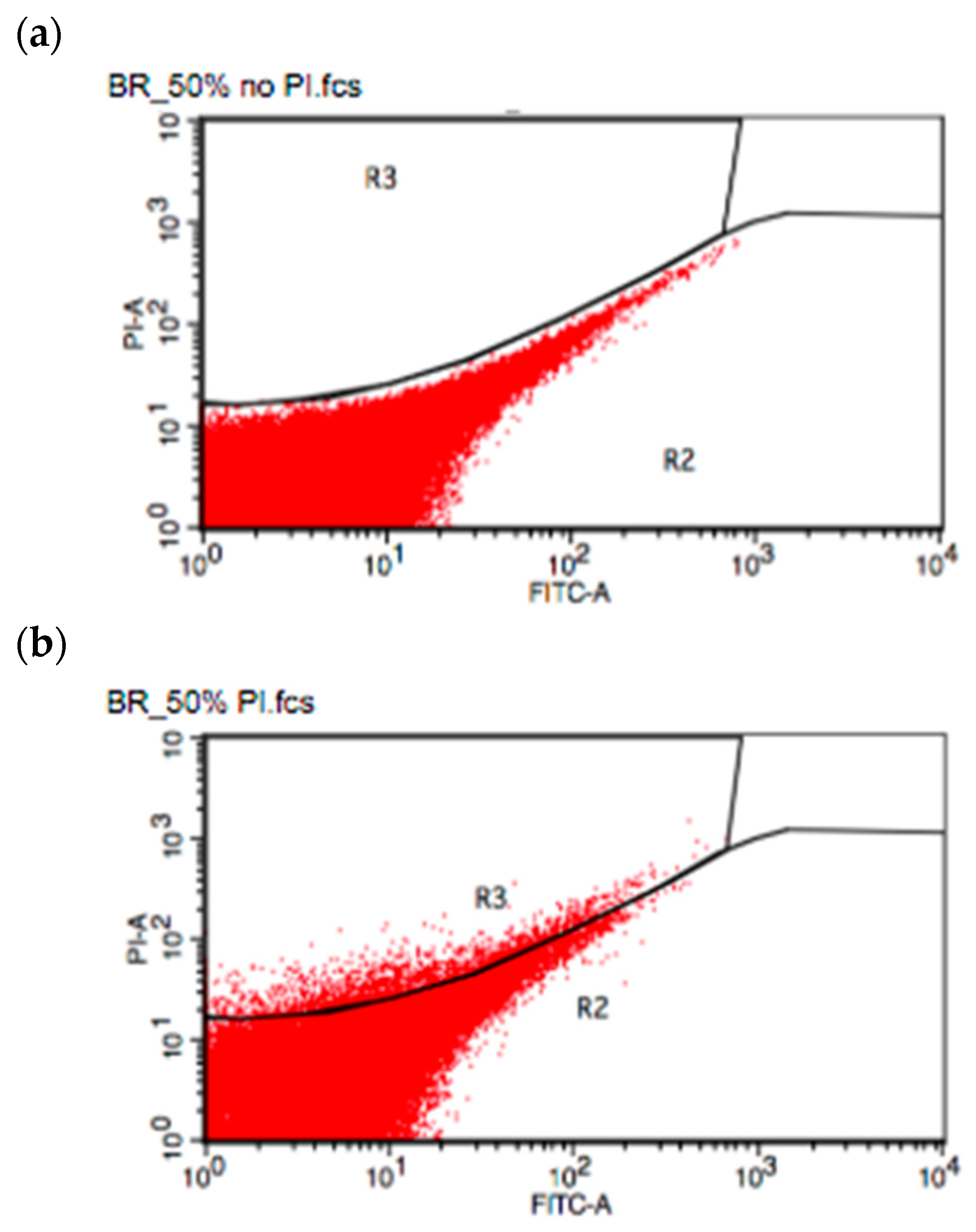
2.5. Flow Cytometry Spore Counting
2.6. Statistical Analysis
3. Results and Discussion
3.1. Cultivation Condition Optimization on Agar Plates
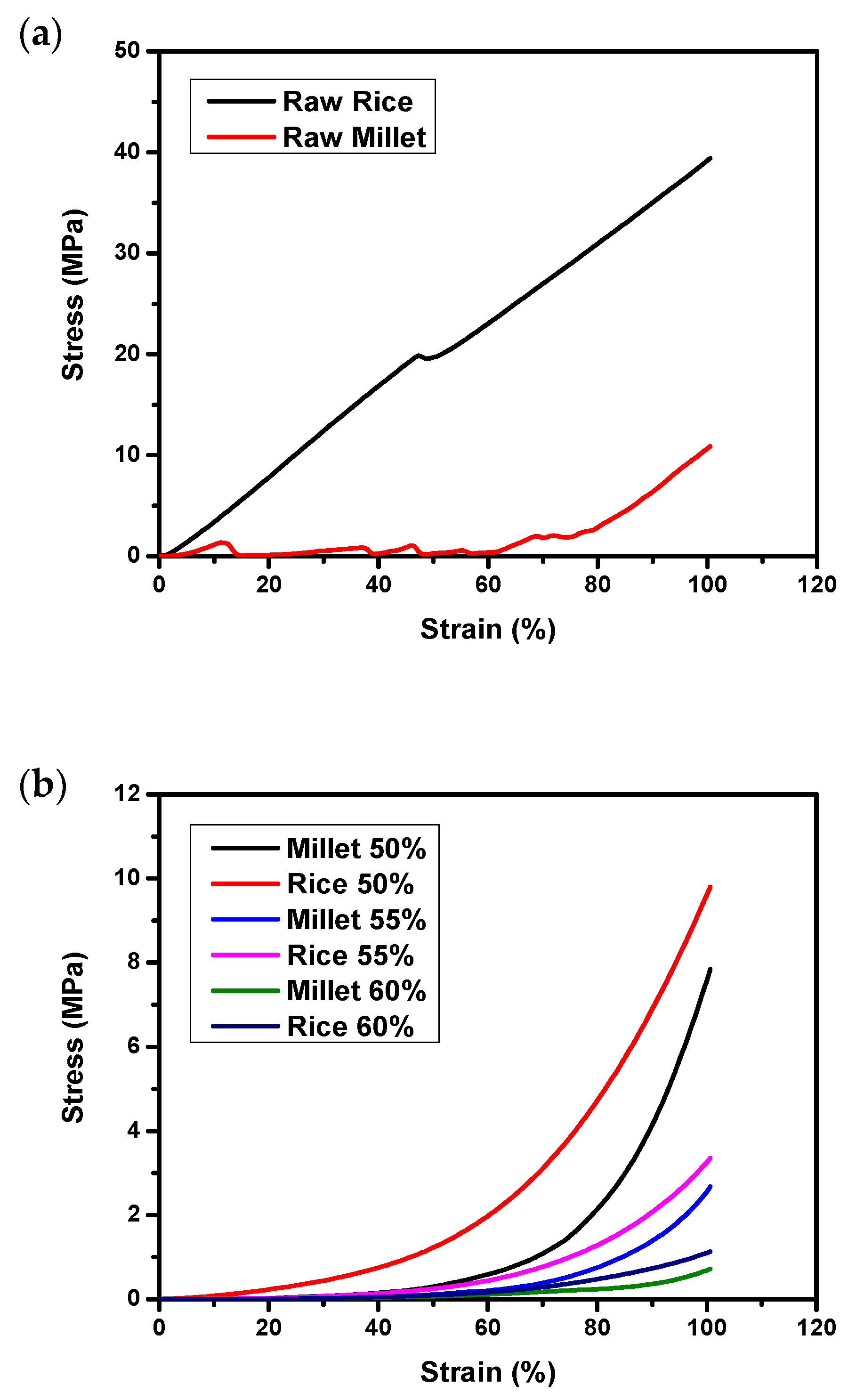
3.2. Texture Profile Analysis (TPA) on Raw and Autoclaved Substrates
3.3. Hardness and Stickiness
3.4. Water Activity
3.5. Effect of Moisture Content and Substrate Type on Spore Production
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Disclaimer
References
- USDA. Oil Crops Yearbook. In Economic Research Service; USDA: Washington, DC, USA, 2020. [Google Scholar]
- Cliff, C.; Hurst, K.; Kirkpatrick, T.; Rupe, J.; Tingle, C.; Trent, M. Asian Soybean Rust; Division of Agriculture, University of Arkansas: Little Rock, AR, USA, 2010. [Google Scholar]
- Schneider, R.; Hollier, C.; Whitam, H.; Palm, M.; McKemy, J.; Hernandez, J.; Levy, L.; DeVries-Paterson, R.; Demonty, I.; Edine, N. First report of soybean rust caused by Phakopsora pachyrhizi in the continental United States. Plant Dis. 2005, 89, 774. [Google Scholar] [CrossRef] [PubMed]
- Langenbach, C.; Campe, R.; Beyer, S.F.; Mueller, A.N.; Conrath, U. Fighting Asian soybean rust. Front. Plant Sci. 2016, 7, 797. [Google Scholar] [CrossRef]
- Giesler, L. Asian Soybean Rust; Institute of Agriculture and Natural Resources, University of Nebraska-Lincoln: Lincoln, NE, USA, 2009. [Google Scholar]
- Sconyers, L.E.; Kemerait, R.C.; Brock, J.; Phillips, D.V.; Jost, P.H.; Sikora, E.J.; Gutierrez-Estrada, A.; Mueller, J.D.; Marois, J.J.; Wright, D.L. Asian soybean rust development in 2005: A perspective from the Southeastern United States. Apsnet Feature Article. 2006. Available online: http://www.Apsnet.Org/Online/Feature/Sbr (accessed on 16 July 2020).
- Rupe, J.; Sconyers, L. Soybean rust. The plant health instructor. In Proceedings of the American Phytopathological Society, St. Paul, MN, USA, 1 April 2008; Available online: https://www.apsnet.org/edcenter/disandpath/fungalbasidio/pdlessons/Pages/SoybeanRust.aspx (accessed on 16 July 2020).
- Wagacha, J.; Muthomi, J.; Mutitu, E.; Mwaura, F. Control of bean rust using antibiotics produced by Bacillus and Streptomyces species-translocation and persistence in snap beans. J. Appl. Sci. Environ. Manag. 2007, 11. [Google Scholar] [CrossRef]
- Hurali, M.K. Eco-Friendly Management of Soybean Rust Caused by Phakopsora Pachyrhizi Syd. Master’s Thesis, University of Agricultural Sciences, Dharwad, India, 2008. [Google Scholar]
- Ward, N.; Robertson, C.; Chanda, A.K.; Schneider, R. Effects of Simplicillium lanosoniveum on Phakopsora pachyrhizi, the soybean rust pathogen, and its use as a biological control agent. Phytopathology 2012, 102, 749–760. [Google Scholar] [CrossRef] [PubMed]
- Serrano, L.; Manker, D.; Brandi, F.; Cali, T. The use of Bacillus subtilis QST 713 and Bacillus pumilus QST 2808 as protectant fungicides in conventional application programs for black leaf streak control. In Proceedings of the VII International Symposium on Banana: ISHS-ProMusa Symposium on Bananas and Plantains: Towards Sustainable Global Production 986, Salvador, Brazil, 10–14 October 2011; pp. 149–155. [Google Scholar]
- Dorighello, D.V.; Bettiol, W.; Maia, N.B.; de Campos Leite, R.M.V.B. Controlling Asian soybean rust (Phakopsora pachyrhizi) with Bacillus spp. and coffee oil. Crop Prot. 2015, 67, 59–65. [Google Scholar] [CrossRef]
- Le Dang, Q.; Shin, T.S.; Park, M.S.; Choi, Y.H.; Choi, G.J.; Jang, K.S.; Kim, I.S.; Kim, J.-C. Antimicrobial activities of novel mannosyl lipids isolated from the biocontrol fungus Simplicillium lamellicola BCP against phytopathogenic bacteria. J. Agric. Food Chem. 2014, 62, 3363–3370. [Google Scholar] [CrossRef]
- Wraight, S.; Jacksonz, M.; De Kock, S. Formulation of Fungal Biocontrol Agents. Fungi Biocontrol Agents Prog. Probl. Potential 2001, 253. [Google Scholar]
- Leggett, M.; Leland, J.; Kellar, K.; Epp, B. Formulation of microbial biocontrol agents—An industrial perspective. Can. J. Plant Pathol. 2011, 33, 101–107. [Google Scholar] [CrossRef]
- Subramaniyam, R.; Vimala, R. Solid state and submerged fermentation for the production of bioactive substances: A comparative study. Int. J. Sci. Nat. 2012, 3, 480–486. [Google Scholar]
- Cawoy, H.; Bettiol, W.; Fickers, P.; Ongena, M. Bacillus-based biological control of plant diseases. Pestic. Mod. World-Pestic. Use Manag. 2011, 273–302. [Google Scholar]
- Shi, J.; Chinn, M.S.; Sharma-Shivappa, R.R. Microbial pretreatment of cotton stalks by solid state cultivation of Phanerochaete chrysosporium. Bioresour. Technol. 2008, 99, 6556–6564. [Google Scholar] [CrossRef] [PubMed]
- Manpreet, S.; Sawraj, S.; Sachin, D.; Pankaj, S.; Banerjee, U. Influence of process parameters on the production of metabolites in solid-state fermentation. Malays. J. Microbiol. 2005, 2, 1–9. [Google Scholar] [CrossRef]
- Mitchell, D.A.; Berovic, M.; Krieger, N. Biochemical engineering aspects of solid state bioprocessing. In New Products and New Areas of Bioprocess Engineering; Springer: Berlin/Heidelberg, Germany, 2000; pp. 61–138. [Google Scholar]
- Georgiou, G.; Shuler, M. A computer model for the growth and differentiation of a fungal colony on solid substrate. Biotechnol. Bioeng. 1986, 28, 405–416. [Google Scholar] [CrossRef] [PubMed]
- Raimbault, M.; Alazard, D. Culture method to study fungal growth in solid fermentation. Eur. J. Appl. Microbiol. Biotechnol. 1980, 9, 199–209. [Google Scholar] [CrossRef]
- Prior, B.; Du Preez, J.; Rein, P. Environmental parameters. Solid Substr. Cultiv. 1992, 65. [Google Scholar]
- Singh, M.; Adedeji, A.; Santra, D. Physico-Chemical and Functional Properties of Nine Proso Millet Cultivars. Trans. Asabe 2018, 61, 1165–1174. [Google Scholar] [CrossRef]
- USDA. Nutrient Lists from Standard Reference Legacy (2018); Nutrient Data Laboratory, USDA: Washington, DC, USA, 2018. [Google Scholar]
- Čihák, M.; Kameník, Z.; Šmídová, K.; Bergman, N.; Benada, O.; Kofroňová, O.; Petříčková, K.; Bobek, J. Secondary metabolites produced during the germination of Streptomyces coelicolor. Front. Microbiol. 2017, 8, 2495. [Google Scholar] [CrossRef]
- Sella, S.R.B.R.; Guizelini, B.P.; Vandenberghe, L.P.d.S.; Medeiros, A.B.P.; Soccol, C.R. Lab-Scale production of Bacillus atrophaeus’ spores by solid state fermentation in fifferent types of bioreactors. Braz. Arch. Biol. Technol. 2009, 52, 159–170. [Google Scholar] [CrossRef][Green Version]
- Kladsuk, S.; Sirisomboon, P. Determination of hardness characteristic of cooked rice samples in rice industry. In Proceedings of the 14th TSAE National Conferences, Hua Hin, Thailand, 1–4 April 2013. [Google Scholar]
- Ogawa, Y.; Wood, D.F.; Whitehand, L.C.; Orts, W.J.; Glenn, G.M. Compression deformation and structural relationships of medium grain cooked rice. Cereal Chem. 2006, 83, 636–640. [Google Scholar] [CrossRef]
- US Department of Agriculture A.R.S. FoodData Central; USDA: Washington, DC, USA, 2019. [Google Scholar]
- Nakayama, A.; Kakugo, A.; Gong, J.P.; Osada, Y.; Takai, M.; Erata, T.; Kawano, S. High Mechanical Strength Double-Network Hydrogel with Bacterial Cellulose. Adv. Funct. Mater. 2004, 14, 1124–1128. [Google Scholar] [CrossRef]
- Panesar, P.; Kaur, S. Rice: Types and Composition. In Encyclopedia of Food and Health; Academic Press: Waltham, MA, USA, 2016; pp. 646–652. [Google Scholar]
- Wang, S.; Li, C.; Copeland, L.; Niu, Q.; Wang, S. Starch retrogradation: A comprehensive review. Compr. Rev. Food Sci. Food Saf. 2015, 14, 568–585. [Google Scholar] [CrossRef]
- Bora, P. Nutritional Properties of Different Millet Types and Their Selected Products. Master’s Thesis, The University of Guelph, Guelph, ON, Canada, 2013. [Google Scholar]
- Krishnakumari, S.; Thayumanavan, B. Content of starch and sugars and in vitro digestion of starch by α-amylase in five minor millets. Plant Foods Hum. Nutr. 1995, 48, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Prakash, S.; Nicholson, T.M.; Fitzgerald, M.A.; Gilbert, R.G. The importance of amylose and amylopectin fine structure for textural properties of cooked rice grains. Food Chem. 2016, 196, 702–711. [Google Scholar] [CrossRef]
- Cuevas, R.P.; Daygon, V.D.; Corpuz, H.M.; Nora, L.; Reinke, R.F.; Waters, D.L.; Fitzgerald, M.A. Melting the secrets of gelatinisation temperature in rice. Funct. Plant Biol. 2010, 37, 439–447. [Google Scholar] [CrossRef]
- Leelayuthsoontorn, P.; Thipayarat, A. Textural and morphological changes of Jasmine rice under various elevated cooking conditions. Food Chem. 2006, 96, 606–613. [Google Scholar] [CrossRef]
- Li, H.; Fitzgerald, M.A.; Prakash, S.; Nicholson, T.M.; Gilbert, R.G. The molecular structural features controlling stickiness in cooked rice, a major palatability determinant. Sci. Rep. 2017, 7, 1–12. [Google Scholar] [CrossRef]
- Nuwamanya, E.; Baguma, Y.; Wembabazi, E.; Rubaihayo, P. A comparative study of the physicochemical properties of starches from root, tuber and cereal crops. Afr. J. Biotechnol. 2011, 10, 12018–12030. [Google Scholar]
- Yu, S.; Ma, Y.; Sun, D.-W. Impact of amylose content on starch retrogradation and texture of cooked milled rice during storage. J. Cereal Sci. 2009, 50, 139–144. [Google Scholar] [CrossRef]
- Liu, D.; Parker, M.L.; Wellner, N.; Kirby, A.R.; Cross, K.; Morris, V.J.; Cheng, F. Structural variability between starch granules in wild type and in ae high-amylose mutant maize kernels. Carbohydr. Polym. 2013, 97, 458–468. [Google Scholar] [CrossRef]
- Hamaker, B.R.; Griffin, V.K. Changing the viscoelastic properties of cooked rice through protein disruption. Cereal Chem. 1990, 67, 261–264. [Google Scholar]
- Robinson, T.; Nigam, P. Bioreactor design for protein enrichment of agricultural residues by solid state fermentation. Biochem. Eng. J. 2003, 13, 197–203. [Google Scholar] [CrossRef]
- Johri, B.N.; Satyanarayana, T.; Olsen, J. Thermophilic Moulds in Biotechnology; Springer Science & Business Media: Dordrecht, The Netherlands, 2013. [Google Scholar]
- Dobraszczyk, B.J.; Vincent, J.F. Measurement of mechanical properties of food materials in relation to texture: The materials approach. Food Texture Meas. Percept. 1999, 99–151. [Google Scholar]
- Pandey, A.; Selvakumar, P.; Soccol, C.R.; Nigam, P. Solid state fermentation for the production of industrial enzymes. Curr. Sci. 1999, 149–162. [Google Scholar]
- Zhao, S.; Xiong, S.; Zhang, S. Paste System on Rice Starch and Its Retrogradation Properties. Chin. Cereals Oils Assoc. 2001, 2. [Google Scholar]
- Berney, M.; Hammes, F.; Bosshard, F.; Weilenmann, H.-U.; Egli, T. Assessment and interpretation of bacterial viability by using the LIVE/DEAD BacLight Kit in combination with flow cytometry. Appl. Environ. Microbiol. 2007, 73, 3283–3290. [Google Scholar] [CrossRef] [PubMed]
- Leiro, J.; Cano, E.; Ubeira, F.M.; Orallo, F.; Sanmartín, M.L. In vitro effects of resveratrol on the viability and infectivity of the microsporidian Encephalitozoon cuniculi. Antimicrob. Agents Chemother. 2004, 48, 2497–2501. [Google Scholar] [CrossRef]
- Leiro, J.M.; Piazzon, C.; Domínguez, B.; Mallo, N.; Lamas, J. Evaluation of some physical and chemical treatments for inactivating microsporidian spores isolated from fish. Int. J. Food Microbiol. 2012, 156, 152–160. [Google Scholar] [CrossRef]
- Green, L.; LeBlanc, P.; Didier, E. Discrimination between viable and dead Encephalitozoon cuniculi (microsporidian) spores by dual staining with Sytox Green and Calcofluor White M2R. J. Clin. Microbiol. 2000, 38, 3811–3814. [Google Scholar] [CrossRef]
- Lopez-Amoros, R.; Comas, J.; Vives-Rego, J. Flow cytometric assessment of Escherichia coli and Salmonella typhimurium starvation-survival in seawater using rhodamine 123, propidium iodide, and oxonol. Appl. Environ. Microbiol. 1995, 61, 2521–2526. [Google Scholar] [CrossRef]
- López-Amorós, R.; Castel, S.; Comas-Riu, J.; Vives-Rego, J. Assessment of E. coli and Salmonella viability and starvation by confocal laser microscopy and flow cytometry using rhodamine 123, DiBAC4 (3), propidium iodide, and CTC. Cytom. J. Int. Soc. Anal. Cytol. 1997, 29, 298–305. [Google Scholar]
- Comas, J.; Vives-Rego, J. Assessment of the effects of gramicidin, formaldehyde, and surfactants on Escherichia coli by flow cytometry using nucleic acid and membrane potential dyes. Cytom. J. Int. Soc. Anal. Cytol. 1997, 29, 58–64. [Google Scholar]
- Vives-Rego, J.; Lebaron, P.; Nebe-von Caron, G. Current and future applications of flow cytometry in aquatic microbiology. Fems Microbiol. Rev. 2000, 24, 429–448. [Google Scholar] [CrossRef] [PubMed]
- Chitarra, L.G. Fluorescence Techniques to Detect and to Assess Viability of Plant Pathogenic Bacteria. Ph.D. Thesis, Wageningen University, Wageningen, The Netherlands, 2001; p. 101. [Google Scholar]
- Deligeorgiev, T.G.; Kaloyanova, S.; Vaquero, J.J. Intercalating cyanine dyes for nucleic acid detection. Recent Pat. Mater. Sci. 2009, 2, 1–26. [Google Scholar] [CrossRef]
- Mathys, A.; Chapman, B.; Bull, M.; Heinz, V.; Knorr, D. Flow cytometric assessment of Bacillus spore response to high pressure and heat. Innov. Food Sci. Emerg. Technol. 2007, 8, 519–527. [Google Scholar] [CrossRef]
- Jue, T. Fundamental Concepts in Biophysics; Springer (Humana Press): Totowa, NJ, USA, 2009; Volome 1. [Google Scholar]
- Baiera, D.; Mathysa, A.; Knorra, D. Identification of different physiological states of bacterial spores and distinction from vegetative cells after high pressure treatments via flow cytometry. In Proceedings of the 11th International congress on engineering and food, Athens, Greece, 22–26 May 2011; pp. 357–358. [Google Scholar]
- Alsharif, R.; Godfrey, W. Bacterial detection and live/dead discrimination by flow cytometry. Microb. Cytom. Appl. Note Bd Biosci. Immunocytometry Syst. 2002. [Google Scholar]



| Parboiled Rice (kPa) | Millets (kPa) | |
|---|---|---|
| Raw | 421.2 | 118.9 |
| 50% MC | 16.8 | 10.1 |
| 55% MC | 11.3 | 5.7 |
| 60% MC | 4.2 | 2.5 |
| Parboiled Rice | Millets | |
|---|---|---|
| Raw | 0.401 | 0.435 |
| 50% MC | 0.921 | 0.916 |
| 55% MC | 0.956 | 0.962 |
| 60% MC | 0.996 | 0.987 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, G.Y.; Li, W.; Chirwa, U.M.; Shi, J. Effect of Substrate Characteristics on the Growth and Sporulation of Two Biocontrol Microorganisms during Solid State Cultivation. Fermentation 2020, 6, 69. https://doi.org/10.3390/fermentation6030069
Lee GY, Li W, Chirwa UM, Shi J. Effect of Substrate Characteristics on the Growth and Sporulation of Two Biocontrol Microorganisms during Solid State Cultivation. Fermentation. 2020; 6(3):69. https://doi.org/10.3390/fermentation6030069
Chicago/Turabian StyleLee, Ga Young, Wenqi Li, Ulalo M. Chirwa, and Jian Shi. 2020. "Effect of Substrate Characteristics on the Growth and Sporulation of Two Biocontrol Microorganisms during Solid State Cultivation" Fermentation 6, no. 3: 69. https://doi.org/10.3390/fermentation6030069
APA StyleLee, G. Y., Li, W., Chirwa, U. M., & Shi, J. (2020). Effect of Substrate Characteristics on the Growth and Sporulation of Two Biocontrol Microorganisms during Solid State Cultivation. Fermentation, 6(3), 69. https://doi.org/10.3390/fermentation6030069







