Review on Non-Dairy Probiotics and Their Use in Non-Dairy Based Products
Abstract
1. Introduction
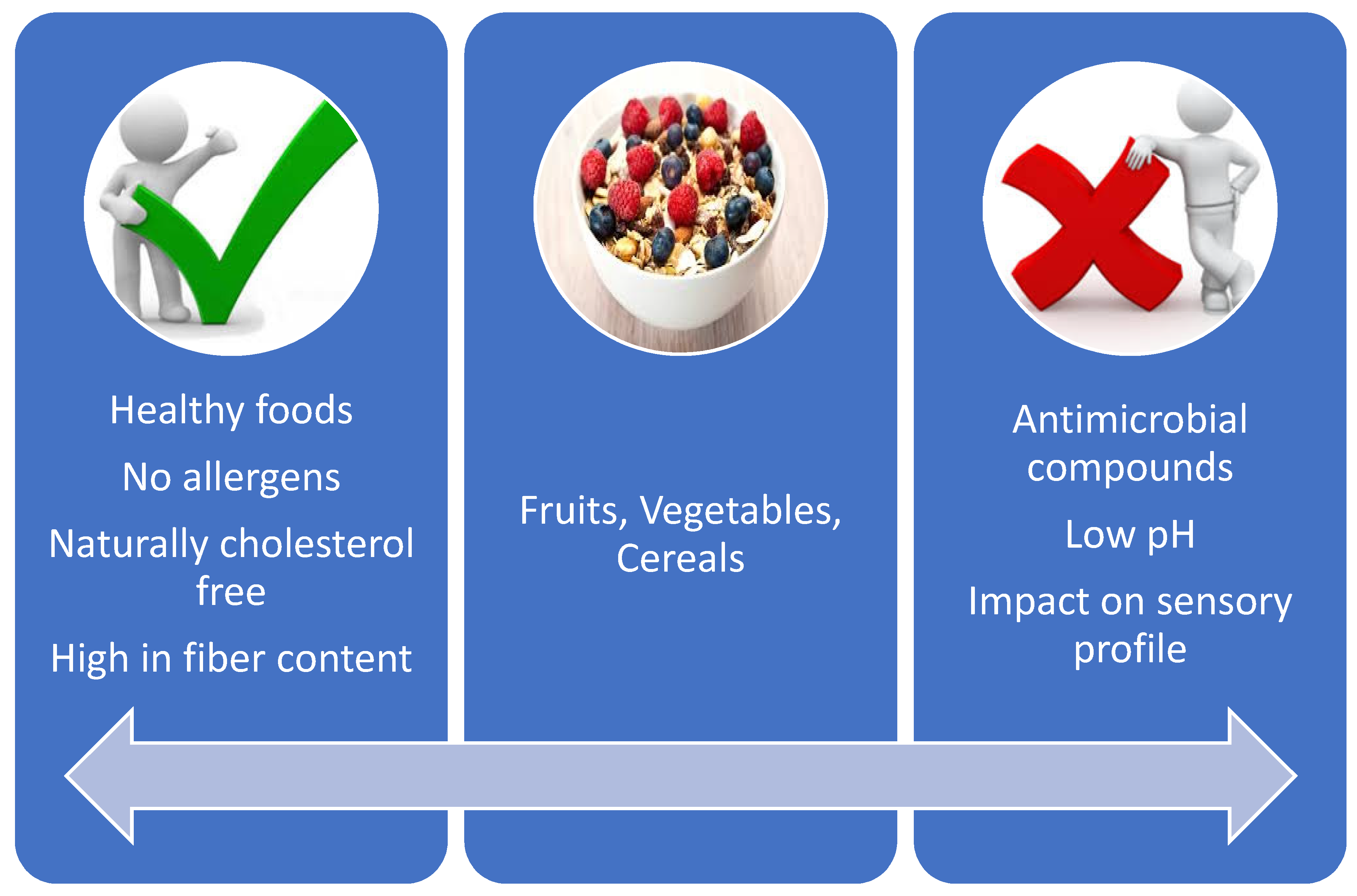
2. Why Do We Need Non-Dairy Probiotics?
3. Market Potential for Non-Dairy Probiotics
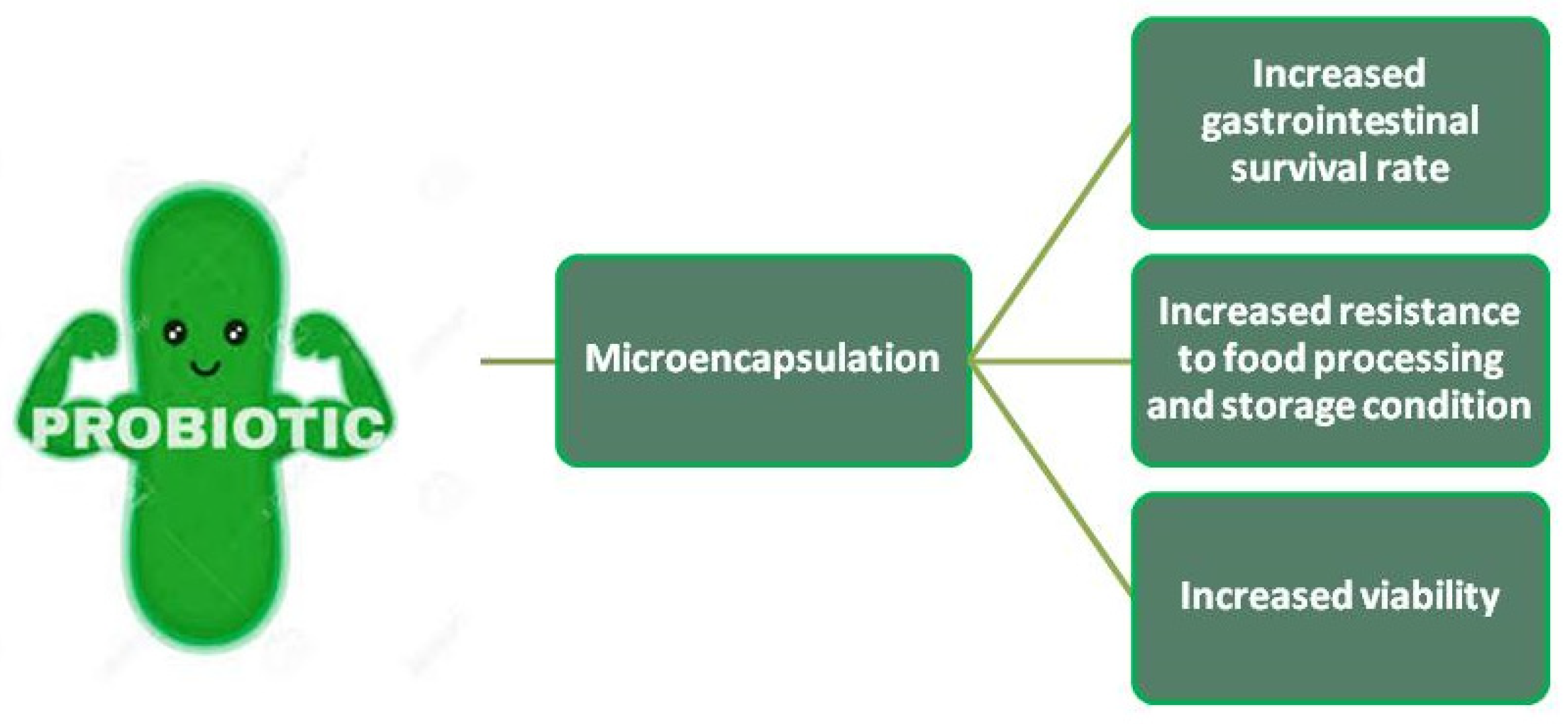
4. Probiotic Preparation and Viability
5. Challenges of Probiotics in Non-Dairy Products
6. Sensory and Overall Acceptance of Non-Dairy Probiotics
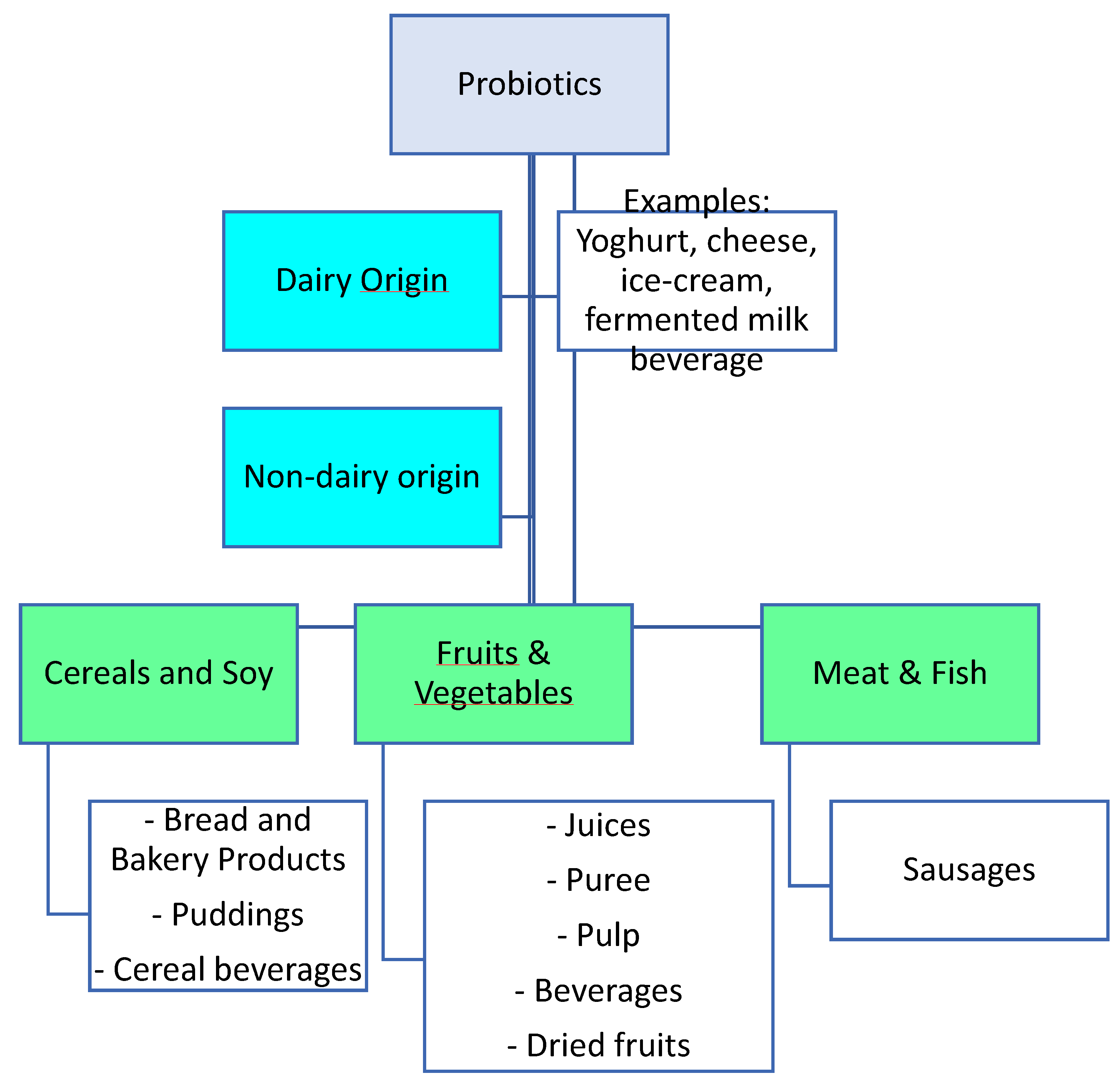
7. Examples of Non-Dairy Probiotics
7.1. Fruits and Vegetables
7.2. Cereals
7.3. Meat Products
7.4. Chocolate
8. Conclusions
Funding
Conflicts of Interest
References
- Shori, A.B. The potential applications of probiotics on dairy and non-dairy foods focusing on viability during storage. Biocatal. Agric. Biotechnol. 2015, 4, 423–431. [Google Scholar] [CrossRef]
- Siro, I.; Kápolna, E.; Kápolna, B.; Lugasi, A. Functional food. Product development, marketing and consumer acceptance—A review. Appetite 2008, 51, 456–467. [Google Scholar] [CrossRef] [PubMed]
- Cencic, A.; Chingwaru, W. The role of functional foods, nutraceuticals, and food supplements in intestinal health. Nutrients 2010, 2, 611–625. [Google Scholar] [CrossRef] [PubMed]
- Kerry, R.G.; Patra, J.K.; Gouda, S.; Park, Y.; Shin, H.-S.; Das, G. Benefaction of probiotics for human health: A review. J. Food Drug Anal. 2018, 26, 927–939. [Google Scholar] [CrossRef]
- Soccol, C.R.; Vandenberghe, L.P.d.S.; Spier, M.R.; Medeiros, A.B.P.; Yamaguishi, C.T.; Lindner, J.D.D.; Pandey, A.; Thomaz-Soccol, V. The potential of probiotics: A review. Food Technol. Biotechnol. 2010, 48, 413–434. [Google Scholar]
- Achi, O.K.; Asamudo, N.U. Cereal-based fermented foods of Africa as functional foods. Bioact. Mol. Food 2018, 1–32. [Google Scholar]
- Granato, D.; Branco, G.F.; Nazzaro, F.; Cruz, A.G.; Faria, J.A. Functional foods and nondairy probiotic food development: Trends, concepts, and products. Compr. Rev. Food Sci. Food Saf. 2010, 9, 292. [Google Scholar] [CrossRef]
- Martins, E.M.F.; Ramos, A.M.; Vanzela, E.S.L.; Stringheta, P.C.; de Oliveira Pinto, C.L.; Martins, J.M. Products of vegetable origin: A new alternative for the consumption of probiotic bacteria. Food Res. Int. 2013, 51, 764–770. [Google Scholar] [CrossRef]
- Tripathi, M.K.; Giri, S.K. Probiotic functional foods: Survival of probiotics during processing and storage. J. Funct. Foods 2014, 9, 225–241. [Google Scholar] [CrossRef]
- Buriti, F.C.A.; de Souza, C.H.B.; Saad, S.M.I. Cheese as probiotic carrier: Technological aspects and benefits. In Handbook of Animal-Based Fermented Food and Beverage Technology; CRC Press: Boca Raton, FL, USA, 2016; pp. 764–799. [Google Scholar]
- Bansal, S.; Mangal, M.; Sharma, S.K.; Gupta, R.K. Non-dairy based probiotics: A healthy treat for intestine. Crit. Rev. Food Sci. Nutr. 2016, 56, 1856–1867. [Google Scholar] [CrossRef]
- Molin, G. Probiotics in foods not containing milk or milk constituents, with special reference to Lactobacillus plantarum 299v. Am. J. Clin. Nutr. 2001, 73, 380s–385s. [Google Scholar] [CrossRef] [PubMed]
- Panghal, A.; Janghu, S.; Virkar, K.; Gat, Y.; Kumar, V.; Chhikara, N. Potential non-dairy probiotic products—A healthy approach. Food Biosci. 2018, 21, 80–89. [Google Scholar] [CrossRef]
- Dornblaser, L. Probiotics and prebiotics: What in the world is going on? Cereal Foods World 2007, 52, 20. [Google Scholar] [CrossRef][Green Version]
- Bhat, A.; Irorere, V.; Bartlett, T.; Hill, D.; Kedia, G.; Charalampopoulos, D.; Nualkaekul, S.; Radecka, I. Improving survival of probiotic bacteria using bacterial poly-γ-glutamic acid. Int. J. Food Microbiol. 2015, 196, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Shori, A.B. Influence of food matrix on the viability of probiotic bacteria: A review based on dairy and non-dairy beverages. Food Biosci. 2016, 13, 1–8. [Google Scholar] [CrossRef]
- Iravani, S.; Korbekandi, H.; Mirmohammadi, S.V. Technology and potential applications of probiotic encapsulation in fermented milk products. J. Food Sci. Technol. 2015, 52, 4679–4696. [Google Scholar] [CrossRef]
- Perricone, M.; Bevilacqua, A.; Altieri, C.; Sinigaglia, M.; Corbo, M.R. Challenges for the production of probiotic fruit juices. Beverages 2015, 1, 95–103. [Google Scholar] [CrossRef]
- Maleki, D.; Azizi, A.; Vaghef, E.; Balkani, S.; Homayouni, A. Methods of increasing probiotic survival in food and gastrointestinal conditions. Prensa Med. Argent. 2015, 101, 1–9. [Google Scholar]
- González-Ferrero, C.; Irache, J.; González-Navarro, C. Soybean protein-based microparticles for oral delivery of probiotics with improved stability during storage and gut resistance. Food Chem. 2018, 239, 879–888. [Google Scholar] [CrossRef]
- El-Salam, M.H.A.; El-Shibiny, S. Preparation and properties of milk proteins-based encapsulated probiotics: A review. Dairy Sci. Technol. 2015, 95, 393–412. [Google Scholar] [CrossRef]
- Ying, D.; Schwander, S.; Weerakkody, R.; Sanguansri, L.; Gantenbein-Demarchi, C.; Augustin, M.A. Microencapsulated Lactobacillus rhamnosus GG in whey protein and resistant starch matrices: Probiotic survival in fruit juice. J. Funct. Foods 2013, 5, 98–105. [Google Scholar] [CrossRef]
- Martín, M.J.; Lara-Villoslada, F.; Ruiz, M.A.; Morales, M.E. Microencapsulation of bacteria: A review of different technologies and their impact on the probiotic effects. Innov. Food Sci. Emerg. Technol. 2015, 27, 15–25. [Google Scholar] [CrossRef]
- Kavitake, D.; Kandasamy, S.; Devi, P.B.; Shetty, P.H. Recent developments on encapsulation of lactic acid bacteria as potential starter culture in fermented foods—A review. Food Biosci. 2018, 21, 34–44. [Google Scholar] [CrossRef]
- Calabuig-Jiménez, L.; Betoret, E.; Betoret, N.; Patrignani, F.; Barrera, C.; Seguí, L.; Lanciotti, R.; Dalla Rosa, M. High pressures homogenization (HPH) to microencapsulate L. salivarius spp. salivarius in mandarin juice. Probiotic survival and in vitro digestion. J. Food Eng. 2019, 240, 43–48. [Google Scholar] [CrossRef]
- Olivares, A.; Soto, C.; Caballero, E.; Altamirano, C. Survival of microencapsulated Lactobacillus casei (prepared by vibration technology) in fruit juice during cold storage. Electron. J. Biotechnol. 2019, 42, 42–48. [Google Scholar] [CrossRef]
- Gandomi, H.; Abbaszadeh, S.; Misaghi, A.; Bokaie, S.; Noori, N. Effect of chitosan-alginate encapsulation with inulin on survival of Lactobacillus rhamnosus GG during apple juice storage and under simulated gastrointestinal conditions. LWT-Food Sci. Technol. 2016, 69, 365–371. [Google Scholar] [CrossRef]
- Haffner, F.B.; Pasc, A. Freeze-dried alginate-silica microparticles as carriers of probiotic bacteria in apple juice and beer. LWT 2018, 91, 175–179. [Google Scholar] [CrossRef]
- Dias, C.O.; de Almeida, J.d.S.O.; Pinto, S.S.; de Oliveira Santana, F.C.; Verruck, S.; Müller, C.M.O.; Prudêncio, E.S.; Amboni, R.D.d.M.C. Development and physico-chemical characterization of microencapsulated bifidobacteria in passion fruit juice: A functional non-dairy product for probiotic delivery. Food Biosci. 2018, 24, 26–36. [Google Scholar] [CrossRef]
- Mokhtari, S.; Jafari, S.M.; Khomeiri, M. Survival of encapsulated probiotics in pasteurized grape juice and evaluation of their properties during storage. Food Sci. Technol. Int. 2019, 25, 120–129. [Google Scholar] [CrossRef]
- Vinderola, G.; Burns, P.; Reinheimer, J. Probiotics in nondairy products. In Vegetarian and Plant-Based Diets in Health and Disease Prevention; Elsevier: Amsterdam, The Netherlands, 2017; pp. 809–835. [Google Scholar]
- Min, M.; Bunt, C.R.; Mason, S.L.; Hussain, M.A. Non-dairy probiotic food products: An emerging group of functional foods. Crit. Rev. Food Sci. Nutr. 2019, 59, 2626–2641. [Google Scholar] [CrossRef]
- Luckow, T.; Delahunty, C. Which juice is ‘healthier’? A consumer study of probiotic non-dairy juice drinks. Food Qual. Prefer. 2004, 15, 751–759. [Google Scholar] [CrossRef]
- Lebaka, V.R.; Wee, Y.J.; Narala, V.R.; Joshi, V.K. Development of new probiotic foods—A case study on probiotic juices. In Therapeutic Probiotic Unconv. Foods; Elsevier: Amsterdam, The Netherlands, 2018; pp. 55–78. [Google Scholar]
- Luckow, T.; Sheehan, V.; Fitzgerald, G.; Delahunty, C. Exposure, health information and flavour-masking strategies for improving the sensory quality of probiotic juice. Appetite 2006, 47, 315–323. [Google Scholar] [CrossRef]
- Slavin, J.L.; Lloyd, B. Health benefits of fruits and vegetables. Adv. Nutr. 2012, 3, 506–516. [Google Scholar] [CrossRef]
- Kandylis, P.; Pissaridi, K.; Bekatorou, A.; Kanellaki, M.; Koutinas, A.A. Dairy and non-dairy probiotic beverages. Curr. Opin. Food Sci. 2016, 7, 58–63. [Google Scholar] [CrossRef]
- Rivera-Espinoza, Y.; Gallardo-Navarro, Y. Non-dairy probiotic products. Food Microbiol. 2010, 27, 1–11. [Google Scholar] [CrossRef]
- de Souza Neves Ellendersen, L.; Granato, D.; Bigetti Guergoletto, K.; Wosiacki, G. Development and sensory profile of a probiotic beverage from apple fermented with Lactobacillus casei. Eng. Life Sci. 2012, 12, 475–485. [Google Scholar] [CrossRef]
- Li, Z.; Teng, J.; Lyu, Y.; Hu, X.; Zhao, Y.; Wang, M. Enhanced Antioxidant Activity for Apple Juice Fermented with Lactobacillus plantarum ATCC14917. Molecules 2019, 24, 51. [Google Scholar] [CrossRef] [PubMed]
- Bujna, E.; Farkas, N.A.; Tran, A.M.; Sao Dam, M.; Nguyen, Q.D. Lactic acid fermentation of apricot juice by mono-and mixed cultures of probiotic Lactobacillus and Bifidobacterium strains. Food Sci. Biotechnol. 2018, 27, 547–554. [Google Scholar] [CrossRef] [PubMed]
- Tsen, J.H.; Lin, Y.P.; King, V.A.E. Response surface methodology optimisation of immobilised Lactobacillus acidophilus banana puree fermentation. Int. J. Food Sci. Technol. 2009, 44, 120–127. [Google Scholar] [CrossRef]
- Yoon, K.Y.; Woodams, E.E.; Hang, Y.D. Fermentation of beet juice by beneficial lactic acid bacteria. LWT-Food Sci. Technol. 2005, 38, 73–75. [Google Scholar] [CrossRef]
- de Oliveira Ribeiro, A.P.; dos Santos Gomes, F.; dos Santos, K.M.O.; da Matta, V.M.; de Araujo Santiago, M.C.P.; Conte, C.; de Oliveira Costa, S.D.; de Oliveira Ribeiro, L.; de Oliveira Godoy, R.L.; Walter, E.H.M. Development of a probiotic non-fermented blend beverage with juçara fruit: Effect of the matrix on probiotic viability and survival to the gastrointestinal tract. LWT 2019, 108756. [Google Scholar] [CrossRef]
- Xu, X.; Bao, Y.; Wu, B.; Lao, F.; Hu, X.; Wu, J. Chemical analysis and flavor properties of blended orange, carrot, apple and Chinese jujube juice fermented by selenium-enriched probiotics. Food Chem. 2019, 289, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Yoon, K.Y.; Woodams, E.E.; Hang, Y.D. Production of probiotic cabbage juice by lactic acid bacteria. Bioresour. Technol. 2006, 97, 1427–1430. [Google Scholar] [CrossRef] [PubMed]
- Valero-Cases, E.; Roy, N.C.; Frutos, M.J.; Anderson, R.C. Influence of the fruit juice carriers on the ability of Lactobacillus plantarum DSM20205 to improve in vitro intestinal barrier integrity and its probiotic properties. J. Agric. Food Chem. 2017, 65, 5632–5638. [Google Scholar] [CrossRef]
- Kaprasob, R.; Kerdchoechuen, O.; Laohakunjit, N.; Somboonpanyakul, P. B vitamins and prebiotic fructooligosaccharides of cashew apple fermented with probiotic strains Lactobacillus spp., Leuconostoc mesenteroides and Bifidobacterium longum. Process Biochem. 2018, 70, 9–19. [Google Scholar] [CrossRef]
- Pereira, A.L.F.; Maciel, T.C.; Rodrigues, S. Probiotic beverage from cashew apple juice fermented with Lactobacillus casei. Food Res. Int. 2011, 44, 1276–1283. [Google Scholar] [CrossRef]
- Fonteles, T.V.; Costa, M.G.M.; de Jesus, A.L.T.; Rodrigues, S. Optimization of the fermentation of cantaloupe juice by Lactobacillus casei NRRL B-442. Food Bioprocess Technol. 2012, 5, 2819–2826. [Google Scholar] [CrossRef]
- Ricci, A.; Cirlini, M.; Maoloni, A.; Del Rio, D.; Calani, L.; Bernini, V.; Galaverna, G.; Neviani, E.; Lazzi, C. Use of Dairy and Plant-Derived Lactobacilli as Starters for Cherry Juice Fermentation. Nutrients 2019, 11, 213. [Google Scholar] [CrossRef]
- Mantzourani, I.; Nouska, C.; Terpou, A.; Alexopoulos, A.; Bezirtzoglou, E.; Panayiotidis, M.; Galanis, A.; Plessas, S. Production of a novel functional fruit beverage consisting of Cornelian cherry juice and probiotic bacteria. Antioxidants 2018, 7, 163. [Google Scholar] [CrossRef]
- Rodgers, S. Novel applications of live bacteria in food services: Probiotics and protective cultures. Trends Food Sci. Technol. 2008, 19, 188–197. [Google Scholar] [CrossRef]
- Saw, L.K.; Chen, S.; Wong, S.H.; Tan, S.A.; Goh, K. Fermentation of tropical fruit juices by lactic acid bacteria. In Proceedings of the 12th Asean Food Conference, Bangkok, Thailand, 16–18 June 2011. [Google Scholar]
- Zheng, X.; Yu, Y.; Xiao, G.; Xu, Y.; Wu, J.; Tang, D.; Zhang, Y. Comparing product stability of probiotic beverages using litchi juice treated by high hydrostatic pressure and heat as substrates. Innov. Food Sci. Emerg. Technol. 2014, 23, 61–67. [Google Scholar] [CrossRef]
- Maldonado, R.R.; da Costa Araújo, L.; da Silva Dariva, L.C.; Rebac, K.N.; de Souza Pinto, I.A.; Prado, J.P.R.; Saeki, J.K.; Silva, T.S.; Takematsu, E.K.; Tiene, N.V. Potential application of four types of tropical fruits in lactic fermentation. LWT 2017, 86, 254–260. [Google Scholar] [CrossRef]
- Vanajakshi, V.; Vijayendra, S.; Varadaraj, M.; Venkateswaran, G.; Agrawal, R. Optimization of a probiotic beverage based on Moringa leaves and beetroot. LWT-Food Sci. Technol. 2015, 63, 1268–1273. [Google Scholar] [CrossRef]
- Nualkaekul, S.; Deepika, G.; Charalampopoulos, D. Survival of freeze dried Lactobacillus plantarum in instant fruit powders and reconstituted fruit juices. Food Res. Int. 2012, 48, 627–633. [Google Scholar] [CrossRef]
- Sheehan, V.M.; Ross, P.; Fitzgerald, G.F. Assessing the acid tolerance and the technological robustness of probiotic cultures for fortification in fruit juices. Innov. Food Sci. Emerg. Technol. 2007, 8, 279–284. [Google Scholar] [CrossRef]
- Ankolekar, C.; Pinto, M.; Greene, D.; Shetty, K. In vitro bioassay based screening of antihyperglycemia and antihypertensive activities of Lactobacillus acidophilus fermented pear juice. Innov. Food Sci. Emerg. Technol. 2012, 13, 221–230. [Google Scholar] [CrossRef]
- Nguyen, B.T.; Bujna, E.; Fekete, N.; Tran, A.T.; Rezessy-Szabo, J.M.; Prasad, R.; Nguyen, Q.D. Probiotic beverage from pineapple juice fermented with Lactobacillus and Bifidobacterium strains. Front. Nutr. 2019, 6, 54. [Google Scholar] [CrossRef]
- Sheela, T.; Suganya, R. Studies on anti-diarrhoeal activity of synbiotic plums juice. Int. J. Sci. Res. Publ. 2012, 2, 1–5. [Google Scholar]
- Mantzourani, I.; Kazakos, S.; Terpou, A.; Alexopoulos, A.; Bezirtzoglou, E.; Bekatorou, A.; Plessas, S. Potential of the Probiotic Lactobacillus Plantarum ATCC 14917 Strain to Produce Functional Fermented Pomegranate Juice. Foods 2019, 8, 4. [Google Scholar] [CrossRef]
- Mousavi, Z.; Mousavi, S.; Razavi, S.; Emam-Djomeh, Z.; Kiani, H. Fermentation of pomegranate juice by probiotic lactic acid bacteria. World J. Microbiol. Biotechnol. 2011, 27, 123–128. [Google Scholar] [CrossRef]
- Semjonovs, P.; Denina, I.; Fomina, A.; Sakirova, L.; Auzina, L.; Patetko, A.; Upite, D. Evaluation of Lactobacillus reuteri strains for pumpkin (Cucurbita pepo L.) juice fermentation. Biotechnology 2013, 12, 202–208. [Google Scholar] [CrossRef]
- Vivek, K.; Mishra, S.; Pradhan, R.C.; Jayabalan, R. Effect of probiotification with Lactobacillus plantarum MCC 2974 on quality of Sohiong juice. LWT 2019, 108, 55–60. [Google Scholar] [CrossRef]
- Lu, Y.; Tan, C.W.; Chen, D.; Liu, S.Q. Potential of three probiotic lactobacilli in transforming star fruit juice into functional beverages. Food Sci. Nutr. 2018, 6, 2141–2150. [Google Scholar] [CrossRef] [PubMed]
- Lavermicocca, P.; Valerio, F.; Lonigro, S.L.; De Angelis, M.; Morelli, L.; Callegari, M.L.; Rizzello, C.G.; Visconti, A. Study of adhesion and survival of lactobacilli and bifidobacteria on table olives with the aim of formulating a new probiotic food. Appl. Environ. Microbiol. 2005, 71, 4233–4240. [Google Scholar] [CrossRef]
- Hurtado, A.; Reguant, C.; Bordons, A.; Rozès, N. Lactic acid bacteria from fermented table olives. Food Microbiol. 2012, 31, 1–8. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, H.; Chen, W.; Zhong, Q.; Zhang, G.; Chen, W. Beneficial Effects of Tomato Juice Fermented by Lactobacillus Plantarum and Lactobacillus Casei: Antioxidation, Antimicrobial Effect, and Volatile Profiles. Molecules 2018, 23, 2366. [Google Scholar] [CrossRef]
- Yoon, K.Y.; Woodams, E.E.; Hang, Y.D. Probiotication of tomato juice by lactic acid bacteria. J. Microbiol. (Seoul, Korea) 2004, 42, 315–318. [Google Scholar]
- Profir, A.; Vizireanu, C. Effect of the preservation processes on the storage stability of juice made from carrot, celery and beetroot. J. Agroaliment. Process. Technol. 2013, 19, 99–104. [Google Scholar]
- Yang, X.; Zhou, J.; Fan, L.; Qin, Z.; Chen, Q.; Zhao, L. Antioxidant properties of a vegetable–fruit beverage fermented with two Lactobacillus plantarum strains. Food Sci. Biotechnol. 2018, 27, 1719–1726. [Google Scholar] [CrossRef]
- Lee, S.Y.; Ganesan, P.; Ahn, J.; Kwak, H.-S. Lactobacillus acidophilus fermented yam (Dioscorea opposita Thunb.) and its preventive effects on gastric lesion. Food Sci. Biotechnol. 2011, 20, 927. [Google Scholar] [CrossRef]
- Pereira, A.L.F.; Rodrigues, S. Turning Fruit Juice Into Probiotic Beverages. In Fruit Juices; Elsevier: Amsterdam, The Netherlands, 2018; pp. 279–287. [Google Scholar]
- Patel, A. Probiotic fruit and vegetable juices-recent advances and future perspective. Int. Food Res. J. 2017, 24, 1850–1857. [Google Scholar]
- Ding, W.; Shah, N.P. Survival of free and microencapsulated probiotic bacteria in orange and apple juices. Int. Food. Res. J. 2008, 15, 219–232. [Google Scholar]
- Rakin, M.; Vukasinovic, M.; Siler-Marinkovic, S.; Maksimovic, M. Contribution of lactic acid fermentation to improved nutritive quality vegetable juices enriched with brewer’s yeast autolysate. Food Chem. 2007, 100, 599–602. [Google Scholar] [CrossRef]
- Saarela, M.; Virkajärvi, I.; Nohynek, L.; Vaari, A.; Mättö, J. Fibres as carriers for Lactobacillus rhamnosus during freeze-drying and storage in apple juice and chocolate-coated breakfast cereals. Int. J. Food Microbiol. 2006, 112, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Kun, S.; Rezessy-Szabó, J.M.; Nguyen, Q.D.; Hoschke, Á. Changes of microbial population and some components in carrot juice during fermentation with selected Bifidobacterium strains. Process Biochem. 2008, 43, 816–821. [Google Scholar] [CrossRef]
- Wang, C.-Y.; Ng, C.-C.; Su, H.; Tzeng, W.-S.; Shyu, Y.-T. Probiotic potential of noni juice fermented with lactic acid bacteria and bifidobacteria. Int. J. Food Sci. Nutr. 2009, 60, 98–106. [Google Scholar] [CrossRef]
- Nagpal, R.; Kumar, A.; Kumar, M. Fortification and fermentation of fruit juices with probiotic lactobacilli. Ann. Microbiol. 2012, 62, 1573–1578. [Google Scholar] [CrossRef]
- Kumar, B.V.; Vijayendra, S.V.N.; Reddy, O.V.S. Trends in dairy and non-dairy probiotic products—A review. J. Food Sci. Technol. 2015, 52, 6112–6124. [Google Scholar] [CrossRef]
- Prado, F.C.; Parada, J.L.; Pandey, A.; Soccol, C.R. Trends in non-dairy probiotic beverages. Food Res. Int. 2008, 41, 111–123. [Google Scholar] [CrossRef]
- Sridharan, S.; Das, K.M.S. A Study on Suitable Non Dairy Food Matrix for Probiotic Bacteria—A Systematic Review. Curr. Res. Nutr. Food Sci. J. 2019, 7, 5–16. [Google Scholar]
- Heperkan, D.; Daskaya-Dikmen, C.; Bayram, B. Evaluation of lactic acid bacterial strains of boza for their exopolysaccharide and enzyme production as a potential adjunct culture. Process Biochem. 2014, 49, 1587–1594. [Google Scholar] [CrossRef]
- Muyanja, C.; Narvhus, J.A.; Treimo, J.; Langsrud, T. Isolation, characterisation and identification of lactic acid bacteria from bushera: A Ugandan traditional fermented beverage. Int. J. Food Microbiol. 2003, 80, 201–210. [Google Scholar] [CrossRef]
- Blandino, A.; Al-Aseeri, M.; Pandiella, S.; Cantero, D.; Webb, C. Cereal-based fermented foods and beverages. Food Res. Int. 2003, 36, 527–543. [Google Scholar] [CrossRef]
- Zannini, E.; Mauch, A.; Galle, S.; Gänzle, M.; Coffey, A.; Arendt, E.K.; Taylor, J.P.; Waters, D.M. Barley malt wort fermentation by exopolysaccharide-forming W eissella cibaria MG 1 for the production of a novel beverage. J. Appl. Microbiol. 2013, 115, 1379–1387. [Google Scholar] [CrossRef] [PubMed]
- Coda, R.; Lanera, A.; Trani, A.; Gobbetti, M.; Di Cagno, R. Yogurt-like beverages made of a mixture of cereals, soy and grape must: Microbiology, texture, nutritional and sensory properties. Int. J. Food Microbiol. 2012, 155, 120–127. [Google Scholar] [CrossRef]
- Gao, Y.; Hamid, N.; Gutierrez-Maddox, N.; Kantono, K.; Kitundu, E. Development of a probiotic beverage using breadfruit flour as a substrate. Foods 2019, 8, 214. [Google Scholar] [CrossRef] [PubMed]
- Freire, A.L.; Ramos, C.L.; Schwan, R.F. Effect of symbiotic interaction between a fructooligosaccharide and probiotic on the kinetic fermentation and chemical profile of maize blended rice beverages. Food Res. Int. 2017, 100, 698–707. [Google Scholar] [CrossRef] [PubMed]
- Duru, K.C.; Kovaleva, E.; Danilova, I.; Belousova, A. Production and assessment of novel probiotic fermented oat flour enriched with isoflavones. LWT 2019, 111, 9–15. [Google Scholar] [CrossRef]
- Gupta, M.; Bajaj, B.K. Development of fermented oat flour beverage as a potential probiotic vehicle. Food Biosci. 2017, 20, 104–109. [Google Scholar] [CrossRef]
- Świeca, M.; Kordowska-Wiater, M.; Pytka, M.; Gawlik-Dziki, U.; Bochnak, J.; Złotek, U.; Baraniak, B. Lactobacillus plantarum 299V improves the microbiological quality of legume sprouts and effectively survives in these carriers during cold storage and in vitro digestion. PLoS ONE 2018, 13, e0207793. [Google Scholar] [CrossRef]
- Menezes, A.G.T.; Ramos, C.L.; Dias, D.R.; Schwan, R.F. Combination of probiotic yeast and lactic acid bacteria as starter culture to produce maize-based beverages. Food Res. Int. 2018, 111, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Rathore, S.; Salmerón, I.; Pandiella, S.S. Production of potentially probiotic beverages using single and mixed cereal substrates fermented with lactic acid bacteria cultures. Food Microbiol. 2012, 30, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Di Stefano, E.; White, J.; Seney, S.; Hekmat, S.; McDowell, T.; Sumarah, M.; Reid, G. A novel millet-based probiotic fermented food for the developing world. Nutrients 2017, 9, 529. [Google Scholar] [CrossRef]
- Gao, F.; Cai, S.; Robert Nout, M.; Wang, Y.; Xia, Y.; Li, Y.; Ji, B. Production of oat-based synbiotic beverage by two-stage fermentation with Rhizopus oryzae and Lactobacillus acidophilus. J. Food Agric. Environ. 2012, 10, 175–179. [Google Scholar]
- Angelov, A.; Gotcheva, V.; Kuncheva, R.; Hristozova, T. Development of a new oat-based probiotic drink. Int. J. Food Microbiol. 2006, 112, 75–80. [Google Scholar] [CrossRef]
- Kabeir, B.M.; Yazid, A.M.; Hakim, M.N.; Khahatan, A.; Shaborin, A.; Mustafa, S. Survival of Bifidobacterium pseudocatenulatum G4 during the storage of fermented peanut milk (PM) and skim milk (SM) products. Afr. J. Food Sci. 2009, 3, 151–155. [Google Scholar]
- Donkor, O.N.; Henriksson, A.; Vasiljevic, T.; Shah, N. α-Galactosidase and proteolytic activities of selected probiotic and dairy cultures in fermented soymilk. Food Chem. 2007, 104, 10–20. [Google Scholar] [CrossRef]
- İçier, F.; Gündüz, G.T.; Yılmaz, B.; Memeli, Z. Changes on some quality characteristics of fermented soy milk beverage with added apple juice. LWT-Food Sci. Technol. 2015, 63, 57–64. [Google Scholar] [CrossRef]
- Sharma, M.; Mridula, D.; Gupta, R. Development of sprouted wheat based probiotic beverage. J. Food Sci. Technol. 2014, 51, 3926–3933. [Google Scholar] [CrossRef][Green Version]
- Leboš-Pavunc, A.; Penava, L.; Ranilović, J.; Novak, J.; Banić, M.; Butorac, K.; Petrović, E.; Mihaljević-Herman, V.; Bendelja, K.; Savić-Mlakar, A. Influence of Dehydrated Wheat/Rice Cereal Matrices on Probiotic Activity of Bifidobacterium animalis ssp. lactis BB-12® §. Food Technol. Biotechnol. 2019, 57, 147. [Google Scholar] [CrossRef]
- Helland, M.H.; Wicklund, T.; Narvhus, J.A. Growth and metabolism of selected strains of probiotic bacteria, in maize porridge with added malted barley. Int. J. Food Microbiol. 2004, 91, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Kedia, G.; Vázquez, J.A.; Pandiella, S.S. Fermentability of whole oat flour, PeriTec flour and bran by Lactobacillus plantarum. J. Food Eng. 2008, 89, 246–249. [Google Scholar] [CrossRef][Green Version]
- Charalampopoulos, D.; Pandiella, S.S. Survival of human derived Lactobacillus plantarum in fermented cereal extracts during refrigerated storage. LWT-Food Sci. Technol. 2010, 43, 431–435. [Google Scholar] [CrossRef]
- Zubaidah, E.; Nurcholis, M.; Wulan, S.N.; Kusuma, A. Comparative study on synbiotic effect of fermented rice bran by probiotic lactic acid bacteria Lactobacillus casei and newly isolated Lactobacillus plantarum B2 in Wistar rats. APCBEE Procedia 2012, 2, 170–177. [Google Scholar] [CrossRef]
- Ai, J.; Li, A.L.; Su, B.X.; Meng, X.C. Multi-Cereal Beverage Fermented by Lactobacillus Helveticus and Saccharomyces Cerevisiae. J. Food Sci. 2015, 80, M1259–M1265. [Google Scholar] [CrossRef]
- Arihara, K.; Ohata, M. Functional meat products. In Functional Foods; Elsevier: Amsterdam, The Netherlands, 2011; pp. 512–533. [Google Scholar]
- Rouhi, M.; Sohrabvandi, S.; Mortazavian, A. Probiotic fermented sausage: Viability of probiotic microorganisms and sensory characteristics. Crit. Rev. Food Sci. Nutr. 2013, 53, 331–348. [Google Scholar] [CrossRef]
- Bis-Souza, C.; Barba, F.; Lorenzo, J.; Penna, A.B.; Barretto, A. New strategies for the development of innovative fermented meat products: A review regarding the incorporation of probiotics and dietary fibers. Food Rev. Int. 2019, 35, 467–484. [Google Scholar] [CrossRef]
- Cavalheiro, C.P.; Ruiz-Capillas, C.; Herrero, A.M.; Jiménez-Colmenero, F.; de Menezes, C.R.; Fries, L.L.M. Application of probiotic delivery systems in meat products. Trends Food Sci. Technol. 2015, 46, 120–131. [Google Scholar] [CrossRef]
- Slima, S.B.; Ktari, N.; Triki, M.; Trabelsi, I.; Abdeslam, A.; Moussa, H.; Makni, I.; Herrero, A.M.; Jiménez-Colmenero, F.; Ruiz-Capillas, C. Effects of probiotic strains, Lactobacillus plantarum TN8 and Pediococcus acidilactici, on microbiological and physico-chemical characteristics of beef sausages. LWT 2018, 92, 195–203. [Google Scholar] [CrossRef]
- Blaiotta, G.; Murru, N.; Di Cerbo, A.; Romano, R.; Aponte, M. Production of probiotic bovine salami using Lactobacillus plantarum 299v as adjunct. J. Sci. Food Agric. 2018, 98, 2285–2294. [Google Scholar] [CrossRef]
- Coelho, S.R.; Lima, Í.A.; Martins, M.L.; Júnior, A.A.B.; de Almeida Torres Filho, R.; Ramos, A.d.L.S.; Ramos, E.M. Application of Lactobacillus paracasei LPC02 and lactulose as a potential symbiotic system in the manufacture of dry-fermented sausage. LWT 2019, 102, 254–259. [Google Scholar] [CrossRef]
- Pavli, F.G.; Argyri, A.A.; Chorianopoulos, N.G.; Nychas, G.-J.E.; Tassou, C.C. Evaluation of Lactobacillus plantarum L125 strain with probiotic potential on physicochemical, microbiological and sensorial characteristics of dry-fermented sausages. LWT 2019, 118, 108810. [Google Scholar] [CrossRef]
- Sidira, M.; Mitropoulou, G.; Galanis, A.; Kanellaki, M.; Kourkoutas, Y. Effect of Sugar Content on Quality Characteristics and Shelf-Life of Probiotic Dry-Fermented Sausages Produced by Free or Immobilized Lactobacillus casei ATCC 393. Foods 2019, 8, 219. [Google Scholar] [CrossRef] [PubMed]
- Wójciak, K.M.; Libera, J.; Stasiak, D.M.; Kołożyn-Krajewska, D. Technological Aspect of Lactobacillus acidophilus Bauer, Bifidobacterium animalis BB-12 and Lactobacillus rhamnosus LOCK900 USE in Dry-Fermented Pork Neck and Sausage. J. Food Process. Preserv. 2017, 41, e12965. [Google Scholar] [CrossRef]
- Arihara, K.; Ota, H.; Itoh, M.; Kondo, Y.; Sameshima, T.; Yamanaka, H.; Akimoto, M.; Kanai, S.; Miki, T. Lactobacillus acidophilus group lactic acid bacteria applied to meat fermentation. J. Food Sci. 1998, 63, 544–547. [Google Scholar] [CrossRef]
- Bis-Souza, C.V.; Pateiro, M.; Domínguez, R.; Lorenzo, J.M.; Penna, A.L.B.; da Silva Barretto, A.C. Volatile profile of fermented sausages with commercial probiotic strains and fructooligosaccharides. J. Food Sci. Technol. 2019, 56, 5465–5473. [Google Scholar] [CrossRef] [PubMed]
- Song, M.-Y.; Van-Ba, H.; Park, W.-S.; Yoo, J.-Y.; Kang, H.-B.; Kim, J.-H.; Kang, S.-M.; Kim, B.-M.; Oh, M.-H.; Ham, J.-S. Quality Characteristics of Functional Fermented Sausages Added with Encapsulated Probiotic Bifidobacterium longum KACC 91563. Korean J. Food Sci. Anim. Resour. 2018, 38, 981. [Google Scholar] [CrossRef] [PubMed]
- Rubio, R.; Jofré, A.; Aymerich, T.; Guàrdia, M.D.; Garriga, M. Nutritionally enhanced fermented sausages as a vehicle for potential probiotic lactobacilli delivery. Meat Sci. 2014, 96, 937–942. [Google Scholar] [CrossRef]
- Ruiz, J.N.; Villanueva, N.D.M.; Favaro-Trindade, C.S.; Contreras-Castillo, C.J. Physicochemical, microbiological and sensory assessments of Italian salami sausages with probiotic potential. Sci. Agric. 2014, 71, 204–211. [Google Scholar] [CrossRef]
- Sameshima, T.; Magome, C.; Takeshita, K.; Arihara, K.; Itoh, M.; Kondo, Y. Effect of intestinal Lactobacillus starter cultures on the behaviour of Staphylococcus aureus in fermented sausage. Int. J. Food Microbiol. 1998, 41, 1–7. [Google Scholar] [CrossRef]
- Bis-Souza, C.V.; Pateiro, M.; Domínguez, R.; Penna, A.L.; Lorenzo, J.M.; Barretto, A.C.S. Impact of fructooligosaccharides and probiotic strains on the quality parameters of low-fat Spanish Salchichón. Meat Sci. 2020, 159, 107936. [Google Scholar] [CrossRef] [PubMed]
- Jaworska, D.; Neffe, K.; Kołożyn-Krajewska, D.; Dolatowski, Z. Survival during storage and sensory effect of potential probiotic lactic acid bacteria Lactobacillus acidophilus Bauer and Lactobacillus casei Bif3′/IV in dry fermented pork loins. Int. J. Food Sci. Technol. 2011, 46, 2491–2497. [Google Scholar] [CrossRef]
- Ruiz-Moyano, S.; Martín, A.; Benito, M.J.; Hernández, A.; Casquete, R.; de Guia Córdoba, M. Application of Lactobacillus fermentum HL57 and Pediococcus acidilactici SP979 as potential probiotics in the manufacture of traditional Iberian dry-fermented sausages. Food Microbiol. 2011, 28, 839–847. [Google Scholar] [CrossRef] [PubMed]
- Kemsawasd, V.; Chaikham, P.; Rattanasena, P. Survival of immobilized probiotics in chocolate during storage and with an in vitro gastrointestinal model. Food Biosci. 2016, 16, 37–43. [Google Scholar] [CrossRef]
- Nebesny, E.; Żyżelewicz, D.; Motyl, I.; Libudzisz, Z. Dark chocolates supplemented with Lactobacillus strains. Eur. Food Res. Technol. 2007, 225, 33–42. [Google Scholar] [CrossRef]
- Aragon-Alegro, L.C.; Alegro, J.H.A.; Cardarelli, H.R.; Chiu, M.C.; Saad, S.M.I. Potentially probiotic and synbiotic chocolate mousse. LWT-Food Sci. Technol. 2007, 40, 669–675. [Google Scholar] [CrossRef]
- Klindt-Toldam, S.; Larsen, S.K.; Saaby, L.; Olsen, L.R.; Svenstrup, G.; Müllertz, A.; Knøchel, S.; Heimdal, H.; Nielsen, D.S.; Zielińska, D. Survival of Lactobacillus acidophilus NCFM® and Bifidobacterium lactis HN019 encapsulated in chocolate during in vitro simulated passage of the upper gastrointestinal tract. LWT 2016, 74, 404–410. [Google Scholar] [CrossRef]
- Zarić, D.B.; Bulatović, M.L.; Rakin, M.B.; Krunić, T.Ž.; Lončarević, I.S.; Pajin, B.S. Functional, rheological and sensory properties of probiotic milk chocolate produced in a ball mill. RSC Adv. 2016, 6, 13934–13941. [Google Scholar] [CrossRef]
- Mirković, M.; Seratlić, S.; Kilcawley, K.; Mannion, D.; Mirković, N.; Radulović, Z. The Sensory Quality and Volatile Profile of Dark Chocolate Enriched with Encapsulated Probiotic Lactobacillus plantarum Bacteria. Sensors 2018, 18, 2570. [Google Scholar] [CrossRef]
| Probiotic Product | Type | Probiotic Microorganisms | Company |
|---|---|---|---|
| Avenly velle | Oat based drink | Lactobacillus and Bifidobacterium | Avenly Oy Ltd., Finland |
| Biola | Fruit Juice | Lacctobacillus rhamnosus GG | Tine BA, Norway |
| Bioprofit | Fruit Juice | Lactobacillus rhamnosus GG, Probionibacterium freudenreichii, Shermanii JS | Valio Ltd., Finland |
| Bravo Friscus | Fruit Juice | Lactobacillus plantarum HEAL9, Lactobacillus paracasei 8700:2 | Skanemajerier, Sweden |
| Gefilus | Fruit Juice | Lactobacillus rhamnosus GG | Valio Ltd., Finland |
| GoodBelly drink | Fruit Juice | Lactobacillus plantarum 299v | NextFoods, Colorado |
| Grainfields wholegrain liquid | Grains, beans and seeds | Lactobacillus acidophilus, Lactobacillus delbreukki, Saccharomyces boulardii, Saccharomyces cerevisiae | AGM Foods Pvt. Ltd., Australia |
| Healthy life probiotics | Fruit Juice | Lactobacillus paracasei 8700:2, Lactobacillus plantarum Hea19 | Golden circle, Australia |
| Kefir Soy | Soya beans | Kluyveromyces marxianus, Kluyveromyces lactis, Lactobacillus brevis, Lactobacillus. kefir, Leuconostoc mesenteroides, Lactobacillus helveticus | Life way, Greek |
| KeVita | Sparkling lemon and ginger drink | Bacillus coagulans GBI-306086, Lactobacillus paracasei 8700:2, Lactobacillus plantarum HEAL 9 | H-E-B, USA |
| Mucilon | Oat and Rice | Bifidus BL | Nestle |
| Proviva | Fermented fruit drink with oatmeal | Lactobacillus plantarum 299v | Skane Dairy, Sweden |
| Rela | Fruit Juice | Lactobacillus reuteri MM53 | Biogaia, Sweden |
| Probiotic Beverage | Probiotic Bacteria | Encapsulation Material | Encapsulation Technique | Reference |
|---|---|---|---|---|
| Mandarin Juice | Lactobacillus salivarius spp. salivarius CECT 4063 | Alginate | Emulsion | [25] |
| Pineapple, raspberry and orange juice | Lactobacillus casei (DSM 20011) | Alginate | Vibration technology | [26] |
| Apple Juice | Lactobacillus rhamnosus GG | Chitosan-alginate with/without inulin | Extrusion | [27] |
| Lactobacillus rhamnosus GG | Alginate-Silica | Freeze drying | [28] | |
| Passion Fruit Juice | Bifidobacterium animalis ssp. lactis BB-12 | Maltodextrin and/or inulin | Spay drying | [29] |
| Grape Juice | Lactobacillus acidophilus and Bifidobacterium bifidum | Alginate | Internal gelation | [30] |
| Product | Probiotic Microorganism | Reference |
|---|---|---|
| Apple | Lactobacillus casei | [39] |
| Lactobacillus plantarum ATCC14917 | [40] | |
| Apricot Juice | Bifidobacterium lactis Bb-12, Bifidobacterium longum Bb-46, Lactobacillus casei 01 and Lactobacillus acidophilus La-5 | [41] |
| Banana puree | Lactobacillus acidophilus | [42] |
| Beet Juice | Lactobacillus plantarum, Lactobacillus casei, Lactobacillus delbrueckii | [43] |
| Beverage with juçara fruit | Bifidobacterium animalis subsp. lactis BB-12 and Lactobacillus acidophilus LA-5 | [44] |
| Blended orange, carrot, apple and Chinese jujube juice | Lactobacillus plantarum CICC20265, Bifidobacterium breve CICC6184, and Streptococcus thermophilus CICC6220 | [45] |
| Cabbage Juice | Lactobacillus plantarum C3, Lactobacillus delbrueckii D7 | [46] |
| Carrot and orange juice | Lactobacillus plantarum | [47] |
| Cashew apple juice | Lactobacillus plantarum | [48] |
| Lactobacillus casei | [49] | |
| Cantaloupe juice | Lactobacillus casei NRRL B-442 | [50] |
| Cherry juice | Lactobacillus plantarum, Lactobacillus casei, Lactobacillus paracasei and Lactobacillus rhamnosus | [51] |
| Cornelian cherry juice | Lactobacillus plantarum ATCC 14917 | [52] |
| Fruit smoothies | Lactobacillus acidophilus LA-5, Bifidobacterium animalis spp. lactis BB-12 | [53] |
| Honeydew melon juice | Lactobacillus casei NCIMB 4114 | [54] |
| Litchi juice | Lactobacillus casei | [55] |
| Mango and guava juice | Lactobacillus casei, Streptococcus thermophillus, Lactobacilluc bulgaricus | [56] |
| Moringa leaves and beetroot beverage | Lactobacillus plantarum, Enterococcus hirae | [57] |
| Orange, grapefruit, black currant, pineapple, pomegranate, cranberry and lemon juice | Lactobacillus plantarum | [58] |
| Orange, pineapple and cranberry juice | Lactobacillus casei DN 114001, Lactobacillus rhamnosus GG, Lactobacillus paracasei NFBC 43338, Bifidobacterium lactis BB-12 | [59] |
| Passion fruit juice | Bifidobacterium animalis subsp. lactis BB-12 | [29] |
| Pear Juice | Lactobacillus acidophilus | [60] |
| Pineapple Juice | Lactobacillus plantarum 299V, Lactobacillus acidophilus La5, Bifidobacterium lactis Bb-12 | [61] |
| Plum Juice | Lactobacillus kefiranofaciens, Candida kefir, Saccharomyces boluradii | [62] |
| Pomegranate juice | Lactobacillus plantarum ATCC 14917 | [63] |
| Lactobacillus plantarum, Lactobacillus delbrueckii, Lactobacillus acidophilus, Lactobacillus paracasei | [64] | |
| Pumpkin juice | Lactobacillus reuteri | [65] |
| Sohiong juice | Lactobacillus plantarum MCC 2974 | [66] |
| Star fruit juice | Lactobacillus helveticus L10, Lactobacillus paracasei L26, and Lactobacillus rhamnosus HN001 | [67] |
| Table olives | Lactobacillus GG, Lactobacillus paracasei | [68] |
| Lactobacillus plantarum | [69] | |
| Tomato Juice | Lactobacillus plantarum and Lactobacillus casei | [70] |
| Lactobacillus acidophilus LA39, Lactobacillus plantarum C3, Lactobacillus casei A4, Lactobacillus delbrueckii D7 | [71] | |
| Vegetable probiotic beverage (beetroot, carrot, celery, honey) | Lactobacillus acidophilus, Lactobacillus casei, Saccharomyces boulardii | [72] |
| Vegetable-fruit beverage | Lactobacillus plantarum | [73] |
| Yam | Lactobacillus acidophilus | [74] |
| Cereal Probiotic Product | Probiotic Strains | Viable Cell Counts | Reference |
|---|---|---|---|
| Barley malt fermented beverage | Weissella cibaria | 107 cfu/mL | [89] |
| Beverage from rice, barley, oats, wheat, soy flour and red grape juice | Lactobacillus plantarum 6E and M6 | 108 cfu/mL | [90] |
| Breadfruit Flour beverage | Lactobacillus plantarum DPC 206, Lactobacillus acidophilus “de Winkel”, Lactobacillus casei Shirota | 107–108 cfu/mL | [91] |
| Cashew juice | Lactobacillus casei NRRL B 442 | 108 cfu/mL | [49] |
| Fermented beverage from maize and rice | Lactobacillus plantarum, Torulaspora delbrueckii, Lactobacillus acidophilus | 107 cfu/mL | [92] |
| Fermented oat flour | Streptococcus thermophilus TH-4, Lactobacillus acidophilus LA-5 | 106 cfu/g | [93] |
| Fermented oat flour beverage | Lactobacillus plantarum | 1014 cfu/mL | [94] |
| Legume sprouts | Lactobacilllus plantarum 299V | 109 cfu/mL | [95] |
| maize-based substrate | Lactobacillus paracasei LBC-81, Saccharomyces cerevisiae CCMA 0731, Saccharomyces cerevisiae CCMA 0732 and Pichia kluyveri CCMA 0615 | 106 cfu/mL | [96] |
| Malt beverage | Lactobacillus plantarum NCIMB 8826, Lactobacillus acidophilus NCIMB 8821 | 108 cfu/mL | [97] |
| Millet-Based Probiotic Fermented Food | Lactobacillus rhamnosus GR-1 and Streptococcus thermophilus C106 | 106 cfu/g | [98] |
| Oat based symbiotic drink | Rhizopus oryzae, L. acidophilus | 108 cfu/mL | [99] |
| Oat-based probiotic drink | Lactobacillus plantarum B28; Lactobacillus reuteri ATCC 55730 | 108–109 cfu/mL | [100] |
| Peanut milk | Bifidobacterium pseudocatenulatum G4 | 108 cfu/mL | [101] |
| Soymilk | Lactobacillus acidophilus | 108 cfu/mL | [102] |
| Soymilk with apple juice | Lactobacillus acidophilus | 109 cfu/mL | [103] |
| Wheat based probiotic beverage | Lactobacillus acidophilus NCDC-14, Lactobacillus acidophilus NCDC-16 | 108–1010 cfu/mL | [104] |
| Wheat/rice cereal infant products | Bifidobacterium animalis subsp. lactis BB-12® | 106 cfu/g | [105] |
| Meat Probiotic Product | Probiotic Strains | Reference |
|---|---|---|
| Beef sausage | Lactobacillus plantarum TN8 and Pediococcus acidilactici MA 18/5M | [115] |
| Bovine Salami | Lactobacillus plantarum 299v | [116] |
| Dry fermented sausage | Lactobacillus paracasei LPC02 | [117] |
| Lactobacillus plantarum L125 | [118] | |
| Lactobacillus casei ATCC 393 | [119] | |
| Dry-fermented pork neck and sausage | Lactobacillus acidophilus Bauer, Bifidobacterium animalis BB-12 and Lactobacillus rhamnosus LOCK900 | [120] |
| Fermented sausage | Lactobacillus gasseri JCM1131T, Lactobacillus fermentum CTC1693 | [121] |
| Lactobacillus paracasei and Lactobacillus rhamnosus GG | [122] | |
| Bifidobacterium longum KACC 91563 | [123] | |
| Fuet (fermented sausage) | Lactobacillus rhamnosus CTC1679 | [124] |
| Italian salami sausage | Lactobacillus acidophilus, Bifidobacterium lactis | [125] |
| Pork fermented sausage | Lactobacillus rhamnosus FERMP-15120, Lactobacillus paracasei subsp. paracasei FERMP-15121 | [126] |
| Spanish Salchichón | Lactobacillus paracasei, Lactobacillus rhmanosus GG | [127] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aspri, M.; Papademas, P.; Tsaltas, D. Review on Non-Dairy Probiotics and Their Use in Non-Dairy Based Products. Fermentation 2020, 6, 30. https://doi.org/10.3390/fermentation6010030
Aspri M, Papademas P, Tsaltas D. Review on Non-Dairy Probiotics and Their Use in Non-Dairy Based Products. Fermentation. 2020; 6(1):30. https://doi.org/10.3390/fermentation6010030
Chicago/Turabian StyleAspri, Maria, Photis Papademas, and Dimitrios Tsaltas. 2020. "Review on Non-Dairy Probiotics and Their Use in Non-Dairy Based Products" Fermentation 6, no. 1: 30. https://doi.org/10.3390/fermentation6010030
APA StyleAspri, M., Papademas, P., & Tsaltas, D. (2020). Review on Non-Dairy Probiotics and Their Use in Non-Dairy Based Products. Fermentation, 6(1), 30. https://doi.org/10.3390/fermentation6010030







