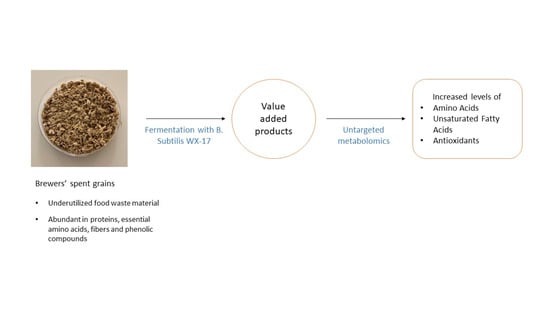
Solid State Fermentation of Brewers’ Spent Grains for Improved Nutritional Profile Using Bacillus subtilis WX-17
Abstract
1. Introduction
2. Materials and Methods
2.1. Microorganism for Fermentation
2.2. Brewer’s Spent Grains and Fermentation Conditions
2.3. Gas Chromatography-Mass Spectrophotometry (GC-MS) Conditions
2.4. Analysis of Amino Acids Using GC-MS
2.5. Derivatization for Metabolomics Study
2.6. Analysis of Fatty Acids Using GC-MS
2.7. Antioxidant Assay
2.8. Statistical Analysis
3. Results
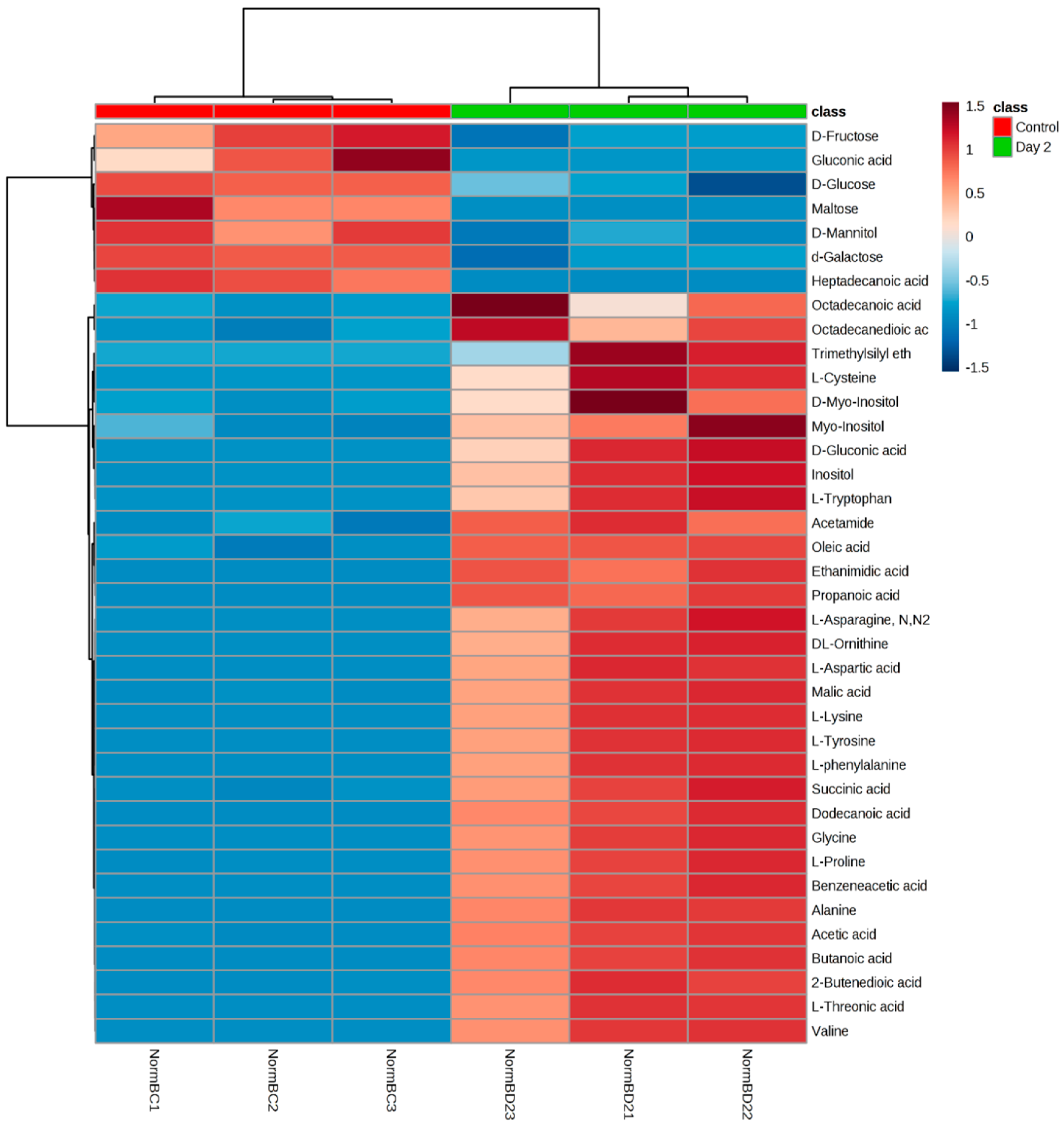
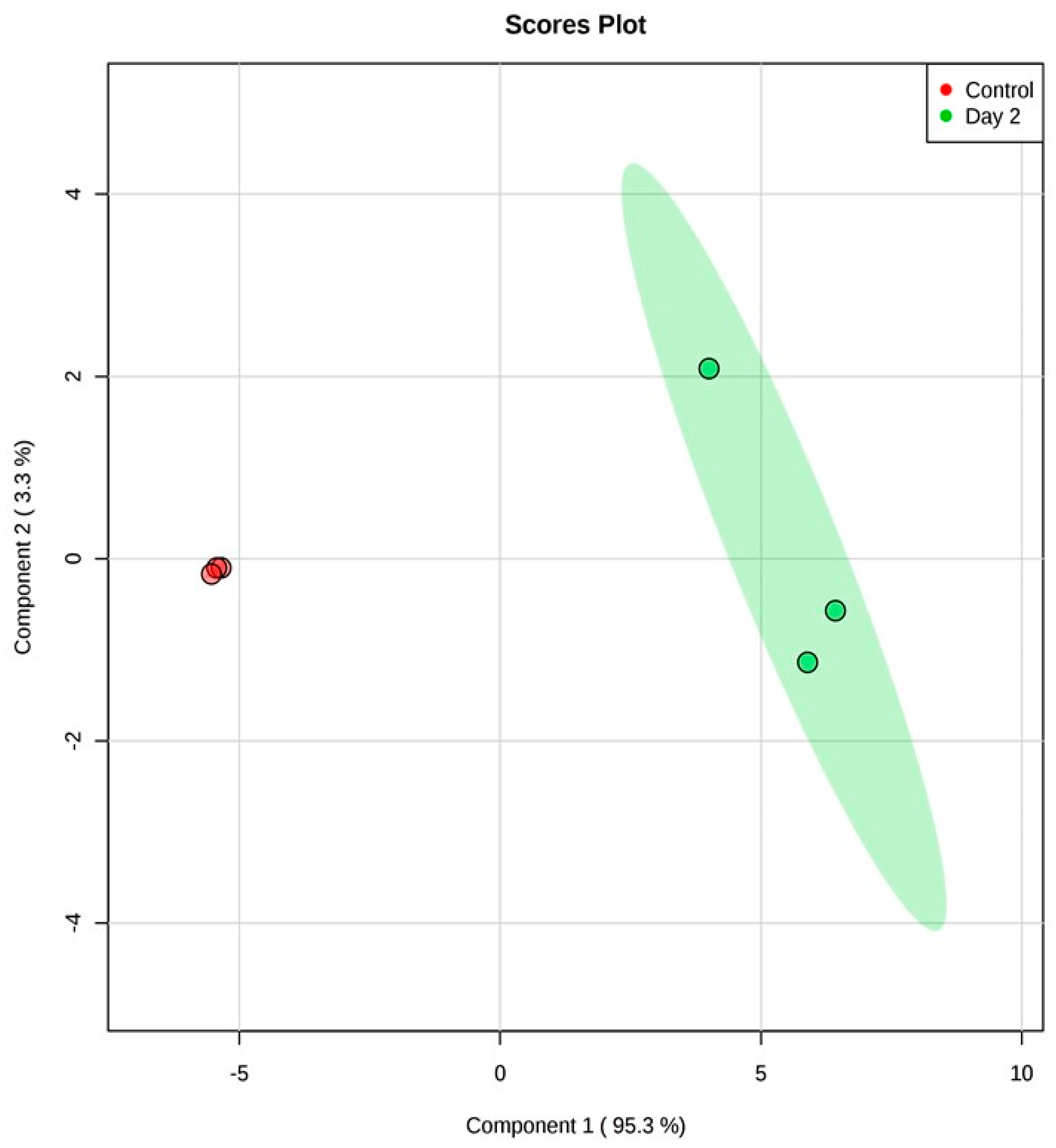
3.1. Analysis on Untargeted Extracellular Metabolic Profiling
3.2. Amino Acids Analysis
3.3. Fatty Acids Analysis
3.4. Carbohydrates Analysis
3.5. Antioxidant Test
4. Discussion
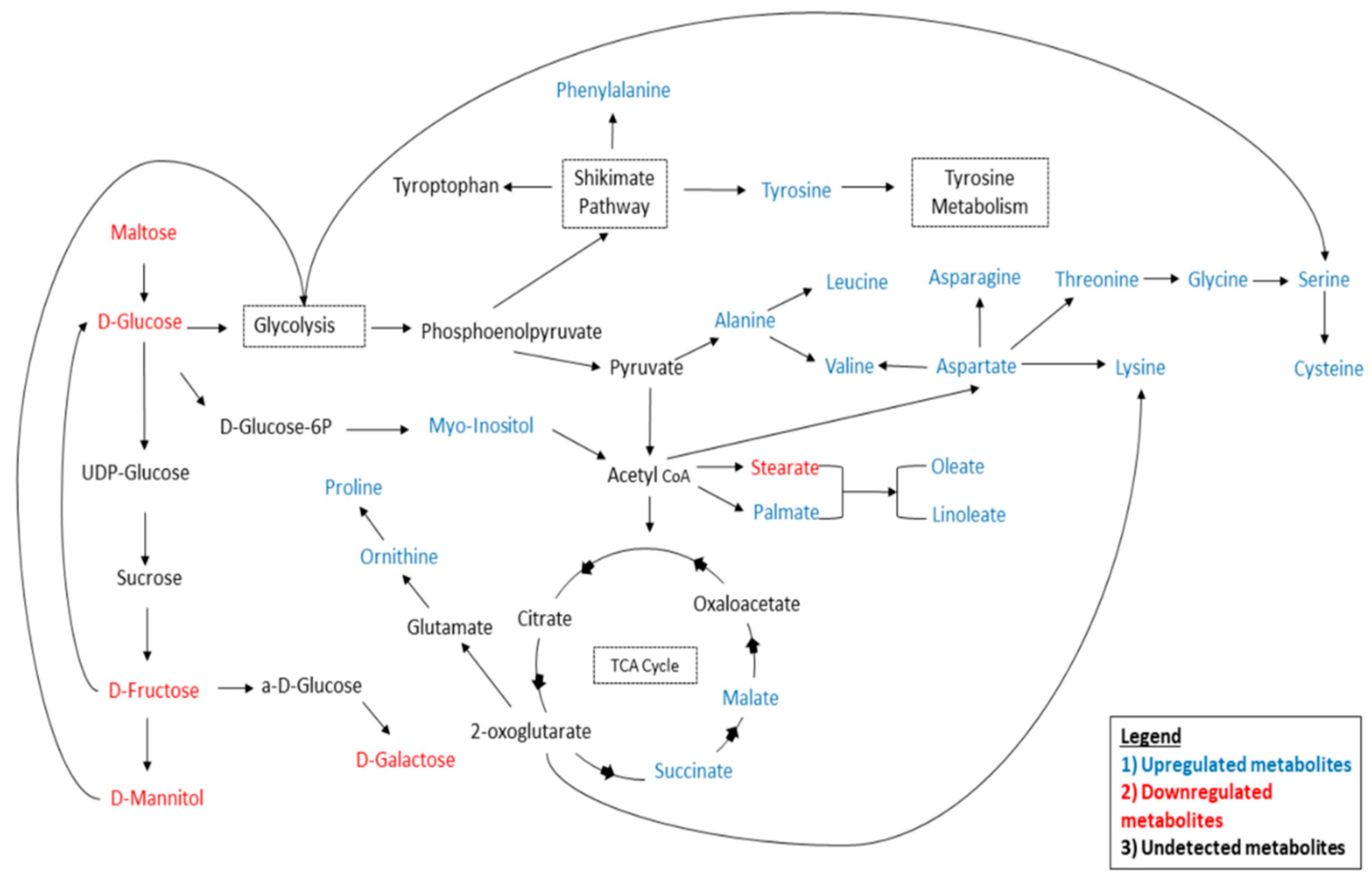
4.1. Carbohydrate Pathway Analysis
4.2. Amino Acids Metabolism Analysis
4.3. TCA Cycle Metabolism Analysis
4.4. Fatty Acid Metabolism Analysis
4.5. Antioxidant Test Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Waqas, M.; Rehan, M.; Khan, M.D.; Nizami, A.-S. Conversion of Food Waste to Fermentation Products. In Encyclopedia of Food Security and Sustainability; Ferranti, P., Berry, E.M., Anderson, J.R., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 501–509. [Google Scholar]
- Mussatto, S.I. Biotechnological Potential of Brewing Industry By-Products. In Biotechnology for Agro-Industrial Residues Utilisation: Utilisation of Agro-Residues; Singh nee’ Nigam, P., Pandey, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2009; pp. 313–326. [Google Scholar]
- Mussatto, S.I. Brewer’s spent grain: A valuable feedstock for industrial applications. J. Sci. Food Agric. 2014, 94, 1264–1275. [Google Scholar] [CrossRef] [PubMed]
- Mathias, T.R.D.S.; Fernandes de Aguiar, P.; de Almeida e Silva, J.B.; Moretzsohn de Mello, P.P.; Camporese Sérvulo, E.F. Brewery Waste Reuse for Protease Production by Lactic Acid Fermentation. Food Technol. Biotechnol. 2017, 55, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Kerby, C.; Vriesekoop, F. An Overview of the Utilisation of Brewery By-Products as Generated by British Craft Breweries. Beverages 2017, 3, 24. [Google Scholar] [CrossRef]
- Mussatto, S.I.; Dragone, G.; Roberto, I.C. Brewers’ spent grain: Generation, characteristics and potential applications. J. Cereal Sci. 2006, 43, 1–14. [Google Scholar] [CrossRef]
- Lynch, K.M.; Steffen, E.J.; Arendt, E.K. Brewers’ spent grain: A review with an emphasis on food and health. J. Inst. Brew. 2016, 122, 553–568. [Google Scholar] [CrossRef]
- Ikram, S.; Huang, L.; Zhang, H.; Wang, J.; Yin, M. Composition and Nutrient Value Proposition of Brewers Spent Grain. J. Food Sci. 2017, 82, 2232–2242. [Google Scholar] [CrossRef]
- Fărcaş, A.C.; Socaci, S.A.; Dulf, F.V.; Tofană, M.; Mudura, E.; Diaconeasa, Z. Volatile profile, fatty acids composition and total phenolics content of brewers’ spent grain by-product with potential use in the development of new functional foods. J. Cereal Sci. 2015, 64, 34–42. [Google Scholar] [CrossRef]
- Salihu, A.; Bala, M. Brewer’s spent grain: A review of its potentials and applications. Afr. J. Biotechnol. 2011, 10, 324–331. [Google Scholar] [CrossRef]
- Behera, S.; Arora, R.; Nandhagopal, N.; Kumar, S. Importance of chemical pretreatment for bioconversion of lignocellulosic biomass. Renew. Sustain. Energy Rev. 2014, 36, 91–106. [Google Scholar] [CrossRef]
- Sindhu, R.; Binod, P.; Pandey, A. Biological pretreatment of lignocellulosic biomass—An overview. Bioresour. Technol. 2016, 199, 76–82. [Google Scholar] [CrossRef]
- Pandey, A. Solid-state fermentation. Biochem. Eng. J. 2003, 13, 81–84. [Google Scholar] [CrossRef]
- Couto, S.R.; Sanromán, M.Á. Application of solid-state fermentation to food industry—A review. J. Food Eng. 2006, 76, 291–302. [Google Scholar] [CrossRef]
- Madamwar, D.; Patel, S.; Parikh, H. Solid state fermentation for cellulases and β-glucosidase production by Aspergillus niger. J. Ferment. Bioeng. 1989, 67, 424–426. [Google Scholar] [CrossRef]
- Queiroz Santos, V.A.; Nascimento, C.G.; Schmidt, C.A.P.; Mantovani, D.; Dekker, R.F.H.; da Cunha, M.A.A. Solid-state fermentation of soybean okara: Isoflavones biotransformation, antioxidant activity and enhancement of nutritional quality. LWT 2018, 92, 509–515. [Google Scholar] [CrossRef]
- Lizardi-Jimenez, M.A.; Hernandez-Martinez, R. Solid state fermentation (SSF): Diversity of applications to valorize waste and biomass. 3 Biotech 2017, 7, 44. [Google Scholar] [CrossRef] [PubMed]
- Tišma, M.; Jurić, A.; Bucić-Kojić, A.; Panjičko, M.; Planinić, M. Biovalorization of brewers’ spent grain for the production of laccase and polyphenols. J. Inst. Brew. 2018, 124, 182–186. [Google Scholar] [CrossRef]
- Nascimento, R.P.; Coelho, R.R.R.; Marques, S.; Alves, L.; Gírio, F.M.; Bon, E.P.S.; Amaral-Collaço, M.T. Production and partial characterisation of xylanase from Streptomyces sp. strain AMT-3 isolated from Brazilian cerrado soil. Enzym. Microb. Technol. 2002, 31, 549–555. [Google Scholar] [CrossRef]
- Schallmey, M.; Singh, A.; Ward, O.P. Developments in the use of Bacillus species for industrial production. Can. J. Microbiol. 2004, 50, 1–17. [Google Scholar] [CrossRef]
- Nishinari, K.; Fang, Y.; Nagano, T.; Guo, S.; Wang, R. 6—Soy as a food ingredient. In Proteins in Food Processing, 2nd ed.; Yada, R.Y., Ed.; Woodhead Publishing: Cambridge, UK, 2018; pp. 149–186. [Google Scholar]
- Zamboni, N.; Fendt, S.-M.; Rühl, M.; Sauer, U. 13C-based metabolic flux analysis. Nat. Protoc. 2009, 4, 878. [Google Scholar] [CrossRef]
- Chen, L.; Chen, W.N. Metabolite and Fatty Acid Analysis of Yeast Cells and Culture Supernatants. Bio-protocol 2014, 4, e1219. [Google Scholar] [CrossRef]
- Wan, C.; Yu, Y.; Zhou, S.; Liu, W.; Tian, S.; Cao, S. Antioxidant activity and free radical-scavenging capacity of Gynura divaricata leaf extracts at different temperatures. Pharmacogn. Mag. 2011, 7, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Chong, J.; Soufan, O.; Li, C.; Caraus, I.; Li, S.; Bourque, G.; Wishart, D.S.; Xia, J. MetaboAnalyst 4.0: Towards more transparent and integrative metabolomics analysis. Nucleic Acids Res. 2018, 46, W486–W494. [Google Scholar] [CrossRef] [PubMed]
- Gromski, P.S.; Muhamadali, H.; Ellis, D.I.; Xu, Y.; Correa, E.; Turner, M.L.; Goodacre, R. A tutorial review: Metabolomics and partial least squares-discriminant analysis--a marriage of convenience or a shotgun wedding. Anal. Chim. Acta 2015, 879, 10–23. [Google Scholar] [CrossRef] [PubMed]
- Henseler, J.; Ringle, C.M.; Sinkovics, R.R. The use of partial least squares path modeling in international marketing. In New Challenges to International Marketing; Emerald Group Publishing Limited: Bingley, UK, 2009; Volume 20, pp. 277–319. [Google Scholar]
- Lunn, J.; Theobald, H.E. The health effects of dietary unsaturated fatty acids. Nutr. Bull. 2006, 31, 178–224. [Google Scholar] [CrossRef]
- Lincoln, L.; More, S.S. Bacterial invertases: Occurrence, production, biochemical characterization, and significance of transfructosylation. J. Basic Microbiol. 2017, 57, 803–813. [Google Scholar] [CrossRef] [PubMed]
- Raul, D.; Biswas, T.; Mukhopadhyay, S.; Kumar Das, S.; Gupta, S. Production and Partial Purification of Alpha Amylase from Bacillus subtilis (MTCC 121) Using Solid State Fermentation. Biochem. Res. Int. 2014, 2014, 5. [Google Scholar] [CrossRef]
- Sethi, S.; Datta, A.; Gupta, B.L.; Gupta, S. Optimization of Cellulase Production from Bacteria Isolated from Soil. Isrn Biotechnol. 2013, 2013, 7. [Google Scholar] [CrossRef]
- Ahlawat, S.; Dhiman, S.S.; Battan, B.; Mandhan, R.P.; Sharma, J. Pectinase production by Bacillus subtilis and its potential application in biopreparation of cotton and micropoly fabric. Process Biochem. 2009, 44, 521–526. [Google Scholar] [CrossRef]
- Kanehisa, M.; Furumichi, M.; Tanabe, M.; Sato, Y.; Morishima, K. KEGG: New perspectives on genomes, pathways, diseases and drugs. Nucleic Acids Res. 2016, 45. [Google Scholar] [CrossRef]
- Oyeleke, S.B.; Oyewole, O.; Egwim, E. Production of Protease and Amylase from Bacillus subtilis and Aspergillus niger Using Parkia biglobossa (Africa Locust Beans) as Substrate in Solid State Fermentation. Adv. Life Sci. 2012, 1, 49–53. [Google Scholar] [CrossRef]
- Lee, D.E.; Shin, G.R.; Lee, S.; Jang, E.S.; Shin, H.W.; Moon, B.S.; Lee, C.H. Metabolomics reveal that amino acids are the main contributors to antioxidant activity in wheat and rice gochujangs (Korean fermented red pepper paste). Food Res. Int. (Ott. Ont.) 2016, 87, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Bayitse, R.; Hou, X.; Laryea, G.; Bjerre, A.-B. Protein enrichment of cassava residue using Trichoderma pseudokoningii (ATCC 26801). AMB Express 2015, 5, 80. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, P.K.; Jones, L.J.; Craven, G.S.; Somerset, S.M.; Palmer, C. Amino acid profiles of kinema, a soybean-fermented food. Food Chem. 1997, 59, 69–75. [Google Scholar] [CrossRef]
- Song, H.; Lee, S.Y. Production of succinic acid by bacterial fermentation. Enzym. Microb. Technol. 2006, 39, 352–361. [Google Scholar] [CrossRef]
- Suci, M.; Arbianti, R.; Hermansyah, H. Lipase production from Bacillus subtilis with submerged fermentation using waste cooking oil. In Proceedings of the IOP Conference Series: Earth and Environmental Science, Bali, Indonesia, 3 October 2017. [Google Scholar]
- Lesuisse, E.; Schanck, K.; Colson, C. Purification and preliminary characterization of the extracellular lipase of Bacillus subtilis 168, an extremely basic pH-tolerant enzyme. Eur. J. Biochem. 1993, 216, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Kanghae, A.; Eungwanichayapant, P.D.; Chukeatirote, E. Fatty acid profiles of fermented soybean prepared by Bacillus subtilis and Rhizopus oligosporus. Environ. Exp. Biol. 2017, 15, 173–176. [Google Scholar] [CrossRef]
- Jangbua, P.; Laoteng, K.; Kitsubun, P.; Nopharatana, M.; Tongta, A. Gamma-linolenic acid production of Mucor rouxii by solid-state fermentation using agricultural by-products. Lett. Appl. Microbiol. 2009, 49, 91–97. [Google Scholar] [CrossRef]
- McCarthy, A.; O’Callaghan, Y.; Piggott, C.; FitzGerald, R.; O’Brien, N.M. Brewers’ spent grain; Bioactivity of phenolic component, its role in animal nutrition and potential for incorporation in functional foods: A review. Proc. Nutr. Soc. 2012, 72, 1–9. [Google Scholar] [CrossRef]
- Meneses, N.G.T.; Martins, S.; Teixeira, J.A.; Mussatto, S.I. Influence of extraction solvents on the recovery of antioxidant phenolic compounds from brewer’s spent grains. Sep. Purif. Technol. 2013, 108, 152–158. [Google Scholar] [CrossRef]
- Martins, S.; Mussatto, S.I.; Martínez-Avila, G.; Montañez-Saenz, J.; Aguilar, C.N.; Teixeira, J.A. Bioactive phenolic compounds: Production and extraction by solid-state fermentation. A review. Biotechnol. Adv. 2011, 29, 365–373. [Google Scholar] [CrossRef]
- Weng, Y.; Yao, J.; Sparks, S.; Wang, K.Y. Nattokinase: An Oral Antithrombotic Agent for the Prevention of Cardiovascular Disease. Int. J. Mol. Sci. 2017, 18, 523. [Google Scholar] [CrossRef] [PubMed]
- Mani, V.; Ming, L.C. Chapter 19—Tempeh and Other Fermented Soybean Products Rich in Isoflavones. In Fermented Foods in Health and Disease Prevention; Frias, J., Martinez-Villaluenga, C., Peñas, E., Eds.; Academic Press: Cambridge, MA, USA, 2017; pp. 453–474. [Google Scholar]

| Amino Acids | Unfermented Brewers’ Spent Grains (BSG) | Fermented Brewers’ Spent Grains (BSG) (Day 2) | Fold Change |
|---|---|---|---|
| Leucine | 0.113 ± 0.031 | 0.134 ± 0.098 | 1.185 |
| Serine | 0.015 ± 0.005 | 0.017 ± 0.009 | 1.812 |
| Aspartic Acid | 0.024 ± 0.003 | 0.034 ± 0.007 | 1.542 |
| Threonine | 0.015 ± 0.005 | 0.026 ± 0.016 | 2.092 |
| Phenylalanine | 0.021 ± 0.003 | 0.027 ± 0.015 | 2.133 |
| Proline | 0.349 ± 0.182 | 1.230 ± 0.568 | 3.527 |
| Glutamic Acid | 0.304 ± 0.20 | 0.407 ± 0.376 | 1.625 |
| Lysine | 0.012 ± 0.006 | 0.011 ± 0.010 | 1.200 |
| Tyrosine | 0.006 ± 0.001 | 0.009 ± 0.001 | 1.560 |
| Total amino acids | 0.859 ± 0.049 | 1.894 ± 0.125 | 2.204 |
| Fatty Acids | Unfermented BSG | Fermented BSG (Day 2) | Fold Change |
|---|---|---|---|
| Palmitic Acid | 1.805 ± 0.003 | 1.523 ± 0.205 | 0.844 |
| Linoleic Acid | 0.445 ± 0.100 | 0.731 ± 0.220 | 1.643 |
| Oleic Acid | 0.041 ± 0.006 | 0.097 ± 0.053 | 2.366 |
| Stearic Acid | 4.596 ± 0.091 | 4.734 ± 0.131 | 1.03 |
| Total Fatty Acids | 6.89 ± 0.055 | 7.085 ± 0.152 | 1.028 |
| Samples | Signal Inhibition % | Weight of Trolox (µg/g BSG) |
|---|---|---|
| Unfermented BSG (Control) | 4.72 | 1.2 ± 0.03 |
| Fermented BSG (Day 2) | 28.27 | 6.94 ± 0.21 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, Y.X.; Mok, W.K.; Lee, J.; Kim, J.; Chen, W.N. Solid State Fermentation of Brewers’ Spent Grains for Improved Nutritional Profile Using Bacillus subtilis WX-17. Fermentation 2019, 5, 52. https://doi.org/10.3390/fermentation5030052
Tan YX, Mok WK, Lee J, Kim J, Chen WN. Solid State Fermentation of Brewers’ Spent Grains for Improved Nutritional Profile Using Bacillus subtilis WX-17. Fermentation. 2019; 5(3):52. https://doi.org/10.3390/fermentation5030052
Chicago/Turabian StyleTan, Yong Xing, Wai Kit Mok, Jaslyn Lee, Jaejung Kim, and Wei Ning Chen. 2019. "Solid State Fermentation of Brewers’ Spent Grains for Improved Nutritional Profile Using Bacillus subtilis WX-17" Fermentation 5, no. 3: 52. https://doi.org/10.3390/fermentation5030052
APA StyleTan, Y. X., Mok, W. K., Lee, J., Kim, J., & Chen, W. N. (2019). Solid State Fermentation of Brewers’ Spent Grains for Improved Nutritional Profile Using Bacillus subtilis WX-17. Fermentation, 5(3), 52. https://doi.org/10.3390/fermentation5030052