Kinetic Study on Heterotrophic Growth of Acetobacterium woodii on Lignocellulosic Substrates for Acetic Acid Production
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Experimental Design
2.3. Inoculum
2.4. Substrate
2.5. Batch Fermentation in Serum Vials
2.6. Analytical Methods
2.6.1. Measurement of Substrate Utilization and Product Formation Using HPLC Analysis
2.6.2. OD Measurement and Cell Dry Weight
2.7. Calculations
Determination of Kinetic Constants
3. Results
3.1. Growth of A. woodii on C6 (glucose) and C5 (xylose) Sugars
3.2. Effect of Different Glucose Concentrations on the Kinetics of A. woodii
3.3. Monod Growth Kinetics
3.4. Studies on Lignocellulosic Feedstock Hydrolysates
3.5. Calculation of Yield Coefficients
4. Discussions
Author Contributions
Funding
Conflicts of Interest
References
- Micromarket Monitor. North America Acetic Acid Market by Application (Vinyl Acetate Monomer (VAM), Purified Terephthalic Acid (PTA), Acetic Anhydride, Ester Solvents & Others) & by Country–Trends & Forecasts to 2019. Report Code AC 1086. 2015. Available online: http://www.micromarketmonitor.com/market/north-america-acetic-acid-9541396535.html?utm_source=NL-NAAAM&utm_medium=NL-NAAAM&utm_campaign=NL-NAAAM (accessed on 25 May 2015).
- Renewable Chemicals Market–Alcohols (Ethanol, Methanol), Biopolymers (Starch Blends, Regenerated Cellulose, PBS, bio-PET, PLA, PHA, bio-PE, and Others), Platform Chemicals & Others-Global Trends & Forecast to 2020. Report Code CH 2063. 2015. Available online: http://www.marketsandmarkets.com/Market-Reports/renewable-chemical-274.html (accessed on 1 October 2015).
- Christodoulou, X.; Velasquez-orta, S.B. Microbial electrosynthesis and anaerobic fermentation: An economic Evaluation for Acetic Acid Production from CO2 and CO. Environ. Sci. Technol. 2016, 50, 11234–11242. [Google Scholar] [CrossRef] [PubMed]
- Sustainable Development Goals. Global Chemicals Outlook—Towards Sound Management of Chemicals. 2013. Available online: https://sustainabledevelopment.un.org/index.php?page=view&type=400&nr=1966&menu=35 (accessed on 14 June 2018).
- Raj, P.T. Plant Design for Manufacturing Acetic Acid; Bachelor Project Report; Jawaharlal Nehru Technological University: Kakinada, India, 2014. [Google Scholar]
- Gullo, M.; Verzelloni, E.; Canonico, M. Aerobic submerged fermentation by acetic acid bacteria for vinegar production: Process and biotechnological aspects. Process Biochem. 2014, 49, 1571–1579. [Google Scholar] [CrossRef]
- Liew, F.M.; Kӧpke, M.; Simpson, S.D. Gas Fermentation for Commercial Biofuels Production Liquid. In Gaseous and Solid Biofuels-Conversion Techniques; Licensee InTech: London, UK, 2013; Chapter 5; pp. 125–173. [Google Scholar]
- Müller, V. Energy conservation in acetogenic bacteria. Appl. Environ. Microbiol. 2003, 69, 6345–6353. [Google Scholar] [CrossRef] [PubMed]
- Schiel-bengelsdorf, B.; Dürre, P. Pathway engineering and synthetic biology using acetogens. FEBS Lett. 2012, 586, 2191–2198. [Google Scholar] [CrossRef] [PubMed]
- Schuchmann, K.; Müller, V. Energetics and application of heterotrophy in acetogenic bacteria. Appl. Environ. Microbiol. 2016, 82, 4056–4069. [Google Scholar] [CrossRef] [PubMed]
- Ragsdale, S.W. The eastern and the western branch of the Wood/Ljungdahl pathway: How the east and west were won. Biofactors 1997, 6, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Ragsdale, S.W. Enzymology of the acetyl-CoA pathway of autotrophic CO2 fixation. CRC Crit. Rev. Biochem. Mol. Biol. 1991, 26, 261–300. [Google Scholar] [CrossRef] [PubMed]
- Schuchmann, K.; Müller, V. Autotrophy at the thermodynamic limit of life: A model for energy conservation in acetogenic bacteria. Nat. Rev. Microbiol. 2014, 12, 809–821. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, G. CO2 fixation in acetogenic bacteria: Variations on a theme. FEMS Microbiol. Rev. 1986, 39, 181–213. [Google Scholar] [CrossRef]
- Hugenholtz, J.; Ljungdahl, L.G. Metabolism and energy generation in homoacetogenic clostridia. FEMS Microbiol. Rev. 1990, 87, 383–389. [Google Scholar] [CrossRef]
- Ljungdahl, L.G. The autotrophic pathway of acetate synthesis in acetogenic bacteria. Ann. Rev. Microbiol. 1986, 40, 415–450. [Google Scholar] [CrossRef] [PubMed]
- Drake, H.L.; Gӧßner, A.S.; Daniel, S. Old Acetogens, New Light. Ann. N.Y. Acad. Sci. 2008, 1125, 100–128. [Google Scholar] [CrossRef] [PubMed]
- Fontaine, F.E.; Peterson, W.H.; McCOY, E.; Johnson, M.J.; Ritter, G.J. A new type of glucose fermentation by Clostridium thermoaceticum N. sp. J. Bacteriol. 1941, 43, 701–715. [Google Scholar]
- Ljungdahl, L.G. The Acetyl-CoA Pathway and the Chemiosmotic Generation of ATP during Acetogenesis; Chapman and Hall Microbiology Series; Drake HL: New York, NY, USA, 1994; pp. 63–87. [Google Scholar]
- Ragsdale, S.W.; Pierce, E. Acetogenesis and the Wood-Ljungdahl pathway of CO2 fixation. Biochem. Biophys. Acta 2008, 1784, 1873–1898. [Google Scholar] [CrossRef] [PubMed]
- Wieringa, K.T. The formation of acetic acid from carbon dioxide and hydrogen by anaerobic spore-forming bacteria. Antonie Leeuwenhoek 1939, 6, 251–262. [Google Scholar] [CrossRef]
- Balasubramanian, N.; Kim, J.S.; Lee, Y.Y. Fermentation of xylose into acetic acid by Clostridium thermoaceticum. Appl. Biochem. Biotechnol. 2001, 91, 367–376. [Google Scholar] [CrossRef]
- Ehsanipour, M.; Suko, A.V.; Bura, R. Fermentation of lignocellulosic sugars to acetic acid by Morella thermoacetica. J. Ind. Microbiol. Biotechnol. 2016, 43, 807–816. [Google Scholar] [CrossRef]
- Aufurth, S.; Schӓgger, H.; Müller, V. Identification of subunits a, b, and c1 from Acetobacterium woodii Na+-F1F0-ATPase. Subunits c1, c2, and c3 constitute a mixed c-oligomer. J. Biol. Chem. 2000, 275, 33297–33301. [Google Scholar] [CrossRef]
- Heise, R.; Müller, V.; Gottschalk, G. Presence of a sodium-translocating ATPase in membrane vesicles of the homoacetogenic bacterium Acetobacterium woodii. Eur. J. Biochem. 1992, 206, 553–557. [Google Scholar] [CrossRef]
- Heise, R.; Reidlinger, J.; Müller, V.; Gottschalk, G. A sodium-stimulated ATP synthase in the acetogenic bacterium Acetobacterium woodii. FEBS Lett. 1991, 295, 119–122. [Google Scholar] [CrossRef]
- Balch, W.E.; Schoberth, S.; Tanner, R.S. Acetobacterium, a new genus of hydrogen-oxidizing, carbon dioxide-reducing, anaerobic bacteria. Int. J. Syst. Bacteriol. 1977, 27, 355–361. [Google Scholar] [CrossRef]
- Groher, A.; Weuster-Botz, D. Comparative reaction engineering analysis of different acetogenic bacteria for gas fermentation. J. Biotechnol. 2016, 228, 82–94. [Google Scholar] [CrossRef] [PubMed]
- Jansen, R. Integrated Production and Extraction of Acetic Acid from CO2 Using Microbial Electrosynthesis: Creating Reactor Platform. Master’s Thesis, Gent University, Ghent, Belgium, 2016. [Google Scholar]
- Kantzow, C.; Mayer, A.; Weuster-Botz, D. Continuous gas fermentation by Acetobacterium woodii in a submerged membrane reactor with full cell retention. J. Biotechnol. 2015, 212, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Kantzow, C.; Weuster-Botz, D. Effects of hydrogen partial pressure on autotrophic growth and product formation of Acetobacterium woodii. Bioprocess Biosyst. Eng. 2015, 39, 1325–1330. [Google Scholar] [CrossRef] [PubMed]
- Morinaga, T.; Kawada, N. The production of acetic acid from carbon dioxide and hydrogen by an anaerobic bacterium. J. Biotechnol. 1990, 14, 187–194. [Google Scholar] [CrossRef]
- Schmidt, S.; Biegel, E.; Müller, V. The ins and outs of Na+ bioenergetics in Acetobacterium woodii. Biochem. Biophys. Acta 2009, 1787, 691–696. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Q.; Yu, S.J.; Zhang, F.; Xia, X.Y. Enhancement of acetate productivity in a thermophilic (55 °C) hollow-fiber membrane biofilm reactor with mixed culture syngas (H2/CO2) fermentation. Appl. Microbiol. Biotechnol. 2017, 101, 2619–2627. [Google Scholar] [CrossRef]
- Müller, V. Bacterial Fermentation; John Wiley & Sons Ltd.: Chichester, UK, 2008. [Google Scholar]
- Biegel, E.; Mu, V. A Na+ translocating pyrophosphatase in the acetogenic bacterium Acetobacterium woodii. J. Biol. Chem. 2011, 286, 6080–6084. [Google Scholar] [CrossRef]
- Lange, J.P. Lignocellulose conversion: An introduction to chemistry, process and economics. Biofuels Bioprod. Bioref. 2007, 1, 39–48. [Google Scholar] [CrossRef]
- Ahring, B.K.; Biswas, R.; Ahamed, A.; Teller, P.J.; Uellendahl, H. Making lignin accessible for anaerobic digestion by wet-explosion pretreatment. Bioresour. Technol. 2015, 175, 182–188. [Google Scholar] [CrossRef]
- Angelidaki, I.; Petersen, S.P.; Ahring, B.K. Effects of lipids on thermophilic anaerobic digestion and reduction of lipid inhibition upon addition of bentonite. Appl. Microbiol. Biotechnol. 1990, 33, 469–472. [Google Scholar] [CrossRef] [PubMed]
- Biswas, R.; Uellendahl, H.; Ahring, B.K. Wet Explosion: A universal and efficient pretreatment process for lignocellulosic biorefineries. Bioenerg. Res. 2015, 8, 1101–1116. [Google Scholar] [CrossRef]
- Jӧnsson, L.J.; Martín, C. Pretreatment of lignocellulose: Formation of inhibitory byproducts and strategies for minimizing their effects. Bioresour. Technol. 2016, 199, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Moreno, A.D.; Olsson, L. Pretreatment of Lignocellulosic Feedstocks, Extremophilic Enzymatic Processing of Lignocellulosic Feedstocks to Bioenergy; Springer: New York, NY, USA, 2017; pp. 31–52. [Google Scholar]
- Murali, N.; Srinivas, K.; Ahring, B.K. Biochemical production and separation of carboxylic acids for biorefinery applications. Ferment 2018, 3, 22. [Google Scholar] [CrossRef]
- Nielsen, J. Microbial Process Kinetics. In Basic Biotechnology, 2nd ed.; Ratledge, C., Kristiansen, B., Eds.; Cambridge University Press: Cambridge, UK, 2002; Chapter 6. [Google Scholar]
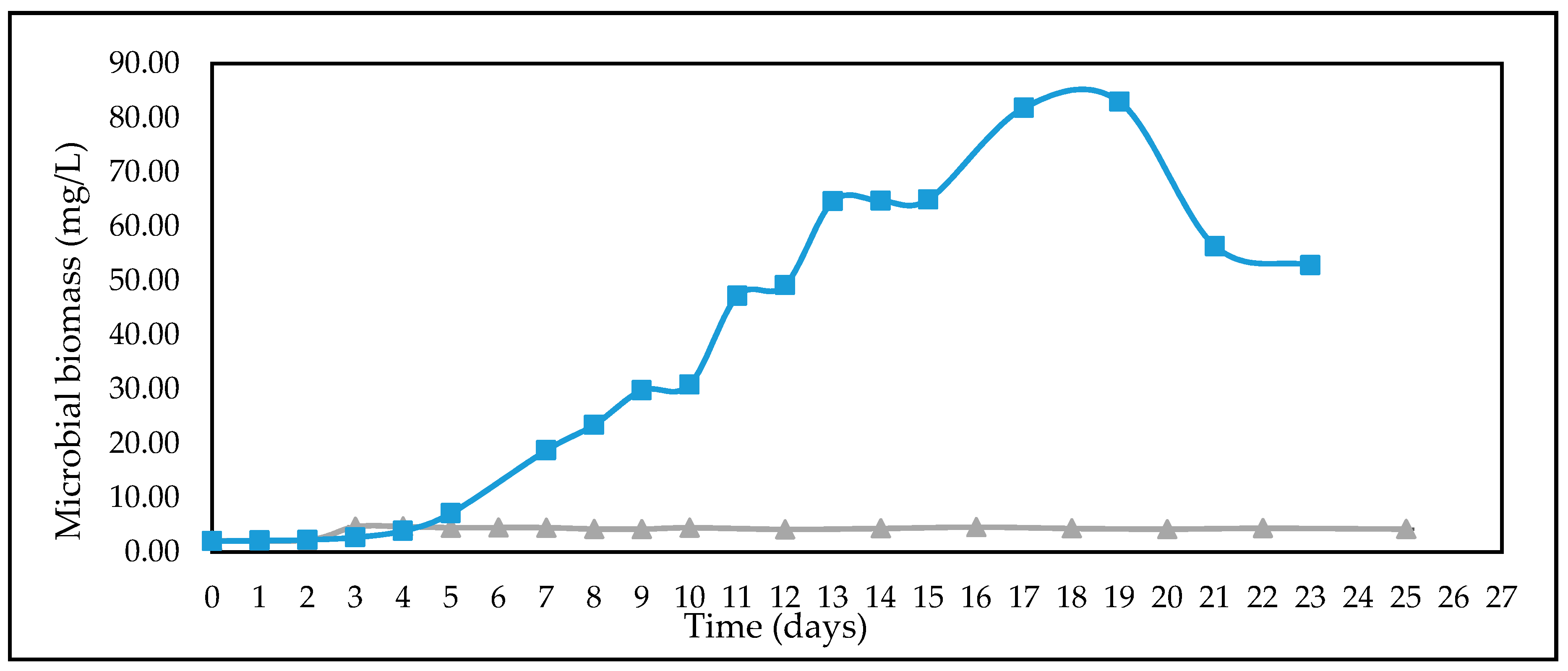
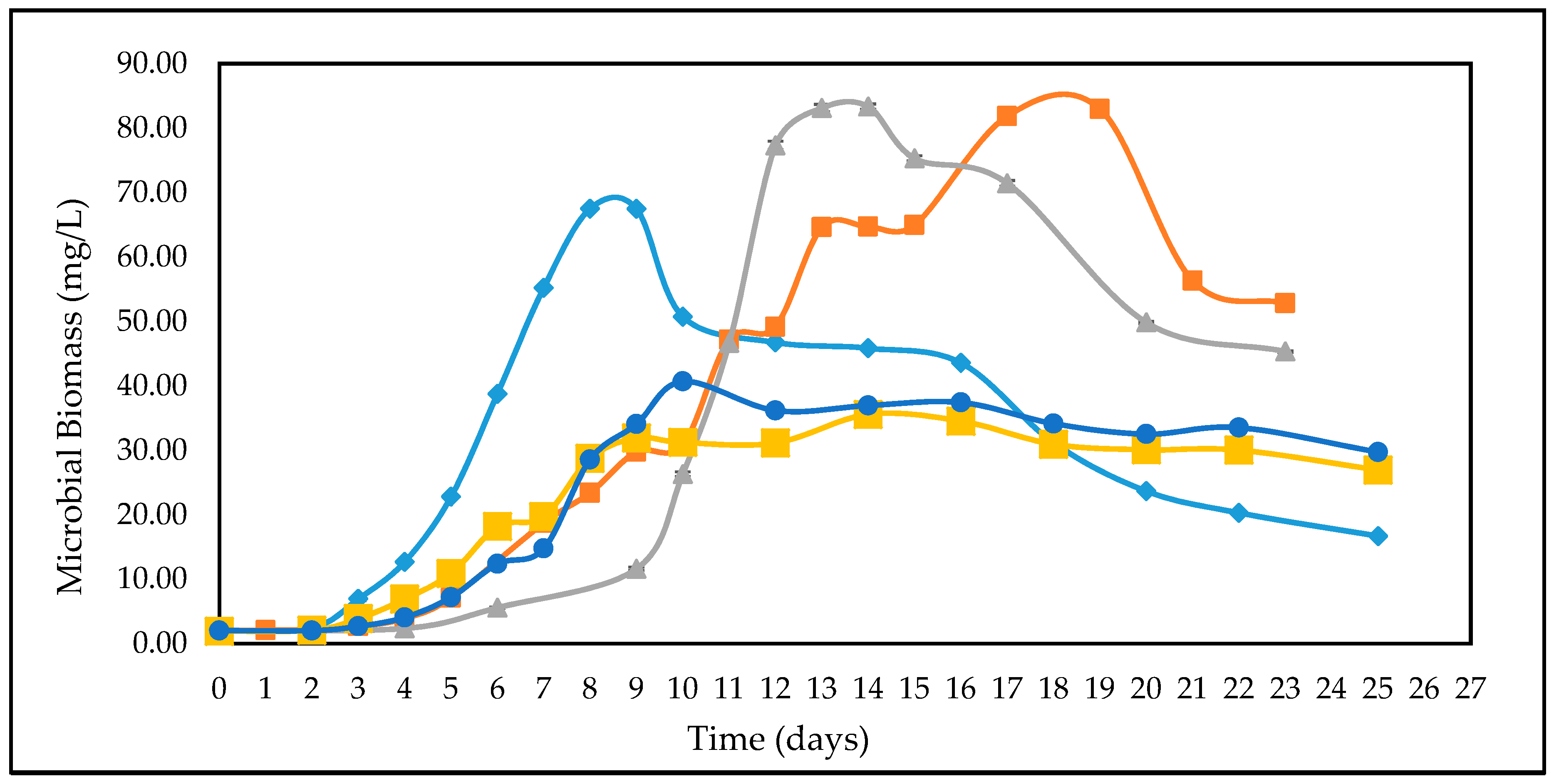
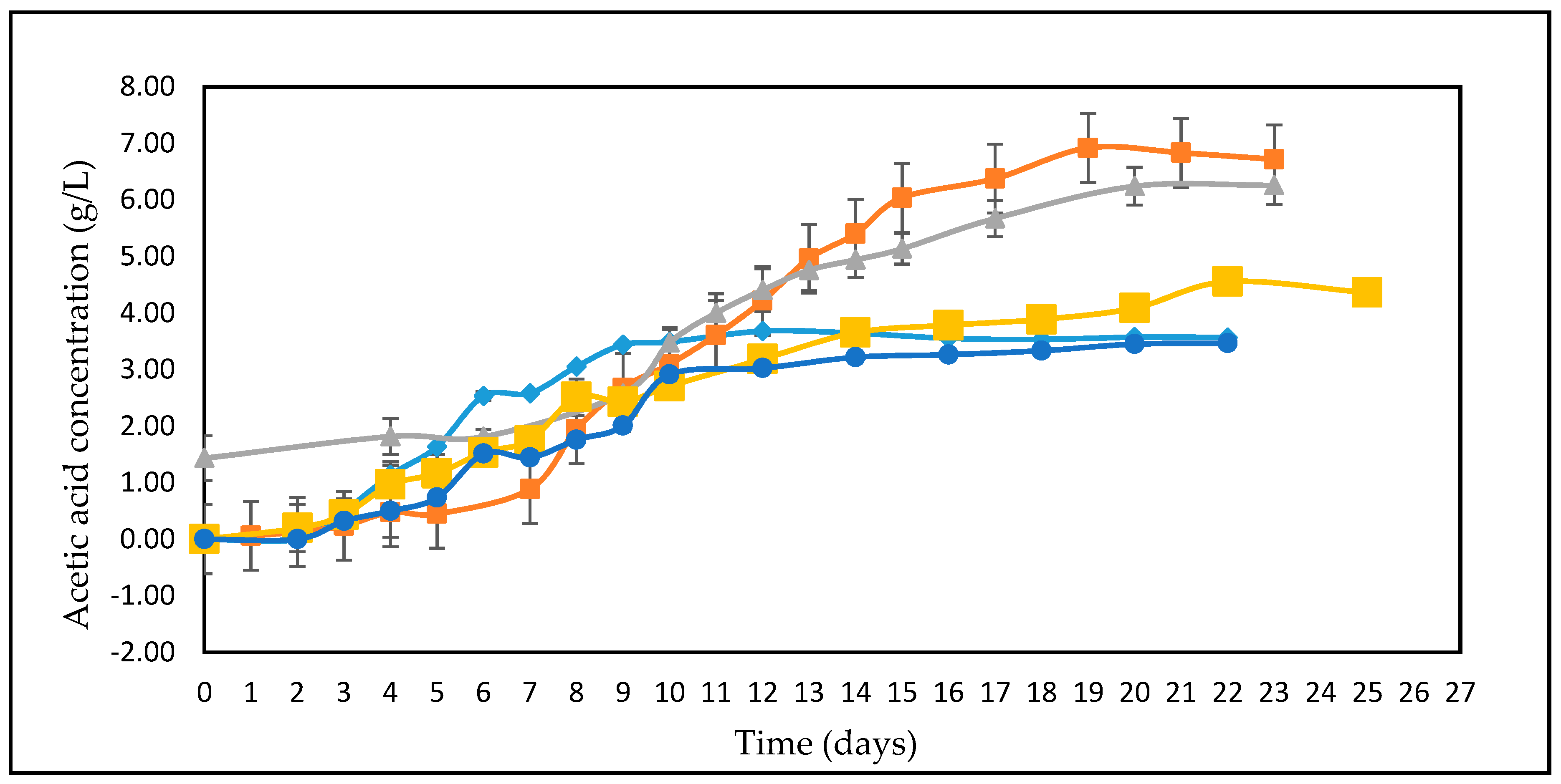
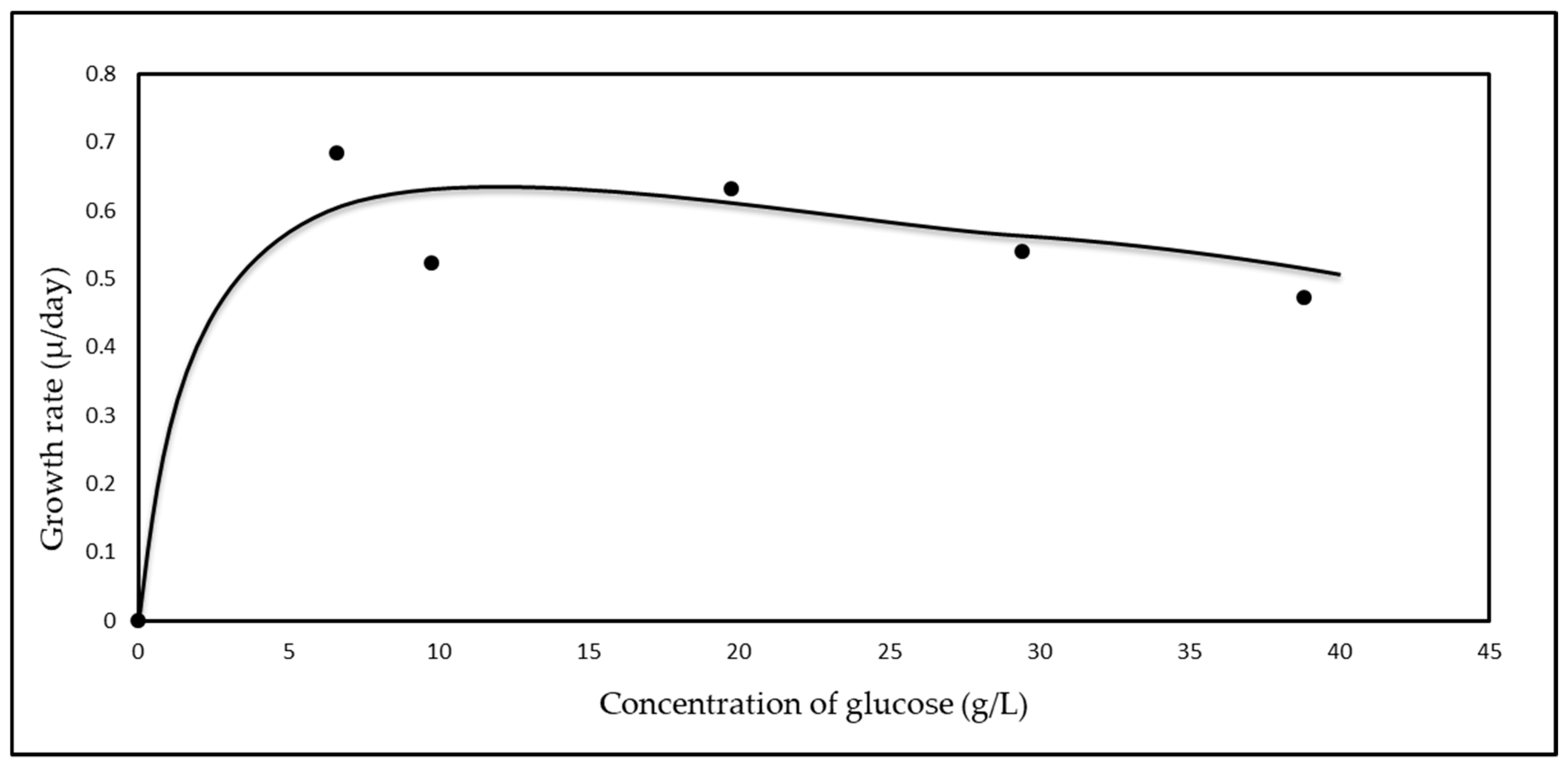
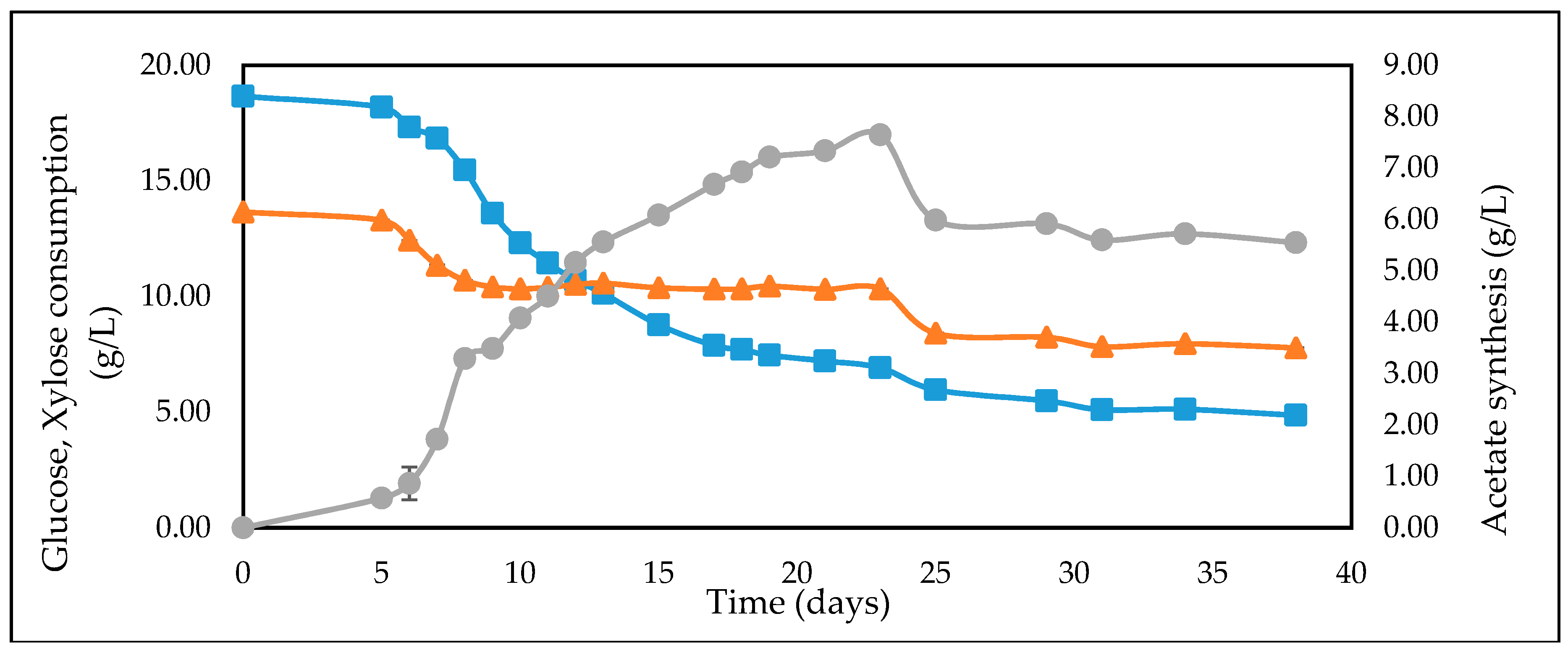
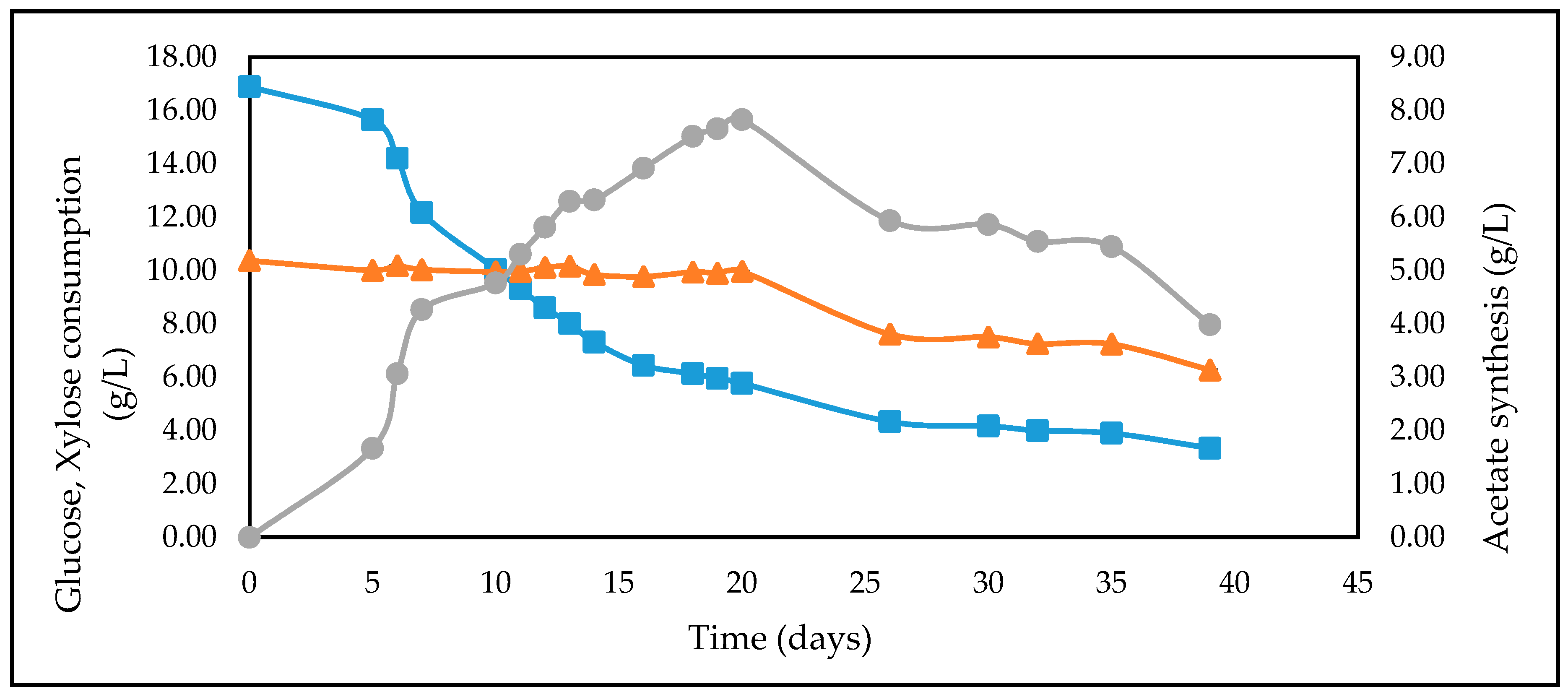
| Feedstock | * Measured Concentration of Batch Feed before Inoculation (g/L) | ||
|---|---|---|---|
| Glucose | Xylose | Acetic Acid | |
| 5 g/L glucose | 6.63 | - | 0 |
| 10 g/L glucose | 9.87 | - | 0 |
| 20 g/L glucose | 20.79 | - | 0 |
| 30 g/L glucose | 31.23 | - | 0 |
| 40 g/L glucose | 41.10 | - | 0 |
| 10 g/L xylose | - | 10.28 | - |
| WSH | 18.58 | 13.45 | 3.31 |
| CSH | 16.66 | 10.02 | 5.16 |
| Glucose Concentration (g/L) | Maximum Growth Rate (µg) (day−1) |
|---|---|
| 6.63 | 0.69 |
| 9.87 | 0.52 |
| 20.79 | 0.63 |
| 31.23 | 0.54 |
| 41.10 | 0.47 |
| Concentration of Glucose (g/L) | Yp/s |
|---|---|
| 6.63 | 0.59 |
| 9.87 | 0.76 |
| 20.79 | 0.44 |
| 31.23 | 0.40 |
| 41.10 | 0.33 |
| WSH (18.58) | 0.70 |
| CSH (16.66) | 0.65 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karekar, S.C.; Srinivas, K.; Ahring, B.K. Kinetic Study on Heterotrophic Growth of Acetobacterium woodii on Lignocellulosic Substrates for Acetic Acid Production. Fermentation 2019, 5, 17. https://doi.org/10.3390/fermentation5010017
Karekar SC, Srinivas K, Ahring BK. Kinetic Study on Heterotrophic Growth of Acetobacterium woodii on Lignocellulosic Substrates for Acetic Acid Production. Fermentation. 2019; 5(1):17. https://doi.org/10.3390/fermentation5010017
Chicago/Turabian StyleKarekar, Supriya C., Keerthi Srinivas, and Birgitte K. Ahring. 2019. "Kinetic Study on Heterotrophic Growth of Acetobacterium woodii on Lignocellulosic Substrates for Acetic Acid Production" Fermentation 5, no. 1: 17. https://doi.org/10.3390/fermentation5010017
APA StyleKarekar, S. C., Srinivas, K., & Ahring, B. K. (2019). Kinetic Study on Heterotrophic Growth of Acetobacterium woodii on Lignocellulosic Substrates for Acetic Acid Production. Fermentation, 5(1), 17. https://doi.org/10.3390/fermentation5010017





