Abstract
Glycerol carbonate (GC) is a value-added product originating from the valorization of widely available glycerol (Gly), a side stream from the production of biodiesel. Here we approach the production of this chemical comparing two reactions based on the transesterification of Gly with dimethyl carbonate (DMC) and ethylene carbonate (EC). When using DMC, it was observed that the free enzyme CALB (lipase B from Candida antarctica) gave the best results, whereas Eversa Transform (a genetic modification of Thermomyces lanuginosus lipase) performed better than the rest if EC was the reagent. With the selected catalysts, their immobilized analogous enzymes Novozym 435 and Lypozyme TL IM, respectively, were also tested. Observing that the yields for the reaction with EC were significantly faster, other operating variables were evaluated, resulting the best performance using a closed system, tert-butanol as solvent, a concentration of enzyme Eversa Transform of 3% w/w, a molar excess of EC:Gly of 9:1 and a temperature of 60 °C. Finally, several runs were conducted at different temperatures and molar ratios of EC:Gly, fitting a kinetic model to all experimental data for the reaction catalyzed with Eversa Transform. This model included the bimolecular transesterification reaction of Gly and EC catalyzed by the lipase and a reversible ring-opening polymerization of EC.
1. Introduction
Valorization of glycerol (Gly) is a blossoming topic of research given the need to make good use of the abundant surplus caused by the development of the biodiesel industry [1], which, in the end, has led to a marked decline in the retail prices in spite of its use in the food, healthcare, pharmaceutical, or tobacco industries [2]. Although the valorization of Gly for energy related purposes has been pursued [3,4], it has not proved as successful as using this compound as a platform chemical owing to its rich reactivity that can lead to a very wide array of products of interest for several industries [5,6].
One of the many products that can be obtained is glycerol carbonate (GC), which has attracted a lot of interest in the last years due to its outstanding physicochemical characteristics. It has been used mainly as a green solvent for purposes that include chemical reaction media, CO2 separation with membranes or Li-ion batteries, but also in the formulation of products in the food and cosmetic industry [7].
Several reviews have described extensively the methods of production of GC [7,8,9,10], which could be summarized in the following reaction pathways: (i) addition of CO or CO2 to Gly under pressurized conditions; (ii) reaction of urea and Gly under vacuum conditions so as to remove the ammonia generated as by-product; and (iii) transesterification of Gly with organic carbonates (OC).
For the latter route, the authors of the present work have conducted extensive research using both dialkyl carbonates like dimethyl carbonate (DMC) [11,12] or cyclic carbonates like ethylene carbonate (EC) [13,14,15,16], or more recently, propylene and butylene carbonate [17]. The advantage of this reaction pathway is that alcohols or glycols can be concomitantly produced when dialkyl or cyclic carbonates, respectively, are used as co-substrates along with Gly.
Whilst the synthesis of GC from Gly by any of the methods described above has mostly been attained with homogeneous and heterogeneous catalysts, the use of enzymes for this process has received far less attention. Thus far, the reaction between Gly and DMC has been reported in the literature mainly using Novozym 435 (immobilized Candida antarctica Lipase B) [18,19,20,21], although some efforts have also been made with lipase from Aspergillus niger supported on magnetic nanoparticles [22]. In addition, enzymes have also been used successfully for the transesterification of Gly with diethyl carbonate [23] or the interesting reaction for the simultaneous production of GC and biodiesel via the reaction of DMC and different oils, thus avoiding the generation of Gly [24,25,26,27]. In all cases, the conversions attained were quantitative and with high selectivities to the desired product of almost 100%.
The free enzyme Eversa Transform (a genetic modification of Thermomyces lanuginosus lipase) is another product commercialized by Novozymes that has shown very promising applications in transesterification reactions, for it has been used to yield biodiesel from different oils as feedstock [28,29] or to concentrate unsaturated monoacylglycerols from fish oil [30]. Lipozyme TL 100 (Thermomyces lanuginosus lipase) is yet another free enzyme that has been tested for the synthesis of sucrose-6-acetate, an intermediate to the synthesis of sucralose, a sweetener; in addition, its immobilized form Lipozyme TL IM was also tried in this study [31]. The latter has also been reported in interesterification reactions to obtain trans-free fats from canola oil and palm oil oleins or hydrogenated soybean oil blends [32] or in acetylation reactions to obtain eugenyl acetate, with antimicrobial activity [33].
Given the limited miscibility between Gly and organic carbonates [13,15], many authors have tried performing the respective transesterifications in the presence of solvents to favor the mutual solubility of the compounds [34,35]. This approach has also been tried in enzymatic reactions, where the solvation of enzymes is known to affect positively on the activity of enzymes like CALB (lipase B from Candida antarctica) [36].
The aim of this work is to study the synthesis of GC by transesterification of Gly with EC (reaction 1) and DMC (reaction 2) with the liquid lipase preparations CALB, Lypozyme TL 100, and Eversa Transform as well as the immobilized lipase preparations Novozyme 435 and Lypozyme TL IM. A progressive optimization of the operational conditions with the best preparations is performed afterwards, selecting the most productive system to perform its kinetic study, finally.
2. Materials and Methods
2.1. Chemicals
The reactants used for experimental work were glycerol (purity > 99.9%) from Fisher Chemical, dimethyl carbonate (purity = 99%) from Acros Organics Ltd. and ethylene carbonate (purity = 99%), supplied by Scharlau. As solvents, tert-butanol (purity = 99%) from Alfa Aesar and tetrahydrofuran (purity > 99.9%) from Fisher Chemical were used. For calibration of the HPLC method, the following were used: methanol (purity > 99.9 %) from Scharlau, ethylene glycol (anhydrous, purity = 99.8%) and glycerol carbonate (purity 99%) from Sigma-Aldrich in addition to citric acid (purity = 99%) from Sigma Aldrich.
2.2. Enzymes
The following free enzymes were employed throughout the experimental work: CALB (Candida antarctica lipase B) and Lypozyme TL 100 L (Thermomyces lanuginosus lipase) and Eversa Transform 2.0 (lipase from a genetic modification of Thermomyces lanuginosus), which were a kind gift from Novo Nordisk Bioindustry. For stability purposes, the commercial formulation of such free enzymes contains glycerol and water; thus, considering the small amounts of the limiting reactant Gly used in the experiments, HPLC analysis of the enzymes was made so as to calculate accurately the amount of Gly to be added in each of the experiments. It was found that the Gly content was 0.37, 0.23 and 0.03 g/L for Eversa Transform, CALB and Lypozyme TL 100 L, respectively.
As for immobilized enzymes, Novozym 435 (Candida antarctica lipase B immobilized on a macroporous acrylic resin) and Lypozyme TL IM (Thermomyces lanuginosus lipase immobilized on silica gel) were tested here.
2.3. Transesterification Runs and Analytical Method
Kinetic runs were performed in 50 mL round-bottom flasks magnetically stirred and heated in glycerol baths placed on IKA heating plate RCT basic with controlled magnetic agitation and PDI temperature control, working in initial reaction volumes of 25 mL. After mixing the organic carbonate and Gly, the cosolvent (either THF or tert-butanol) was added till getting only one liquid phase. Afterwards, the addition of the biocatalyst was made after the contents of the flasks reached the desired temperature. Two different operation modes were tried using the reaction flask open to the atmosphere or closed with a lid to check for volume changes before and after the experiments. These volume changes were monitored by volumetric and gravimetric measurements, while chemical composition of the remaining liquid was determined by HPLC as explained in the next paragraph.
For runs with free enzyme, samples were withdrawn at reaction volumes of 100 µL at a time and then prepared for analysis in 900 µL of an aqueous solution of 5 g/L of citric acid. For runs using immobilized enzyme, 300 µL were withdrawn and subjected to centrifugation so that 100 µL of the supernatant could be finally sampled and prepared for analysis as described above. Subsequent analysis of samples was performed by a HPLC method following a device and method reported in previous works [14]. Briefly, for the HPLC analysis, a method based on ion-dipole interaction and molecular volume exclusion was employed, with a REZEX H+ 300 × 7.5 mm column for carboxylic acids as stationary phase, placed in an oven at 65 °C, H2SO4 5 mM in Milli-Q water flowing at 0.5 mL min−1 as eluent, and a refractive index equipment set at 45 °C as detector.
2.4. Statistical Methods
Once the most reactive system was chosen, several runs changing two classical operation conditions: temperature and molar ratio of carbonate—in excess—to glycerol. A kinetic model was then proposed on the basis of the temporal evolution of the involved compounds concentrations. This model was fitted to all retrieved data at the same time by using a gradient non-linear regression algorithm (Levenberg-Marquardt) coupled to numerical integration (4th order Runge-Kutta) of the kinetic equations, algorithms that were implemented in software Aspen Custom Modeler v10. The statistical analysis led to optimal values of the kinetic parameters in the equations, together with their error intervals and a series of goodness-of-fit parameters that indicate the adequacy of the kinetic model to represent the change of the system chemical composition with time. The basic goodness-of-fit parameter is SQR: the sum of quadratic residues, which should tend to zero as the fitting improves; Other goodness-of-fit parameters are calculated from it: the residual mean squared error (RMSE) corresponds to the square root of SQR divided by the degrees of freedom, for which, again, a trend to zero would be indicative of better fittings; and finally Fisher F, with a tendency to infinity for the best fit, as SQR is in its denominator. They were calculated with these equations:
where Ci,exp is the molar concentration of each compound, Ci,calc is the concentration of compound i estimated by the kinetic model with the optimal values of the kinetic parameters, N is the number of data, and K is the number of kinetic constants in the model (3, for the proposed model).
3. Results and Discussion
3.1. Preselection of Reaction, Enzyme, and Operating Mode
3.1.1. Selection of Enzymes for Each Reaction
The three free enzymes CALB, Lypozyme TL 100 L, and Eversa Transform were tested to investigate their performance at a molecular level in the transesterification of Gly with EC and DMC. The conditions selected for this screening experiments considered a molar ratio of OC to Gly of 2:1 and a temperature of 60 °C in a closed system, which have been tested previously for the same reactions catalyzed with potassium methoxide (Esteban et al., 2015a). Figure 1 and Figure 2 show the conversion of Gly reached as well as the yields to each of the products achieved after 24 and 48 h for reactions A and B, respectively.
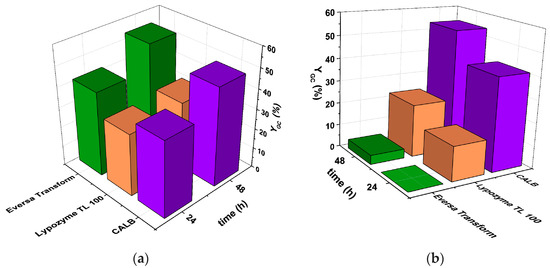
Figure 1.
Glycerol carbonate yields using lipases CALB, Lypozyme TL 100 L and Eversa Transform in the transesterification of glycerol with (a) EC and (b) DMC. Type of reactor: batch closed system; T = 60 °C; Carbonate: Gly = 2:1; enzyme concentration: 3% v/v; solvent: tert-butanol (30% v/v) agitation speed: 400 rpm.
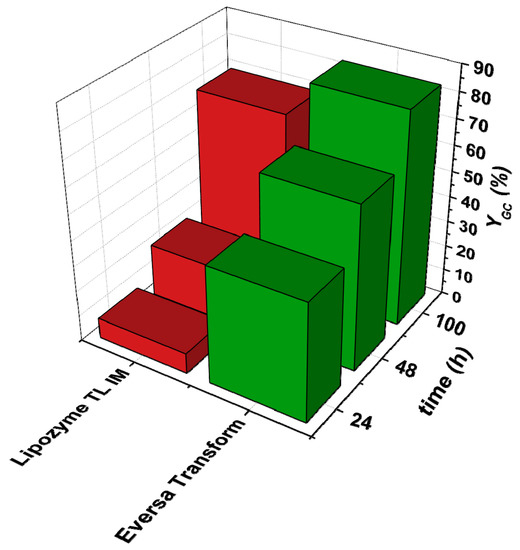
Figure 2.
Comparison of the use of the Eversa Transform and Lipozyme TL IM for the reaction between glycerol with ethylene carbonate. Reaction conditions: closed system; T = 60 °C; DMC:Gly = 3:1; amount of enzyme: 3% v/v; solvent: tert-butanol (30% v/v) agitation speed: 400 rpm.
The results from Figure 1a show that Eversa Transform 2.0, an enzyme formulated by Novozymes A/S for biodiesel production from waste oil, is the most active free enzyme for the reaction between Gly and EC, reaching a yield to GC of 42% and 54% after 24 and 48 h, respectively, compared to 38% and 49% for CALB (lipase B from Candida antarctica) after the same periods or 31% and 33% for Lypozyme TL 100, an enzyme preparation containing the lipase of Thermomyces lanuginosus, a thermophilic fungus normally found in compost heaps and employed in detergent formulation.
Figure 1b depicts that the performance of Eversa Transform in the case of reaction 2 is definitely much lower, practically not observing any product in the first 24 h and reaching only a conversion of 4% after 48 h, which agrees with the fact that no products were observed using the immobilized form Lipozyme TL IM when performing this reaction [18]. On the other hand, Lipozyme TL 100 showed a remarkable improvement reaching 15% of yield after 24 h and 23% after 48 h, although CALB was by far the best for this reaction, obtaining 41% and 54% of yield after the same periods. The latter fact indeed is a good indicator of why it is the enzyme of choice of previous works for this reaction, although it was used in its supported form Novozyme 435 [18,19,20,21].
For this reason, with the two best performing enzymes Eversa Transform and CALB, further experiments were conducted for up to 100 h employing this time a molar ratio of OC to Gly of 3:1. As Figure 2 and Figure 3 depict, yields to GC observed were 83% and 81% for reactions A and B, respectively. Bearing these results in mind, these long run experiments were extended to commercially available immobilized enzymes corresponding to TL 100 L and CALB, i.e., Lipozyme TL IM and Novozyme 435 for the two reactions. The aforementioned figures display the yields to product observed with immobilized enzymes, which are comparatively lower than those with the free enzyme, which is more than presumably ascribable to the fact that there are strong internal mass transfer limitations within the particles of the supported enzyme (it is important to state that the amount of active enzyme in the solids is very high, 10 times higher than in liquid enzyme preparations). When observing results of reaction 2, these are comparable to those obtained by Jung et al. under the same conditions of temperature, molar ratio of DMC to Gly of 3:1 and using THF as a solvent after 48 h, where they reached approximately 49% of yield compared to our 42% employing tert-butanol [18].
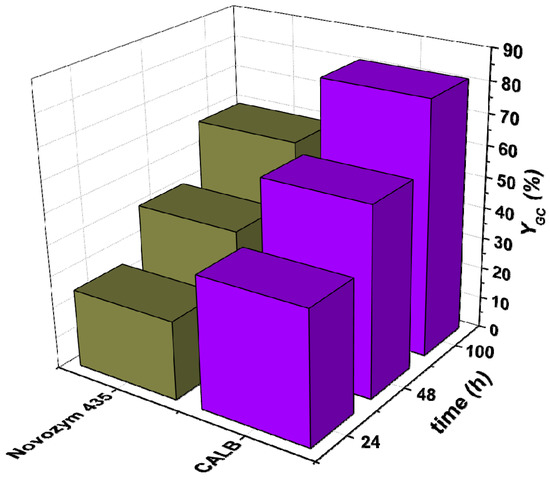
Figure 3.
Comparison of the use of the enzymes CALB (lipase B from Candida antarctica) and Novozyme 435 in the transesterification of glycerol with dimethyl carbonate. Reaction conditions: closed system; T = 60 °C; DMC:Gly = 3:1; amount of enzyme: 3% w/w; solvent: tert-butanol (30% v/v) agitation speed: 400 rpm.
3.1.2. Selection of Open vs. Closed System
Throughout the experiments it was observed that the initial 25 mL reacting mixture underwent certain degree of volume loss; subsequently, the two most active free enzymes were tested under the same conditions in and open (without lid) and closed (with lid) systems for comparison.
Figure 4 represents the yields to product and volume losses obtained operating with Eversa Transform in reaction 1 for the open and closed systems. In Figure 5 it can be seen that after 100 h of operation, the type of system has been found to exert a slight influence on the performance of Eversa Transform in reaction 1: the yield to GC decreased from 83% to 77% and the loss of volume increased from 4% to 10% when an open system was employed.
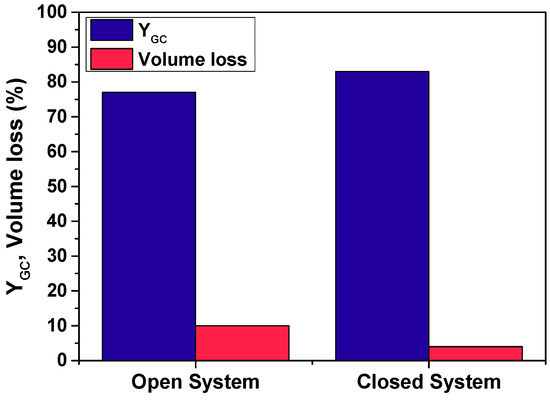
Figure 4.
Yield to product and volume loss in reaction 1 under an open and a closed system using Eversa Transform. Conditions: T = 60 °C; EC:Gly = 3:1; enzyme load: 3% w/w; solvent: tert-butanol (30% v/v) agitation speed: 400 rpm.
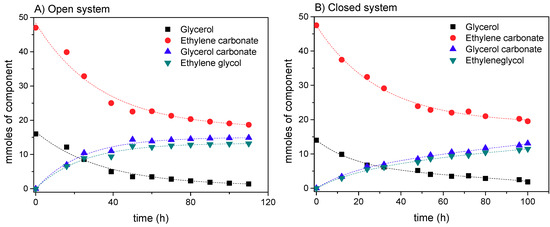
Figure 5.
Evolution of the presence of reactants and products in the reaction mixture of reaction 1 for (A) the open system and (B) the closed system. Conditions: T = 60 °C; EC:Gly = 3:1; enzyme load: 3% w/w; solvent: tert-butanol (30% v/v) agitation speed: 400 rpm.
Figure 5 depicts the kinetic evolution of each of the species in the reaction in the (A) open and (B) closed systems, respectively, where it can be seen that the molar amount of carbonate that disappears is higher than that due to its reaction with Gly. This fact is more evident in the closed system, where a fast reduction in carbonate and Gly is observed at the beginning of the process. However, the temporal evolutions of the products are faster in the open system, suggesting that a certain shift in the equilibrium towards the products is obtained in this system or, at least, that the presence of some water from the environment could result in an enhancement of the transcarboxylation activity of the enzyme.
For reaction 2, Figure 6 shows a much more significant difference of performance as the yield to product practically doubled from 41% to 81% when using the closed system with respect to the open one, while the volume loss amounted to 45% with the latter setup in contrast to 20% with the closed system. This seems to be related to the higher volatility of DMC compared to that of EC. In fact, the reduction in the concentration of DMC, a reagent, results in a lower yield to GC. This is verified in trends observed in Figure 7: the concentration of DMC declines much more rapidly in an open system.
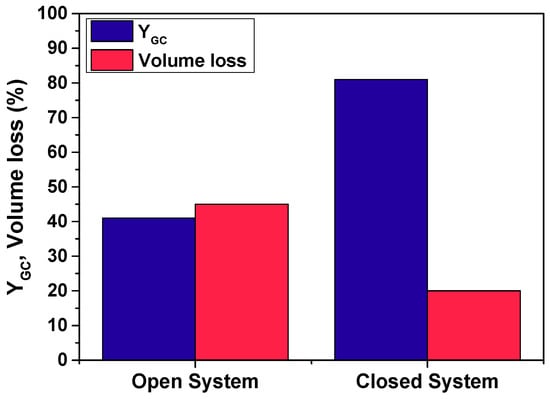
Figure 6.
Yield to product and volume loss in reaction 2 using an open and a closed system with CALB as catalyst. Conditions: T = 60 °C; DMC:Gly = 3:1; enzyme load: 3% w/w; solvent: tert-butanol (30% v/v) agitation speed: 400 rpm.
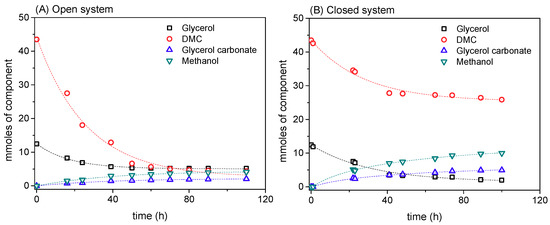
Figure 7.
Evolution of the presence of reactants and products in the reaction mixture of reaction 2 for (A) the open system and (B) the closed system. Conditions: T = 60 °C; EC:Gly = 3:1; enzyme load: 3% w/w; solvent: tert-butanol (30% v/v) agitation speed: 400 rpm.
For reaction 2, the significant volume loss could be easily ascribable to the removal of the methanol generated during the reaction, whose boiling point is 64.7 °C with the reaction taking place at the nearby temperature of 60 °C. Additionally, tert-butanol and DMC have boiling points of 83 and 90 °C, respectively, which could lead to some depletion of this component, too.
Based on the results described throughout Section 3.1, it can be concluded that to obtain the best yields to GC without incurring in too much loss of reaction volume, the best approach is to conduct the transesterification of Gly with EC (reaction 1) catalyzed by Eversa Transform in a closed reactor, the system employed in the following sections.
3.2. Effect of Variables on the Transesterification of Glycerol with Ethylene Carbonate
3.2.1. Effect of the Solvent
The effect of the solvent is tested for the transesterification of Gly with EC for the most active enzyme, i.e., Eversa Transform. For this, the effect of another hydrophilic solvent like tetrahydrofuran (THF) has been tested due to the promising performance shown in the past for the transesterification with DMC [18,19] and compared to tert-butanol, which had already been tested for the transesterification of soybean oil with DMC (Seong et al., 2011). The yield to GC after 100 h in tert-butanol was of 83% as described in Figure 3, whereas only 57% was obtained with THF in the medium under the same conditions. Curiously, the productivity in the first case (in the first six hours, that is, initial conditions) is 6 mmoles of GC·h−1·L−1, while, when using THF as solvent, the value of this parameter is 5 mmoles GC·h−1·L−1, so, possibly, enzyme deactivation due to THF happens.
3.2.2. Effect of the Enzyme Concentration
This effect was studied by trying three different levels of loading, namely 2, 3, and 4% w/w. Again, the runs were conducted for 100 h, after which yields to GC of 60%, 83%, and 85% were attained for the three concentrations of enzyme. As shown in Figure 8, there is a saturating behavior of the concentration of catalyst, which is observed using a concentration of enzyme higher that 3% w/w. This effect has already been observed in literature for this reaction [18,37] or, and even in some cases, it appears that increasing higher amounts of enzyme can be detrimental to the performance of the reaction [22]. As turbidity is observed when adding the enzyme, an emulsion should be created and the enzymatic action should take place mainly at the liquid-liquid interface and its surroundings.
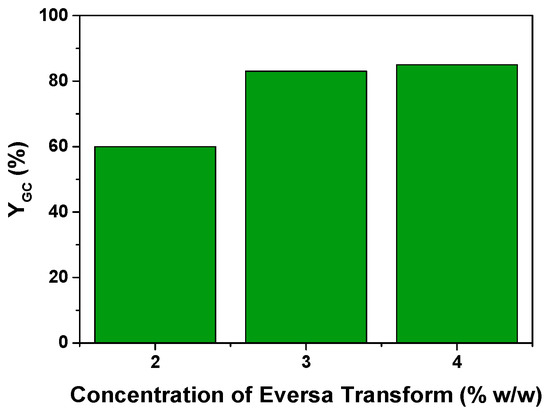
Figure 8.
Effect of the amount of the Eversa Transform enzymatic preparation used in the transesterification of Gly with EC on the yield to GC. Conditions: T = 60 °C; EC:Gly = 3:1; solvent: tert-butanol (30% v/v); t = 100 h; agitation speed: 400 rpm.
3.2.3. Effect of the Molar Ratio of Reactants and Temperature
The transesterification of Gly with EC had been tested employing relatively low molar ratios ranging from 1.5:1 to 3:1, although in the absence of any solvents in all cases [13,14,16,38,39]. The molar ratio of reactants may affect the equilibrium conversion and the kinetics of the reaction. Considering the low rates of reaction in enzymatic reactions in contrast with chemically catalyzed reactions and also the fact that here this reaction has been performed in the presence of a solvent, thus diluting the concentration of the species, it has been decided to make use of larger molar excess of organic carbonate ranging from 3:1 to 9:1. Additionally, the reaction was evaluated at 50, 60, and 70 °C, as this is the temperature range that has been considered in kinetic studies [11,16].
Figure 9 shows that better results are obtained as the molar excess increases from 3:1 to 9:1 at all temperatures, which agrees with the fact that even larger excesses of 17:1 and 21:1 of diethyl carbonate to Gly were reported previously as a strategy to reach conversions of 84% or 97% [40,41]. On the other hand, temperature does not show a clear positive effect on performance at 100 h reaction time, but there is a sharp increase in initial reaction rate when calculating it for the first 6 h of reaction, as expected.
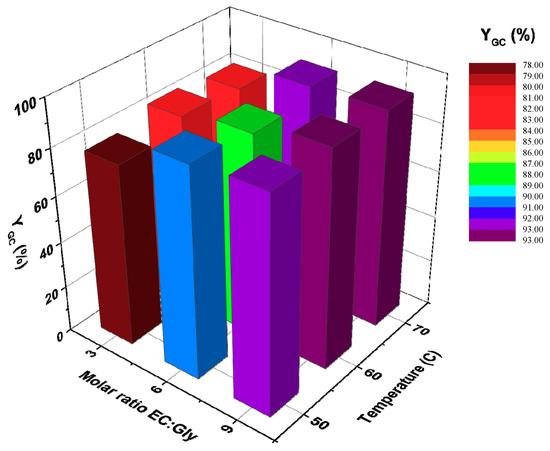
Figure 9.
Effect of the molar ratio of EC to Gly and temperature on the yield to GC. Conditions: enzyme load: 3% w/w; solvent: tert-butanol (30% v/v); t = 100 h; agitation speed: 400 rpm.
3.3. Kinetic Modeling of Enzymatic Transesterification of Glycerol With Ethylene Carbonate
To study the kinetics of this reacting system, the evolution of each of the components has been monitored from the experiments performed at different values of temperature (50, 60, and 70 °C) and molar ratio of EC to Gly (3:1 to 9:1), at a constant enzyme concentration (3% w/w). To avoid problems due to solvent and EC evaporation (they seemed to form a low-boiling point azeotrope), runs were performed in closed reactors provided with an adequate sampling system.
It can be observed that the evolution of EC cannot be explained only on the basis of the transesterification reaction. At the same time, both products (GC and EG) are obtained in equimolar amounts, thus avoiding a possible further reaction of GC with Gly and a polymerization of EC and EG or Gly driven by the lipases (condensation polymerization). However, ring-opening polymerization of ethylene carbonate driven by Eversa Transform 2.0 is possible [42].
where r1 (mol·L−1·min−1) is the reaction rate for the Gly transesterification driven by the lipase; k1 (L·mol−1·min−1) is a second-order kinetic constant, nGly is the molar concentration of Gly and nEC the molar concentration of EC.
Here, r2 (mol L−1·s−1) is the reaction rate for the ring-opening polymerization of EC; and r3 is the inverse reaction (ring-closing depolymerization to EC) (mol L−1·s−1). Their kinetic constants are k2 (L·mol−1·min−1) and k3 (min−1); nEC is the molar concentration of EC and nPEC is the molar concentration of the polymer, whose production can account for the increasing turbidity with time molar excess of EC and reaction temperature.
The suggested model with three reactions happening in parallel was fitted to data from runs performed with different excesses of EC and at temperatures ranging from 50 to 70 °C.
Results shown in Figure 10 and Table 1 indicate that the excess of EC only helps slightly the formation of GC and EG. As expected, values for the kinetic constants do not depend on the excess of EC, taking into account the respective absolute errors for such constants. The kinetic model fits perfectly to retrieved experimental data, as indicated by the very high values for Fisher’s F parameter, and very low values of SQR and RMSE, together with the evident goodness of fit shown by lines (calculated with the model) and experimental points in the figure. Finally, the maximum error intervals at 95% confidence for the kinetic constants are very small, rendering very narrow confidence intervals for such constants.
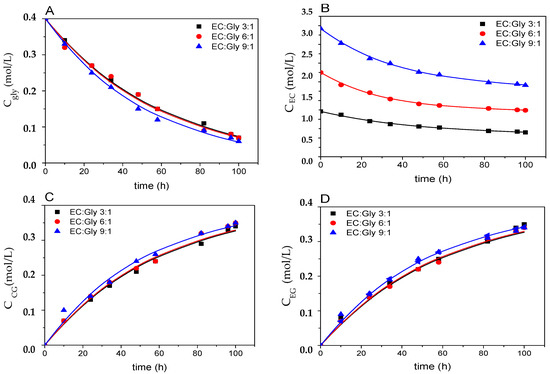
Figure 10.
Fitting of the kinetic model proposed to runs 1, 2, and 3 (molar ratios EC:Gly 1:3, 1:6, and 1:9, respectively). Subfigure (A) shows results for glycerol, (B) for ethylene carbonate, (C) for glycerol carbonate, and (D) for ethylene glycol. Other conditions: enzyme load: 3% w/w; solvent: tert-butanol (30% v/v); closed stirred batch reactor; agitation speed: 400 rpm; T = 50 °C.

Table 1.
Kinetic and goodness-of-fit parameters for the proposed model and the different runs.
From the kinetic model, it can be inferred that the effect of increasing temperature would seem to affect both parallel reactions (transesterification and the couple polymerization-depolymerization of ethylene carbonate). Results of the fit are shown in Figure 11 and Table 1, and they indicate that temperatures as high as 70 °C affect all reaction rates dramatically, while 50 and 60 °C lead to very similar results (slightly higher reaction rates for 60 °C). In general, these observations correspond to the relatively high activation energies for transesterification (55.21 kJ·mol−1), polymerization by ring-opening (63.88 kJ·mol−1) and depolymerization by ring-closing (77.96 kJ·mol−1). It is interesting to observe that the latter reaction tends to disappear at high temperatures, so the lipase effectively does not act as a good catalyst at high temperatures for this reaction in particular. It is unusual to observe negative activation energies, a phenomenon that can be related to a notably negative enthalpy change with temperature [43], to protein folding dynamics [44] or to deactivation or denaturation of the enzyme, which can happen at least partially [45]. In this latter case, the effect could affect only some of the reactions catalyzed by the enzyme. It should be considered that all kinetic constants here calculated include the activity of the enzyme towards the reaction under consideration.
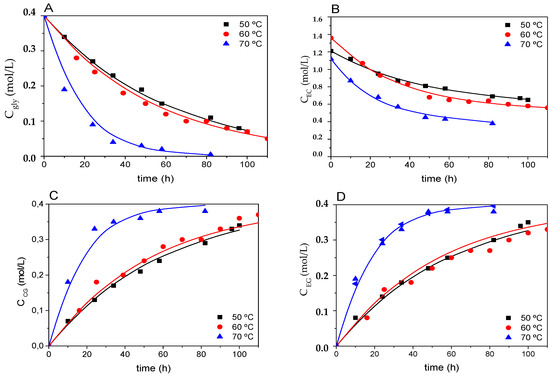
Figure 11.
Fitting of the kinetic model proposed to runs 1, 4, and 5 (50 °C, 60 °C, and 70 °C, respectively). Subfigure (A) shows results for glycerol, (B) for ethylene carbonate, (C) for glycerol carbonate, and (D) for ethylene glycol. Other conditions: enzyme load: 3% w/w; solvent: tert-butanol (30% v/v); closed stirred batch reactor; agitation speed: 400 rpm; molar ratio EC:Gly 1:3.
4. Conclusions
Glycerol carbonate can be synthesized from glycerol and organic carbonates like dimethyl carbonate and ethylene carbonate employing enzymatic processes. In fact, the latter reaction is presented for the first time here using an enzymatic transesterification reaction. The two reactions are first conducted with different free and immobilized enzymes and their performance was compared. For the transesterification with dimethyl carbonate, CALB was the free enzyme of choice, whereas Eversa Transform 2.0 performed better for the reaction with ethylene carbonate, a reaction, that in the end, showed a much better performance.
For this reason, subsequent studies with this reaction and enzyme optimized the operation conditions using tert-butanol as solvent in a closed system to minimize possible evaporation, a concentration of enzyme Eversa Transform 2.0 of 3% w/w, a molar excess of EC:Gly from 3:1 to 9:1 and a temperature from 50 to 70 °C. The evolution of the concentration of the components involved was determined by ion exclusion HPLC, while a kinetic model was proposed to fit data retrieved in runs performed in a closed reactor at several molar ratios (runs 1 to 3) and at several temperatures (runs 1, 4, and 5). The model proposed and validated included a transesterification reaction taking place in parallel to a polymerization-depolymerization reaction affecting EC. The first one was very influenced by temperature, as its reaction rate rocketed between 60 and 70 °C.
Author Contributions
M.L. and J.E. conceived the research; M.L. designed the experiments; A G.-L.; D.V. and D.E.B. performed the experiments and analyzed the samples; J.E. developed the original analytical procedures, while A.G.-L. and D.V. developed a modified analytical HPLC procedure for this reacting system and analyzed the retrieved data, including the preliminary statistical analysis; J.E., P.Y. and M.L. performed the final statistical analysis and wrote the paper. All the authors read and approved the final manuscript.
Funding
This work has been supported by MINECO (Ministerio de Economía y Competitividad, Spain) under contracts CTQ2013-45970-C2-1-R and PCIN-2013-021-C02-01 and by the Complutense University through BSCH-UCM, GR35/10-A 910134.
Acknowledgments
The kind gift of Lipozyme 435, Lipozyme TL IM, Lipozyme TL 100L, Lipozyme CALB L, and Eversa Transform 2.0 by Ramiro Martinez (Novozymes Spain, Pozuelo de Alarcon-Madrid-Spain) is gratefully acknowledged. This work was funded by MICINN under contracts CTQ-2013-45970-C2-1-R and PCIN-2013-021-C02-01.
Conflicts of Interest
The authors declare no conflict of interest. The founding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
References
- Lamers, P.; Hamelinck, C.; Junginger, M.; Faaij, A. International bioenergy trade—A review of past developments in the liquid biofuel market. Renew. Sustain. Energy Rev. 2011, 15, 2655–2676. [Google Scholar] [CrossRef]
- Quispe, C.A.G.; Coronado, C.J.R.; Carvalho, J.A. Glycerol: Production, consumption, prices, characterization and new trends in combustion. Renew. Sustain. Energy Rev. 2013, 27, 475–493. [Google Scholar] [CrossRef]
- He, Q.; McNutt, J.; Yang, J. Utilization of the residual glycerol from biodiesel production for renewable energy generation. Renew. Sustain. Energy Rev. 2017, 71, 63–76. [Google Scholar] [CrossRef]
- Thompson, J.C.; He, B.B. Characterization of crude glycerol from biodiesel production from multiple feedstocks. Appl. Eng. Agric. 2006, 22, 261–265. [Google Scholar] [CrossRef]
- Anitha, M.; Kamarudin, S.K.; Kofli, N.T. The potential of glycerol as a value-added commodity. Chem. Eng. J. 2016, 295, 119–130. [Google Scholar] [CrossRef]
- Behr, A.; Eilting, J.; Irawadi, K.; Leschinski, J.; Lindner, F. Improved utilisation of renewable resources: New important derivatives of glycerol. Green Chem. 2008, 10, 13–30. [Google Scholar] [CrossRef]
- Sonnati, M.O.; Amigoni, S.; de Givenchy, E.P.T.; Darmanin, T.; Choulet, O.; Guittard, F. Glycerol carbonate as a versatile building block for tomorrow: Synthesis, reactivity, properties and applications. Green Chem. 2013, 15, 283–306. [Google Scholar] [CrossRef]
- Ishak, Z.I.; Sairi, N.A.; Alias, Y.; Aroua, M.K.T.; Yusoff, R. A review of ionic liquids as catalysts for transesterification reactions of biodiesel and glycerol carbonate production. Catal. Rev. Sci. Eng. 2017, 59, 44–93. [Google Scholar] [CrossRef]
- Ochoa-Gomez, J.R.; Gomez-Jimenez-Aberasturi, O.; Ramirez-Lopez, C.; Belsue, M. A Brief Review on Industrial Alternatives for the Manufacturing of Glycerol Carbonate, a Green Chemical. Org. Process Res. Dev. 2012, 16, 389–399. [Google Scholar] [CrossRef]
- Teng, W.K.; Ngoh, G.C.; Yusoff, R.; Aroua, M.K. A review on the performance of glycerol carbonate production via catalytic transesterification: Effects of influencing parameters. Energy Convers. Manag. 2014, 88, 484–497. [Google Scholar] [CrossRef]
- Esteban, J.; Dominguez, E.; Ladero, M.; Garcia-Ochoa, F. Kinetics of the production of glycerol carbonate by transesterification of glycerol with dimethyl and ethylene carbonate using potassium methoxide, a highly active catalyst. Fuel Process. Technol. 2015, 138, 243–251. [Google Scholar] [CrossRef]
- Esteban, J.; Fuente, E.; Blanco, A.; Ladero, M.; Garcia-Ochoa, F. Phenomenological kinetic model of the synthesis of glycerol carbonate assisted by focused beam reflectance measurements. Chem. Eng. J. 2015, 260, 434–443. [Google Scholar] [CrossRef]
- Esteban, J.; Ladero, M.; Garcia-Ochoa, F. Liquid-liquid equilibria for the systems ethylene carbonate plus ethylene glycol plus glycerol; ethylene carbonate plus glycerol carbonate plus glycerol and ethylene carbonate plus ethylene glycol plus glycerol carbonate plus glycerol at catalytic reacting temperatures. Chem. Eng. Res. Des. 2015, 94, 440–448. [Google Scholar]
- Esteban, J.; Fuente, E.; Gonzalez-Miquel, M.; Blanco, A.; Ladero, M.; Garcia-Ochoa, F. Sustainable joint solventless coproduction of glycerol carbonate and ethylene glycol via thermal transesterification of glycerol. RSC Adv. 2014, 4, 53206–53215. [Google Scholar] [CrossRef]
- Esteban, J.; Ladero, M.; Molinero, L.; Garcia-Ochoa, F. Liquid-liquid equilibria for the ternary systems DMC-methanol-glycerol, DMC-glycerol carbonate-glycerol and the quaternary system DMC-methanol-glycerol carbonate-glycerol at catalytic reacting temperatures. Chem. Eng. Res. Des. 2014, 92, 2797–2805. [Google Scholar] [CrossRef]
- Esteban, J.; Ladero, M.; Fuente, E.; Blanco, A.; Garcia-Ochoa, F. Experimental and modelling approach to the catalytic coproduction of glycerol carbonate and ethylene glycol as a means to valorise glycerol. J. Taiwan Inst. Chem. Eng. 2016, 63, 89–100. [Google Scholar] [CrossRef]
- Esteban, J.; Vorholt, A.J. Obtaining glycerol carbonate and glycols using thermomorphic systems based on glycerol and cyclic organic carbonates: Kinetic studies. J. Ind. Eng. Chem. 2018, 63, 124–132. [Google Scholar] [CrossRef]
- Jung, H.; Lee, Y.; Kim, D.; Han, S.O.; Kim, S.W.; Lee, J.; Kim, Y.H.; Park, C. Enzymatic production of glycerol carbonate from by-product after biodiesel manufacturing process. Enzyme Microb. Technol. 2012, 51, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.C.; Kim, Y.H.; Lee, H.; Yoon, D.Y.; Song, B.K. Lipase-catalyzed synthesis of glycerol carbonate from renewable glycerol and dimethyl carbonate through transesterification. J. Mol. Catal. B Enzym. 2007, 49, 75–78. [Google Scholar] [CrossRef]
- Lee, K.H.; Park, C.H.; Lee, E.Y. Biosynthesis of glycerol carbonate from glycerol by lipase in dimethyl carbonate as the solvent. Bioprocess Biosyst. Eng. 2010, 33, 1059–1065. [Google Scholar] [CrossRef] [PubMed]
- Waghmare, G.V.; Vetal, M.D.; Rathod, V.K. Ultrasound assisted enzyme catalyzed synthesis of glycerol carbonate from glycerol and dimethyl carbonate. Ultrason. Sonochem. 2015, 22, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Tudorache, M.; Negoi, A.; Protesescu, L.; Parvulescu, V.I. Biocatalytic alternative for bio-glycerol conversion with alkyl carbonates via a lipase-linked magnetic nano-particles assisted process. Appl. Catal. B Environ. 2014, 145, 120–125. [Google Scholar] [CrossRef]
- Tudorache, M.; Negoi, A.; Tudora, B.; Parvulescu, V.I. Environmental-friendly strategy for biocatalytic conversion of waste glycerol to glycerol carbonate. Appl. Catal. B Environ. 2014, 146, 274–278. [Google Scholar] [CrossRef]
- Go, A.R.; Lee, Y.; Kim, Y.H.; Park, S.; Choi, J.; Lee, J.; Han, S.O.; Kim, S.W.; Park, C. Enzymatic coproduction of biodiesel and glycerol carbonate from soybean oil in solvent-free system. Enzyme Microb. Technol. 2013, 53, 154–158. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Lee, J.H.; Yang, H.J.; Jane, M.; Kim, J.R.; Byun, E.H.; Lee, J.; Na, J.G.; Kim, S.W.; Park, C. Efficient simultaneous production of biodiesel and glycerol carbonate via statistical optimization. J. Ind. Eng. Chem. 2017, 51, 49–53. [Google Scholar] [CrossRef]
- Min, J.Y.; Lee, E.Y. Lipase-catalyzed simultaneous biosynthesis of biodiesel and glycerol carbonate from corn oil in dimethyl carbonate. Biotechnol. Lett. 2011, 33, 1789–1796. [Google Scholar] [CrossRef] [PubMed]
- Seong, P.J.; Jeon, B.W.; Lee, M.; Cho, D.H.; Kim, D.K.; Jung, K.S.; Kim, S.W.; Han, S.O.; Kim, Y.H.; Park, C. Enzymatic coproduction of biodiesel and glycerol carbonate from soybean oil and dimethyl carbonate. Enzyme Microb. Technol. 2011, 48, 505–509. [Google Scholar] [CrossRef] [PubMed]
- Adewale, P.; Vithanage, L.N.; Christopher, L. Optimization of enzyme-catalyzed biodiesel production from crude tall oil using Taguchi method. Energy Convers. Manag. 2017, 154, 81–91. [Google Scholar] [CrossRef]
- Andrade, T.A.; Errico, M.; Christensen, K.V. Evaluation of Reaction Mechanisms and Kinetic Parameters for the Transesterification of Castor Oil by Liquid Enzymes. Ind. Eng. Chem. Res. 2017, 56, 9478–9488. [Google Scholar] [CrossRef]
- He, Y.J.; Li, J.B.; Kodali, S.; Balle, T.; Chen, B.L.; Guo, Z. Liquid lipases for enzymatic concentration of n-3 polyunsaturated fatty acids in monoacylglycerols via ethanolysis: Catalytic specificity and parameterization. Bioresour. Technol. 2017, 224, 445–456. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.e.; Zheng, P.; Ni, Y.; Sun, Z. Highly efficient biosynthesis of sucrose-6-acetate with cross-linked aggregates of Lipozyme TL 100 L. J. Biotechnol. 2012, 161, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Farmani, J.; Safari, M.; Hamedi, M. Trans-free fats through interesterification of canola oil/palm olein or fully hydrogenated soybean oil blends. Eur. J. Lipid Sci. Technol. 2009, 111, 1212–1220. [Google Scholar] [CrossRef]
- Silva, M.J.A.; Loss, R.A.; Laroque, D.A.; Lerin, L.A.; Pereira, G.N.; Thon, E.; Oliveira, J.V.; Ninow, J.L.; Hense, H.; Oliveira, D. Lipozyme TL IM as Catalyst for the Synthesis of Eugenyl Acetate in Solvent-Free Acetylation. Appl. Biochem. Biotechnol. 2015, 176, 782–795. [Google Scholar] [CrossRef] [PubMed]
- Li, J.B.; Wang, T. On the deactivation of alkali solid catalysts for the synthesis of glycerol carbonate from glycerol and dimethyl carbonate. React. Kinet. Mech. Catal. 2011, 102, 113–126. [Google Scholar] [CrossRef]
- Takagaki, A.; Iwatani, K.; Nishimura, S.; Ebitani, K. Synthesis of glycerol carbonate from glycerol and dialkyl carbonates using hydrotalcite as a reusable heterogeneous base catalyst. Green Chem. 2010, 12, 578–581. [Google Scholar] [CrossRef]
- Ravelo, M.; Esteban, J.; Ladero, M.; Garcia-Ochoa, F. Enzymatic synthesis of ibuprofen monoglycerides catalyzed by free Candida antarctica lipase B in a toluene-glycerol biphasic medium. RSC Adv. 2016, 6, 69658–69669. [Google Scholar] [CrossRef]
- Gao, J.; Wang, Y.; Du, Y.J.; Zhou, L.Y.; He, Y.; Ma, L.; Yin, L.Y.; Kong, W.X.; Jiang, Y.J. Construction of biocatalytic colloidosome using lipase-containing dendritic mesoporous silica nanospheres for enhanced enzyme catalysis. Chem. Eng. J. 2017, 317, 175–186. [Google Scholar] [CrossRef]
- Climent, M.J.; Corma, A.; De Frutos, P.; Iborra, S.; Noy, M.; Velty, A.; Concepcion, P. Chemicals from biomass: Synthesis of glycerol carbonate by transesterification and carbonylation with urea with hydrotalcite catalysts. The role of acid-base pairs. J. Catal. 2010, 269, 140–149. [Google Scholar] [CrossRef]
- Cho, H.J.; Kwon, H.M.; Tharun, J.; Park, D.W. Synthesis of glycerol carbonate from ethylene carbonate and glycerol using immobilized ionic liquid catalysts. J. Ind. Eng. Chem. 2010, 16, 679–683. [Google Scholar] [CrossRef]
- Alvarez, M.G.; Segarra, A.M.; Contreras, S.; Sueiras, J.E.; Medina, F.; Figueras, F. Enhanced use of renewable resources: Transesterification of glycerol catalyzed by hydrotalcite-like compounds. Chem. Eng. J. 2010, 161, 340–345. [Google Scholar] [CrossRef]
- Alvarez, M.G.; Pliskova, M.; Segarra, A.M.; Medina, F.; Figueras, F. Synthesis of glycerol carbonates by transesterification of glycerol in a continuous system using supported hydrotalcites as catalysts. Appl. Catal. B Environ. 2012, 113, 212–220. [Google Scholar] [CrossRef]
- Kobayashi, S. Lipase-catalyzed polyester synthesis—A green polymer chemistry. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2010, 86, 338–365. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Lee, R.; Chen, T.; Luo, J.; Lu, Y.; Huang, K.-W. Kinetic Evidence of an Apparent Negative Activation Enthalpy in an Organocatalytic Process. Sci. Rep. 2013, 3, 2557. [Google Scholar] [CrossRef] [PubMed]
- Oliveberg, M.; Tan, Y.J.; Fersht, A.R. Negative activation enthalpies in the kinetics of protein folding. Proc. Natl. Acad. Sci. USA 1995, 92, 8926–8929. [Google Scholar] [CrossRef] [PubMed]
- Silverstein, T.P. Falling Enzyme Activity as Temperature Rises: Negative Activation Energy or Denaturation? J. Chem. Educ. 2012, 89, 1097–1099. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).











