Effect of Operating Variables and Kinetics of the Lipase Catalyzed Transesterification of Ethylene Carbonate and Glycerol
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Enzymes
2.3. Transesterification Runs and Analytical Method
2.4. Statistical Methods
3. Results and Discussion
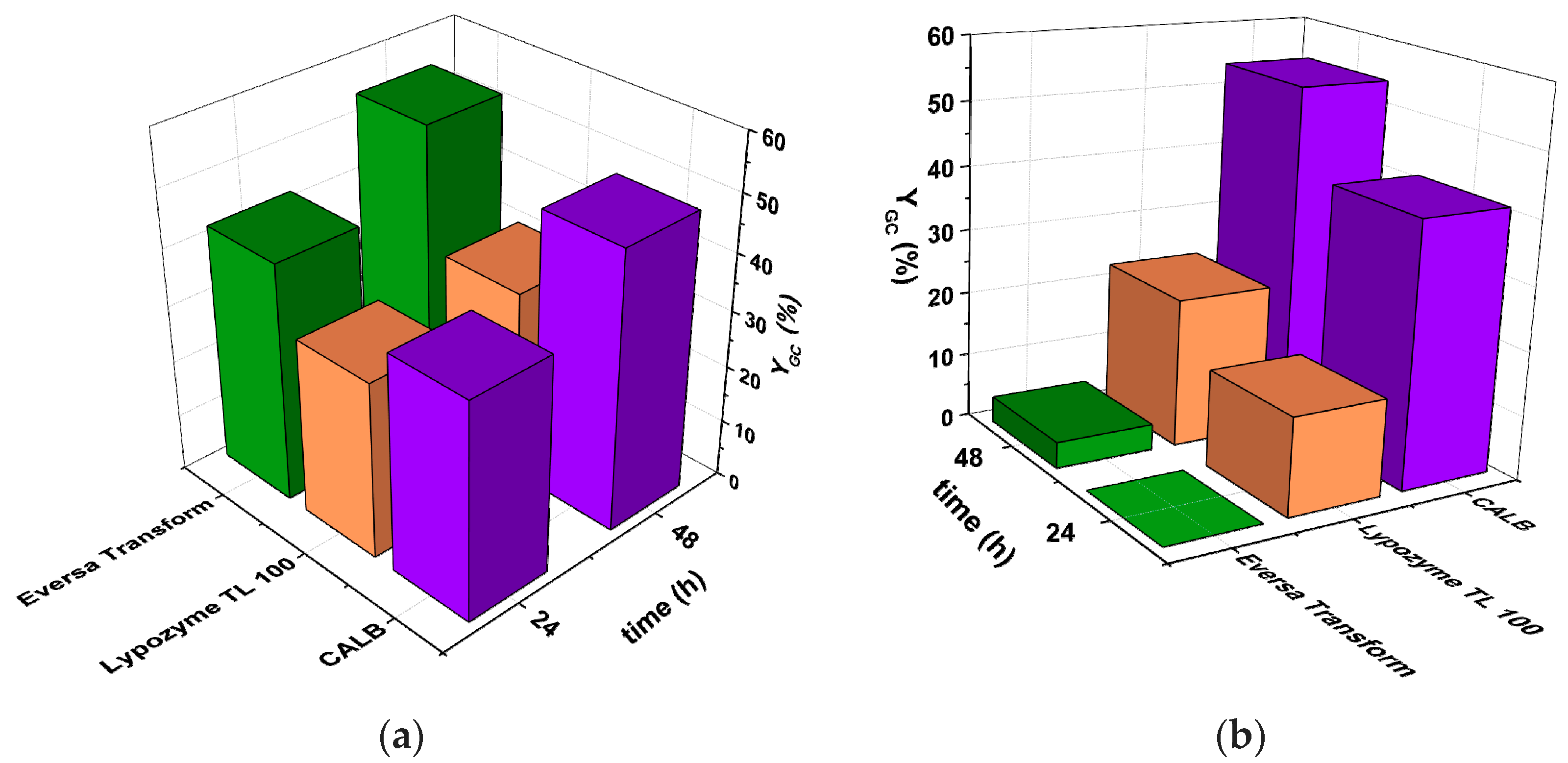
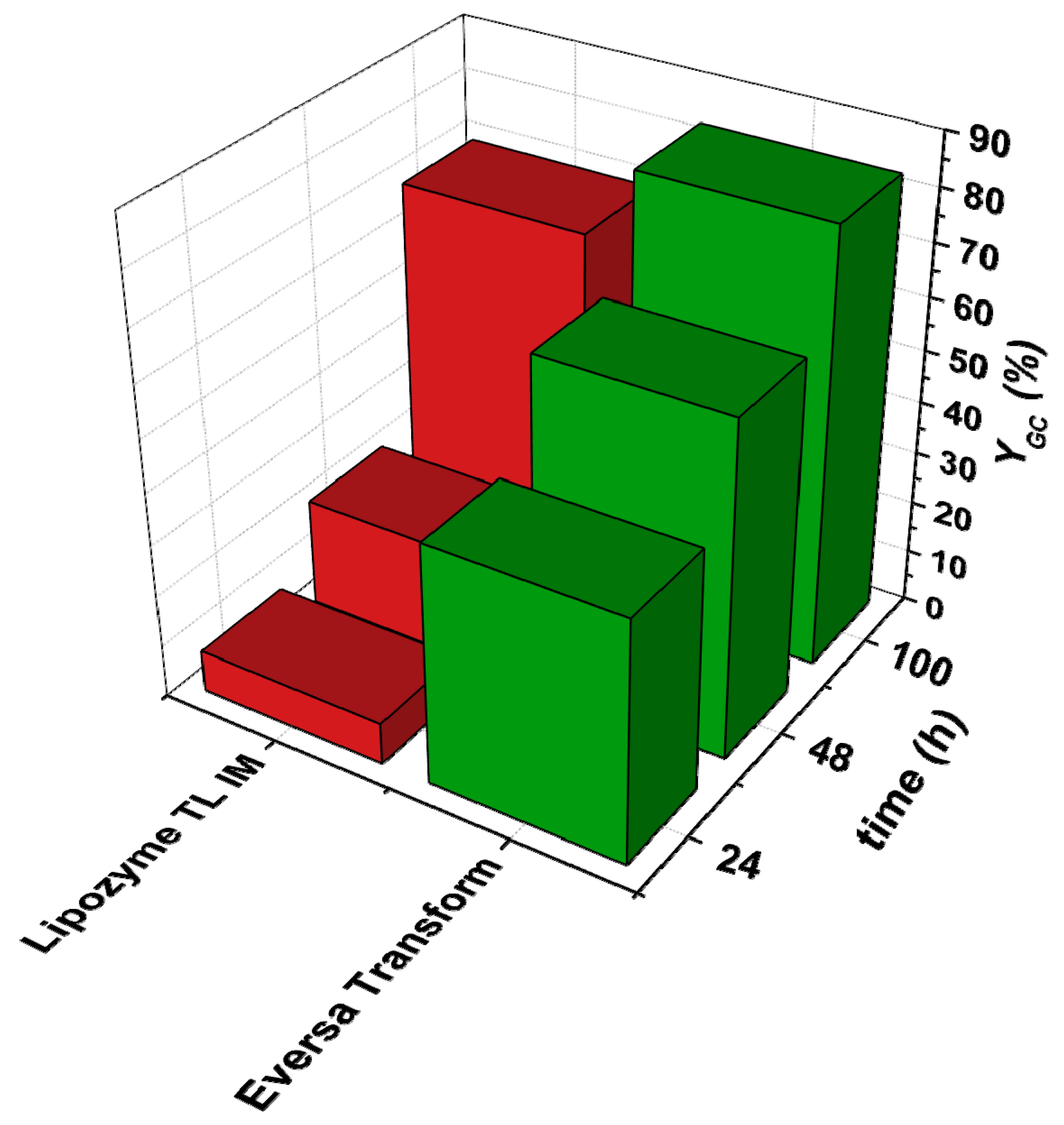
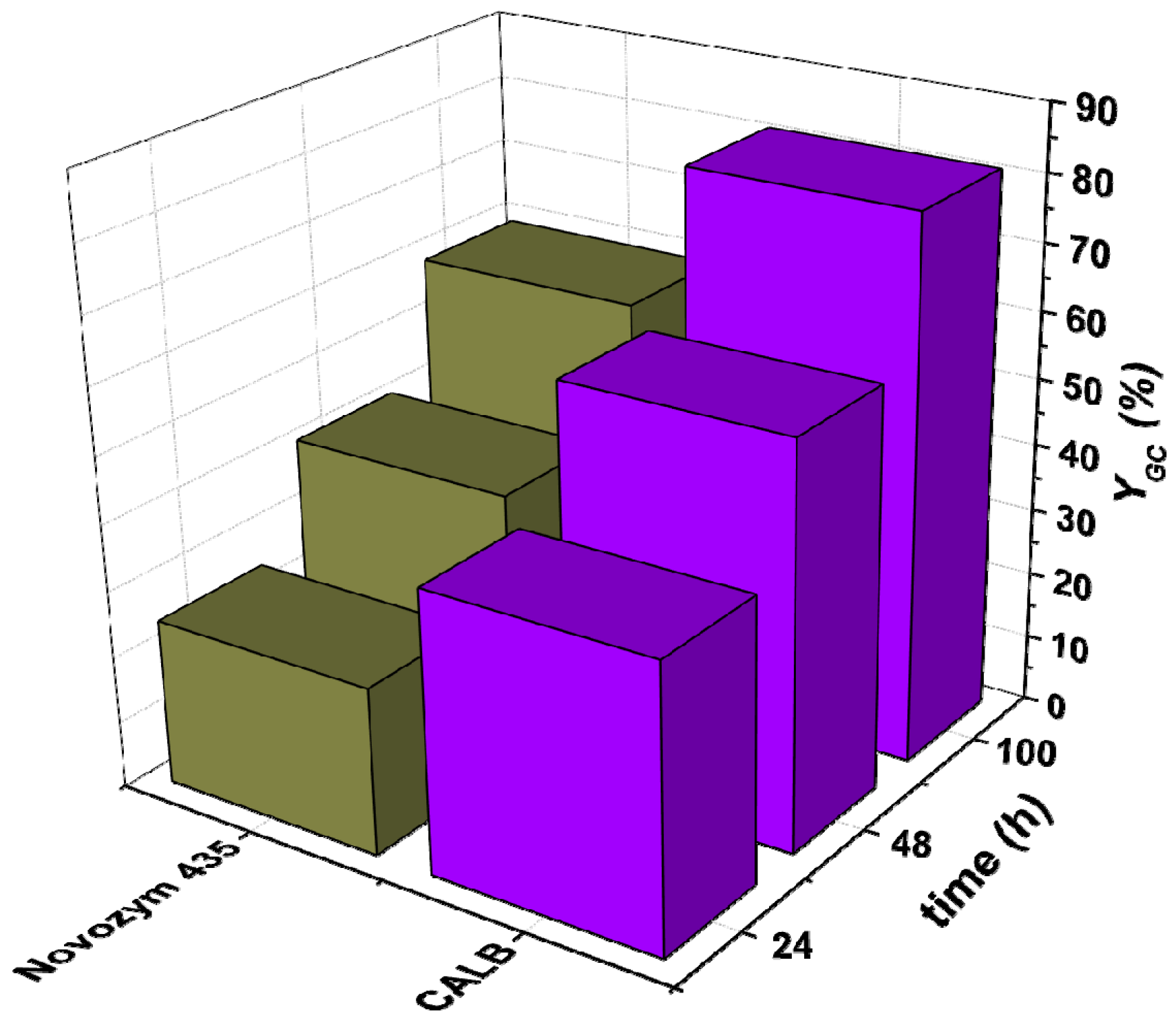
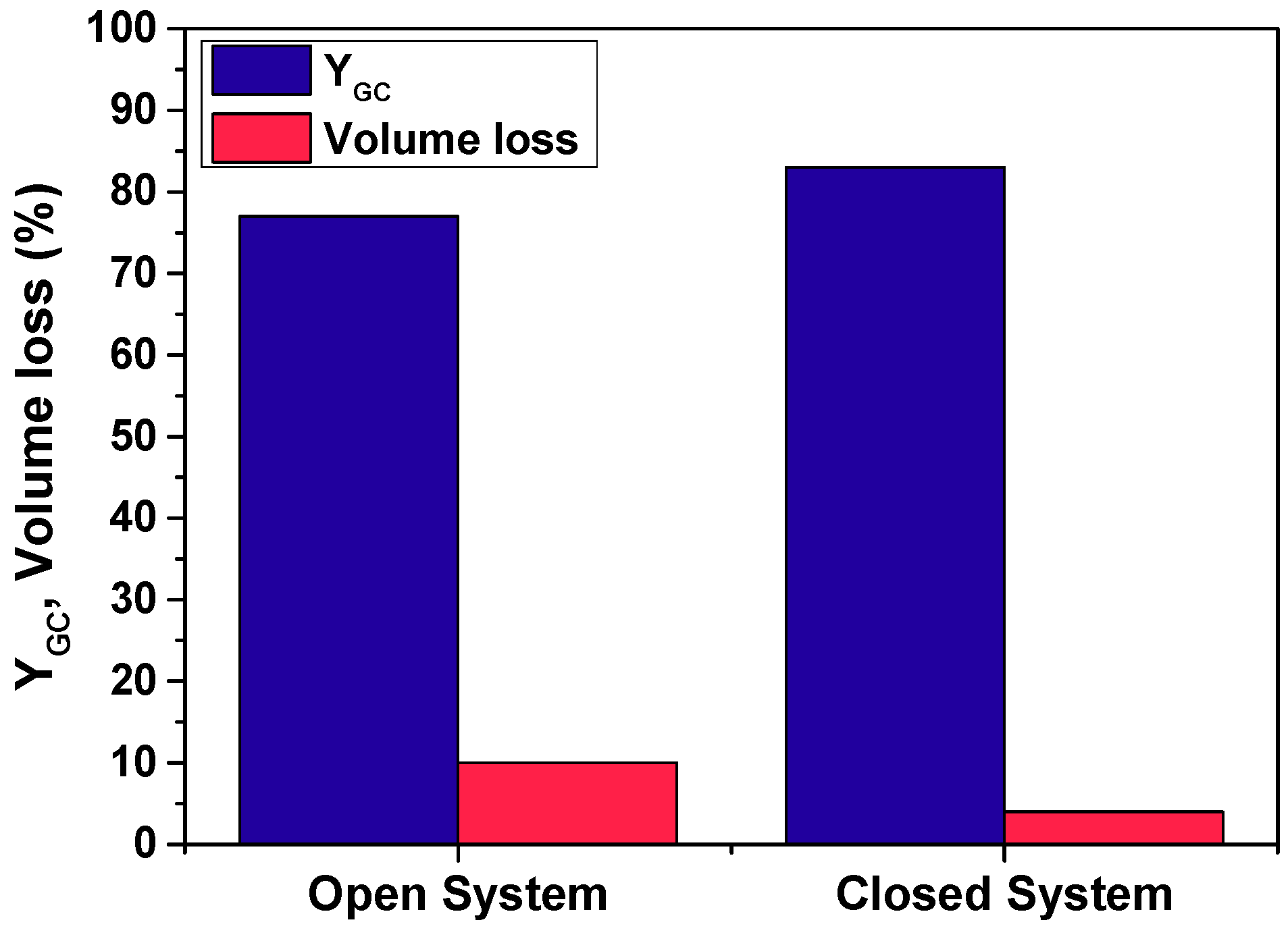
3.1. Preselection of Reaction, Enzyme, and Operating Mode
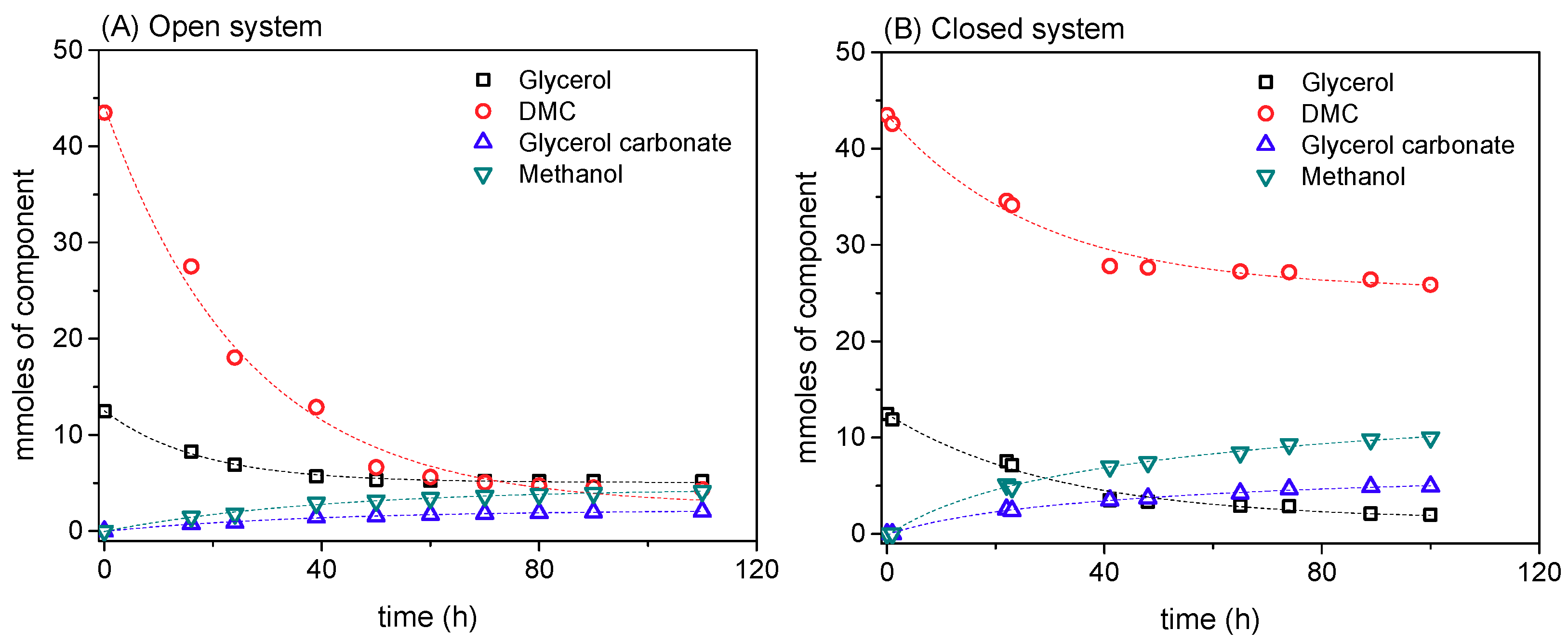
3.1.1. Selection of Enzymes for Each Reaction
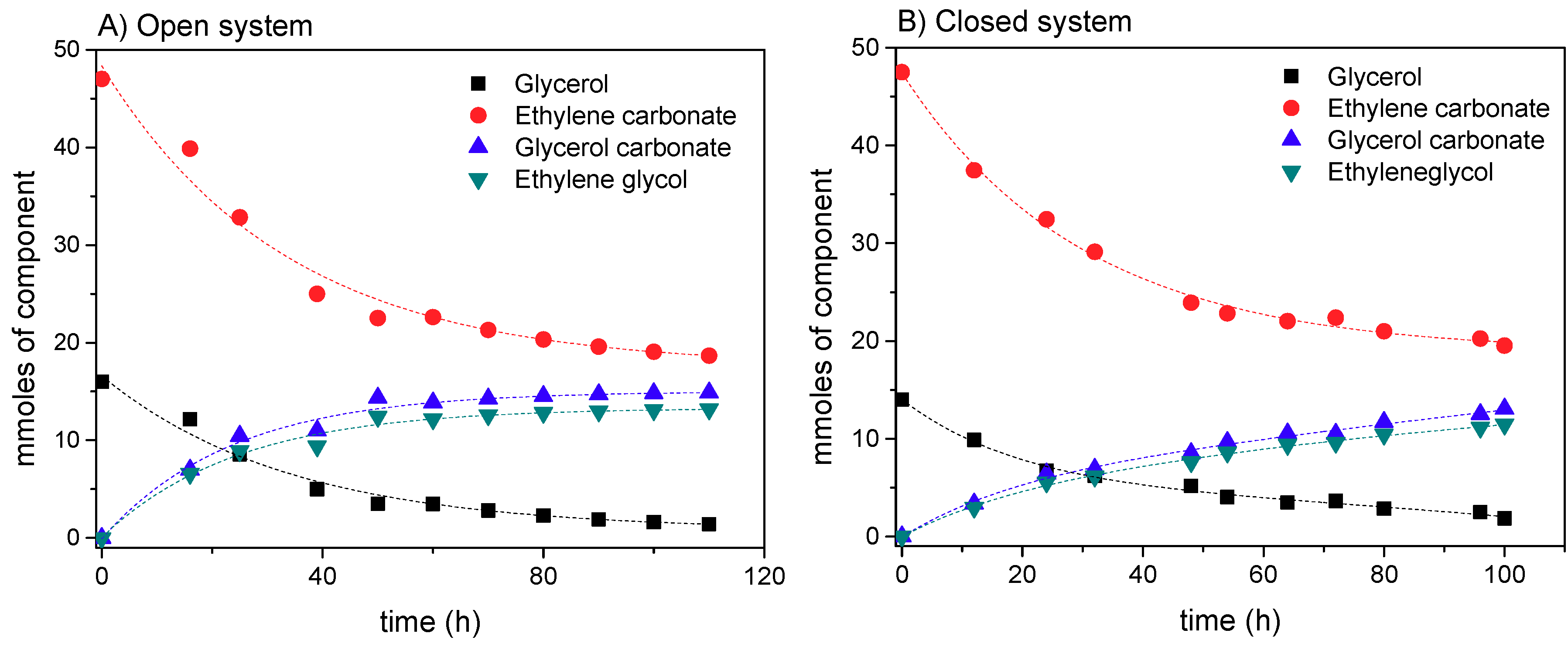
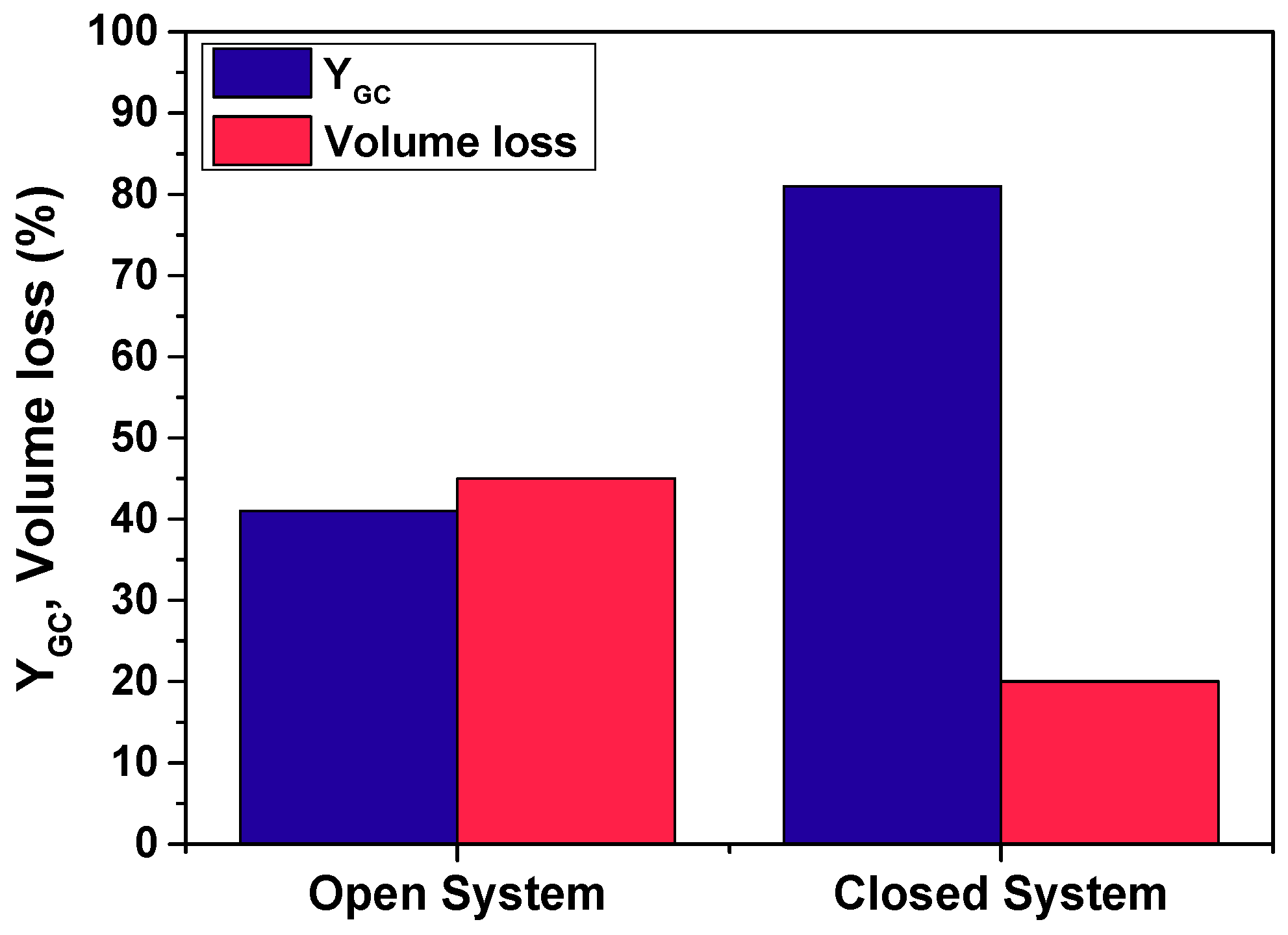
3.1.2. Selection of Open vs. Closed System
3.2. Effect of Variables on the Transesterification of Glycerol with Ethylene Carbonate
3.2.1. Effect of the Solvent
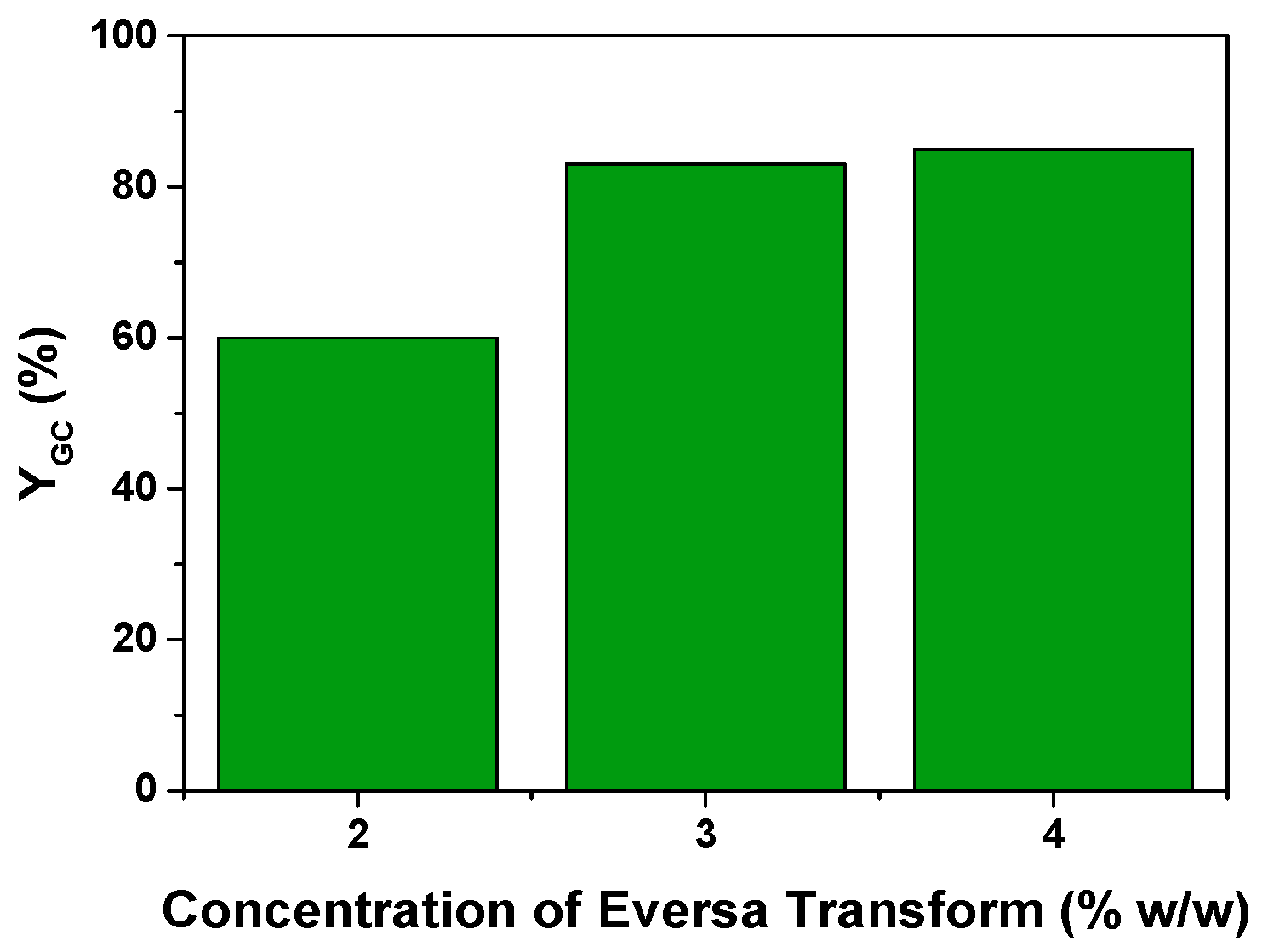
3.2.2. Effect of the Enzyme Concentration
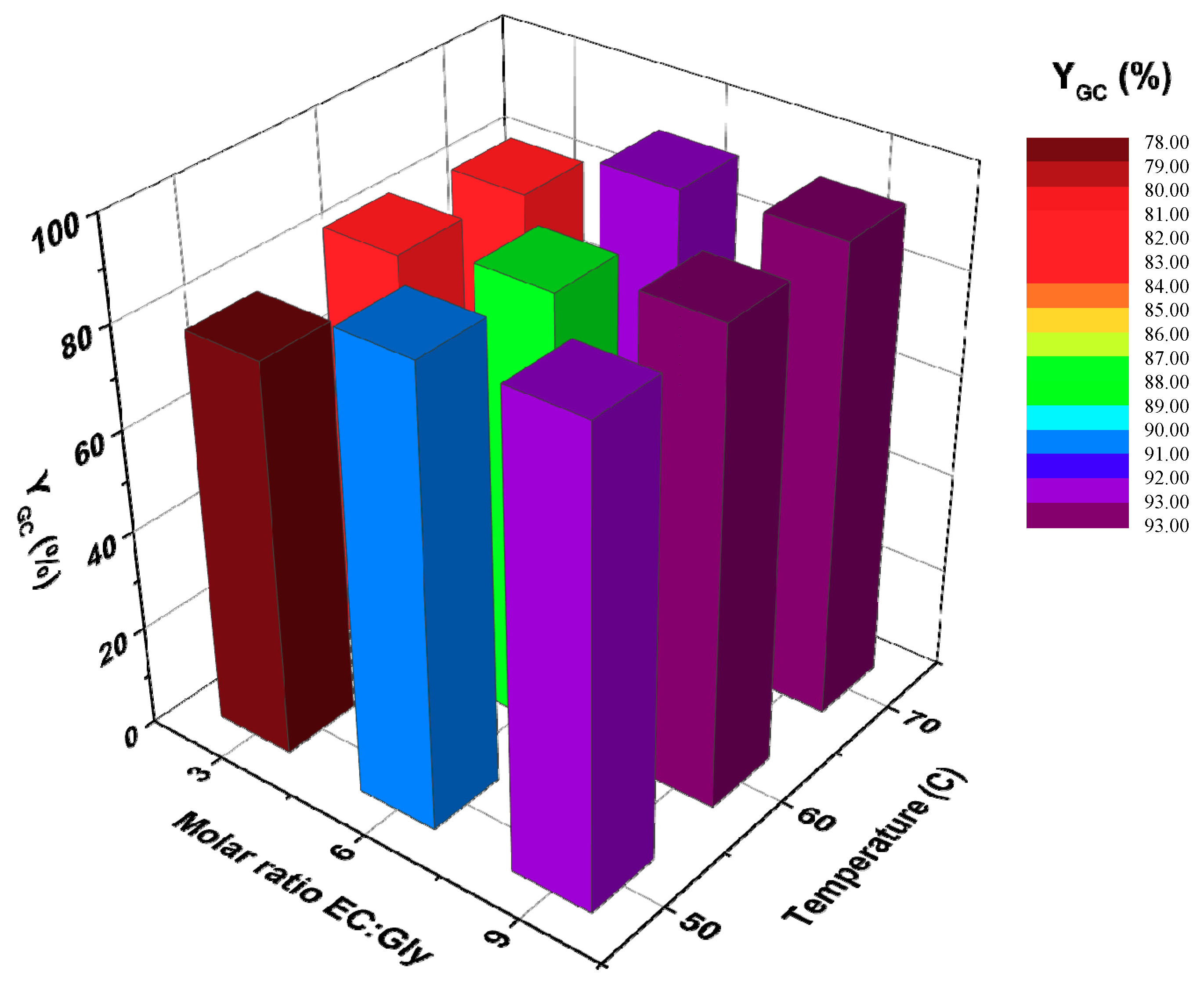
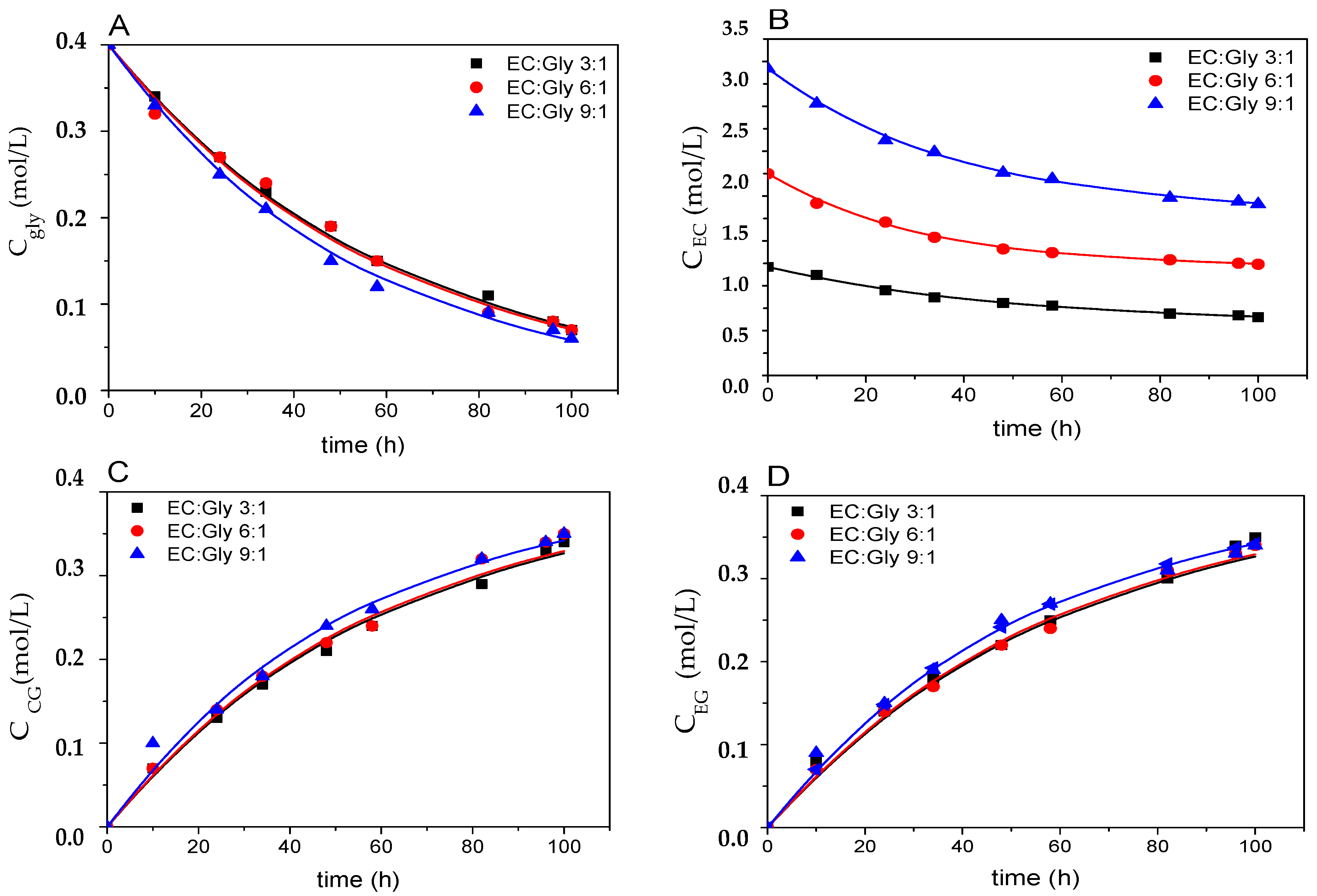
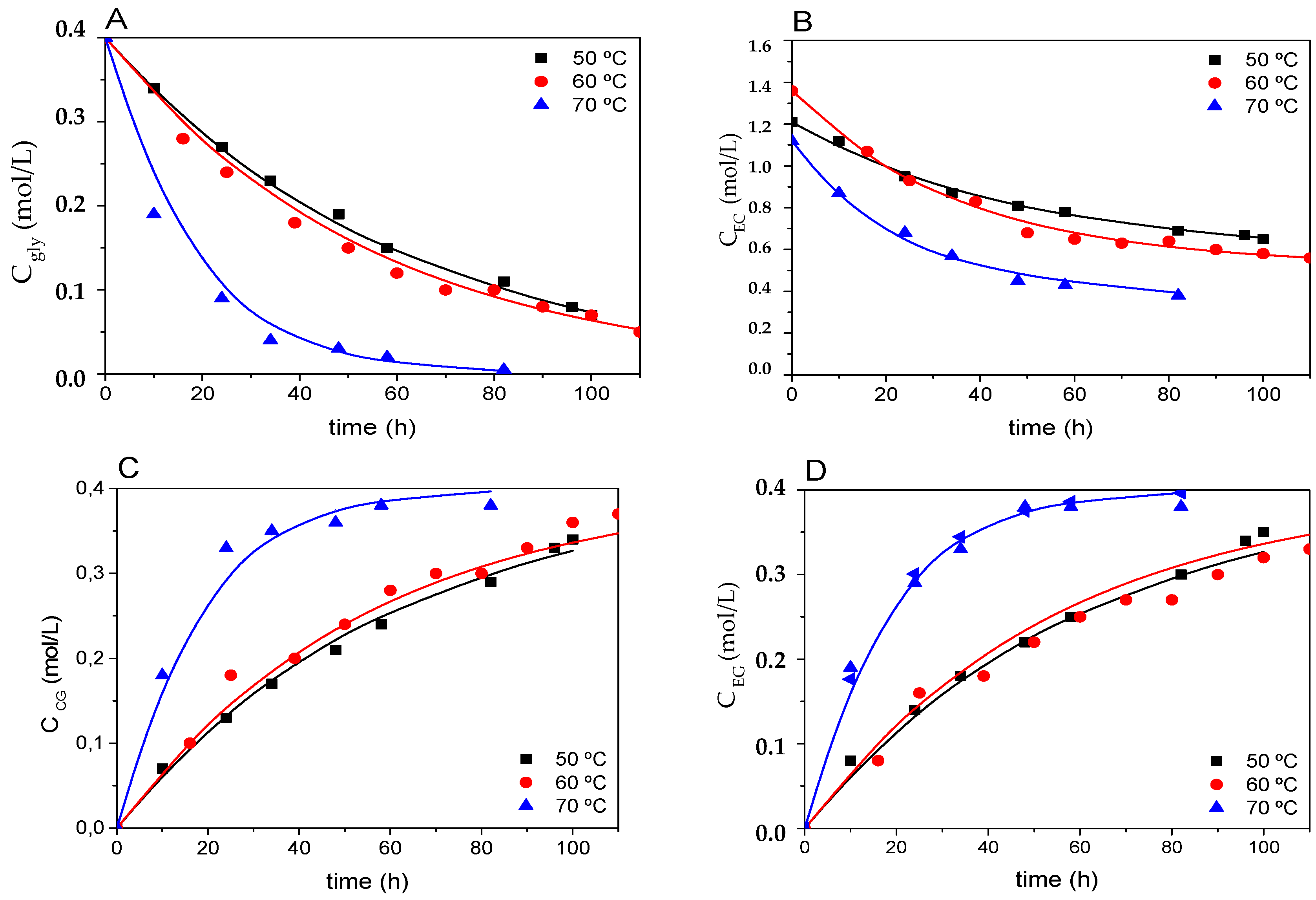
3.2.3. Effect of the Molar Ratio of Reactants and Temperature
3.3. Kinetic Modeling of Enzymatic Transesterification of Glycerol With Ethylene Carbonate
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lamers, P.; Hamelinck, C.; Junginger, M.; Faaij, A. International bioenergy trade—A review of past developments in the liquid biofuel market. Renew. Sustain. Energy Rev. 2011, 15, 2655–2676. [Google Scholar] [CrossRef]
- Quispe, C.A.G.; Coronado, C.J.R.; Carvalho, J.A. Glycerol: Production, consumption, prices, characterization and new trends in combustion. Renew. Sustain. Energy Rev. 2013, 27, 475–493. [Google Scholar] [CrossRef]
- He, Q.; McNutt, J.; Yang, J. Utilization of the residual glycerol from biodiesel production for renewable energy generation. Renew. Sustain. Energy Rev. 2017, 71, 63–76. [Google Scholar] [CrossRef]
- Thompson, J.C.; He, B.B. Characterization of crude glycerol from biodiesel production from multiple feedstocks. Appl. Eng. Agric. 2006, 22, 261–265. [Google Scholar] [CrossRef]
- Anitha, M.; Kamarudin, S.K.; Kofli, N.T. The potential of glycerol as a value-added commodity. Chem. Eng. J. 2016, 295, 119–130. [Google Scholar] [CrossRef]
- Behr, A.; Eilting, J.; Irawadi, K.; Leschinski, J.; Lindner, F. Improved utilisation of renewable resources: New important derivatives of glycerol. Green Chem. 2008, 10, 13–30. [Google Scholar] [CrossRef]
- Sonnati, M.O.; Amigoni, S.; de Givenchy, E.P.T.; Darmanin, T.; Choulet, O.; Guittard, F. Glycerol carbonate as a versatile building block for tomorrow: Synthesis, reactivity, properties and applications. Green Chem. 2013, 15, 283–306. [Google Scholar] [CrossRef]
- Ishak, Z.I.; Sairi, N.A.; Alias, Y.; Aroua, M.K.T.; Yusoff, R. A review of ionic liquids as catalysts for transesterification reactions of biodiesel and glycerol carbonate production. Catal. Rev. Sci. Eng. 2017, 59, 44–93. [Google Scholar] [CrossRef]
- Ochoa-Gomez, J.R.; Gomez-Jimenez-Aberasturi, O.; Ramirez-Lopez, C.; Belsue, M. A Brief Review on Industrial Alternatives for the Manufacturing of Glycerol Carbonate, a Green Chemical. Org. Process Res. Dev. 2012, 16, 389–399. [Google Scholar] [CrossRef]
- Teng, W.K.; Ngoh, G.C.; Yusoff, R.; Aroua, M.K. A review on the performance of glycerol carbonate production via catalytic transesterification: Effects of influencing parameters. Energy Convers. Manag. 2014, 88, 484–497. [Google Scholar] [CrossRef]
- Esteban, J.; Dominguez, E.; Ladero, M.; Garcia-Ochoa, F. Kinetics of the production of glycerol carbonate by transesterification of glycerol with dimethyl and ethylene carbonate using potassium methoxide, a highly active catalyst. Fuel Process. Technol. 2015, 138, 243–251. [Google Scholar] [CrossRef]
- Esteban, J.; Fuente, E.; Blanco, A.; Ladero, M.; Garcia-Ochoa, F. Phenomenological kinetic model of the synthesis of glycerol carbonate assisted by focused beam reflectance measurements. Chem. Eng. J. 2015, 260, 434–443. [Google Scholar] [CrossRef]
- Esteban, J.; Ladero, M.; Garcia-Ochoa, F. Liquid-liquid equilibria for the systems ethylene carbonate plus ethylene glycol plus glycerol; ethylene carbonate plus glycerol carbonate plus glycerol and ethylene carbonate plus ethylene glycol plus glycerol carbonate plus glycerol at catalytic reacting temperatures. Chem. Eng. Res. Des. 2015, 94, 440–448. [Google Scholar]
- Esteban, J.; Fuente, E.; Gonzalez-Miquel, M.; Blanco, A.; Ladero, M.; Garcia-Ochoa, F. Sustainable joint solventless coproduction of glycerol carbonate and ethylene glycol via thermal transesterification of glycerol. RSC Adv. 2014, 4, 53206–53215. [Google Scholar] [CrossRef]
- Esteban, J.; Ladero, M.; Molinero, L.; Garcia-Ochoa, F. Liquid-liquid equilibria for the ternary systems DMC-methanol-glycerol, DMC-glycerol carbonate-glycerol and the quaternary system DMC-methanol-glycerol carbonate-glycerol at catalytic reacting temperatures. Chem. Eng. Res. Des. 2014, 92, 2797–2805. [Google Scholar] [CrossRef]
- Esteban, J.; Ladero, M.; Fuente, E.; Blanco, A.; Garcia-Ochoa, F. Experimental and modelling approach to the catalytic coproduction of glycerol carbonate and ethylene glycol as a means to valorise glycerol. J. Taiwan Inst. Chem. Eng. 2016, 63, 89–100. [Google Scholar] [CrossRef]
- Esteban, J.; Vorholt, A.J. Obtaining glycerol carbonate and glycols using thermomorphic systems based on glycerol and cyclic organic carbonates: Kinetic studies. J. Ind. Eng. Chem. 2018, 63, 124–132. [Google Scholar] [CrossRef]
- Jung, H.; Lee, Y.; Kim, D.; Han, S.O.; Kim, S.W.; Lee, J.; Kim, Y.H.; Park, C. Enzymatic production of glycerol carbonate from by-product after biodiesel manufacturing process. Enzyme Microb. Technol. 2012, 51, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.C.; Kim, Y.H.; Lee, H.; Yoon, D.Y.; Song, B.K. Lipase-catalyzed synthesis of glycerol carbonate from renewable glycerol and dimethyl carbonate through transesterification. J. Mol. Catal. B Enzym. 2007, 49, 75–78. [Google Scholar] [CrossRef]
- Lee, K.H.; Park, C.H.; Lee, E.Y. Biosynthesis of glycerol carbonate from glycerol by lipase in dimethyl carbonate as the solvent. Bioprocess Biosyst. Eng. 2010, 33, 1059–1065. [Google Scholar] [CrossRef] [PubMed]
- Waghmare, G.V.; Vetal, M.D.; Rathod, V.K. Ultrasound assisted enzyme catalyzed synthesis of glycerol carbonate from glycerol and dimethyl carbonate. Ultrason. Sonochem. 2015, 22, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Tudorache, M.; Negoi, A.; Protesescu, L.; Parvulescu, V.I. Biocatalytic alternative for bio-glycerol conversion with alkyl carbonates via a lipase-linked magnetic nano-particles assisted process. Appl. Catal. B Environ. 2014, 145, 120–125. [Google Scholar] [CrossRef]
- Tudorache, M.; Negoi, A.; Tudora, B.; Parvulescu, V.I. Environmental-friendly strategy for biocatalytic conversion of waste glycerol to glycerol carbonate. Appl. Catal. B Environ. 2014, 146, 274–278. [Google Scholar] [CrossRef]
- Go, A.R.; Lee, Y.; Kim, Y.H.; Park, S.; Choi, J.; Lee, J.; Han, S.O.; Kim, S.W.; Park, C. Enzymatic coproduction of biodiesel and glycerol carbonate from soybean oil in solvent-free system. Enzyme Microb. Technol. 2013, 53, 154–158. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Lee, J.H.; Yang, H.J.; Jane, M.; Kim, J.R.; Byun, E.H.; Lee, J.; Na, J.G.; Kim, S.W.; Park, C. Efficient simultaneous production of biodiesel and glycerol carbonate via statistical optimization. J. Ind. Eng. Chem. 2017, 51, 49–53. [Google Scholar] [CrossRef]
- Min, J.Y.; Lee, E.Y. Lipase-catalyzed simultaneous biosynthesis of biodiesel and glycerol carbonate from corn oil in dimethyl carbonate. Biotechnol. Lett. 2011, 33, 1789–1796. [Google Scholar] [CrossRef] [PubMed]
- Seong, P.J.; Jeon, B.W.; Lee, M.; Cho, D.H.; Kim, D.K.; Jung, K.S.; Kim, S.W.; Han, S.O.; Kim, Y.H.; Park, C. Enzymatic coproduction of biodiesel and glycerol carbonate from soybean oil and dimethyl carbonate. Enzyme Microb. Technol. 2011, 48, 505–509. [Google Scholar] [CrossRef] [PubMed]
- Adewale, P.; Vithanage, L.N.; Christopher, L. Optimization of enzyme-catalyzed biodiesel production from crude tall oil using Taguchi method. Energy Convers. Manag. 2017, 154, 81–91. [Google Scholar] [CrossRef]
- Andrade, T.A.; Errico, M.; Christensen, K.V. Evaluation of Reaction Mechanisms and Kinetic Parameters for the Transesterification of Castor Oil by Liquid Enzymes. Ind. Eng. Chem. Res. 2017, 56, 9478–9488. [Google Scholar] [CrossRef]
- He, Y.J.; Li, J.B.; Kodali, S.; Balle, T.; Chen, B.L.; Guo, Z. Liquid lipases for enzymatic concentration of n-3 polyunsaturated fatty acids in monoacylglycerols via ethanolysis: Catalytic specificity and parameterization. Bioresour. Technol. 2017, 224, 445–456. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.e.; Zheng, P.; Ni, Y.; Sun, Z. Highly efficient biosynthesis of sucrose-6-acetate with cross-linked aggregates of Lipozyme TL 100 L. J. Biotechnol. 2012, 161, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Farmani, J.; Safari, M.; Hamedi, M. Trans-free fats through interesterification of canola oil/palm olein or fully hydrogenated soybean oil blends. Eur. J. Lipid Sci. Technol. 2009, 111, 1212–1220. [Google Scholar] [CrossRef]
- Silva, M.J.A.; Loss, R.A.; Laroque, D.A.; Lerin, L.A.; Pereira, G.N.; Thon, E.; Oliveira, J.V.; Ninow, J.L.; Hense, H.; Oliveira, D. Lipozyme TL IM as Catalyst for the Synthesis of Eugenyl Acetate in Solvent-Free Acetylation. Appl. Biochem. Biotechnol. 2015, 176, 782–795. [Google Scholar] [CrossRef] [PubMed]
- Li, J.B.; Wang, T. On the deactivation of alkali solid catalysts for the synthesis of glycerol carbonate from glycerol and dimethyl carbonate. React. Kinet. Mech. Catal. 2011, 102, 113–126. [Google Scholar] [CrossRef]
- Takagaki, A.; Iwatani, K.; Nishimura, S.; Ebitani, K. Synthesis of glycerol carbonate from glycerol and dialkyl carbonates using hydrotalcite as a reusable heterogeneous base catalyst. Green Chem. 2010, 12, 578–581. [Google Scholar] [CrossRef]
- Ravelo, M.; Esteban, J.; Ladero, M.; Garcia-Ochoa, F. Enzymatic synthesis of ibuprofen monoglycerides catalyzed by free Candida antarctica lipase B in a toluene-glycerol biphasic medium. RSC Adv. 2016, 6, 69658–69669. [Google Scholar] [CrossRef]
- Gao, J.; Wang, Y.; Du, Y.J.; Zhou, L.Y.; He, Y.; Ma, L.; Yin, L.Y.; Kong, W.X.; Jiang, Y.J. Construction of biocatalytic colloidosome using lipase-containing dendritic mesoporous silica nanospheres for enhanced enzyme catalysis. Chem. Eng. J. 2017, 317, 175–186. [Google Scholar] [CrossRef]
- Climent, M.J.; Corma, A.; De Frutos, P.; Iborra, S.; Noy, M.; Velty, A.; Concepcion, P. Chemicals from biomass: Synthesis of glycerol carbonate by transesterification and carbonylation with urea with hydrotalcite catalysts. The role of acid-base pairs. J. Catal. 2010, 269, 140–149. [Google Scholar] [CrossRef]
- Cho, H.J.; Kwon, H.M.; Tharun, J.; Park, D.W. Synthesis of glycerol carbonate from ethylene carbonate and glycerol using immobilized ionic liquid catalysts. J. Ind. Eng. Chem. 2010, 16, 679–683. [Google Scholar] [CrossRef]
- Alvarez, M.G.; Segarra, A.M.; Contreras, S.; Sueiras, J.E.; Medina, F.; Figueras, F. Enhanced use of renewable resources: Transesterification of glycerol catalyzed by hydrotalcite-like compounds. Chem. Eng. J. 2010, 161, 340–345. [Google Scholar] [CrossRef]
- Alvarez, M.G.; Pliskova, M.; Segarra, A.M.; Medina, F.; Figueras, F. Synthesis of glycerol carbonates by transesterification of glycerol in a continuous system using supported hydrotalcites as catalysts. Appl. Catal. B Environ. 2012, 113, 212–220. [Google Scholar] [CrossRef]
- Kobayashi, S. Lipase-catalyzed polyester synthesis—A green polymer chemistry. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2010, 86, 338–365. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Lee, R.; Chen, T.; Luo, J.; Lu, Y.; Huang, K.-W. Kinetic Evidence of an Apparent Negative Activation Enthalpy in an Organocatalytic Process. Sci. Rep. 2013, 3, 2557. [Google Scholar] [CrossRef] [PubMed]
- Oliveberg, M.; Tan, Y.J.; Fersht, A.R. Negative activation enthalpies in the kinetics of protein folding. Proc. Natl. Acad. Sci. USA 1995, 92, 8926–8929. [Google Scholar] [CrossRef] [PubMed]
- Silverstein, T.P. Falling Enzyme Activity as Temperature Rises: Negative Activation Energy or Denaturation? J. Chem. Educ. 2012, 89, 1097–1099. [Google Scholar] [CrossRef]
| Parameter | Run 1 | Run 2 | Run 3 | Run 4 | Run 5 |
|---|---|---|---|---|---|
| k1 | 0.0170 ± 0.0006 | 0.0174 ± 0.0006 | 0.0193 ± 0.0011 | 0.0284 ± 0.0022 | 0.058 ± 0.0051 |
| k2 | 0.0046 ± 0.0007 | 0.0058 ± 0.0007 | 0.0032 ± 0.0013 | 0.0089 ± 0.0013 | 0.019 ± 0.008 |
| k3 | 0.0068 ± 0.0033 | 0.0132 ± 0.0016 | 0.0082 ± 0.0008 | 0.0036 ± 0.0024 | 0.0012 ± 0.0006 |
| SQR | 0.000336 | 0.0000518 | 0.000552 | 0.0018 | 0.00838 |
| RMSE | 0.0105 | 0.012 | 0.0012 | 0.021 | 0.017 |
| F-value | 23140 | 51179 | 113139 | 6883 | 4883 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gutierrez-Lazaro, A.; Velasco, D.; Boldrini, D.E.; Yustos, P.; Esteban, J.; Ladero, M. Effect of Operating Variables and Kinetics of the Lipase Catalyzed Transesterification of Ethylene Carbonate and Glycerol. Fermentation 2018, 4, 75. https://doi.org/10.3390/fermentation4030075
Gutierrez-Lazaro A, Velasco D, Boldrini DE, Yustos P, Esteban J, Ladero M. Effect of Operating Variables and Kinetics of the Lipase Catalyzed Transesterification of Ethylene Carbonate and Glycerol. Fermentation. 2018; 4(3):75. https://doi.org/10.3390/fermentation4030075
Chicago/Turabian StyleGutierrez-Lazaro, Ana, Daniel Velasco, Diego E. Boldrini, Pedro Yustos, Jesus Esteban, and Miguel Ladero. 2018. "Effect of Operating Variables and Kinetics of the Lipase Catalyzed Transesterification of Ethylene Carbonate and Glycerol" Fermentation 4, no. 3: 75. https://doi.org/10.3390/fermentation4030075
APA StyleGutierrez-Lazaro, A., Velasco, D., Boldrini, D. E., Yustos, P., Esteban, J., & Ladero, M. (2018). Effect of Operating Variables and Kinetics of the Lipase Catalyzed Transesterification of Ethylene Carbonate and Glycerol. Fermentation, 4(3), 75. https://doi.org/10.3390/fermentation4030075







