Saccharomycodes ludwigii, Control and Potential Uses in Winemaking Processes
Abstract
1. Introduction
2. Saccharomycodes ludwigii
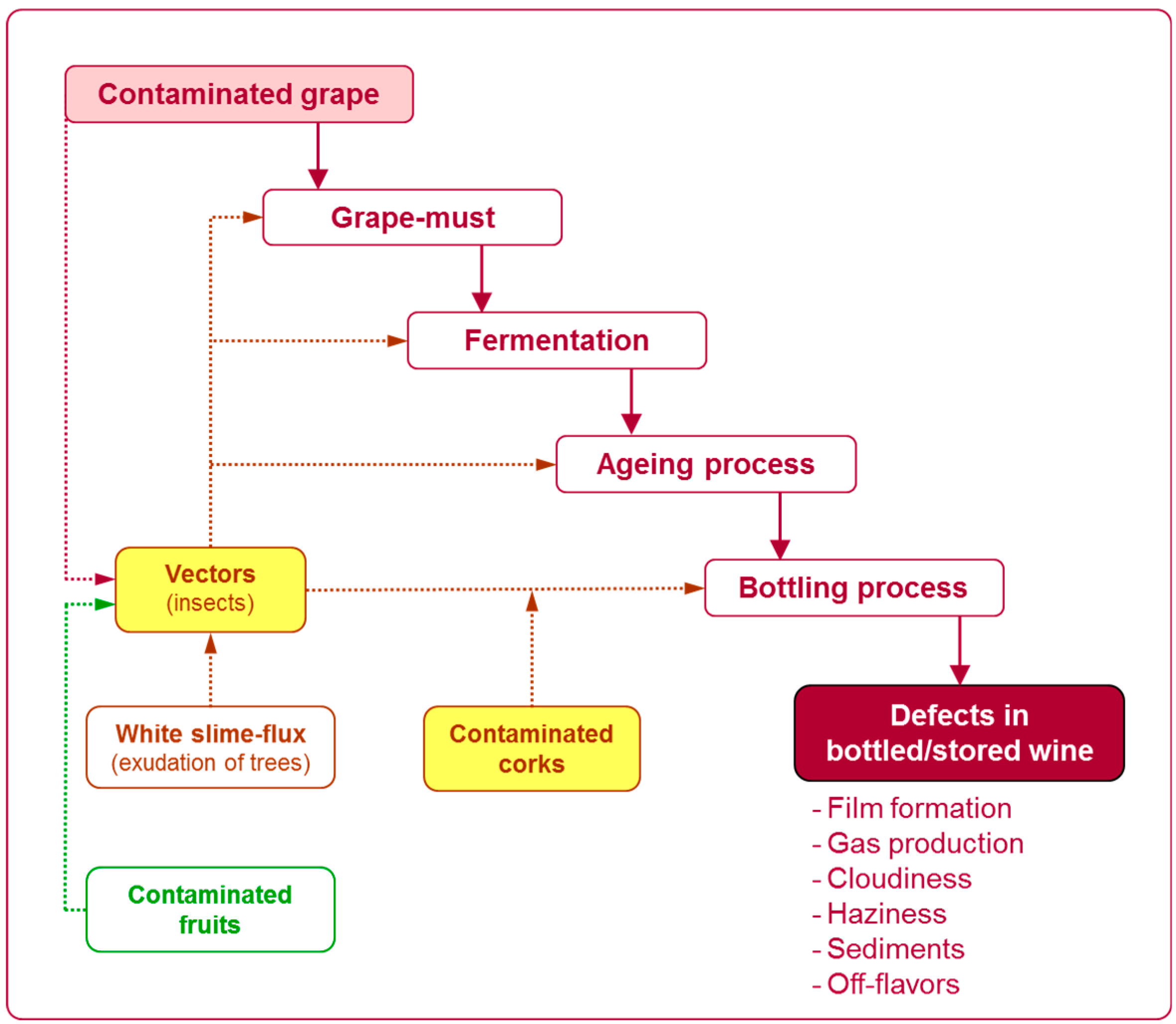
2.1. Sources of Contamination by Saccharomycodes ludwigii
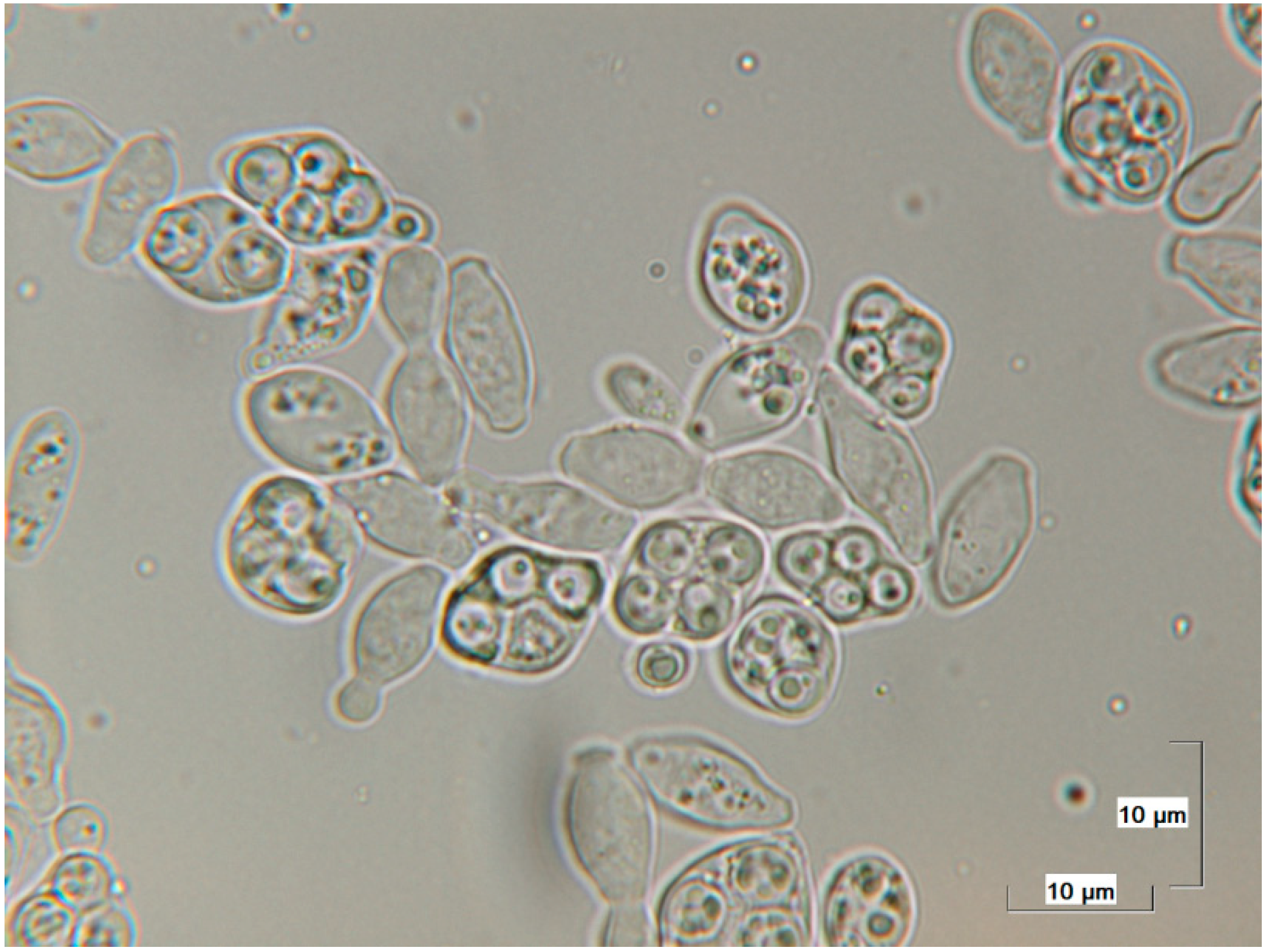
2.2. Detection of Saccharomycodes ludwigii
2.3. Disadvantages of “Sulfiting” and Resistance of Saccharomycodes ludwigii
3. Control by Chemical Treatments
3.1. Dimethyl Dicarbonate
3.2. Chitosan
4. Biological Controllers
5. Control by Physical Treatments
5.1. Pulsed Electric Fields
5.2. Gamma Radiation
6. Other Applications of Saccharomycodes ludwigii
6.1. Aromatic Profile Improvement in Wines
6.2. Reduction of Alcohol Content in Wine
6.3. Release of Polysaccharides in Red Wines
6.4. Combined Treatments: Aging-on-Lees with Ultrasound
6.5. Non-Wine Fermentations
7. Future Perspectives
7.1. Adaptation to Harsh Conditions
7.2. Emerging Technologies for Controlling S’codes ludwigii
7.3. Considerations about Chemical Preservatives
7.4. Selection of S’codes ludwigii Strains with Differentiated Characteristics
7.5. Production of Other Fermented Beverages
8. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Ribéreau-Gayon, P.; Dubourdieu, D.; Donèche, B.; Lonvaud, A. The Microbiology of Wine and Vinification. In Handbook of Enology, 2nd ed.; John Wiley and Sons Ltd.: Chichester, UK, 2006; Volume 1, pp. 193–221. ISBN 978-0-470-01034-1. [Google Scholar]
- Ciani, M.; Maccarelli, F. Oenological properties of non-Saccharomyces yeasts associated with wine-making. World J. Microb. Biot. 1997, 14, 199–203. [Google Scholar] [CrossRef]
- Granchi, L.; Ganucci, D.; Messini, A.; Vincenzini, M. Oenological properties of Hanseniaspora osmophila and Kloeckera cortices from wines produced by spontaneous fermentations of normal and dried grapes. FEMS Yeast Res. 2002, 2, 403–407. [Google Scholar] [CrossRef] [PubMed]
- Loureiro, V.; Malfeito-Ferreira, M. Spoilage yeasts in the wine industry. Int. J. Food Microbiol. 2003, 86, 23–50. [Google Scholar] [CrossRef]
- Lachance, M.A.; Gilbert, G.D.; Starmer, W.T. Yeast communities associated with Drosophila species and related flies in an eastern oak-pine forest: A comparison with western communities. J. Ind. Microbiol. 1995, 14, 484–494. [Google Scholar] [CrossRef] [PubMed]
- Boundy-Mills, K.; Stratford, M.; Miller, M.W. Saccharomycodes E.C. Hansen (1904). In The Yeasts, a Taxonomic Study, 5th ed.; Kurtzman, C.P., Fell, J.W., Boekhout, T., Eds.; Elsevier: London, UK, 2011; pp. 747–750. [Google Scholar]
- Malfeito-Ferreira, M.; Tareco, M.; Loureiro, V. Fatty acid profiling: A feasible typing system to trace yeast contamination in wine bottling plants. Int. J. Food Microbiol. 1997, 38, 143–155. [Google Scholar] [CrossRef]
- Miller, M.W.; Phaff, H.J. Saccharomycodes E.C. Hansen. In The Yeasts. A Taxonomic Study, 4th ed.; Kurtzman, C.P., Fell, J.W., Eds.; Elsevier: New York, NY, USA, 1998; pp. 372–373. [Google Scholar]
- Yamazaki, T.; Oshima, Y. Saccharomycodes ludwigii has seven chromosomes. Yeast 1996, 12, 237–240. [Google Scholar] [CrossRef]
- Domizio, P.; Romani, C.; Lencioni, L.; Comitini, F.; Gobbi, M.; Mannazzu, I.; Ciani, M. Outlining a future for non-Saccharomyces yeasts: Selection of putative spoilage wine strains to be used in association with Saccharomyces cerevisiae for grape juice fermentation. Int. J. Food Microbiol. 2011, 147, 170–180. [Google Scholar] [CrossRef] [PubMed]
- Romano, P.; Marchese, R.; Laurita, C.; Saleano, G.; Turbanti, L. Biotechnological suitability of Saccharomycodes ludwigii for fermented beverages. World J. Microb. Biot. 1999, 15, 451–454. [Google Scholar] [CrossRef]
- Stratford, M.; Morgan, P.; Rose, A.H. Sulphur dioxide resistance in Saccharomyces cerevisiae and Saccharomycodes ludwigii. J. Gen. Microbiol. 1987, 133, 2173–2179. [Google Scholar] [CrossRef]
- Thomas, D.S. Yeasts as spoilage organisms in beverages. In The yeasts. Yeast Technology, 2nd ed.; Rose, A.H., Harrison, J.S., Eds.; Academic Press: London, UK, 1993; Volume 5, pp. 517–561. ISBN 0-12-596415-3. [Google Scholar]
- Warth, A.D. Resistance of yeast species to benzoic and sorbic acid and sulphur dioxide. J. Food Prot. 1985, 48, 564–569. [Google Scholar] [CrossRef]
- Domizio, P.; Liu, Y.; Bisson, L.F.; Barile, D. Use of non-Saccharomyces wine yeasts as novel sources of mannoproteins in wine. Food Microbiol. 2014, 43, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Romano, P.; Fiore, C.; Paraggio, M.; Caruso, M.; Cepece, A. Function of yeast species and strains in wine flavour. Int. J. Food Microbiol. 2003, 86, 169–180. [Google Scholar] [CrossRef]
- Fugelsang, K.C.; Edwards, C.G. Yeasts. In Wine Microbiology Practical Applications and Procedures, 2nd ed.; Fugelsang, K.C., Edwards, C.G., Eds.; Springer Science+Business Media: New York, NY, USA, 2007; pp. 3–14. ISBN 978-0-387-33349-6. [Google Scholar]
- Remize, F.; Roustan, J.L.; Sablayrolles, J.M.; Barre, P.; Dequin, S. Glycerol overproduction by engineered Saccharomyces cerevisiae wine yeast strains leads to substantial changes in byproduct formation and to a stimulation of fermentation rate in stationary phase. Appl. Environ. Microb. 1999, 65, 143–149. [Google Scholar]
- Santos, A.; Marquina, D. Ion channel activity by Pichia membranifaciens killer toxin. Yeast 2004, 21, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Gschaedler, A. Contribution of non-conventional yeasts in alcoholic beverages. Curr. Opin. Food Sci. 2017, 13, 73–77. [Google Scholar] [CrossRef]
- Costa, A.; Barata, A.; Malfeito-Ferreira, M.; Loureiro, V. Evaluation of the inhibitory effect of dimethyl dicarbonate (DMDC) against wine microorganisms. Food Microbiol. 2008, 25, 422–427. [Google Scholar] [CrossRef] [PubMed]
- Roller, S.; Covill, N. The antifungal properties of chitosan in laboratory media and apple juice. Int. J. Food Microbiol. 1999, 47, 67–77. [Google Scholar] [CrossRef]
- Lachance, M.A. Yeast communities in a natural tequila fermentation. Antonie van Leeuwenhoek 1995, 68, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Barnett, J.A.; Payne, R.W.; Yarrow, D. Yeasts: Characteristics and Identification, 3rd ed.; Cambridge University Press: Cambridge, UK, 2000. [Google Scholar]
- Ogilvie, L. Observations on the “slime-fluxes” of trees. Trans. Br. Mycol. Soc. 1924, 9, 167–182. [Google Scholar] [CrossRef]
- Stringini, M.; Comitini, F.; Taccari, M.; Ciani, M. Yeast diversity during tapping and fermentation of palm wine from Cameroon. Food Microbiol. 2009, 26, 415–420. [Google Scholar] [CrossRef] [PubMed]
- Beech, F.W.; Carr, J.G. Cider and perry. In Economic Microbiology, Volume 1, Alcoholic beverages; Rose, A.H., Ed.; Academic Press: London, UK, 1977; pp. 139–313. ISBN 978-0125965507. [Google Scholar]
- Morata, A.; Vejarano, R.; Ridolfi, G.; Benito, S.; Palomero, F.; Uthurry, C.; Tesfaye, W.; González, C.; Suárez-Lepe, J.A. Reduction of 4-ethylphenol production in red wines using HCDC+ yeasts and cinnamyl esterases. Enzyme Microb. Technol. 2013, 52, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Jarvis, B.; Lea, A.G.H. Sulphite binding in ciders. Int. J. Food Sci. Technol. 2000, 35, 113–127. [Google Scholar] [CrossRef]
- OIV. Compendium of International Methods of Wine and Must Analysis, 2018 ed.; International Organization of Vine and Wine (OIV): Paris, France, 2018; Volume II, OIV-MA-C1-01: R2011; ISBN 979-10-91799-79-9. Available online: http://www.oiv.int/public/medias/5773/compendium-2018-en-vol2.pdf (accessed on 25 July 2018).
- Delfini, C.; Gaia, P.; Schellino, R.; Strano, M.; Pagliara, A.; Ambró, S. Fermentability of grape must after inhibition with dimethyl dicarbonate (DMDC). J. Agric. Food Chem. 2002, 50, 5605–5611. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.C.; Nunes, C.; Saraiva, J.A.; Coimbra, M.A. Chemical and physical methodologies for the replacement/reduction of sulfur dioxide use during winemaking: Review of their potentialities and limitations. Eur. Food Res. Technol. 2012, 234, 1–12. [Google Scholar] [CrossRef]
- Vally, H.; Misso, N.L.A.; Madan, V. Clinical effects of sulphite additives. Clin. Exp. Allergy 2009, 39, 1643–1651. [Google Scholar] [CrossRef] [PubMed]
- Bech-Larsen, T.; Scholderer, J. Functional foods in Europe: Consumer research, market experiences and regulatory aspects. Trends Food Sci. Technol. 2007, 18, 231–234. [Google Scholar] [CrossRef]
- Cravero, F.; Englezos, V.; Torchio, F.; Giacosa, S.; Río Segade, S.; Gerbi, V.; Rantsiou, K.; Rolle, L.; Cocolin, L. Post-harvest control of wine-grape mycobiota using electrolyzed water. Innov. Food Sci. Emerg. 2016, 35, 21–28. [Google Scholar] [CrossRef]
- Hinze, H.; Holzer, H. Analysis of the energy metabolism after incubation of Saccharomyces cerevisiae with sulfite or nitrite. Arch. Microbiol. 1986, 145, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Stratford, M.; Rose, A.H. Transport of sulphide dioxide by Saccharomyces cerevisiae. J. Gen. Microbiol. 1986, 132, 1–6. [Google Scholar] [CrossRef]
- Kaneko, H.; Hosahara, M.; Tanaka, M.; Itoh, T. Lipid composition of 30 species of yeast. Lipids 1976, 11, 837–844. [Google Scholar] [CrossRef] [PubMed]
- Terrell, F.R.; Morris, J.R.; Johnson, M.G.; Gbur, E.E.; Makus, D.J. Yeast inhibition in grape juice containing sulfur dioxide, sorbic acid, and dimethyldicarbonate. J. Food. Sci. 1993, 58, 1132–1134. [Google Scholar] [CrossRef]
- Threlfall, R.T.; Morris, J.R. Using dimethyldicarbonate to minimize sulfur dioxide for prevention of fermentation from excessive yeast contamination in juice and semi-sweet wine. J. Food Sci. 2002, 67, 2758–2762. [Google Scholar] [CrossRef]
- Sawant, A.D.; Abdelal, A.T.; Ahearn, D.G. Purification and characterization of the anti-Candida toxin of Pichia anomala WC 65. Antimicrob. Agents Chemother. 1989, 33, 48–52. [Google Scholar] [CrossRef] [PubMed]
- Palpacelli, V.; Ciani, M.; Rosini, G. Activity of different ‘killer’ yeasts on strains of yeast species undesirable in the food industry. FEMS Microbiol. Lett. 1991, 84, 75–78. [Google Scholar] [CrossRef]
- Oro, L.; Ciani, M.; Comitini, F. Antimicrobial activity of Metschnikowia pulcherrima on wine yeasts. J. Appl. Microbiol. 2014, 116, 1209–1217. [Google Scholar] [CrossRef] [PubMed]
- Youssef, B.M.; Asker, A.A.; El-Samahy, S.K.; Swailam, H.M. Combined effect of steaming and gamma irradiation on the quality of mango pulp stored at refrigerated temperature. Food Res. Int. 2002, 35, 1–13. [Google Scholar] [CrossRef]
- González-Arenzana, L.; Portua, J.; López, R.; López, N.; Santamaría, P.; Garde-Cerdán, T.; López-Alfaro, I. Inactivation of wine-associated microbiota by continuous pulsed electric field treatments. Innov. Food Sci. Emerg. 2015, 29, 187–192. [Google Scholar] [CrossRef]
- OIV. International Code of Oenological Practices; Issue 2018; International Organization of Vine and Wine (OIV): Paris, France, 2018; OENO 5/01, OENO 421-2011; ISBN 979-10-91799-88-1. [Google Scholar]
- Peterson, T.W.; Ough, C.S. Dimethyldicarbonate reaction with higher alcohols. Am. J. Enol. Viticult. 1979, 30, 119–123. [Google Scholar]
- Suárez-Lepe, J.A.; Morata, A. New trends in yeast selection for winemaking. Trends Food Sci. Technol. 2012, 23, 39–50. [Google Scholar] [CrossRef]
- Porter, L.T.; Ough, C.S. The effects of ethanol, temperature and dimethyldicarbonate on viability of Saccharomyces cerevisiae Montrachet No 522 in wine. Am. J. Enol. Viticult. 1982, 33, 222–225. [Google Scholar]
- Divol, B.; Strehaiano, P.; Lonvaud-Funel, A. Effectiveness of dimethyldicarbonate to stop alcoholic fermentation in wine. Food Microbiol. 2005, 22, 169–178. [Google Scholar] [CrossRef]
- Feng, M.; Lalor, B.; Hu, S.; Mei, J.; Huber, A.; Kidby, D.; Holbein, B. Inhibition of yeast growth in grape juice through removal of iron and other metals. Int. J. Food Sci. Technol. 1997, 32, 21–28. [Google Scholar] [CrossRef]
- Jackson, S.L.; Heath, I.B. Roles of calcium ions in hyphal tip growth. Microbiol. Mol. Biol. R. 1993, 57, 367–382. [Google Scholar]
- Fang, S.W.; Li, C.F.; Shih, D.Y.C. Antifungal activity of chitosan and its preservative effect on low-sugar candied kumquat. J. Food Protect. 1994, 56, 136–140. [Google Scholar] [CrossRef]
- Leuba, J.L.; Stossel, P. Chitosan and other polyamines: Antifungal activity and interaction with biological membranes. In Chitin in Nature and Technology; Muzzarelli, R., Jeuniaux, C., Gooday, G.W., Eds.; Springer: Boston, MA, USA, 1986; pp. 215–222. [Google Scholar]
- Muzzarelli, R.A.A. Chitosan-based dietary foods. Carbohydr. Polym. 1996, 29, 309–316. [Google Scholar] [CrossRef]
- Ren, J.; Liu, J.; Li, R.; Dong, F.; Guo, Z. Antifungal properties of chitosan salts in laboratory media. J. Appl. Polym. Sci. 2012, 124, 2501–2507. [Google Scholar] [CrossRef]
- Papineau, A.M.; Hoover, D.G.; Knorr, D.; Farkas, D.F. Antimicrobial effect of water-soluble chitosans with high hydrostatic pressure. Food Biotechnol. 1991, 5, 45–57. [Google Scholar] [CrossRef]
- Schmitt, M.; Radler, F. Molecular structure of the cell wall receptor for killer toxin KT28 in Saccharomyces cerevisiae. J. Bacteriol. 1988, 170, 2192–2196. [Google Scholar] [CrossRef] [PubMed]
- Saravanakumar, D.; Ciavorella, A.; Spadaro, D.; Garibaldi, A.; Gullino, M.L. Metschnikowia pulcherrima strain MACH1 outcompetes Botrytis cinerea, Alternaria alternata and Penicillium expansum in apples through iron depletion. Postharvest Biol. Technol. 2008, 49, 121–128. [Google Scholar] [CrossRef]
- Combina, M.; Elia, A.; Mercado, L.; Catania, C.; Ganga, A.; Martinez, C. Dynamics of indigenous yeast populations during spontaneous fermentation of wine from Mendoza, Argentina. Int. J. Food Microbiol. 2005, 99, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Lopes, C.A.; Sangorrín, M.P. Optimization of killer assays for yeast selection protocols. Rev. Argent. Microbiol. 2010, 42, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Türkel, S.; Ener, B. Isolation and characterization of new Metschnikowia pulcherrima strains as producers of the antimicrobial pigment pulcherrimin. Z. Naturforsch. C 2009, 64, 405–410. [Google Scholar] [CrossRef] [PubMed]
- Holmes-Hampton, G.P.; Jhurry, N.D.; McCormick, S.P.; Lindahl, P.A. Iron content of Saccharomyces cerevisiae cells grown under iron-deficient and iron-overload conditions. Biochemistry 2013, 52, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Comitini, F.; Gobbi, M.; Domizio, P.; Romani, C.; Lencioni, L.; Mannazzu, I.; Ciani, M. Selected non-Saccharomyces wine yeasts in controlled multistarter fermentations with Saccharomyces cerevisiae. Food Microbiol. 2011, 28, 873–882. [Google Scholar] [CrossRef] [PubMed]
- Mehlomakulu, N.N.; Setati, M.E.; Divol, B. Characterization of novel killer toxins secreted by wine-related non-Saccharomyces yeasts and their action on Brettanomyces spp. Int. J. Food Microbiol. 2014, 188, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.; Navascués, E.; Bravo, E.; Marquina, D. Ustilago maydis killer toxin as a new tool for the biocontrol of the wine spoilage yeast Brettanomyces bruxellensis. Int. J. Food Microbiol. 2011, 145, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.; Alonso, A.; Belda, I.; Marquina, D. Cell cycle arrest and apoptosis, two alternative mechanisms for PMKT2 killer activity. Fungal Genet. Biol. 2013, 50, 44–54. [Google Scholar] [CrossRef] [PubMed]
- Alonso, A.; Belda, I.; Santos, A.; Navascués, E.; Marquina, D. Advances in the control of the spoilage caused by Zygosaccharomyces species on sweet wines and concentrated grape musts. Food Control. 2015, 51, 129–134. [Google Scholar] [CrossRef]
- Berbegal, C.; Garofalo, C.; Russo, P.; Pati, S.; Capozzi, V.; Spano, G. Use of autochthonous yeasts and bacteria in order to control Brettanomyces bruxellensis in wine. Fermentation 2017, 3, 65. [Google Scholar] [CrossRef]
- Morata, A.; Loira, I.; Vejarano, R.; González, C.; Callejo, M.J.; Suárez-Lepe, J.A. Emerging preservation technologies in grapes for winemaking. Trends Food Sci. Technol. 2017, 67, 36–43. [Google Scholar] [CrossRef]
- Golberg, A. The impact of pulsed electric fields on cells and biomolecules: Comment on “Lightning-triggered electroporation and electrofusion as possible contributors to natural horizontal gene transfer” by Tadej Kotnik. Phys. Life Rev. 2013, 10, 382–383. [Google Scholar] [CrossRef] [PubMed]
- Puértolas, E.; López, N.; Condón, S.; Raso, J.; Álvarez, I. Pulsed electric fields inactivation of wine spoilage yeast and bacteria. Int. J. Food Microbiol. 2009, 130, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Timmermans, R.; Nederhoff, A.; Groot, M.N.; van Boekel, M.; Mastwijk, H. Effect of electrical field strength applied by PEF processing and storage temperature on the outgrowth of yeasts and moulds naturally present in a fresh fruit smoothie. Int. J. Food Microbiol. 2016, 230, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Garde-Cerdán, T.; Arias-Gil, M.; Marsellés-Fontanet, A.R.; Ancín-Azpilicueta, C.; Martín-Belloso, O. Effects of thermal and non-thermal processing treatment on fatty acids and free amino acids of grape juice. Food Control 2007, 18, 473–479. [Google Scholar] [CrossRef]
- Beveridge, J.R.; Wall, K.; MacGregor, S.J.; Anderson, J.G.; Rowan, N.J. Pulsed electric field inactivation of spoilage microorganisms in alcoholic beverages. In Proceedings of the 14th IEEE International Pulsed Power Conference, Dallas, TX, USA, 15–18 June 2003; pp. 1138–1143. [Google Scholar]
- Milani, E.A.; Alkhafaji, S.; Silva, F.V.M. Pulsed electric field continuous pasteurization of different types of beers. Food Control 2015, 50, 223–229. [Google Scholar] [CrossRef]
- Bely, M.; Stoeckle, P.; Masnuef-Pomarède, I.; Dubourdieu, D. Impact of mixed Torulaspora delbrueckii–Saccharomyces cerevisiae culture on high-sugar fermentation. Int. J. Food Microbiol. 2008, 122, 312–320. [Google Scholar] [CrossRef] [PubMed]
- Bovo, B.; Carlot, M.; Lombardi, A.; Lomolino, G.; Lante, A.; Giacomini, A.; Corich, V. Exploring the use of Saccharomyces cerevisiae commercial strain and Saccharomycodes ludwigii natural isolate for grape marc fermentation to improve sensory properties of spirits. Food Microbiol. 2014, 41, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Fia, G.; Giovani, G.; Rosi, I. Study of beta-glucosidase production by wine-related yeasts during alcoholic fermentation. A new rapid fluorimetric method to determine enzymatic activity. J. Appl. Microbiol. 2005, 99, 509–517. [Google Scholar] [CrossRef] [PubMed]
- Ugliano, M.; Bartowsky, E.J.; McCarthy, J.; Moio, L.; Henschke, P.A. Hydrolysis and transformation of grape glycosidically bound volatile compounds during fermentation with three Saccharomyces yeast strains. J. Agric. Food Chem. 2006, 54, 6322–6331. [Google Scholar] [CrossRef] [PubMed]
- Wightman, J.D.; Wrolstad, R.E. β-glucosidase activity in juice-processing enzymes based on anthocyanin analysis. J. Food Sci. 1996, 61, 544–548. [Google Scholar] [CrossRef]
- Vejarano, R.; Morata, A.; Loira, I.; González, M.C.; Suárez-Lepe, J.A. Theoretical considerations about usage of metabolic inhibitors as possible alternative to reduce alcohol content of wines from hot areas. Eur Food Res. Technol. 2013, 237, 281–290. [Google Scholar] [CrossRef]
- Kulkarni, P.; Loira, I.; Morata, A.; Tesfaye, W.; González, M.C.; Suárez-Lepe, J.A. Use of non-Saccharomyces yeast strains coupled with ultrasound treatment as a novel technique to accelerate ageing on lees of red wines and its repercussion in sensorial parameters. LWT-Food Sci. Technol. 2015, 64, 1255–1262. [Google Scholar] [CrossRef]
- Palomero, F.; Morata, A.; Benito, S.; Calderón, F.; Suárez-Lepe, J.A. New genera of yeasts for over-lees aging of red wine. Food Chem. 2009, 112, 432–441. [Google Scholar] [CrossRef]
- Giovani, G.; Rosi, I.; Bertuccioli, M. Quantification and characterization of cell wall polysaccharides released by non-Saccharomyces yeast strains during alcoholic fermentation. Int. J. Food Microbiol. 2012, 160, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Charpentier, C.; N’guyen Van Long, T.; Bonaly, R.C.; Feuillat, M. Alteration of cell wall structure in Saccharomyces cerevisiae and Saccharomyces bayanus during autolysis. Appl. Microbiol. Biotechnol. 1986, 24, 405–413. [Google Scholar] [CrossRef]
- Klis, F.M.; Boorsma, A.; De Groot, P.W.J. Cell wall construction in Saccharomyces cerevisiae. Yeast 2006, 23, 185–202. [Google Scholar] [CrossRef] [PubMed]
- Vidal, S.; Francis, L.; Williams, P.; Kwiatkowski, M.; Gawel, R.; Cheynier, V.; Waters, E. The mouth-feel properties of polysaccharides and anthocyanins in a wine like medium. Food Chem. 2004, 85, 519–525. [Google Scholar] [CrossRef]
- Guadalupe, Z.; Palacios, A.; Ayestarán, B. Maceration enzymes and mannoproteins: A possible strategy to increase colloidal stability and color extraction in red wines. J. Agric. Food Chem 2007, 55, 4854–4862. [Google Scholar] [CrossRef] [PubMed]
- Chalier, P.; Angot, B.; Delteil, D.; Doco, T.; Gunata, Z. Interactions between aroma compounds and whole mannoprotein isolated from Saccharomyces cerevisiae strains. Food Chem. 2007, 100, 22–30. [Google Scholar] [CrossRef]
- Gonzalez-Ramos, D.; Cebollero, E.; Gonzalez, R. A recombinant Saccharomyces cerevisiae strain overproducing mannoproteins stabilizes wine against protein haze. Appl. Environ. Microbiol. 2008, 74, 5533–5540. [Google Scholar] [CrossRef] [PubMed]
- Lubbers, S.; Léger, B.; Charpentier, C.; Feuillat, M. Effet colloïdes protecteurs d’extraits de parois de levures sur la stabilité tartrique d’un vin modèle. J. Int. Sci. Vigne. Vin. 1993, 27, 13–22. [Google Scholar]
- Rodrigues, A.; Ricardo-Da-Silva, J.M.; Lucas, C.; Laureano, O. Effect of commercial mannoproteins on wine colour and tannins stability. Food Chem. 2012, 131, 907–914. [Google Scholar] [CrossRef]
- Iriti, M.; Varoni, E.M. Cardioprotective effects of moderate red wine consumption: Polyphenols vs. Etanol. Review. J. Appl. Biomed. 2014, 12, 193–202. [Google Scholar] [CrossRef]
- Loira, I.; Vejarano, R.; Morata, A.; Ricardo-da-Silva, J.M.; Laureano, O.; González, M.C.; Suárez-Lepe, J.A. Effect of Saccharomyces strains on the quality of red wines aged on lees. Food Chem. 2013, 139, 1044–1051. [Google Scholar] [CrossRef] [PubMed]
- Rosi, I.; Gheri, A.; Domizio, P.; Fia, G. Production de macromolecules parietals de Saccharomyces cerevisiae au cours de la fermentation et leur influence sur la fermentation malolactique. Revue des Œnologues 2000, 94, 18–20. [Google Scholar]
- Moreno-Arribas, V.; Pueyo, E.; Nieto, F.J.; Martín-Álvarez, P.J.; Polo, M.C. Influence of the polysaccharides and the nitrogen compounds on foaming properties of sparkling wines. Food Chem. 2000, 70, 309–317. [Google Scholar] [CrossRef]
- Moruno, E.G.; Sanlorenzo, C.; Boccaccino, B.; Di Stefano, R. Treatment with yeast to reduce the concentration of ochratoxin A. in red wine. Am. J. Enol. Viticult. 2005, 56, 73–76. [Google Scholar]
- Vejarano, R.; Siche, R.; Tesfaye, W. Evaluation of biological contaminants in foods by hyperspectral imaging (HSI): A. review. Int. J. Food Prop. 2017, 20, 1264–1297. [Google Scholar] [CrossRef]
- Morata, A.; Gómez-Cordovés, M.C.; Suberviola, J.; Bartolomé, B.; Colomo, B.; Suárez-Lepe, J.A. Adsorption of anthocyanins by yeast cell walls during the fermentation of red wines. J. Agric. Food Chem. 2003, 51, 4084–4088. [Google Scholar] [CrossRef] [PubMed]
- Morata, A.; Gómez-Cordovés, M.C.; Calderón, F.; Suárez-Lepe, J.A. Effects of pH, temperature and SO2 on the formation of pyranoanthocyanins during red wine fermentation with two species of Saccharomyces. Int. J. Food Microbiol. 2006, 106, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Rokhina, E.V.; Piet, L.; Virkutyte, J. Low-frequency ultrasound in biotechnology: State of the art. Trends Biotechnol. 2009, 27, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Koda, S.; Miyamoto, M.; Toma, M.; Matsuoka, T.; Maebayashi, M. Inactivation of Escherichia coli and Streptococcus mutants by ultrasound at 500 kHz. Ultrason. Sonochem. 2009, 16, 655–659. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Ma, H. Ultrasonic degradation of polysaccharide from a red algae (Porphyra yezoensis). J. Agric. Food Chem. 2006, 54, 2223–2228. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.Q.; Aung, M.T.; Lee, P.R.; Yu, B. Yeast and volatile evolution in cider co-fermentation with Saccharomyces cerevisiae and Williopsis saturnus. Ann. Microbiol. 2016, 66, 307–315. [Google Scholar] [CrossRef]
- Lu, Y.; Huang, D.; Lee, P.R.; Liu, S.Q. Assessment of volatile and non-volatile compounds in durian wines fermented with four commercial non-Saccharomyces yeasts. J. Sci. Food Agric. 2016, 96, 1511–1521. [Google Scholar] [CrossRef] [PubMed]
- Fugelsang, K. Population dynamics and effects of Brettanomyces bruxellensis strains on Pinot noir (Vitis vinifera L.) wines. Am. J. Enol. Viticult. 2003, 54, 294–300. [Google Scholar]
- Crauwels, S.; Van Opstaele, F.; Jaskula-Goiris, B.; Steensels, J.; Verreth, C.; Bosmans, L.; Paulussen, C.; Herrera-Malaver, B.; de Jonge, R.; De Clippeleer, J.; et al. Fermentation assays reveal differences in sugar and (off-) flavor metabolism across different Brettanomyces bruxellensis strains. FEMS Yeast Res. 2017, 17, fow105. [Google Scholar] [CrossRef] [PubMed]
- Smith, B.D.; Divol, B. Brettanomyces bruxellensis, a survivalist prepared for the wine apocalypse and other beverages. Food Microbiol. 2016, 59, 161–175. [Google Scholar] [CrossRef] [PubMed]
- Nardi, T.; Remize, F.; Alexandre, H. Adaptation of yeasts Saccharomyces cerevisiae and Brettanomyces bruxellensis to winemaking conditions: A comparative study of stress genes expression. Appl. Microbiol. Biotechnol. 2010, 88, 925–937. [Google Scholar] [CrossRef] [PubMed]
- Avramova, M.; Vallet-Courbin, A.; Maupeu, J.; Masneuf-Pomarede, I.; Albertin, W. Molecular diagnosis of Brettanomyces bruxellensis’ sulfur dioxide sensitivity through genotype specific method. Front Microbiol. 2018, 9, 1260. [Google Scholar] [CrossRef] [PubMed]
- Capozzi, V.; Di Toro, M.R.; Grieco, F.; Michelotti, V.; Salma, M.; Lamontanara, A.; Russo, P.; Orrù, L.; Alexandre, H.; Spano, G. Viable but not culturable (VBNC) state of Brettanomyces bruxellensis in wine: New insights on molecular basis of VBNC behaviour using a transcriptomic approach. Food Microbiol. 2016, 59, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Avramova, M.; Cibrario, A.; Peltier, E.; Coton, M.; Coton, E.; Schacherer, J.; Spano, G.; Capozzi, V.; Blaiotta, G.; Salin, F.; et al. Brettanomyces bruxellensis population survey reveals a diploid-triploid complex structured according to substrate of isolation and geographical distribution. Sci. Rep. 2018, 8, 4136. [Google Scholar] [CrossRef] [PubMed]
- Guerrero, R.F.; Cantos-Villar, E. Demonstrating the efficiency of sulphur dioxide replacements in wine: A. parameter review. Trends Food Sci. Technol. 2015, 42, 27–43. [Google Scholar] [CrossRef]
- Saldaña, G.; Puértolas, E.; Álvarez, I.; Meneses, N.; Knorr, D.; Raso, J. Evaluation of a static treatment chamber to investigate kinetics of microbial inactivation by pulsed electric fields at different temperatures at quasi-isothermal conditions. J. Food Eng. 2010, 100, 349–356. [Google Scholar] [CrossRef]
- Marsellés-Fontanet, À.R.; Puig, A.; Olmos, P.; Mínguez-Sanz, S.; Martín-Belloso, O. Optimising the inactivation of grape juice spoilage organisms by pulse electric fields. Int. J. Food Microbiol. 2009, 130, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Portugal, C.; Sáenz, Y.; Rojo-Bezares, B.; Zarazaga, M.; Torres, C.; Cacho, J.; Ruiz-Larrea, F. Brettanomyces susceptibility to antimicrobial agents used in winemaking: In vitro and practical approaches. Eur. Food Res. Technol. 2014, 238, 641–652. [Google Scholar] [CrossRef]
- Bizri, J.N.; Wahem, I.A. Citric acid and antimicrobial affect microbiological stability and quality of tomato juice. J. Food Sci. 1994, 59, 130–134. [Google Scholar] [CrossRef]
- Barceloux, D.G.; Bond, G.R.; Krenzelok, E.P.; Cooper, H.; Vale, J.A. American Academy of Clinical Toxicology practice guidelines on treatment of methanol poisoning. J. Toxicol. Clin. Toxicol. 2002, 40, 415–446. [Google Scholar] [CrossRef] [PubMed]
- Cravero, F.; Englezos, V.; Rantsiou, K.; Torchio, F.; Giacosa, S.; Río Segade, S.; Gerbi, V.; Rolle, L.; Cocolin, L. Control of Brettanomyces bruxellensis on wine grapes by post-harvest treatments with electrolyzed water, ozonated water and gaseous ozone. Innov. Food Sci. Emerg. Technol. 2018, 47, 309–316. [Google Scholar] [CrossRef]
- Charpentier, C.; Escot, S.; González, E.; Dulau, L.; Feuillat, M. The influence of yeast glycosylated proteins on tannins aggregation in model solution. Int. J. Vine Wine Sci. 2004, 38, 209–218. [Google Scholar] [CrossRef]
- Suárez-Lepe, J.A.; Morata, A. Nuevo Método de Crianza Sobre Lías. Patente P200602423, 25 September 2006. [Google Scholar]
| Sample | Applied Treatment | Reference |
|---|---|---|
| Grape must | DMDC | [31,39] |
| DMDC + SO2 | [39,40] | |
| DMDC + sorbic acid | [39] | |
| Toxin of Pichia anomala WC65 | [41] | |
| Toxin KpKt | [42] | |
| Biological control: Metschnikowia pulcherrima | [43] | |
| Apple juice | Chitosan | [22] |
| Mango pulp | Gamma radiation Gamma radiation + steaming | [44] |
| Wine | DMDC in red wine | [21] |
| DMDC in semi-sweet wine | [40] | |
| PEF | [45] |
| Component (%) | S’codes ludwigii | S. pombe | S. cerevisiae | Reference |
|---|---|---|---|---|
| Proteins | 12 | 11 | 24 * | [85] |
| Mannose | 93 | 55 | 88 * | |
| Glucose | 7 | 22 | 12 * | |
| Galactose | - | 23 | - | |
| α (1-3) glucan | Yes | No | [84] ** | |
| β (1-3) glucan | Yes | Yes | ||
| β (1-6) glucan | Yes | Yes | ||
| Chitin (% of dry weight) | 0.5 | 0.1 |
© 2018 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vejarano, R. Saccharomycodes ludwigii, Control and Potential Uses in Winemaking Processes. Fermentation 2018, 4, 71. https://doi.org/10.3390/fermentation4030071
Vejarano R. Saccharomycodes ludwigii, Control and Potential Uses in Winemaking Processes. Fermentation. 2018; 4(3):71. https://doi.org/10.3390/fermentation4030071
Chicago/Turabian StyleVejarano, Ricardo. 2018. "Saccharomycodes ludwigii, Control and Potential Uses in Winemaking Processes" Fermentation 4, no. 3: 71. https://doi.org/10.3390/fermentation4030071
APA StyleVejarano, R. (2018). Saccharomycodes ludwigii, Control and Potential Uses in Winemaking Processes. Fermentation, 4(3), 71. https://doi.org/10.3390/fermentation4030071






