Mitigation of Volatile Fatty Acid Build-Up by the Use of Soft Carbon Felt Electrodes: Evaluation of Anaerobic Digestion in Acidic Conditions
Abstract
1. Introduction
2. Materials and Methods
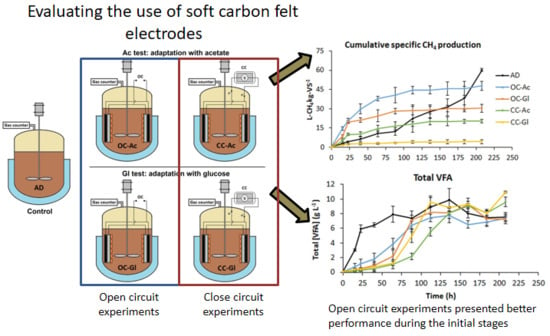
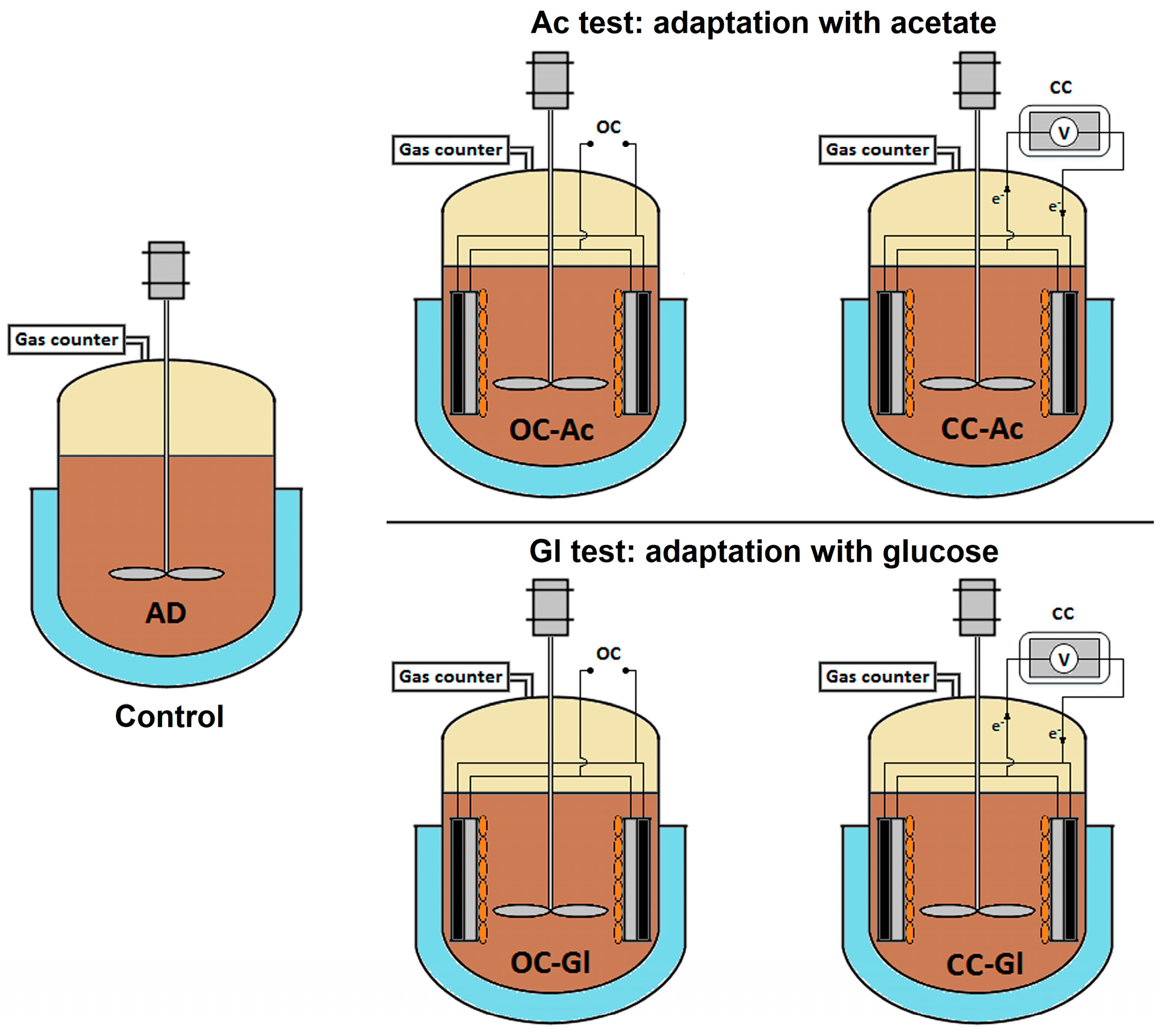
2.1. Reactor Design
2.2. Electrode Preparation and Operation
2.3. Analytical Measurements and Calculations
3. Results and Discussion
3.1. Batch Digestion Tests
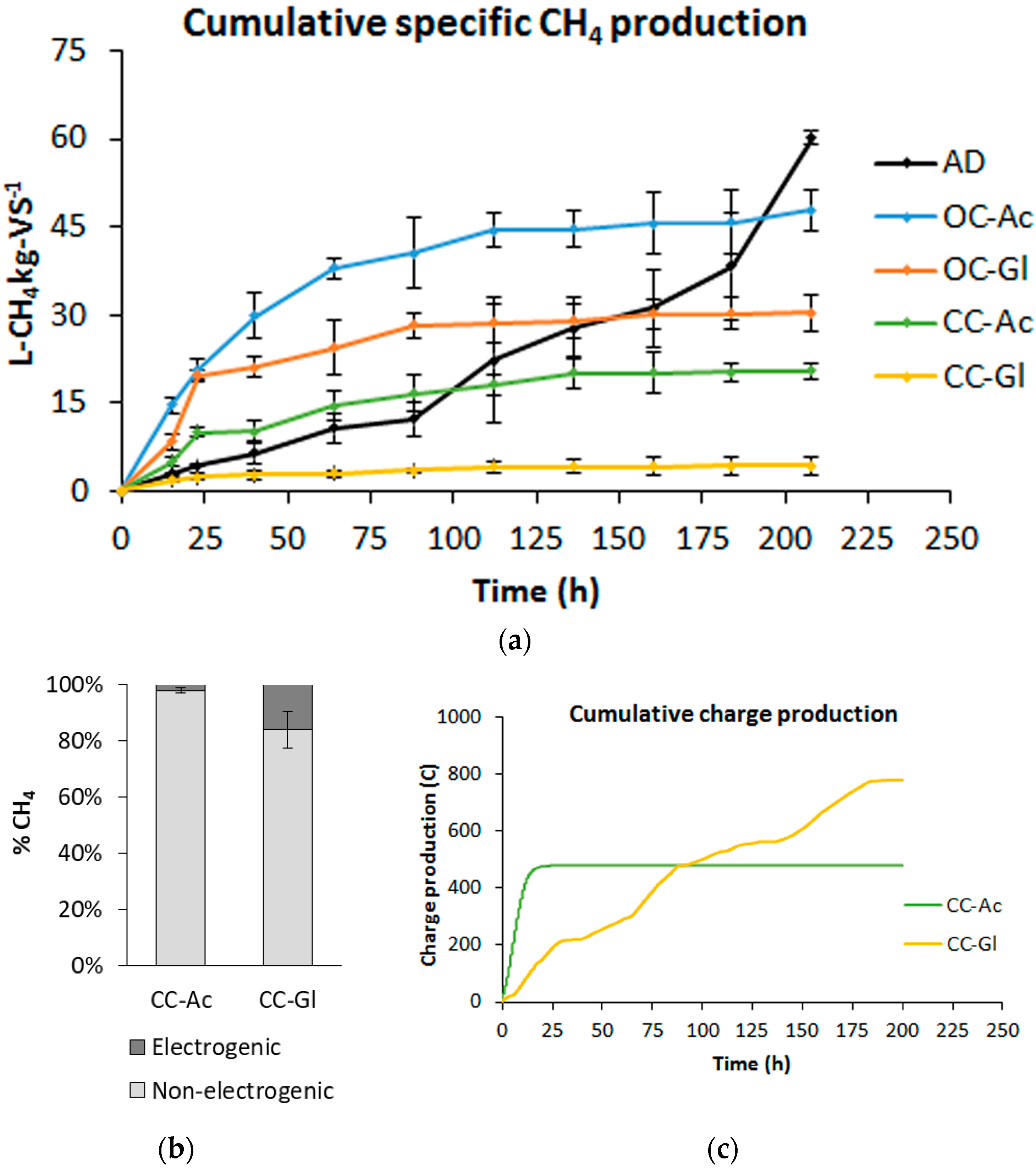
3.2. Combined AD-BES Reactors
3.2.1. Methane Production and Current Profiles
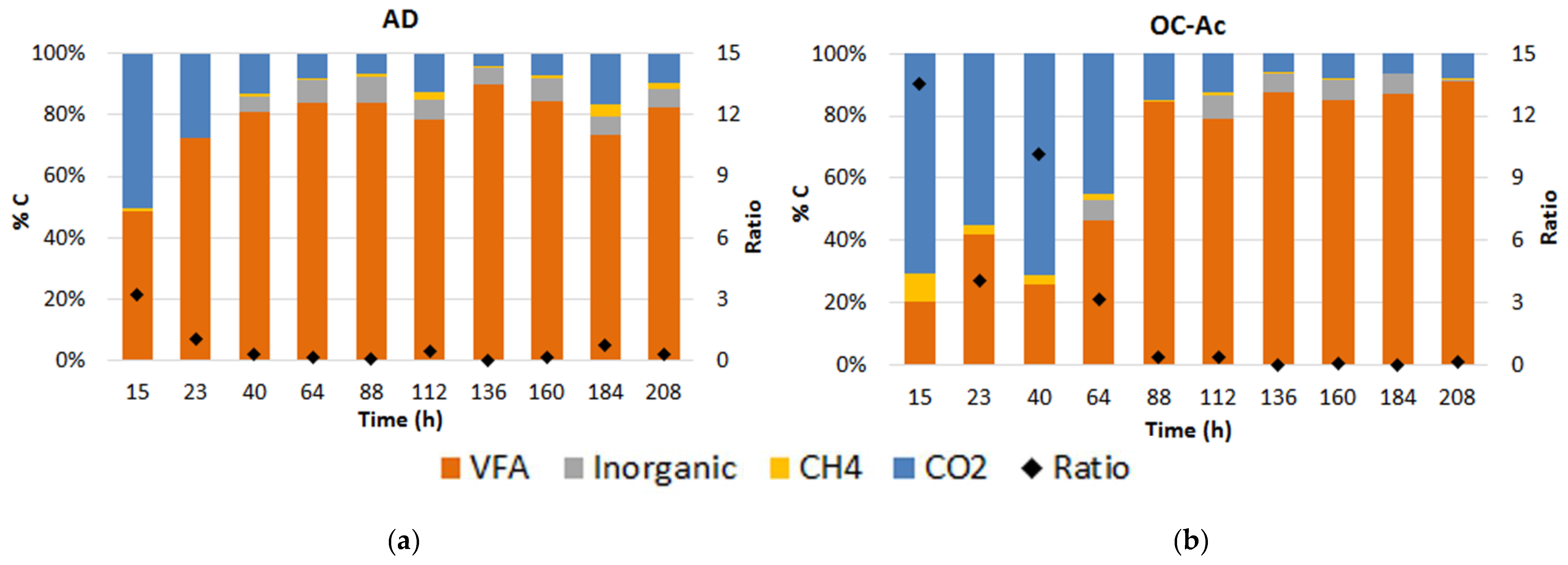
3.2.2. Analysis of Volatile Fatty Acids
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Saleh, B. Parametric and working fluid analysis of a combined organic Rankine-vapor compression refrigeration system activated by low-grade thermal energy. J. Adv. Res. 2016, 7, 651–660. [Google Scholar] [CrossRef] [PubMed]
- Ezeji, T.C. Production of Bio-Derived Fuels and Chemicals. Fermentation 2017, 3, 42. [Google Scholar] [CrossRef]
- Van Meerbeek, K.; Appels, L.; Dewil, R.; Calmeyn, A.; Lemmens, P.; Muys, B.; Hermy, M. Biomass of invasive plant species as a potential feedstock for bioenergy production. Biofuel. Bioprod. Bior. 2015, 9, 273–282. [Google Scholar] [CrossRef]
- Cuetos, M.J.; Martinez, E.J.; Moreno, R.; González, R.; Otero, M.; Gómez, X. Enhancing anaerobic digestion of poultry blood using activated carbon. J. Adv. Res. 2017, 8, 297–307. [Google Scholar] [CrossRef] [PubMed]
- Carlsson, M.; Lagerkvist, A.; Morgan-Sagastume, F. The effects of substrate pre-treatment on anaerobic digestion systems: A review. Waste Manag. 2012, 32, 1634–1650. [Google Scholar] [CrossRef] [PubMed]
- Al bkoor Alrawashdeh, K.; Pugliese, A.; Slopiecka, K.; Pistolesi, V.; Massoli, S.; Bartocci, P.; Fantozzi, F. Codigestion of untreated and treated sewage sludge with the organic fraction of municipal solid wastes. Fermentation 2017, 3, 35. [Google Scholar] [CrossRef]
- Westman, S.Y.; Chandolias, K.; Taherzadeh, M.J. Syngas Biomethanation in a Semi-Continuous Reverse Membrane Bioreactor (RMBR). Fermentation 2016, 2, 8. [Google Scholar] [CrossRef]
- Martin, I.; Pidou, M.; Soares, A.; Judd, S.; Jefferson, B. Modelling the energy demands of aerobic and anaerobic membrane bioreactors for wastewater treatment. Environ. Technol. 2011, 32, 921–932. [Google Scholar] [CrossRef] [PubMed]
- Tartakovsky, B.; Mehta, P.; Bourque, J.S.; Guiot, S.R. Electrolysis-enhanced anaerobic digestion of wastewater. Bioresour. Technol. 2011, 102, 5685–5691. [Google Scholar] [CrossRef] [PubMed]
- Aslanzadeh, S.; Rajendran, K.; Jeihanipour, A.; Taherzadeh, M.J. The effect of effluent recirculation in a semi-continuous two-stage anaerobic digestion system. Energies 2013, 6, 2966–2981. [Google Scholar] [CrossRef]
- Bialek, K.; Cysneiros, D.; O’Flaherty, V. Hydrolysis, acidification and methanogenesis during low-temperature anaerobic digestion of dilute dairy wastewater in an inverted fluidised bioreactor. Appl. Microbiol. Biot. 2014, 98, 8737–8750. [Google Scholar] [CrossRef] [PubMed]
- Angenent, L.T.; Karim, K.; Al-Dahhan, M.H.; Wrenn, B.A.; Domíguez-Espinosa, R. Production of bioenergy and biochemicals from industrial and agricultural wastewater. Trends Biotechnol. 2004, 22, 477–485. [Google Scholar] [CrossRef] [PubMed]
- Ziganshina, E.E.; Belostotskiy, D.E.; Ilinskaya, O.N.; Boulygina, E.A.; Grigoryeva, T.V.; Ziganshin, A.M. Effect of the organic loading rate increase and the presence of zeolite on microbial community composition and process stability during anaerobic digestion of chicken wastes. Microbial. Ecol. 2015, 70, 948–960. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, Y.; Quan, X.; Chen, S. Effects of ferric iron on the anaerobic treatment and microbial biodiversity in a coupled microbial electrolysis cell (MEC)—Anaerobic reactor. Water Res. 2013, 47, 5719–5728. [Google Scholar] [CrossRef] [PubMed]
- Sleutels, T.H.J.A.; Ter Heijne, A.; Buisman, C.J.N.; Hamelers, H.V.M. Bioelectrochemical systems: An outlook for practical applications. ChemSusChem 2012, 5, 1012–1019. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Angelidaki, I. Microbial electrolysis cells turning to be versatile technology: Recent advances and future challenges. Water Res. 2014, 56, 11–25. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Abu-Reesh, I.M.; He, Z. Development of bioelectrochemical systems to promote sustainable agriculture. Agriculture 2015, 5, 367–388. [Google Scholar] [CrossRef]
- Moreno, R.; Escapa, A.; Cara, J.; Carracedo, B.; Gómez, X. A two-stage process for hydrogen production from cheese whey: Integration of dark fermentation and biocatalyzed electrolysis. Int. J. Hydrog. Energy 2015, 40, 168–175. [Google Scholar] [CrossRef]
- ElMekawy, A.; Srikanth, S.; Vanbroekhoven, K.; De Wever, H.; Pant, D. Bioelectro-catalytic valorization of dark fermentation effluents by acetate oxidizing bacteria in bioelectrochemical system (BES). J. Power Sources 2014, 262, 183–191. [Google Scholar] [CrossRef]
- Liu, H.; Leng, F.; Guan, Y.; Yao, Y.; Li, Y.; Xu, S. Simultaneous Pollutant Removal and Electricity Generation in a Combined ABR-MFC-MEC System Treating Fecal Wastewater. Water Air Soil. Pollut. 2017, 228, 179. [Google Scholar] [CrossRef]
- Feng, Y.; Zhang, Y.; Chen, S.; Quan, X. Enhanced production of methane from waste activated sludge by the combination of high-solid anaerobic digestion and microbial electrolysis cell with iron-graphite electrode. Chem. Eng. J. 2015, 259, 787–794. [Google Scholar] [CrossRef]
- Liu, W.; He, Z.; Yang, C.; Zhou, A.; Guo, Z.; Liang, B.; Varrone, C.; Wang, A.-J. Microbial network for waste activated sludge cascade utilization in an integrated system of microbial electrolysis and anaerobic fermentation. Biotechnol. Biofuels 2016, 9, 1. [Google Scholar] [CrossRef] [PubMed]
- De Vrieze, J.; Gildemyn, S.; Arends, J.B.A.; Vanwonterghem, I.; Verbeken, K.; Boon, N.; Verstraete, W.; Tyson, G.W.; Hennebel, T.; Rabaey, K. Biomass retention on electrodes rather than electrical current enhances stability in anaerobic digestion. Water Res. 2014, 54, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.; Ahn, Y.; Logan, B.E. A two-stage microbial fuel cell and anaerobic fluidized bed membrane bioreactor (MFC-AFMBR) system for effective domestic wastewater treatment. Environ. Sci. Technol. 2014, 48, 4199–4206. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Gao, X.; Jin, J.; Vidonish, J.; Zhu, L. A novel bioelectrode and anaerobic sludge coupled system for p-ClNB degradation by magnetite nanoparticles addition. Environ. Sci. Pollut. Res. 2017, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Moreno, R.; San-Martín, M.I.; Escapa, A.; Morán, A. Domestic wastewater treatment in parallel with methane production in a microbial electrolysis cell. Renew. Energy 2016, 93, 442–448. [Google Scholar] [CrossRef]
- Deng, L.; Chen, C.; Zheng, D.; Yang, H.; Liu, Y.; Chen, Z. Effect of temperature on continuous dry fermentation of swine manure. J. Environ. Manag. 2016, 177, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Escapa, A.; San-Martín, M.I.; Mateos, R.; Morán, A. Scaling-up of membraneless microbial electrolysis cells (MECs) for domestic wastewater treatment: Bottlenecks and limitations. Bioresour. Technol. 2015, 180, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Martínez, E.J.; Fierro, J.; Sánchez, M.E.; Gómez, X. Anaerobic co-digestion of FOG and sewage sludge: Study of the process by Fourier transform infrared spectroscopy. Int. Biodeterior. Biodegrad. 2012, 75, 1–6. [Google Scholar] [CrossRef]
- American Public Health Association (APHA); American Water Works Association (AWWA); Water Environmental Federation (WEF). Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 2005. [Google Scholar]
- Møller, H.B.; Sommer, S.G.; Ahring, B.K. Methane productivity of manure, straw and solid fractions of manure. Biomass Bioenergy 2004, 26, 485–495. [Google Scholar] [CrossRef]
- Siegert, I.; Banks, C. The effect of volatile fatty acid additions on the anaerobic digestion of cellulose and glucose in batch reactors. Process Biochem. 2015, 40, 3412–3418. [Google Scholar] [CrossRef]
- Zinder, S.H.; Koch, M. Non-aceticlastic methanogenesis from acetate: Acetate oxidation by a thermophilic syntrophic coculture. Arch. Microbiol. 1984, 138, 263–272. [Google Scholar] [CrossRef]
- Westerholm, M.; Müller, B.; Arthurson, V.; Schnürer, A. Changes in the acetogenic population in a mesophilic anaerobic digester in response to increasing ammonia concentration. Microbes Environ. 2011, 26, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Moestedt, J.; Müller, B.; Westerholm, M.; Schnürer, A. Ammonia threshold for inhibition of anaerobic digestion of thin stillage and the importance of organic loading rate. Microb. Biotechnol. 2016, 9, 180–194. [Google Scholar] [CrossRef] [PubMed]
- Rotaru, A.-E.; Shrestha, P.M.; Liu, F.; Shrestha, M.; Shrestha, D.; Embree, M.; Zengler, K.; Wardman, C.; Nevin, K.P.; Lovley, D.R. A new model for electron flow during anaerobic digestion: Direct interspecies electron transfer to Methanosaeta for the reduction of carbon dioxide to methane. Energy Environ. Sci. 2014, 7, 408–415. [Google Scholar] [CrossRef]
- Ma, J.; Carballa, M.; Van De Caveye, P.; Verstraete, W. Enhanced propionic acid degradation (EPAD) system: Proof of principle and feasibility. Water Res. 2009, 43, 3239–3248. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, Y.; Wang, J.; Meng, L. Effects of volatile fatty acid concentrations on methane yield and methanogenic bacteria. Biomass Bioenergy 2009, 33, 848–853. [Google Scholar] [CrossRef]
- Pullammanappallil, P.C.; Chynoweth, D.P.; Lyberatos, G.; Svoronos, S.A. Stable performance of anaerobic digestion in the presence of a high concentration of propionic acid. Bioresour. Technol. 2001, 78, 165–169. [Google Scholar] [CrossRef]
- Fierro, J.; Martinez, E.J.; Rosas, J.G.; Fernández, R.A.; López, R.; Gómez, X. Co-Digestion of swine manure and crude glycerine: Increasing glycerine ratio results in preferential degradation of labile compounds. Water Air Soil Pollut. 2016, 227, 78. [Google Scholar] [CrossRef]
- Fukuzaki, S.; Nishio, N.; Shobayashi, M.; Nagai, S. Inhibition of the fermentation of propionate to methane by hydrogen, acetate, and propionate. Appl. Environ. Microb. 1990, 56, 719–723. [Google Scholar]
- Cuetos, M.J.; Gómez, X.; Escapa, A.; Moran, A. Evaluation and simultaneous optimization of bio-hydrogen production using 32 factorial design and the desirability function. J. Power Sources 2007, 169, 131–139. [Google Scholar] [CrossRef]
- Fernández, C.; Carracedo, B.; Martínez, E.J.; Gómez, X.; Morán, A. Application of a packed bed reactor for the production of hydrogen from cheese whey permeate: Effect of organic loading rate. J. Environ. Sci. Heal. A 2014, 49, 210–217. [Google Scholar] [CrossRef] [PubMed]

| Parameter | Value |
|---|---|
| pH | 7.36 |
| Organic matter (%) | 1.12 ± 0.05 |
| Total nitrogen (%) | 0.21 ± 0.01 |
| C/N ratio | 3.15 |
| Total solids (g L−1) | 16.71 ± 0.80 |
| Volatile solids (g L−1) | 11.93 ± 0.59 |
| NH4+ (g L−1) | 1.13 ± 0.05 |
| Alcalinity (g-CaCO3 L−1) | 2.32 ± 0.10 |
| VFA (g L−1) | 0.5 ± 0.001 |
| Acetate (g L−1) | 0.030 ± 0.001 |
| PO43− (ppm) | 440 ± 13 |
| Ca2+ (ppm) | 576 ± 17 |
| Mg2+ (ppm) | 108 ± 3 |
| K+ (ppm) | 232 ± 7 |
| Na+ (ppm) | 48.4 ± 1.0 |
| Mn (ppm) | 3.4 ± 0.1 |
| Fe (ppm) | 263 ± 7 |
| Cu (ppm) | 3.4 ± 0.1 |
| Zn (ppm) | 17.1 ± 0.5 |
| Time (h) | Specific CH4 Production (L-CH4 kgVS−1 ) | VFA Production (mg L−1) | |||||
|---|---|---|---|---|---|---|---|
| Ratio 0.25 | Ratio 2.0 | Ratio 0.25 | Ratio 2.0 | ||||
| TVFA | Acetate | Butyrate | Propionate | TVFA | |||
| 0 | 0 ± 0 | 0 ± 0 | 37 | 36 | 6 | 0 | 42 |
| 15 | 105 ± 15 | 11 ± 3 | 225 | 491 | 1043 | 18 | 1560 |
| 23 | 153 ± 24 | 23 ± 2 | 170 | 820 | 2139 | 24 | 2988 |
| 40 | 234 ± 22 | 30 ± 10 | 240 | 1190 | 2372 | 173 | 3814 |
| 64 | 240 ± 15 | 34 ± 2 | 4 | 1496 | 2415 | 364 | 4513 |
| 88 | 245 ± 17 | 37 ± 14 | 3 | 1902 | 2942 | 356 | 5576 |
| 112 | 253 ± 15 | 39 ± 0 | - | - | - | - | - |
| 136 | 268 ± 15 | 43 ± 16 | 84 | 2300 | 3280 | 415 | 6458 |
| 160 | 283 ± 15 | 47 ± 16 | 6 | 2298 | 3325 | 413 | 6383 |
| 184 | 293 ± 25 | 48 ± 18 | n.d. | - | - | - | - |
| 208 | 299 ± 15 | 50 ± 15 | n.d. | - | - | - | - |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moreno, R.; Martínez, E.J.; Escapa, A.; Martínez, O.; Díez-Antolínez, R.; Gómez, X. Mitigation of Volatile Fatty Acid Build-Up by the Use of Soft Carbon Felt Electrodes: Evaluation of Anaerobic Digestion in Acidic Conditions. Fermentation 2018, 4, 2. https://doi.org/10.3390/fermentation4010002
Moreno R, Martínez EJ, Escapa A, Martínez O, Díez-Antolínez R, Gómez X. Mitigation of Volatile Fatty Acid Build-Up by the Use of Soft Carbon Felt Electrodes: Evaluation of Anaerobic Digestion in Acidic Conditions. Fermentation. 2018; 4(1):2. https://doi.org/10.3390/fermentation4010002
Chicago/Turabian StyleMoreno, Rubén, Elia J. Martínez, Adrián Escapa, Olegario Martínez, Rebeca Díez-Antolínez, and Xiomar Gómez. 2018. "Mitigation of Volatile Fatty Acid Build-Up by the Use of Soft Carbon Felt Electrodes: Evaluation of Anaerobic Digestion in Acidic Conditions" Fermentation 4, no. 1: 2. https://doi.org/10.3390/fermentation4010002
APA StyleMoreno, R., Martínez, E. J., Escapa, A., Martínez, O., Díez-Antolínez, R., & Gómez, X. (2018). Mitigation of Volatile Fatty Acid Build-Up by the Use of Soft Carbon Felt Electrodes: Evaluation of Anaerobic Digestion in Acidic Conditions. Fermentation, 4(1), 2. https://doi.org/10.3390/fermentation4010002