Characterizing the Phenotypic Responses of Escherichia coli to Multiple 4-Carbon Alcohols with Raman Spectroscopy
Abstract
:1. Introduction
2. Experimental Section
2.1. Bacteria Strain and Chemicals
2.2. Culture Conditions
2.3. Raman Spectroscopy and Data Analysis
3. Results
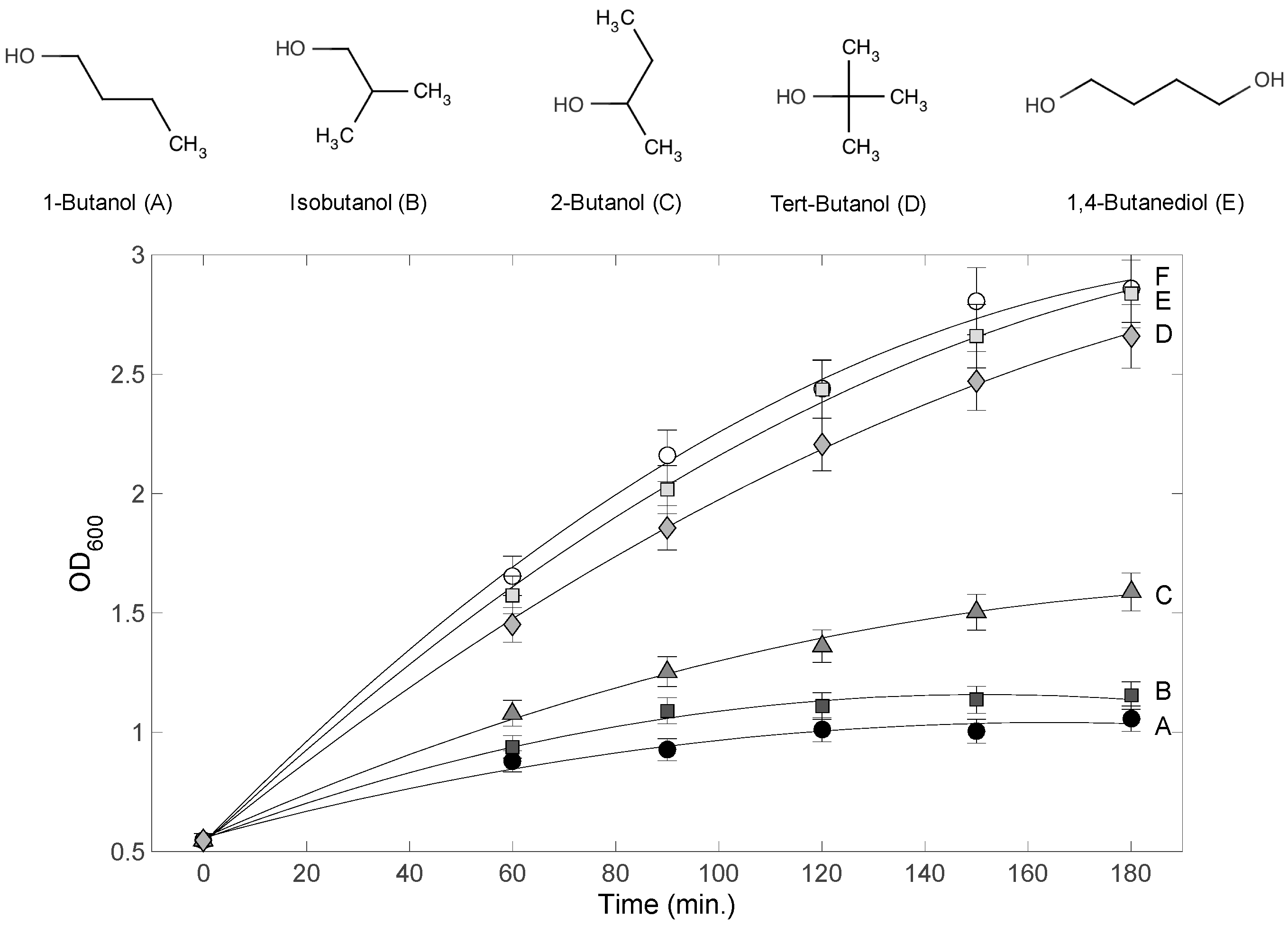
3.1. Variable Inhibitory Effect of 4-Carbon Alcohols on E. coli Cultures
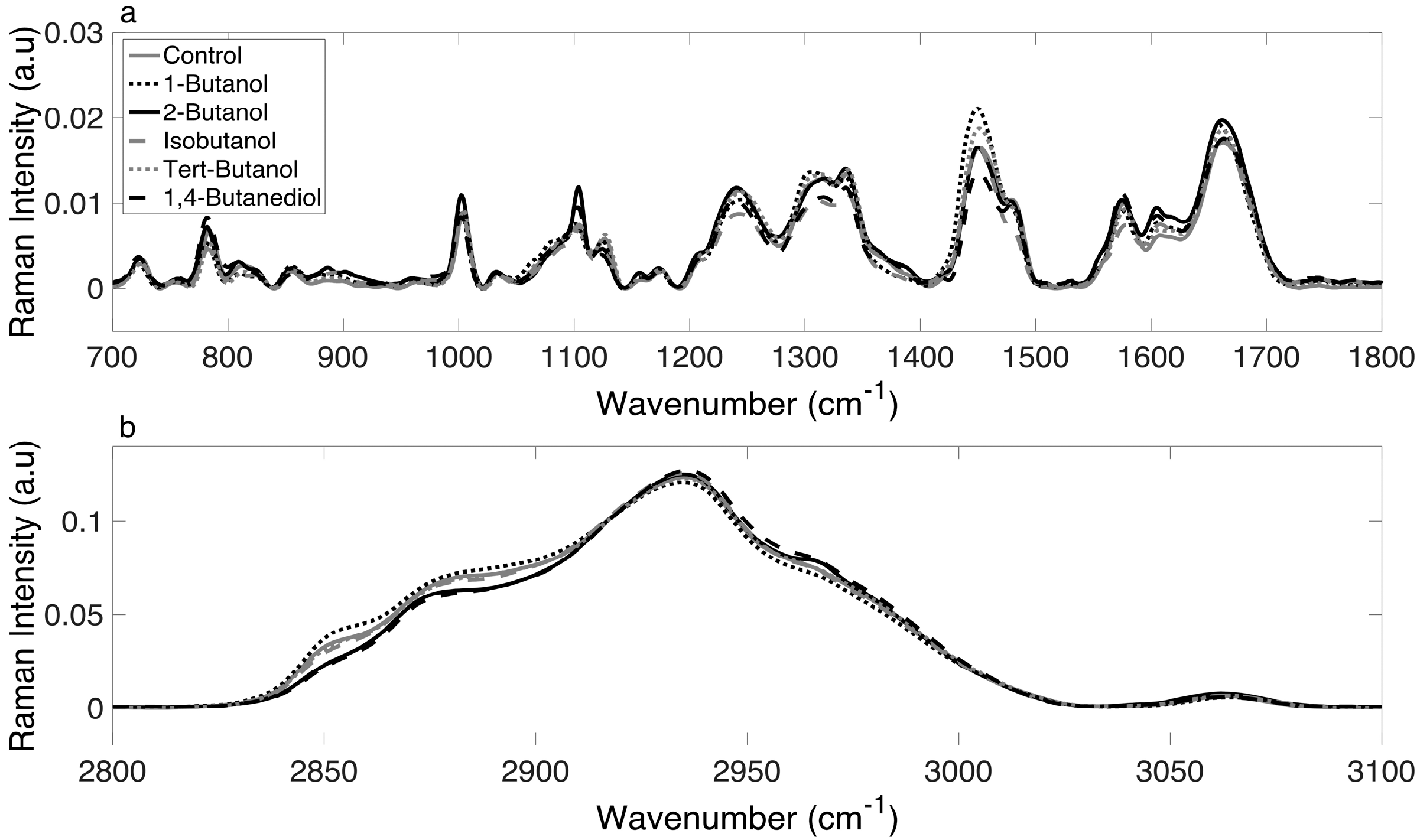
3.2. Raman Spectra of E. coli Cultures Are Consistent and Reliable
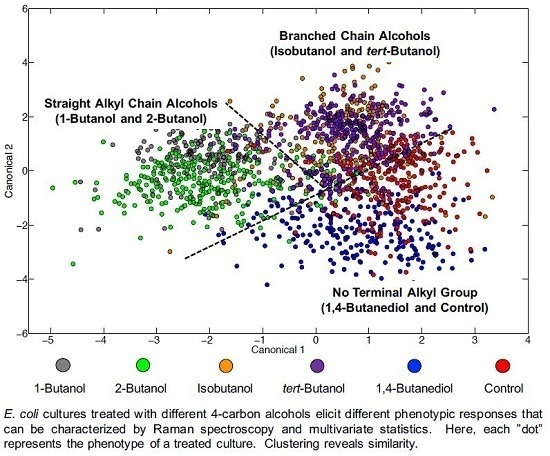
3.3. Chemometric Fingerprinting to Distinguish E. coli Phenotypes (by Alcohol)
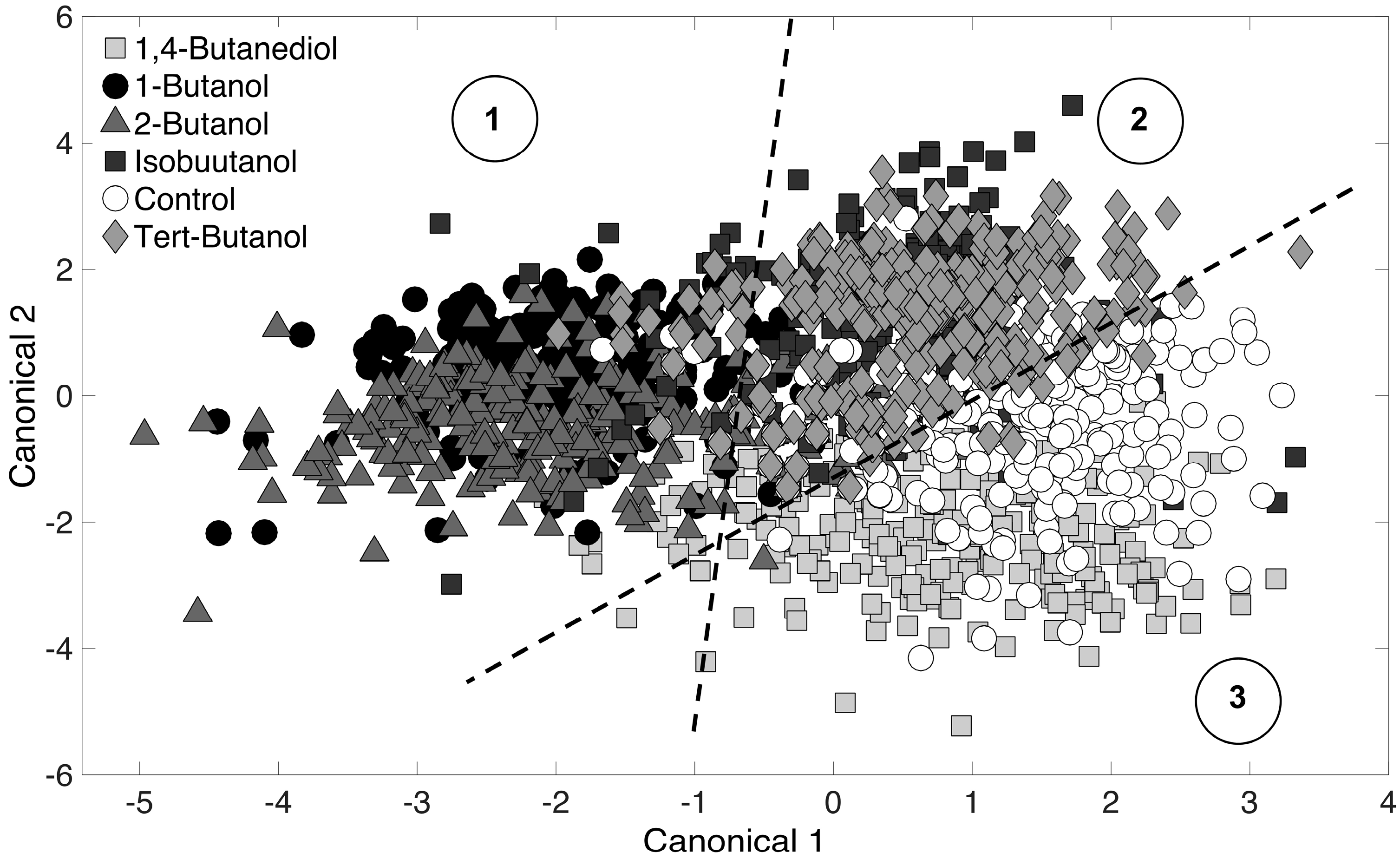
3.4. Chemometric Fingerprinting to Distinguish E. coli Phenotypes (by Time of Exposure)
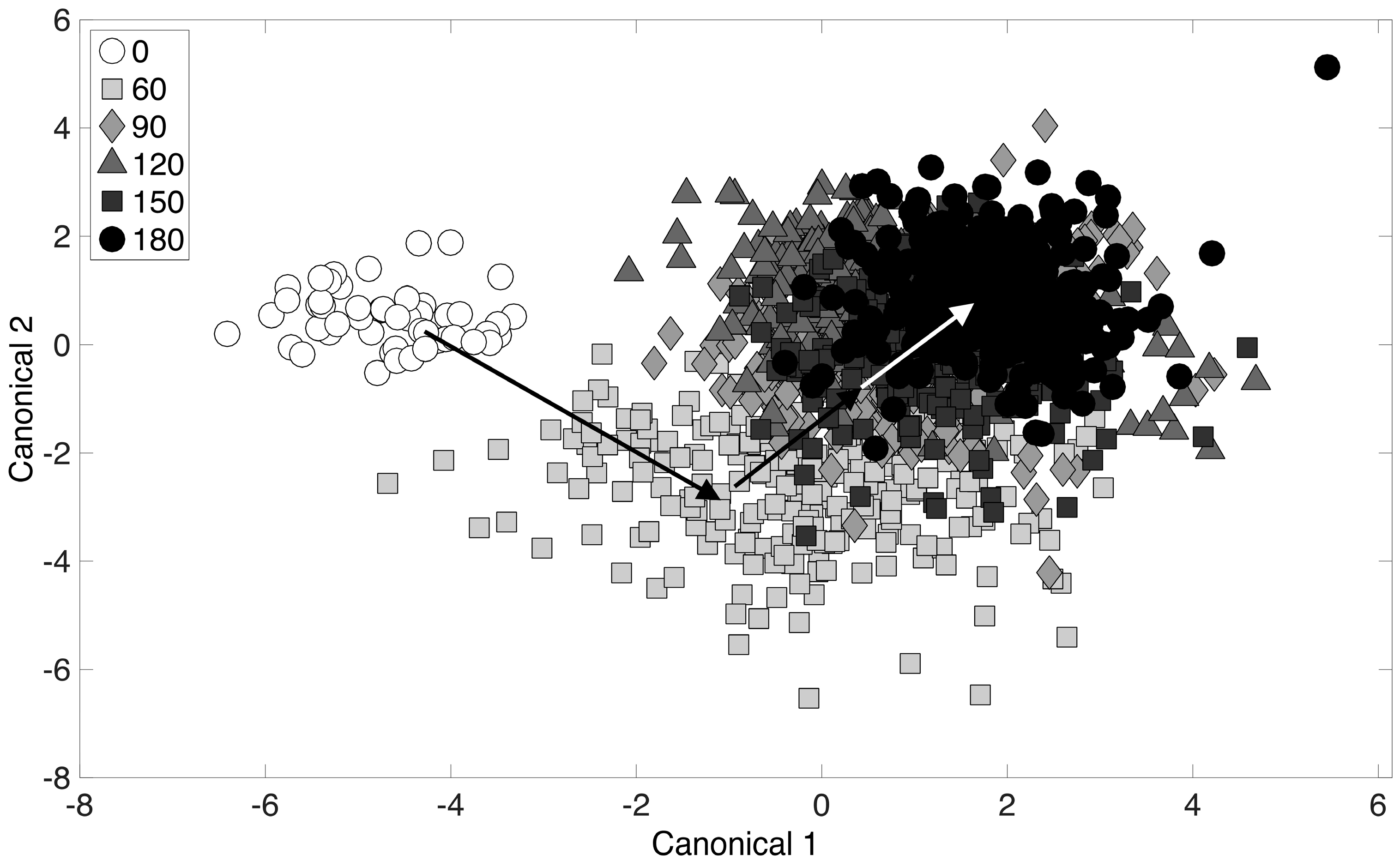
3.5. Chemometric Fingerprinting to Distinguish E. coli Phenotypes (by Alcohol and Time)

4. Discussion
4.1. Monitoring Phenotypic Changes in Near Real-Time
4.2. Effects of Terminal Alkyl Chain Length
4.3. Potential Uses
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Stephanopoulos, G. Challenges in engineering microbes for biofuels production. Science 2007, 315, 801–804. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, N.; Ezeji, T.C. Butanol,“a superior biofuel”production from agricultural residues (renewable biomass): Recent progress in technology. Biofuel Bioprod. Biorefin. 2008, 2, 319–330. [Google Scholar] [CrossRef]
- Dunlop, M.J.; Dossani, Z.Y.; Szmidt, H.L.; Chu, H.C.; Lee, T.S.; Keasling, J.D.; Hadi, M.Z.; Mukhopadhyay, A. Engineering microbial biofuel tolerance and export using efflux pumps. Mol. Syst. Biol. 2011, 7. [Google Scholar] [CrossRef] [PubMed]
- Brynildsen, M.P.; Liao, J.C. An integrated network approach identifies the isobutanol response network of Escherichia coli. Mol. Syst. Biol. 2009, 5. [Google Scholar] [CrossRef] [PubMed]
- Dunlop, M.J.; Keasling, J.D.; Mukhopadhyay, A. A model for improving microbial biofuel production using a synthetic feedback loop. Syst. Synth. Biol. 2010, 4, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Nicolaou, S.A.; Gaida, S.M.; Papoutsakis, E.T. A comparative view of metabolite and substrate stress and tolerance in microbial bioprocessing: From biofuels and chemicals, to biocatalysis and bioremediation. Metab. Eng. 2010, 12, 307–331. [Google Scholar] [CrossRef] [PubMed]
- Sinensky, M. Homeoviscous adaptation—A homeostatic process that regulates the viscosity of membrane lipids in Escherichia coli. Proc. Natl. Acad. Sci. USA 1974, 71, 522–525. [Google Scholar] [CrossRef] [PubMed]
- Reyes, L.H.; Abdelaal, A.S.; Kao, K.C. Genetic determinants for n-butanol tolerance in evolved escherichia coli mutants: Cross adaptation and antagonistic pleiotropy between n-butanol and other stressors. Appl. Environ. Microbiol. 2013, 79, 5313–5320. [Google Scholar] [CrossRef] [PubMed]
- Winkler, J.; Kao, K.C. Transcriptional analysis of lactobacillus brevis to n-butanol and ferulic acid stress responses. PLoS ONE 2011, 6. [Google Scholar] [CrossRef] [PubMed]
- Winkler, J.; Rehmann, M.; Kao, K.C. Novel Escherichia coli hybrids with enhanced butanol tolerance. Biotechnol. Lett. 2010, 32, 915–920. [Google Scholar] [CrossRef] [PubMed]
- Collas, F.; Kuit, W.; Clément, B.; Marchal, R.; López-Contreras, A.M.; Monot, F. Simultaneous production of isopropanol, butanol, ethanol and 2,3-butanediol by Clostridium acetobutylicum ATCC 824 engineered strains. AMB Express 2012, 2. [Google Scholar] [CrossRef] [PubMed]
- Ingram, L.O. Ethanol tolerance in bacteria. Crit. Rev. Biotechnol. 1989, 9, 305–319. [Google Scholar] [CrossRef]
- Huffer, S.; Clark, M.E.; Ning, J.C.; Blanch, H.W.; Clark, D.S. Role of alcohols in growth, lipid composition, and membrane fluidity of yeasts, bacteria, and archaea. Appl. Environ. Microbiol. 2011, 77, 6400–6408. [Google Scholar] [CrossRef] [PubMed]
- Ingram, L.O.; Buttke, T.M. Effects of alcohols on micro-organisms. Adv. Microb. Physiol. 1984, 25, 253–300. [Google Scholar] [PubMed]
- Ingram, L. Microbial tolerance to alcohols: Role of the cell membrane. Trends Biotechnol. 1986, 4, 40–44. [Google Scholar] [CrossRef]
- Kabelitz, N.; Santos, P.M.; Heipieper, H.J. Effect of aliphatic alcohols on growth and degree of saturation of membrane lipids in acinetobacter calcoaceticus. FEMS Microbiol. Lett. 2003, 220, 223–227. [Google Scholar] [CrossRef]
- Ramos, J.L.; Duque, E.; Gallegos, M.T.; Godoy, P.; Ramos-Gonzalez, M.I.; Rojas, A.; Teran, W.; Segura, A. Mechanisms of solvent tolerance in Gram-negative bacteria. Annu. Rev. Microbiol. 2002, 56, 743–768. [Google Scholar] [CrossRef] [PubMed]
- Ingram, L.O. Adaptation of membrane lipids to alcohols. J. Bacteriol. 1976, 125, 670–678. [Google Scholar] [PubMed]
- Rutherford, B.J.; Dahl, R.H.; Price, R.E.; Szmidt, H.L.; Benke, P.I.; Mukhopadhyay, A.; Keasling, J.D. Functional genomic study of exogenous n-butanol stress in Escherichia coli. Appl. Environ. Microbial. 2010, 76, 1935–1945. [Google Scholar] [CrossRef] [PubMed]
- Reyes, L.H.; Almario, M.P.; Kao, K.C. Genomic library screens for genes involved in n-butanol tolerance in Escherichia coli. PLoS ONE 2011, 6, e17678. [Google Scholar] [CrossRef] [PubMed]
- Minty, J.J.; Lesnefsky, A.A.; Lin, F.; Chen, Y.; Zaroff, T.A.; Veloso, A.B.; Xie, B.; McConnell, C.A.; Ward, R.J.; Schwartz, D.R.; et al. Evolution combined with genomic study elucidates genetic bases of isobutanol tolerance in Escherichia coli. Microb. Cell Fact. 2011, 10. [Google Scholar] [CrossRef] [PubMed]
- Zu, T.N.K.; Athamneh, A.I.M.; Wallace, R.S.; Collakova, E.; Senger, R.S. Near real-time analysis of the phenotypic responses of Escherichia coli to 1-butanol exposure using Raman spectroscopy. J. Bacteriol. 2014, 196, 3983–3991. [Google Scholar] [CrossRef] [PubMed]
- Kirschner, C.; Maquelin, K.; Pina, P.; Thi, N.N.; Choo-Smith, L.P.; Sockalingum, G.; Sandt, C.; Ami, D.; Orsini, F.; Doglia, S. Classification and identification of Enterococci: A comparative phenotypic, genotypic, and vibrational spectroscopic study. J. Clin. Microbiol. 2001, 39, 1763–1770. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.W.; Lieu, D.K.; Huser, T.; Li, R.A. Label-free separation of human embryonic stem cells and their cardiac derivatives using Raman spectroscopy. Anal. Chem. 2009, 81, 1324–1331. [Google Scholar] [CrossRef] [PubMed]
- Athamneh, A.I.; Alajlouni, R.A.; Wallace, R.S.; Seleem, M.N.; Senger, R.S. Phenotypic profiling of antibiotic response signatures in Escherichia coli using Raman spectroscopy. Antimicrob. Agents Chemother. 2014, 58, 1302–1314. [Google Scholar] [CrossRef] [PubMed]
- Ewanick, S.M.; Thompson, W.J.; Marquardt, B.J.; Bura, R. Real-time understanding of lignocellulosic bioethanol fermentation by Raman spectroscopy. Biotechnol. Biofuels 2013, 6. [Google Scholar] [CrossRef] [PubMed]
- Sivakesava, S.; Irudayaraj, J.; Ali, D. Simultaneous determination of multiple components in lactic acid fermentation using FT-MIR, NIR, and FT-Raman spectroscopic techniques. Process Biochem. 2001, 37, 371–378. [Google Scholar] [CrossRef]
- Lee, H.L.T.; Boccazzi, P.; Gorret, N.; Ram, R.J.; Sinskey, A.J. In situ bioprocess monitoring of Escherichia coli bioreactions using Raman spectroscopy. Vib. Spectrosc. 2004, 35, 131–137. [Google Scholar] [CrossRef]
- Milne, C.B.; Eddy, J.A.; Raju, R.; Ardekani, S.; Kim, P.J.; Senger, R.S.; Jin, Y.S.; Blaschek, H.P.; Price, N.D. Metabolic network reconstruction and genome-scale model of butanol-producing strain Clostridium beijerinckii NCIMB 8052. BMC Syst. Biol. 2011, 5. [Google Scholar] [CrossRef] [PubMed]
- Senger, R.S.; Papoutsakis, E.T. Genome-scale model for Clostridium acetobutylicum: Part I. Metabolic network resolution and analysis. Biotechnol. Bioeng. 2008, 101, 1036–1052. [Google Scholar] [CrossRef] [PubMed]
- Atsumi, S.; Hanai, T.; Liao, J.C. Non-fermentative pathways for synthesis of branched-chain higher alcohols as biofuels. Nature 2008, 451, 86–89. [Google Scholar] [CrossRef] [PubMed]
- Yim, H.; Haselbeck, R.; Niu, W.; Pujol-Baxley, C.; Burgard, A.; Boldt, J.; Khandurina, J.; Trawick, J.D.; Osterhout, R.E.; Stephen, R.; et al. Metabolic engineering of Escherichia coli for direct production of 1,4-butanediol. Nat. Chem. Biol. 2011, 7, 445–452. [Google Scholar] [CrossRef] [PubMed]
- Movasaghi, Z.; Rehman, S.; Rehman, I.U. Raman spectroscopy of biological tissues. Appl. Spectrosc. Rev. 2007, 42, 493–541. [Google Scholar] [CrossRef]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zu, T.N.K.; Athamneh, A.I.M.; Senger, R.S. Characterizing the Phenotypic Responses of Escherichia coli to Multiple 4-Carbon Alcohols with Raman Spectroscopy. Fermentation 2016, 2, 3. https://doi.org/10.3390/fermentation2010003
Zu TNK, Athamneh AIM, Senger RS. Characterizing the Phenotypic Responses of Escherichia coli to Multiple 4-Carbon Alcohols with Raman Spectroscopy. Fermentation. 2016; 2(1):3. https://doi.org/10.3390/fermentation2010003
Chicago/Turabian StyleZu, Theresah N. K., Ahmad I. M. Athamneh, and Ryan S. Senger. 2016. "Characterizing the Phenotypic Responses of Escherichia coli to Multiple 4-Carbon Alcohols with Raman Spectroscopy" Fermentation 2, no. 1: 3. https://doi.org/10.3390/fermentation2010003
APA StyleZu, T. N. K., Athamneh, A. I. M., & Senger, R. S. (2016). Characterizing the Phenotypic Responses of Escherichia coli to Multiple 4-Carbon Alcohols with Raman Spectroscopy. Fermentation, 2(1), 3. https://doi.org/10.3390/fermentation2010003