Continuous Fermentative Biohydrogen Production from Fruit-Vegetable Waste: A Parallel Approach to Assess Process Reproducibility
Abstract
1. Introduction
2. Materials and Methods
2.1. Biocatalyst
2.2. Substrate
2.3. Setup and Experimental Conditions
2.4. Analytical Methods
2.5. Data Treatment
3. Results and Discussion
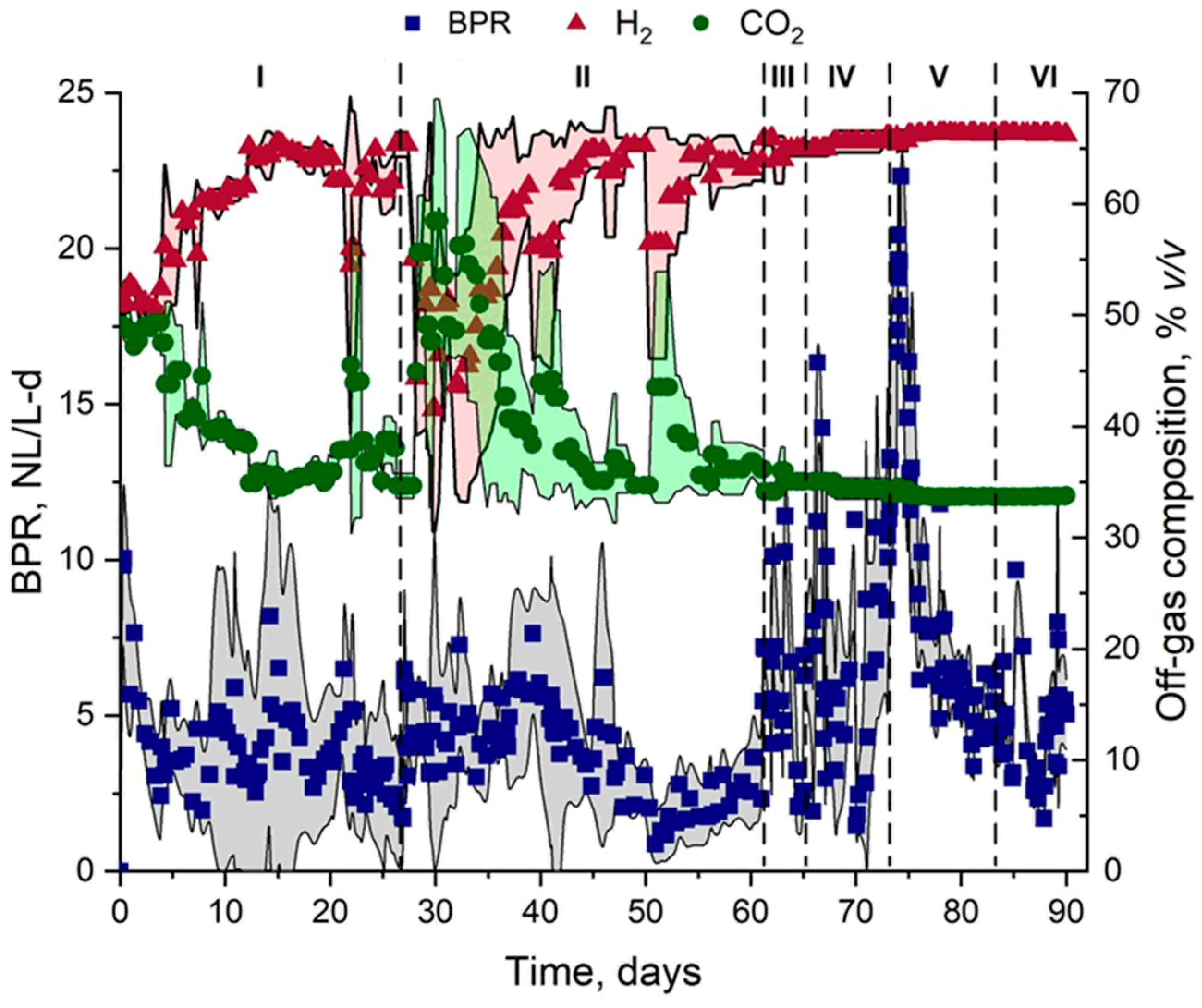
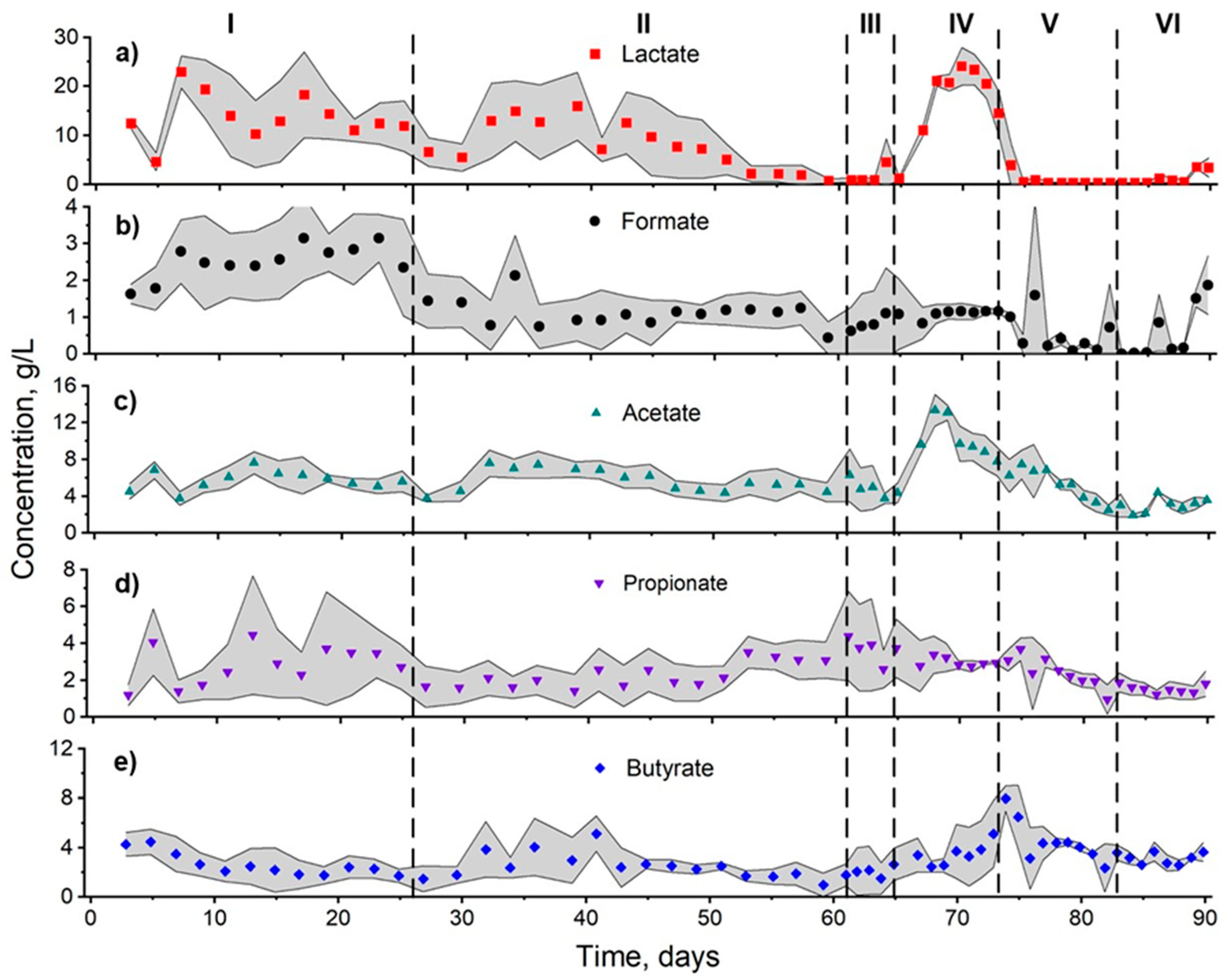
3.1. H2 Production Performance
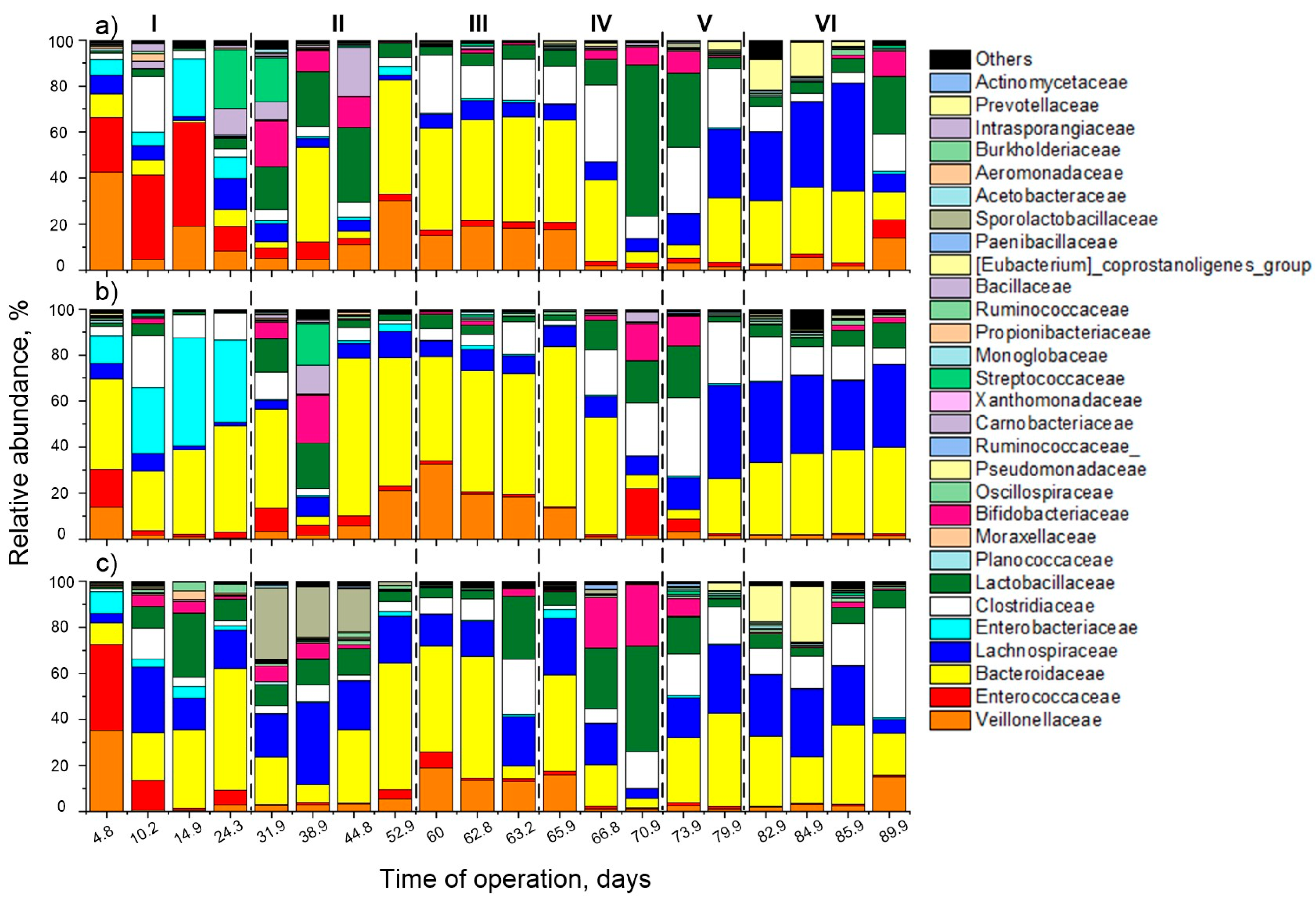
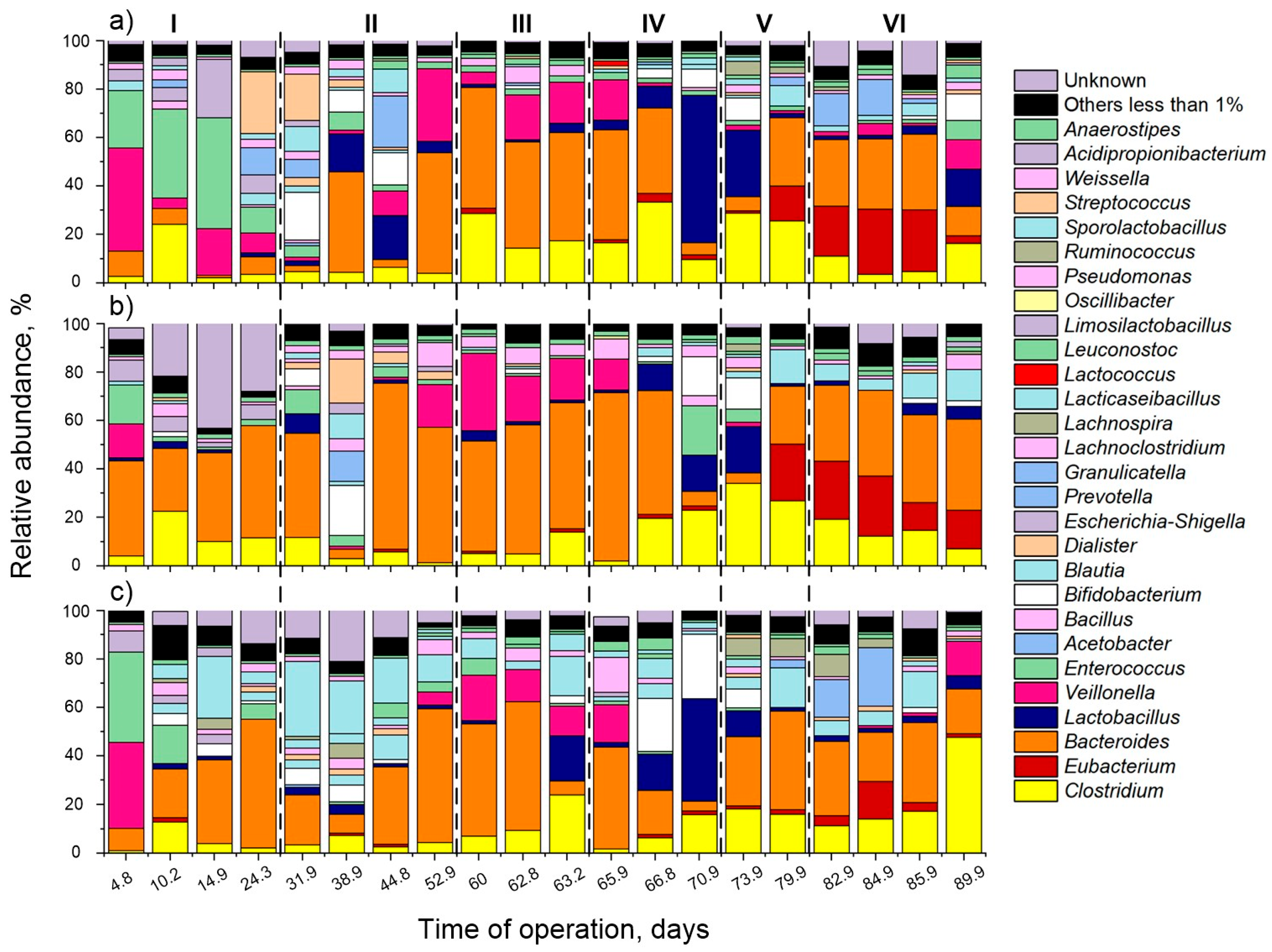
3.2. Microbial Ecology
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Food Waste. Available online: https://food.ec.europa.eu/food-safety/food-waste_en (accessed on 12 August 2025).
- Food Waste and Food Waste Prevention—Estimates. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Food_waste_and_food_waste_prevention_-_estimates (accessed on 12 August 2025).
- FAO. The State of Food and Agriculture 2023—Revealing the True Cost of Food to Transform Agrifood Systems; FAO: Rome, Italy, 2023. [Google Scholar]
- Moonsamy, T.A.; Rajauria, G.; Priyadarshini, A.; Jansen, M.A.K. Food waste: Analysis of the complex and variable composition of a promising feedstock for valorisation. Food Bioprod. Process. 2024, 148, 31–42. [Google Scholar] [CrossRef]
- Adamu, H.; Bello, U.; Yuguda, A.U.; Tafida, U.I.; Jalam, A.M.; Sabo, A.; Qamar, M. Production processes, techno-economic and policy challenges of bioenergy production from fruit and vegetable wastes. Renew. Sustain. Energy Rev. 2023, 186, 113686. [Google Scholar] [CrossRef]
- Taheri, S.; Hosseini, S.S. Waste not, want not: Comprehensive valorization of fruit and vegetable waste from single-product recovery to zero-waste strategies. Clean. Waste Syst. 2025, 11, 100300. [Google Scholar] [CrossRef]
- Zhu, Y.; Luan, Y.; Zhao, Y.; Liu, J.; Duan, Z.; Ruan, R. Current technologies and uses for fruit and vegetable wastes in a sustainable system: A review. Foods 2023, 12, 1949. [Google Scholar] [CrossRef]
- García-Depraect, O.; Vargas-Estrada, L.; Muñoz, R.; Castro-Muñoz, R. Membrane-assisted dark fermentation for integrated biohydrogen production and purification: A comprehensive review. Fermentation 2025, 11, 19. [Google Scholar] [CrossRef]
- Ghimire, A.; Frunzo, L.; Pirozzi, F.; Trably, E.; Escudie, R.; Lens, P.N.L.; Esposito, G. A review on dark fermentative biohydrogen production from organic biomass: Process parameters and use of by-products. Appl. Energy 2015, 144, 73–95. [Google Scholar] [CrossRef]
- Nascimento, T.R.; Cavalcante, W.A.; de Oliveira, G.H.D.; Zaiat, M.; Ribeiro, R. Modeling dark fermentation of cheese whey for H2 and n-butyrate production considering the chain elongation perspective. Bioresour. Technol. Rep. 2022, 17, 100940. [Google Scholar] [CrossRef]
- Bertasini, D.; Battista, F.; Mancini, R.; Frison, N.; Bolzonella, D. Hydrogen and methane production through two stage anaerobic digestion of straw residues. Environ. Res. 2024, 247, 118101. [Google Scholar] [CrossRef] [PubMed]
- Kora, E.; Patrinou, V.; Antonopoulou, G.; Ntaikou, I.; Tekerlekopoulou, A.G.; Lyberatos, G. Dark fermentation of expired fruit juices for biohydrogen production followed by treatment and biotechnological exploitation of effluents towards bioplastics and microbial lipids. Biochem. Eng. J. 2023, 195, 108901. [Google Scholar] [CrossRef]
- Chi, Z.; Zheng, Y.; Ma, J.; Chen, S. Oleaginous yeast Cryptococcus curvatus culture with dark fermentation hydrogen production effluent as feedstock for microbial lipid production. Int. J. Hydrogen Energy 2011, 36, 9542–9550. [Google Scholar] [CrossRef]
- Bettencourt, S.; Miranda, C.; Pozdniakova, T.A.; Sampaio, P.; Franco-Duarte, R.; Pais, C. Single cell oil production by oleaginous yeasts grown in synthetic and waste-derived volatile fatty acids. Microorganisms 2020, 8, 1809. [Google Scholar] [CrossRef]
- Zhao, W.; Zhang, J.; Hou, P.; Zhang, G.; Long, Z. Valorisation of food waste through self-fermentation and photosynthetic bacterial protein production: Efficiency, microbial dynamics and safety assessment. Bioresour. Technol. 2025, 436, 132982. [Google Scholar] [CrossRef] [PubMed]
- Lacroux, J.; Llamas, M.; Dauptain, K.; Avila, R.; Steyer, J.-P.; van Lis, R.; Trably, E. Dark fermentation and microalgae cultivation coupled systems: Outlook and challenges. Sci. Total Environ. 2023, 865, 161136. [Google Scholar] [CrossRef]
- Castello, E.; Ferraz-Junior, A.D.N.; Andreani, C.; Anzola-Rojas, M.P.; Borzacconi, L.; Buitron, G.; Carrillo-Reyes, J.; Gomes, S.D.; Maintinguer, S.I.; Moreno-Andrade, I.; et al. Stability problems in the hydrogen production by dark fermentation: Possible causes and solutions. Renew. Sustain. Energy Rev. 2020, 119, 109602. [Google Scholar] [CrossRef]
- Martínez-Mendoza, L.J.; Lebrero, R.; Muñoz, R.; García-Depraect, O. Influence of key operational parameters on biohydrogen production from fruit and vegetable waste via lactate-driven dark fermentation. Bioresour. Technol. 2022, 364, 128070. [Google Scholar] [CrossRef]
- Martínez-Mendoza, L.J.; García-Depraect, O.; Muñoz, R. Unlocking the high-rate continuous performance of fermentative hydrogen bioproduction from fruit and vegetable residues by modulating hydraulic retention time. Bioresour. Technol. 2023, 373, 128716. [Google Scholar] [CrossRef]
- Rodríguez-Valderrama, S.; Escamilla-Alvarado, C.; Magnin, J.-P.; Rivas-García, P.; Valdez-Vazquez, I.; Ríos-Leal, E. Batch biohydrogen production from dilute acid hydrolyzates of fruits-and-vegetables wastes and corn stover as co-substrates. Biomass Bioenergy 2020, 140, 105666. [Google Scholar] [CrossRef]
- Scotto di Perta, E.; Cesaro, A.; Pindozzi, S.; Frunzo, L.; Esposito, G.; Papirio, S. Assessment of hydrogen and volatile fatty acid production from fruit and vegetable waste: A case study of Mediterranean markets. Energies 2022, 15, 5032. [Google Scholar] [CrossRef]
- Rodríguez-Valderrama, S.; Escamilla-Alvarado, C.; Rivas-García, P.; Magnin, J.-P.; Alcalá-Rodríguez, M.; García-Reyes, R.B. Biorefinery concept comprising acid hydrolysis, dark fermentation, and anaerobic digestion for co-processing of fruit and vegetable wastes and corn stover. Environ. Sci. Pollut. Res. 2020, 27, 28585–28596. [Google Scholar] [CrossRef]
- Andolfi, A.; Bianco, F.; Sannino, M.; Faugno, S.; Race, M. Dark-fermentative biohydrogen production from vegetable residue using wine lees as novel inoculum. Bioresour. Technol. 2025, 429, 132495. [Google Scholar] [CrossRef] [PubMed]
- García-Depraect, O.; Castro-Muñoz, R.; Muñoz, R.; Rene, E.R.; León-Becerril, E.; Valdez-Vazquez, I.; Kumar, G.; Reyes-Alvarado, L.C.; Martínez-Mendoza, L.J.; Carrillo-Reyes, J.; et al. A review on the factors influencing biohydrogen production from lactate: The key to unlocking enhanced dark fermentative processes. Bioresour. Technol. 2021, 324, 124595. [Google Scholar] [CrossRef]
- Chezeau, B.; Fontaine, J.P.; Vial, C. Analysis of liquid-to-gas mass transfer, mixing and hydrogen production in dark fermentation process. Chem. Eng. J. 2019, 372, 715–727. [Google Scholar] [CrossRef]
- Palomo-Briones, R.; Celis, L.B.; Méndez-Acosta, H.O.; Bernet, N.; Trably, E.; Razo-Flores, E. Enhancement of mass transfer conditions to increase the productivity and efficiency of dark fermentation in continuous reactors. Fuel 2019, 254, 115648. [Google Scholar] [CrossRef]
- García-Depraect, O.; Diaz-Cruces, V.F.; Rene, E.R.; León-Becerril, E. Changes in performance and bacterial communities in a continuous biohydrogen-producing reactor subjected to substrate- and pH-induced perturbations. Bioresour. Technol. 2020, 295, 122182. [Google Scholar] [CrossRef]
- Mlinar, S.; Weig, A.R.; Freitag, R. Influence of mixing and sludge volume on stability, reproducibility, and productivity of laboratory-scale anaerobic digestion. Bioresour. Technol. Rep. 2020, 11, 100444. [Google Scholar] [CrossRef]
- Hafner, S.D.; Fruteau de Laclos, H.; Koch, K.; Holliger, C. Improving inter-laboratory reproducibility in measurement of biochemical methane potential (BMP). Water 2020, 12, 1752. [Google Scholar] [CrossRef]
- Lin, Q.; Li, L.; De Vrieze, J.; Li, C.; Fang, X.; Li, X. Functional conservation of microbial communities determines composition predictability in anaerobic digestion. ISME J. 2023, 17, 1920–1930. [Google Scholar] [CrossRef]
- Marín, D.; Méndez, L.; Suero, I.; Díaz, I.; Blanco, S.; Fdz-Polanco, M.; Muñoz, R. Anaerobic digestion of food waste coupled with biogas upgrading in an outdoors algal-bacterial photobioreactor at pilot scale. Fuel 2022, 324, 124554. [Google Scholar] [CrossRef]
- Regueira-Marcos, L.; García-Depraect, O.; Muñoz, R. Continuous two-stage lactate-driven dark fermentation process for enhanced biohydrogen production from food waste. J. Water Process Eng. 2024, 67, 106116. [Google Scholar] [CrossRef]
- Regueira-Marcos, L.; Muñoz, R.; García-Depraect, O. Continuous lactate-driven dark fermentation of restaurant food waste: Process characterization and new insights on transient feast/famine perturbations. Bioresour. Technol. 2023, 385, 129385. [Google Scholar] [CrossRef]
- Leroy-Freitas, D.; Muñoz, R.; Martínez-Mendoza, L.J.; Martínez-Fraile, C.; García-Depraect, O. Enhancing biohydrogen production: The role of iron-based nanoparticles in continuous lactate-driven dark fermentation of powdered cheese whey. Fermentation 2024, 10, 296. [Google Scholar] [CrossRef]
- Water Environmental Federation. American Public Health Association. Standard Methods for the Examination of Water and Wastewater, 21st ed.; APHA: Washington, DC, USA, 2005. [Google Scholar]
- Farveen, M.S.; Muñoz, R.; Narayanan, R.; García-Depraect, O. Batch and semi-batch anaerobic digestion of poly (3-hydroxybutyrate-co-3-hydroxyhexanoate) (PHBH) bioplastic: New kinetic, structural, microbiological and digestate phytotoxicity insights. Sci. Total Environ. 2025, 967, 178794. [Google Scholar] [CrossRef]
- Magoč, T.; Salzberg, S.L. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef]
- Edgar, R.C.; Haas, B.J.; Clemente, J.C.; Quince, C.; Knight, R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 2011, 27, 2194–2200. [Google Scholar] [CrossRef]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2012, 41, D590–D596. [Google Scholar] [CrossRef]
- Edgar, R.C. MUSCLE: A multiple sequence alignment method with reduced time and space complexity. BMC Bioinform. 2004, 5, 113. [Google Scholar] [CrossRef]
- Roslan, E.; Mohamed, H.; Abu Hassan, S.H.; Carrere, H.; Trably, E. Effect of exogenous inoculation on dark fermentation of food waste priorly stored in lactic acid fermentation. Recycling 2025, 10, 11. [Google Scholar] [CrossRef]
- Tian, W.; Khan, E.; Tsang, D.C. Strategy to improve anaerobic fermentation performance of lactate-rich wastewater by combining biochar augmentation and acetate supplementation. Chem. Eng. J. 2025, 506, 159782. [Google Scholar] [CrossRef]
- Luo, L.; Lim, R.; Pradhan, N. Lactic acid-based fermentative hydrogen production from kitchen waste: Mechanisms and taxonomic insights. Chem. Eng. J. 2024, 488, 150854. [Google Scholar] [CrossRef]
- Martínez-Fraile, C.; Muñoz, R.; Simorte, M.T.; Sanz, I.; García-Depraect, O. Biohydrogen production by lactate-driven dark fermentation of real organic wastes derived from solid waste treatment plants. Bioresour. Technol. 2024, 403, 130846. [Google Scholar] [CrossRef]
- Fuess, L.T.; Ferraz Júnior, A.D.N.; Machado, C.B.; Zaiat, M. Temporal dynamics and metabolic correlation between lactate-producing and hydrogen-producing bacteria in sugarcane vinasse dark fermentation: The key role of lactate. Bioresour. Technol. 2018, 247, 426–433. [Google Scholar] [CrossRef]
- Sim, Y.-B.; Kim, D.-Y.; Ko, J.; Jung, J.-H.; Kim, S.-H. Bioaugmentation with Clostridium pasteurianum for high-yield continuous bio-hydrogen production in a dynamic membrane bioreactor. Chem. Eng. J. 2024, 497, 154709. [Google Scholar] [CrossRef]
- Okonkwo, O.; Escudie, R.; Bernet, N.; Mangayil, R.; Lakaniemi, A.-M.; Trably, E. Bioaugmentation enhances dark fermentative hydrogen production in cultures exposed to short-term temperature fluctuations. Appl. Microbiol. Biotechnol. 2020, 104, 439–449. [Google Scholar] [CrossRef]
- Kumar, G.; Bakonyi, P.; Kobayashi, T.; Xu, K.-Q.; Sivagurunathan, P.; Kim, S.-H.; Buitrón, G.; Nemestóthy, N.; Bélafi-Bakó, K. Enhancement of biofuel production via microbial augmentation: The case of dark fermentative hydrogen. Renew. Sustain. Energy Rev. 2016, 57, 879–891. [Google Scholar] [CrossRef]
- Detman, A.; Laubitz, D.; Chojnacka, A.; Kiela, P.R.; Salamon, A.; Barberán, A.; Chen, Y.; Yang, F.; Błaszczyk, M.K.; Sikora, A. Dynamics of dark fermentation microbial communities in the light of lactate and butyrate production. Microbiome 2021, 9, 158. [Google Scholar] [CrossRef]
- Etchebehere, C.; Castelló, E.; Wenzel, J.; Anzola-Rojas, M.P.; Borzacconi, L.; Buitrón, G.; Cabrol, L.; Carminato, V.M.; Carrillo-Reyes, J.; Cisneros-Pérez, C.; et al. Microbial communities from 20 different hydrogen-producing reactors studied by 454 pyrosequencing. Appl. Microbiol. Biotechnol. 2016, 100, 3371–3384. [Google Scholar] [CrossRef]
- Regueira-Marcos, L.; Muñoz, R.; García-Depraect, O. Biogenic hydrogen production from household food waste via lactate-driven dark fermentation: A comparative study of single-stage and two-stage configurations. J. Environ. Chem. Eng. 2025, 13, 117672. [Google Scholar] [CrossRef]
- Sasaki, K.; Sasaki, D.; Tsuge, Y.; Morita, M.; Kondo, A. Changes in the microbial consortium during dark hydrogen fermentation in a bioelectrochemical system increases methane production during a two-stage process. Biotechnol. Biofuels 2018, 11, 173. [Google Scholar] [CrossRef]
- Lu, Z.; Kong, L.; Ren, S.; Aschenbach, J.R.; Shen, H. Acid tolerance of lactate-utilizing bacteria of the order Bacteroidales contributes to prevention of ruminal acidosis in goats adapted to a high-concentrate diet. Anim. Nutr. 2023, 14, 130–140. [Google Scholar] [CrossRef] [PubMed]
- Mutuyemungu, E.; Singh, M.; Liu, S.; Rose, D.J. Intestinal gas production by the gut microbiota: A review. J. Funct. Foods 2023, 100, 105367. [Google Scholar] [CrossRef]
- Valdez-Vazquez, I.; Pérez-Rangel, M.; Tapia, A.; Buitrón, G.; Molina, C.; Hernández, G.; Amaya-Delgado, L. Hydrogen and butanol production from native wheat straw by synthetic microbial consortia integrated by species of Enterococcus and Clostridium. Fuel 2015, 159, 214–222. [Google Scholar] [CrossRef]
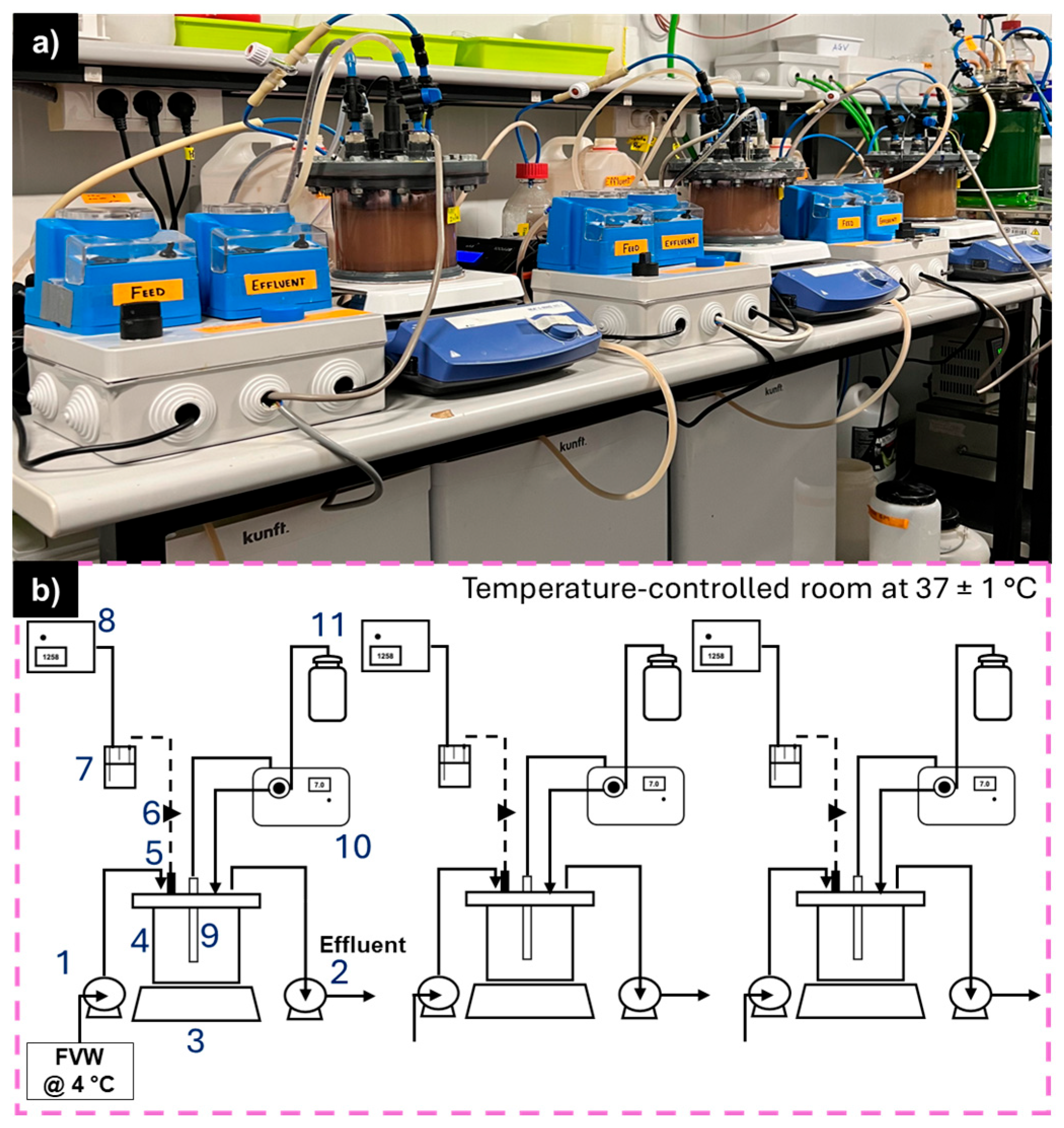



| Operational Stage | ||||||
|---|---|---|---|---|---|---|
| I | II | III | IV | V | VI | |
| Time (days) | 0–25.8 | 25.8–61.0 | 61.0–65.0 | 65.0–73.0 | 73.0–83.0 | 83.0–90.0 |
| Number of cycles (HRTs) | 17.4/25.6 | 27.0–67.8 | 10.7 | 21.3 | 26.7 | 18.7 |
| pH | 7 | 6.5 | 6.5 | 6.5 | 6.5 | 6.5 |
| HRT (h) | 18/12 | 9 | 9 | 9 | 9 | 9 |
| TS concentration (%) | 5 | 5/3 a | 3 | 3 | 3 | 3 |
| OLR (g VS/L-d) | 62.4/93.6 | 124.8/74.4 | 74.4 | 74.4 | 74.4 | 74.4 |
| Bioaugmentation | No | No | Yes | Yes | No | No |
| Nutrient supplementation | No | No | No | Yes | Yes | No |
| Parameter | Reactor | Stage | |||||
|---|---|---|---|---|---|---|---|
| I | II | III | IV | V | VI | ||
| BPR (NL/L-d) | 1 | 1.7 ± 1.4 1.1 ± 0.6 A (60.8/54.5) | 3.4 ± 1.9 A (55.9) | 7.8 ± 4.3 A (55.1) | 7.1 ± 3.2 A (45.1) | 8.9 ± 6.5 A (73.0) | 3.6 ± 1.7 A (47.2) |
| 2 | 7.1 ± 4.2 9.4 ± 4.1 B (59.1/43.6) | 7.2 ± 6.7 B (93.0) | 6.1 ± 2.6 A (42.6) | 5.6 ± 4.4 A (78.6) | 11.2 ± 6.2 A (55.3) | 5.5 ± 3.1 B (56.3) | |
| 3 | 2.3 ± 1.3 3.3 ± 3.4 A (56.5/1) | 2.8 ± 3.3 A (117.8) | 4.8 ± 3.3 A (68.7) | 7.4 ± 6.2 A (83.7) | 10.6 ± 6.2 A (58.5) | 4.9 ± 1.7 B (34.6) | |
| H2 content (% v/v) | 1 | 60.5 ± 2.8 62.7 ± 1.5 A (4.6/2.4) | 59.4 ± 5.5 A (9.2) | 65.3 ± 0.8 A (1.2) | 65.6 ± 0.5 A (0.8) | 66.1 ± 0.7 A (1.1) | 66.3 ± 0.1 A (0.2) |
| 2 | 59.6 ± 4.8 63.3 ± 1.2 A (8.0/1.9) | 60.7 ± 6.8 A (11.2) | 64.9 ± 1.9 A (2.9) | 66.0 ± 0.4 A (0.6) | 66.3 ± 0.1 A (0.2) | 66.3 ± 0.1 A (0.2) | |
| 3 | 55.7 ± 15.9 60.1 ± 8.1 B (28.5/13.4) | 51.6 ± 12.2 B (23.6) | 64.8 ± 0.8 A (1.2) | 64.5 ± 0.3 A (0.5) | 66.1 ± 0.3 A (0.5) | 66.3 ± 0.1 A (0.2) | |
| HPR (NLH2/L-d) | 1 | 1.0 ± 0.6 1.0 ± 0.3 A (60/30) | 1.8 ± 0.9 A (50) | 5.7 ± 2.9 A (50.1) | 4.4 ± 1.8 A (40.9) | 6.0 ± 3.2 A (53.3) | 2.5 ± 1.1 A (44) |
| 2 | 4.5 ± 2.4 4.2 ± 1.3 B (53.3/30.9) | 3.6 ± 1.9 B (52.7) | 3.9 ± 1.8 B (46.1) | 4.5 ± 2.0 A (44.4) | 7.4 ± 3.5 A (47.2) | 3.6 ± 2.0 A (55.5) | |
| 3 | 1.2 ± 0.8 1.9 ± 1.9 A (66.6/100) | 1.3 ± 0.9 A (69.2) | 3.3 ± 2.0 B (60.6) | 5.1 ± 4.1 A (80.3) | 6.7 ± 4.1 A (61.2) | 3.3 ± 1.2 A (36.3) | |
| HY (NmL H2/g VS added) | 1 | 19.9 ± 4.7 12.6 ± 5.3 A (23.6/42.1) | 24.0 ± 13.7 A (57.1) | 71.2 ± 39.5 A (55.4 | 66.4 ± 26.9 A (40.5) | 77.1 ± 41.1 A (53.3) | 34.9 ± 16.1 A (46.1) |
| 2 | 113.5 ± 88.1 76.6 ± 30.6 B (77.6/39.9) | 42.8 ± 24.2 B (56.5) | 55.7 ± 24.1 A (43.2) | 62.0 ± 31.0 A (50) | 101.7 ± 44.3 A (43.5) | 52.6 ± 26.5 B (50.3) | |
| 3 | 33.1 ± 9.1 22.3 ± 11.1 A (27.4/49.7) | 15.4 ± 11.6 A (75.3) | 43.5 ± 30.4 A (69.8) | 71.2 ± 57.5 A (80.7) | 95.2 ± 51.9 A (54.5) | 47.2 ± 17.1 B (36.2) | |
| Carbohydrates removal (%) | 1 | 84.2 ± 3.0 85.0 ± 0.8 A (3.6/0.9) | 85.1 ± 1.3 A (1.5) | 86.7 ± 0.4 A (0.5) | 87.8 ± 0.8 A (0.9) | 92.5 ± 1.9 A (2.0) | 94.8 ± 0.6 A (0.6) |
| 2 | 82.6 ± 2.2 83.8 ± 1.4 A (2.7/1.7) | 86.4 ± 1.7 A (1.9) | 89.6 ± 0.6 A (0.7) | 89.2 ± 1.0 A (1.1) | 92.4 ± 1.6 A (1.7) | 95.4 ± 1.8 A (1.9) | |
| 3 | 84.5 ± 3.2 84.5 ± 1.7 A (3.8/2.0) | 85.8 ± 2.3 A (2.6) | 87.7 ± 1.4 A (1.6) | 89.4 ± 1.4 A (1.6) | 91.7 ± 1.2 A (1.3) | 95.6 ± 1.3 A (1.4) | |
| Stage | Reactor | Lactate (g/L) | Formate (g/L) | Acetate (g/L) | Propionate (g/L) | Butyrate (g/L) |
|---|---|---|---|---|---|---|
| I | 1 | 15.5 ± 7.6 A (49.0) | 2.9 ± 0.6 A (20.7) | 5.0 ± 1.0 A (20.0) | 4.1 ± 1.9 A (46.3) | 1.9 ± 0.7 A (36.8) |
| 2 | 9.4 ± 5.8 A (61.7) | 3.1 ± 0.8 A (25.8) | 5.9 ± 1.3 A (22.0) | 1.2 ± 0.5 B (41.7) | 3.5 ± 1.0 B (28.6) | |
| 3 | 16.5 ± 5.2 A (31.5) | 1.8 ± 0.6 A (33.3) | 6.6 ± 1.3 A (19.7) | 3.5 ± 1.4 B (40.0) | 2.2 ± 1.2 B (54.5) | |
| II | 1 | 11.1 ± 8.0 A (72.1) | 1.2 ± 0.3 AB (25.0) | 5.3 ± 1.5 A (28.3) | 2.4 ± 1.7 A (70.8) | 2.3 ± 1.4 A (60.9) |
| 2 | 5.8 ± 6.1 B (105.2) | 1.3 ± 0.7 A (53.8) | 5.3 ± 1.6 A (30.2) | 2.8 ± 0.9 A (32.1) | 2.6 ± 0.9 B (34.6) | |
| 3 | 5.4 ± 3.5 B (64.8) | 0.7 ± 0.7 B (100) | 6.8 ± 1.1 B (16.2) | 2.1 ± 0.8 A (38.1) | 2.9 ± 1.8 AB (62.1) | |
| III | 1 | 1.7 ± 0.9 A (52.9) | 2.0 ± 0.3 A (15) | 5.2 ± 2.2 A (42.3) | 5.2 ± 1.5 A (28.9) | 3.5 ± 0.6 B (17.1) |
| 2 | 0.8 ± 0.5 A (62.5) | 0.0 ± 0.0 B (0) | 3.2 ± 0.2 A (6.3) | 1.9 ± 0.2 B (10.5) | 1.0 ± 0.2 B (20.0) | |
| 3 | 4.0 ± 5.2 A (130) | 1.0 ± 0.5 C (50) | 4.7 ± 0.6 A (12.8) | 3.2 ± 0.8 AB (25.0) | 2.1 ± 1.5 B (71.4) | |
| IV | 1 | 19.0 ± 5.2 A (27.4) | 1.1 ± 0.1 A (9.1) | 10.3 + 1.8 A (17.5) | 3.0 ± 0.4 A (13.3) | 3.1 ± 1.2 AB (38.7) |
| 2 | 21.3 ± 5.3 A (24.9) | 1.1 ± 0.3 A (27.3) | 11.4 ± 2.3 A (20.2) | 3.0 ± 1.1 A (36.7) | 2.3 ± 0.5 A (21.7) | |
| 3 | 17.7 ± 5.0 A (28.2) | 1.1 ± 0.2 A (18.2) | 9.0 ± 2.7 A (30.0) | 2.9 ± 0.3 A (20.7) | 5.0 ± 2.2 B (44.0) | |
| V | 1 | 0.3 ± 0.0 A (0) | 0.2 ± 0.2 A (100) | 4.5 ± 2.0 A (44.4) | 2.5 ± 1.2 A (48.0) | 4.0 ± 0.5 A (12.5) |
| 2 | 0.5 ± 0.4 A (80) | 0.2 ± 0.2 A (100) | 5.9 ± 2.2 A (37.3) | 2.3 ± 0.5 A (21.7) | 4.9 ± 1.9 A (38.8) | |
| 3 | 0.3 ± 0.1 A (33.3) | 0.9 ± 1.5 A (166.7) | 4.3 ± 2.0 A (46.5) | 2.1 ± 1.2 A (57.1) | 3.2 ± 1.7 A (53.1) | |
| VI | 1 | 1.9 ± 2.2 A (115.8) | 0.9 ± 0.8 A (88.9) | 2.9 ± 0.9 A (31.0) | 1.1 ± 0.2 A (18.2) | 2.9 ± 0.6 A (20.6) |
| 2 | 1.2 ± 1.2 A (100) | 0.6 ± 0.6 A (100) | 3.2 ± 0.9 A (28.1) | 1.4 ± 0.1 A (9.1) | 3.6 ± 0.7 A (19.4) | |
| 3 | 1.7 ± 1.2 A (70.6) | 0.8 ± 1.1 A (137.5) | 3.5 ± 0.7 A (20.0) | 1.9 ± 0.4 B (21.1) | 2.8 ± 0.4 A (14.3) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-Mendoza, L.J.; Muñoz, R.; García-Depraect, O. Continuous Fermentative Biohydrogen Production from Fruit-Vegetable Waste: A Parallel Approach to Assess Process Reproducibility. Fermentation 2025, 11, 545. https://doi.org/10.3390/fermentation11090545
Martínez-Mendoza LJ, Muñoz R, García-Depraect O. Continuous Fermentative Biohydrogen Production from Fruit-Vegetable Waste: A Parallel Approach to Assess Process Reproducibility. Fermentation. 2025; 11(9):545. https://doi.org/10.3390/fermentation11090545
Chicago/Turabian StyleMartínez-Mendoza, Leonardo J., Raúl Muñoz, and Octavio García-Depraect. 2025. "Continuous Fermentative Biohydrogen Production from Fruit-Vegetable Waste: A Parallel Approach to Assess Process Reproducibility" Fermentation 11, no. 9: 545. https://doi.org/10.3390/fermentation11090545
APA StyleMartínez-Mendoza, L. J., Muñoz, R., & García-Depraect, O. (2025). Continuous Fermentative Biohydrogen Production from Fruit-Vegetable Waste: A Parallel Approach to Assess Process Reproducibility. Fermentation, 11(9), 545. https://doi.org/10.3390/fermentation11090545







