Evaluation of the Addition of Polyethylene Glycol in the Enzymatic Hydrolysis of Rice Husk
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Analysis of Lignin, Cellulose, Hemicellulose
2.2.2. Elemental Analysis
2.2.3. Crystallinity of Rice Husk
2.2.4. Enzyme Adsorption Tests
2.2.5. Hydrolysis of Cellulose and Hemicellulose
2.2.6. Scanning Electron Microscopy (SEM)
2.3. Statistical Analysis
Analysis of Variance (ANOVA)
3. Results
3.1. Relative Crystallinity of Rice Husk
3.2. Chemical Characterization of Rice Ash
3.3. Effect of the Addition of PEG on the Interaction of Celluclast 1.5 L with Silicon Dioxide and Lignin
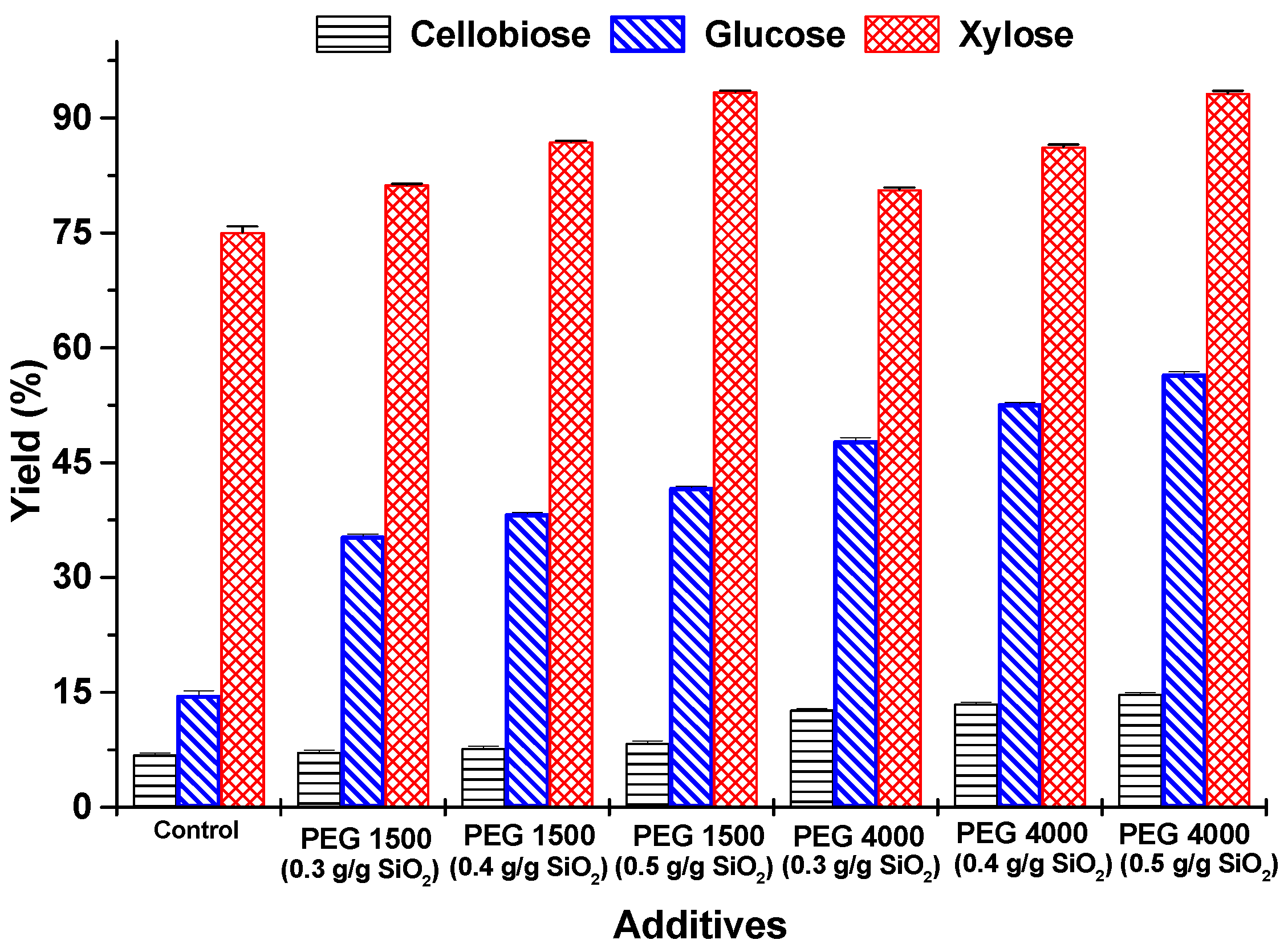
3.4. Enzymatic Hydrolysis of Rice Husk
3.4.1. Partial Hydrolysis of Cellulose
3.4.2. Total Hydrolysis of Cellulose
3.4.3. Hydrolysis of Hemicellulose
3.5. Effect of PEGs (1500 and 4000) on the Conversion of Cellulose and Hemicellulose
Analysis of Variance in Sugar Production
3.6. Qualitative and Quantitative Chemical Microanalysis of PEG 1500 and 4000
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Aa | Amorphous area |
| BSA | Bovine Serum Albumin |
| Ac | Crystalline area |
| CaO | Calcium oxide |
| C | Carbon |
| Cl | Chlorine |
| EH | Enzymatic Hydrolysis |
| EO | Oxyethylene |
| FPU | Filter paper units |
| H | Hydrogen |
| HPLC | High-Performance Liquid Chromatography |
| keV | Kiloelectronvolt |
| K2O | Potassium oxide |
| mA | Milliamps |
| MgO | Magnesium oxide |
| mM | Millimoles |
| μm | Microns |
| N | Nitrogen |
| Na2O | Sodium oxide |
| pH | −log [H+] |
| PEG | Polyethylene glycol |
| RC | Relative crystallinity |
| RH | Rice husk |
| S | Sulfur |
| SEM | Scanning electron microscopy |
| SiO2 | Silicon dioxide |
| w | Weight of dry silicon oxide |
| XRF | X-Ray fluorescence |
| β | Beta |
| Θ | Theta |
References
- FAO. Food Price Index, The Palgrave Encyclopedia of Global Security Studies; Springer: Cham, Switzerland, 2023; pp. 1–6. [Google Scholar] [CrossRef]
- Samsalee, N.; Meerasri, J.; Sothornvit, R. Rice husk nanocellulose: Extraction by high-pressure homogenization, chemical treatments and characterization. Carbohydr. Polym. Technol. Appl. 2023, 6, 100353. [Google Scholar] [CrossRef]
- Cedeno, F.R.P.; de Siqueira, B.B.; Chavez, E.G.S.; Roldán, I.U.M.; Ropelato, L.M.; Galán, J.P.M.; Masarin, F. Recovery of cellulose and lignin from Eucalyptus by-product and assessment of cellulose enzymatic hydrolysis. Renew. Energy 2022, 193, 807–820. [Google Scholar] [CrossRef]
- Miao, H.; Jin, X.; Wang, Y.; Gu, X.; Zheng, Z.; Ouyang, J. Restoring the promoting implications of expansin on enzymatic hydrolysis of lignocellulosic biomass by polyethylene glycol. Ind. Crops Prod. 2024, 219, 119072. [Google Scholar] [CrossRef]
- Xin, L. Advances in 2nd Generation of Bioethanol Production. In Overview of Technologies and Strategies for the Conversion of Lignocellulosic Biomass; Elsevier: Amsterdam, The Netherlands, 2021. [Google Scholar]
- Zhong, C.; Wei, P.; Zhang, Y.-H.P. A kinetic model of one-pot rapid biotransformation of cellobiose from sucrose catalyzed by three thermophilic enzymes. Chem. Eng. Sci. 2017, 161, 159–166. [Google Scholar] [CrossRef]
- Gouveia, P.; Quilles, J.; Alcântara, L.; Boscolo, M.; Gomes, L.; Minim, L.; Da Silva, R. β-glucosidase: An overview on immobilization and some aspects of structure, function, applications and cost. Process. Biochem. 2023, 130, 26–39. [Google Scholar] [CrossRef]
- Gao, D.; Haarmeyer, C.; Balan, V.; Whitehead, T.; Dale, B.E.; Chundawat, S.P. Lignin triggers irreversible cellulase loss during pretreated lignocellulosic biomass saccharification. Biotechnol. Biofuels 2014, 7, 175. [Google Scholar] [CrossRef]
- Olajide, O.; Nokken, M.; Sánchez, L. Alkali–Silica Reactions: Literature Review on the Influence of Moisture and Temperature and the Knowledge Gap. Materials 2024, 17, 10. [Google Scholar] [CrossRef]
- Lou, H.; Wang, M.; Lai, H.; Lin, X.; Zhou, M.; Yang, D.; Qiu, X. Reducing non-productive adsorption of cellulase and enhancing enzymatic hydrolysis of lignocelluloses by noncovalent modification of lignin with lignosulfonate. Bioresour. Technol. 2013, 146, 478–484. [Google Scholar] [CrossRef]
- Talukder, M.; Goh, H.; Puah, S. Interaction of silica with cellulase and minimization of its inhibitory effect on cellulose hydrolysis. Biochem. Eng. J. 2017, 118, 91–96. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, H.; Huang, Z.; Yin, Z.; Wen, R.; Min, X.; Huang, Y.; Liu, Y.; Fang, M.; Wu, X. Preparation and characterization of the properties of polyethylene glycol @ Si3N4 nanowires as phase-change materials. Chem. Eng. J. 2016, 301, 229–237. [Google Scholar] [CrossRef]
- Dai, C.; Xiong, F.; He, R.; Zhang, W.; Ma, H. Effects of low-intensity ultrasound on the growth, cell membrane permeability and ethanol tolerance of Saccharomyces cerevisiae. Ultrason. Sonochem. 2017, 36, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Kim, J.E.; Kim, H.E.; Yu, J.-H.; Cha, Y.-L.; Kim, K.H. Enhanced enzymatic hydrolysis of hydrothermally pretreated empty fruit bunches at high solids loadings by the synergism of hemicellulase and polyethylene glycol. Process. Biochem. 2017, 58, 211–216. [Google Scholar] [CrossRef]
- Ahmad, A.; Waheed, S.; Khan, S.M.; Gul, S.; Shafiq, M.; Farooq, M.; Sanaullah, K.; Jamil, T. Effect of silica on the properties of cellulose acetate/polyethylene glycol membranes for reverse osmosis. Desalination 2015, 355, 1–10. [Google Scholar] [CrossRef]
- Zhang, H.; Ding, X.; Chen, X.; Ma, Y.; Wang, Z.; Zhao, X. A new method of utilizing rice husk: Consecutively preparing d-xylose, organosolv lignin, ethanol and amorphous superfine silica. J. Hazard. Mater. 2015, 291, 65–73. [Google Scholar] [CrossRef]
- Sánchez-Muñoz, S.; Balbino, T.; de Oliveira, F.; Rocha, T.; Barbosa, F.; Vélez-Mercado, M.; Marcelino, P.; Antunes, F.; Moraes, E.; dos Santos, J. Surfactantes, biosurfactantes y proteínas no catalíticas como moléculas clave para mejorar la hidrólisis enzimática de la biomasa lignocelulósica. Moléculas 2022, 27, 8180. [Google Scholar] [CrossRef]
- Nogueira, C.; Padilha, C.; Dos Santos, E. Boosting second-generation ethanol titers from green coconut fiber by using high-concentration polyethylene glycol. Ind. Crop. Prod. 2021, 166, 113494. [Google Scholar] [CrossRef]
- Maryudi, M.; Rahayu, A.; Hakika, D.C. Efectividad de la sílice recubierta de polietilenglicol en la adsorción de iones en aguas residuales industriales. Polimery 2023, 68, 259–263. [Google Scholar] [CrossRef]
- Wang, T.; Xing, K.; Wei, Z.; Quan, X. Nucleophilic Substitution Reaction of Pyrimidin-2-yl Phosphates Using Amines and Thiols as Nucleophiles Mediated by PEG-400 as an Environmentally Friendly Solvent. Synthesis 2015, 47, 3925–3935. [Google Scholar] [CrossRef]
- Templeton, A.; Sluiter, B.; Hames, R.; Ruiz, C.; Scarlata, J.; Sluiter, D. Determination of Sugars, Byproducts, and Degradation Products in Liquid Fraction Process Samples; NREL/TP-510-42623; NREL: Golden, CO, USA, 2008; pp. 1–14. [Google Scholar]
- Singhania, J.; Saini, A.; Kumar, A.; Mukund, R. Cellulase adsorption on lignin: A roadblock for economic hydrolysis of biomass. Renew. Energy 2016, 98, 29–42. [Google Scholar] [CrossRef]
- Wei, F.; Jin, H. Transition-metal-free, ambient-pressure carbonylative cross-coupling reactions of aryl halides with potassium aryltrifluoroborates. Chem. Commun. 2015, 51, 9133–9136. [Google Scholar] [CrossRef]
- Wang, W.; Liu, F. Polyethylene glycol (PEG) promoted hydrodehalogenation of aryl halides. Tetrahedron Lett. 2017, 58, 1673–1676. [Google Scholar] [CrossRef]
- Hii, A.; Lim, B.; Chin, Z.; Jawad, K. Kinetic Analysis of Rice Husk Pyrolysis Using Kissinger-Akahira-Sunose (KAS) Method. Procedia Eng. 2016, 148, 1247–1251. [Google Scholar] [CrossRef]
- Thipchai, P.; Winita, P.; Kittisak, J.; Sarinthip, T.; Sasina, H.; Kanticha, P.; Gopinath, K.; Pornchai, R. Preparation and Characterization of Cellulose Nanocrystals from Bamboos and Their Application in Cassava Starch-Based Film. Polymers 2023, 15, 2622. [Google Scholar] [CrossRef]
- Mozafari, M.; Danaei, M.; Dehghankhold, S.; Ataei, D.; Hasanzadeh, R.; Javanmard, A.; Dokhani, S.; Khorasani, M. Impact of particle size and polydispersity index on the clinical applications of lipidic nanocarrier systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef]
- Barduco Ferreira, M.T.; Zuccari, T.B.B.; Garcia, W.; de Oliveira Neto, M. Studies of adsorptive capacity of bacterial β-glucosidases on lignocresol aiming the enzymatic recycling in bioprocesses. Biotechnol. Rep. 2019, 23, e00326. [Google Scholar] [CrossRef]
- Lebaz, N.; Arnaud, C.; Mathieu, S.; Alain, L.; Morchain, J. Application of the Direct Quadrature Method of Moments for the modelling of the enzymatic hydrolysis of cellulose: II. Case of insoluble substrate. Chem. Eng. Sci. 2016, 149, 322–333. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, T.; Jiang, B.; Mu, W. Enzymatic approaches to rare sugar production. Biotechnol. Adv. 2017, 35, 267–274. [Google Scholar] [CrossRef]
- Ko, J.; Um, Y.; Park, Y.; Seo, H.; Kim, K. Compounds inhibiting the bioconversion of hydrothermally pretreated lignocellulose. Appl. Microbiol. Biotechnol. 2015, 99, 4201–4212. [Google Scholar] [CrossRef]
- El-Reefy, H.; Gad, M.; Hamed, H.; Eldahab, M.; Moustafa, S. Radiation-induced grafting copolymerization of resin onto the surface of silica extracted from rice husk ash for adsorption of gadolinium. J. Mol. Liq. 2017, 231, 45–55. [Google Scholar] [CrossRef]
- Oliveira, J.P.; Bruni, G.P.; Lima, K.O.; Halal, S.L.M.E.; Rosa, G.S.D.; Dias, A.R.G.; Zavareze, E.D.R. Cellulose fibers extracted from rice and oat husks and their application in hydrogel. Food Chem. 2017, 221, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Rouau, G.; Silva, M.; Couturier, J.; Berrin, A.; Buléon, X. Effects of grinding processes on enzymatic degradation of wheat straw. Bioresour. Technol. 2012, 103, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Vij, A.; Beniwal, P.; Saini, A.; Kokkiligadda, S. Use of silicon dioxide nanoparticles for β-galactosidase immobilization and modulated ethanol production by co-immobilized K. marxianus and S. cerevisiae in deproteinized cheese whey. LWT 2018, 87, 553–561. [Google Scholar] [CrossRef]
- Nilesh, S.; Richa, K.; Kumar, K. Lignocellulosic Ethanol: Feedstocks and Bioprocessing, Bioethanol Production from Food Crops; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar] [CrossRef]
- Huang, C.; Zhao, X.; Zheng, Y.; Lin, W.; Lai, L.; Yong, Q.; Ragauskas, A.; Meng, X. Revealing the mechanism of surfactant-promoted enzymatic hydrolysis of dilute acid pretreated bamboo. Bioresour. Technol. 2022, 360, 127524. [Google Scholar] [CrossRef]
- Saravanabhupathy, S.; Dutta, S.; Das, B.; Anusha, R.; Rajak, R.; Banerjee, P.; Dikshit, C.; Padigala, A.; Kim, B.S. Recent Developments in Lignocellulosic Biofuel Production with Nanotechnological Intervention: An Emphasis on Ethanol. Catalysts 2023, 13, 1439. [Google Scholar] [CrossRef]
- Pihlajaniemi, V.; Kallioinen, A.; Sipponen, M.H.; Nyyssölä, A. Modeling and optimization of polyethylene glycol (PEG) addition for cost-efficient enzymatic hydrolysis of lignocellulose. Biochem. Eng. J. 2021, 167, 107894. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Y.; Linru, T. Effect of PEG4000 on cellulase catalysis in the lignocellulose saccharification processes. J. Chem. Technol. Biotechnol. 2011, 86, 115–120. [Google Scholar] [CrossRef]
- Zheng, X.; Li, Y. Lignin-enzyme interaction: Mechanism, mitigation approach, modeling, and research prospects. Biotechnol. Adv. 2017, 35, 466–489. [Google Scholar] [CrossRef]
- Ouyang, J.; Dong, Z.; Song, X.; Lee, X.; Chen, M.; Yong, Q. Improved enzymatic hydrolysis of microcrystalline cellulose (Avicel PH101) by polyethylene glycol addition. Bioresour. Technol. 2010, 101, 6685–6691. [Google Scholar] [CrossRef]
- Rocha-Martín, J.; Martinez-Bernal, C.; Pérez-Cobas, Y.; Reyes-Sosa, F.M.; García, B.D. Additives enhancing enzymatic hydrolysis of lignocellulosic biomass. Bioresour. Technol. 2017, 244, 48–56. [Google Scholar] [CrossRef]
- Li, J.; Li, S.; Fan, C.; Yan, Z. The mechanism of poly(ethylene glycol) 4000 effect on enzymatic hydrolysis of lignocellulose. Colloids Surf. B Biointerfaces 2012, 89, 203–210. [Google Scholar] [CrossRef]
- Szentner, K.; Waśkiewicz, A.; Imbiorowicz, R.; Borysiak, S. The Effect of Polyethylene Glycol Addition on Improving the Bioconversion of Cellulose. Molecules 2024, 29, 5785. [Google Scholar] [CrossRef] [PubMed]
- Song, G.; Hu, W.; Zhang, E.; Gupta, V.K.; Wu, Q.; Madadi, M.; Sun, C.; Sun, F. Polyethylene glycol-driven lignin-first biorefinery: A pathway for efficient conversion of lignocellulose to ethanol, furfural, and lignin-derived solid electrolytes. Chem. Eng. J. 2025, 515, 163530. [Google Scholar] [CrossRef]
- Li, H.; Wang, C.; Xiao, W.; Yang, Y.; Hu, P.; Dai, Y.; Jiang, Z. Dissecting the effect of polyethylene glycol on the enzymatic hydrolysis of diverse lignocellulose. Int. J. Biol. Macromol. 2019, 131, 676–681. [Google Scholar] [CrossRef] [PubMed]
- Chu, Q.; Huang, Y.; Li, X.; Fan, Y.; Jin, Y.; Yu, S.; Yong, Q. Improved Enzymatic Hydrolysis of Corn Stover by Green Liquor Pretreatment and a Specialized Enzyme Cocktail. Bioresourses 2014, 9, 4489–4502. [Google Scholar] [CrossRef]
- Xin, D.; Yin, H.; Ran, G. Integrated alkaline pretreatment and surfactant-assisted hydrolysis for high-yield Manno-oligosaccharides production from spent coffee grounds. Food Chem X 2025, 29, 102892. [Google Scholar] [CrossRef]
- Ascencio, J.J.; Magalhães, L.S.; Ferreira, F.B.; Heinz, O.; Ferraz, A.; Chandel, A.K. Surfactant-Enhanced Enzymatic Hydrolysis of Eucalyptus Kraft Pulp: The Interrelationship Between Lignin Reduction and Sugar Recovery. Catalysts 2025, 15, 47. [Google Scholar] [CrossRef]
- Dutta, S.K.; Chakraborty, S. Mixing effects on the kinetics and the dynamics of two-phase enzymatic hydrolysis of hemicellulose for biofuel production. Bioresour. Technol. 2018, 259, 276–285. [Google Scholar] [CrossRef]
- Shuang, L.; Yang, X.; Yang, S.; Zhu, M.; Wang, X. Technology Prospecting on Enzymes: Application, Marketing and Engineering. Comput. Struct. Biotechnol. J. 2012, 2, e201209017. [Google Scholar] [CrossRef]
- Kellock, M.; Rahikainen, J.; Marjamaa, K.; Kruus, K. Lignin-derived inhibition of monocomponent cellulases and a xylanase in the hydrolysis of lignocellulosics. Bioresour. Technol. 2017, 232, 183–191. [Google Scholar] [CrossRef]
- Pedroso, G.B.; Philippsen, M.R.; Saldanha, L.F.; Araujo, R.B.; Martins, A.F. Strategies for Fermentable Sugar Production by Using Pressurized Acid Hydrolysis for Rice Husks. Rice Sci. 2019, 26, 319–330. [Google Scholar] [CrossRef]
- Draszewski, C.; Bragato, C.; Lachos-Perez, D.; Celante, D.; Frizzo, C.; Castilhos, F.; Tres, M.; Zabot, G.; Abaide, E.; Mayer, F. Subcritical water hydrolysis of rice husks pretreated with deep eutectic solvent for enhance fermentable sugars production. J. Supercrit. Fluids 2021, 178, 105355. [Google Scholar] [CrossRef]
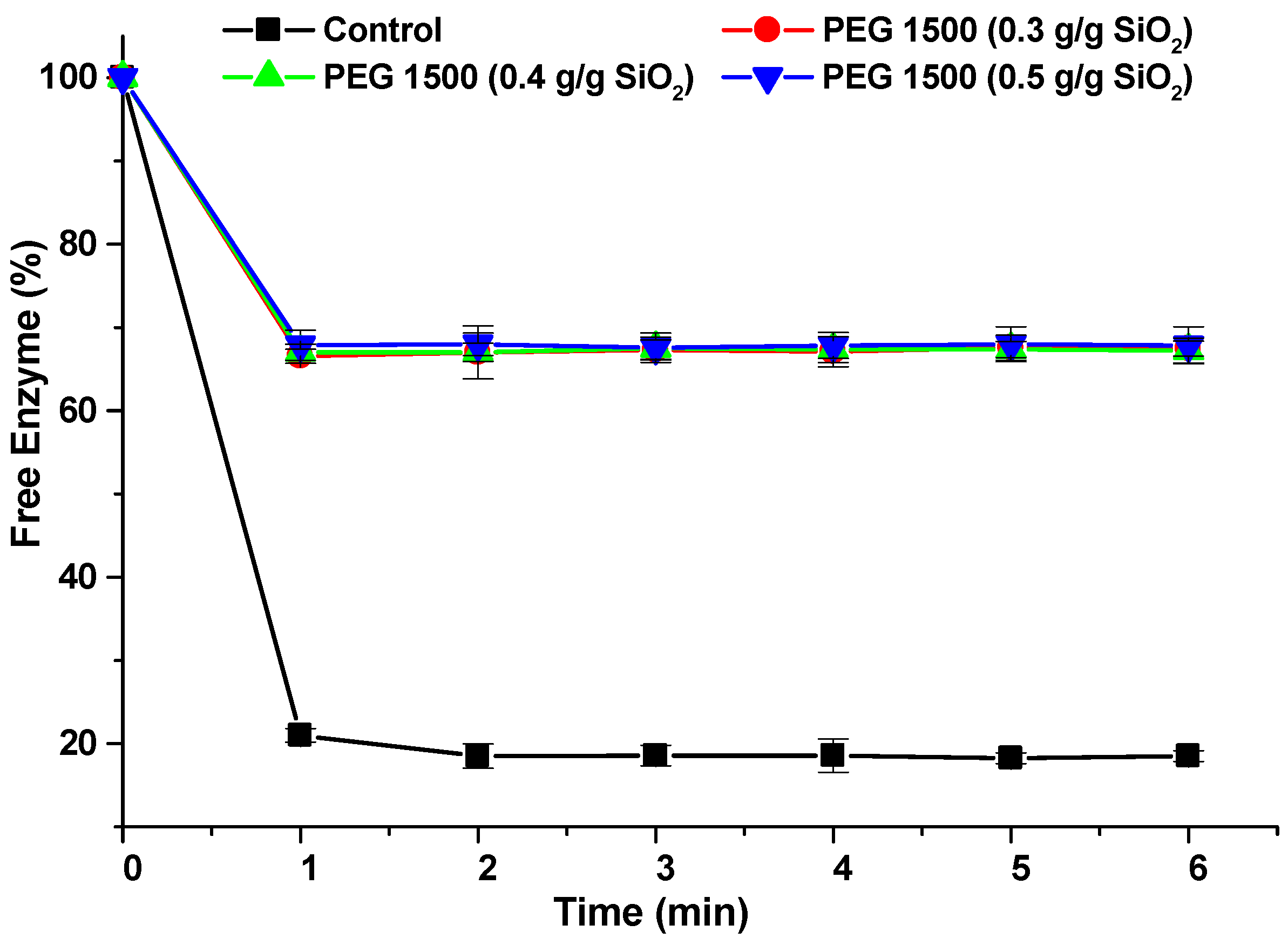
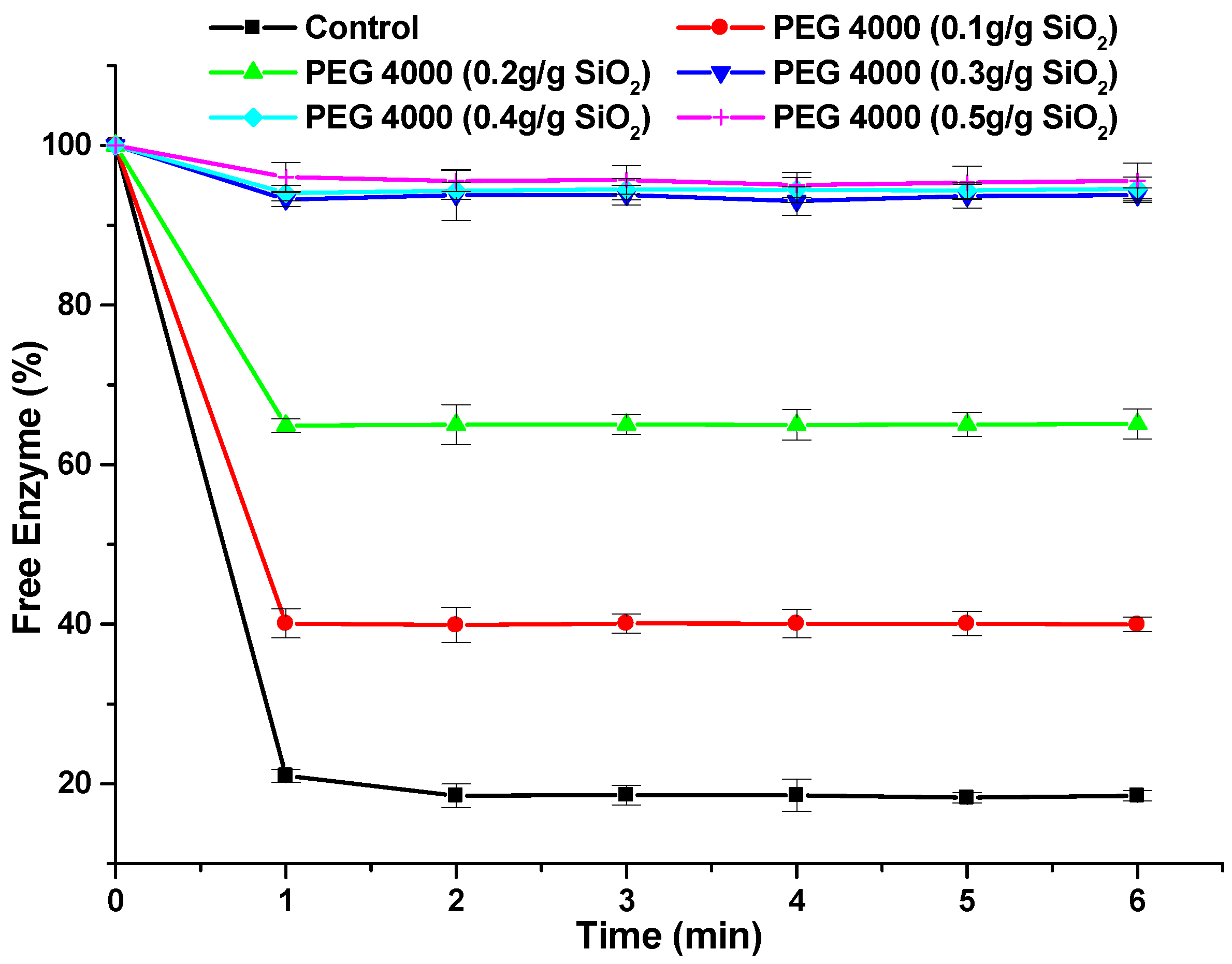

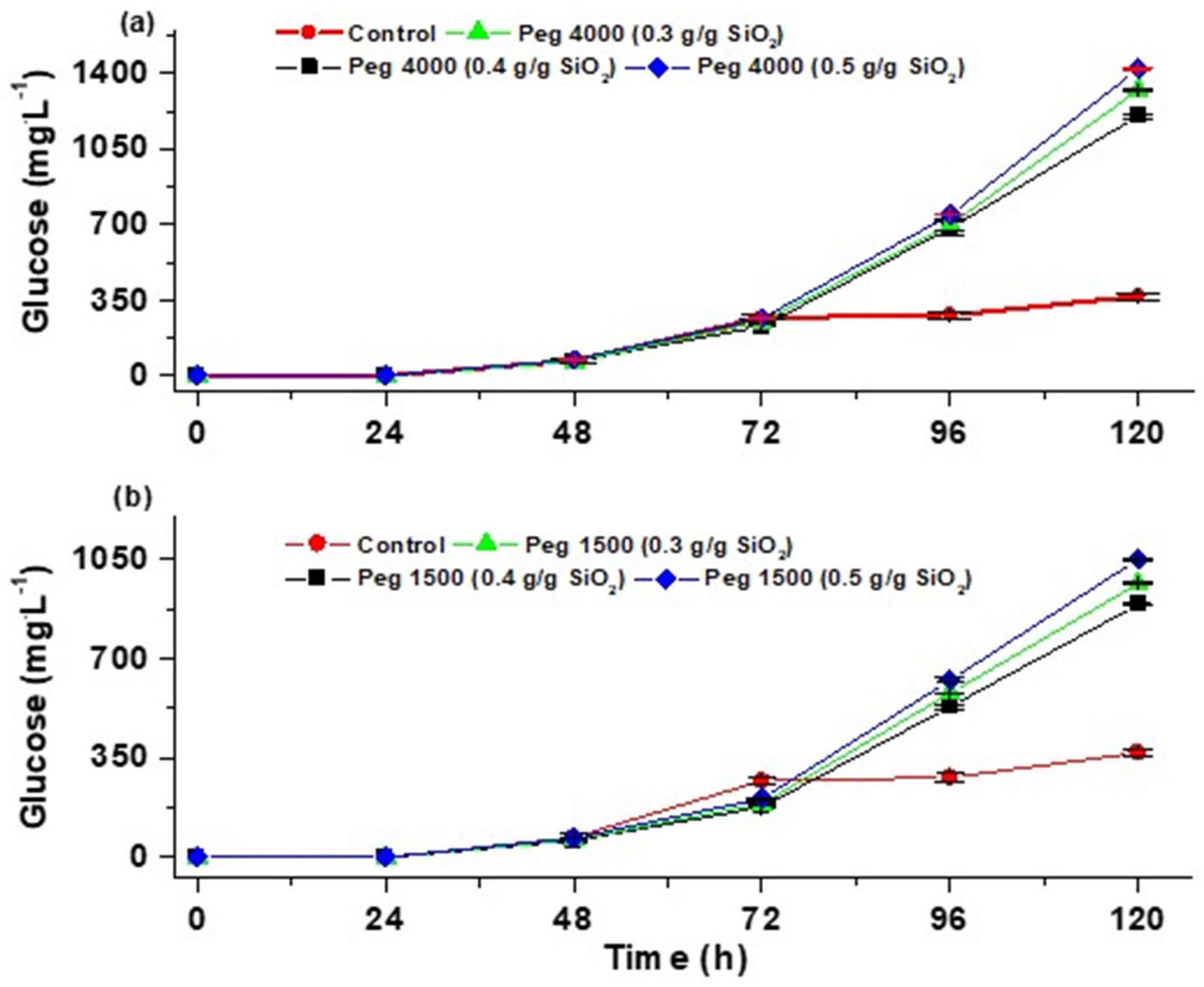
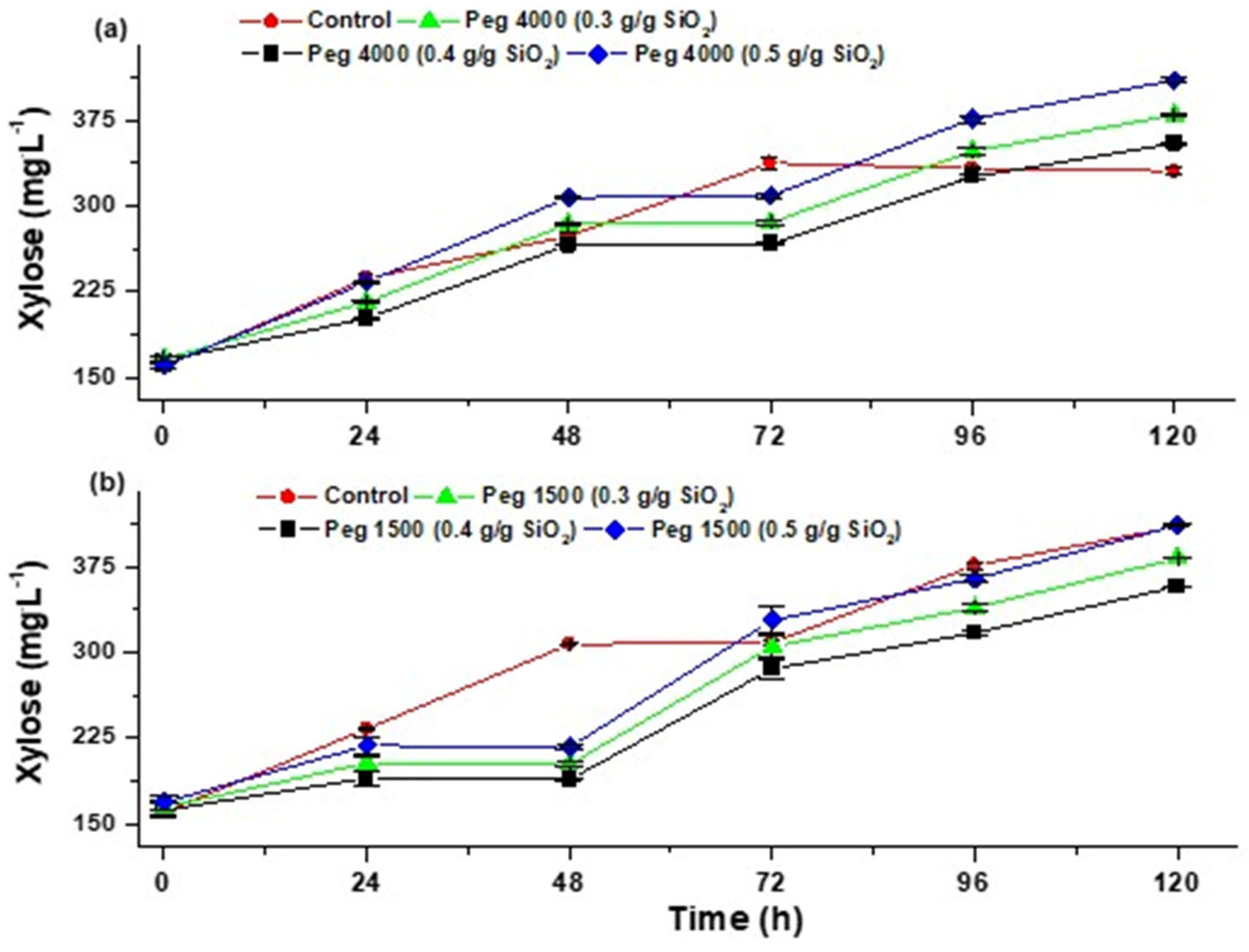
| Data | Mean | Variance | N |
|---|---|---|---|
| Control | 31.29 | 1.20423 | 3 |
| PEG 1500 (0.3 g/g SiO2) | 48.38 | 0.39083 | 3 |
| PEG 1500 (0.4 g/g SiO2) | 52.24 | 0.52923 | 3 |
| PEG 1500 (0.5 g/g SiO2) | 56.73 | 0.1351 | 3 |
| PEG 4000 (0.3 g/g SiO2) | 63.9 | 1.0924 | 3 |
| PEG 4000 (0.4 g/g SiO2) | 69.05 | 1.71903 | 3 |
| PEG 4000 (0.5 g/g SiO2) | 74.79 | 0.35583 | 3 |
| F = 7473.04684 | |||
| p = 0 |
| Substrate | Tipe PEG | PEG Concentration | Implementation Phase | Conversion | Reference |
|---|---|---|---|---|---|
| Rice Husk | 4000 | 5 mg/mL | Co-addition in saccharification | 21.46% | [45] |
| Sugarcane bagasse | 4000 | no report | Pre-treatment | 52% | [46] |
| Rice straw | 4000 | 0.05–0.2 g/g substrate | Saccharification | 0.288 mg/mL | [47] |
| Corn stover | 6000 | 0.05 g/g substrate | Saccharification | 50.14% | [48] |
| Lignocellulosic waste | 6000 | 5 mg/mL | Pre-treatment | 62.3% | [49] |
| Eucalyptus kraft pulp | 400 | 75 mg/g substrate | Saccharification | 65 g/L | [50] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Armijos, H.A.; Veiga, M.C. Evaluation of the Addition of Polyethylene Glycol in the Enzymatic Hydrolysis of Rice Husk. Fermentation 2025, 11, 544. https://doi.org/10.3390/fermentation11090544
Armijos HA, Veiga MC. Evaluation of the Addition of Polyethylene Glycol in the Enzymatic Hydrolysis of Rice Husk. Fermentation. 2025; 11(9):544. https://doi.org/10.3390/fermentation11090544
Chicago/Turabian StyleArmijos, Humberto Ayala, and María C. Veiga. 2025. "Evaluation of the Addition of Polyethylene Glycol in the Enzymatic Hydrolysis of Rice Husk" Fermentation 11, no. 9: 544. https://doi.org/10.3390/fermentation11090544
APA StyleArmijos, H. A., & Veiga, M. C. (2025). Evaluation of the Addition of Polyethylene Glycol in the Enzymatic Hydrolysis of Rice Husk. Fermentation, 11(9), 544. https://doi.org/10.3390/fermentation11090544








