Gas Endeavour Device for the Real-Time In Vitro Measurement of Carbon Dioxide and Methane Emissions Associated with Sheep Diets with Prickly Pear By-Products
Abstract
1. Introduction
2. Materials and Methods
2.1. In Vivo Trial
2.2. In Vitro Study
2.2.1. Preliminary Preparation of the Test
2.2.2. Rumen Liquor Sampling
2.2.3. Gas Endeavour Trial
2.2.4. Repeatability and Reproducibility of Gas Endeavour Measurements
2.2.5. Nutrient Degradability
2.2.6. Kinetics Study of Total Gas and Methane Emissions
2.2.7. DNA Extraction, Amplification and 16S rRNA Sequencing
2.3. Statistical Analyses
3. Results
3.1. Gage Repeatability and Reproducibility
3.2. Gas Production and Their Kinetics
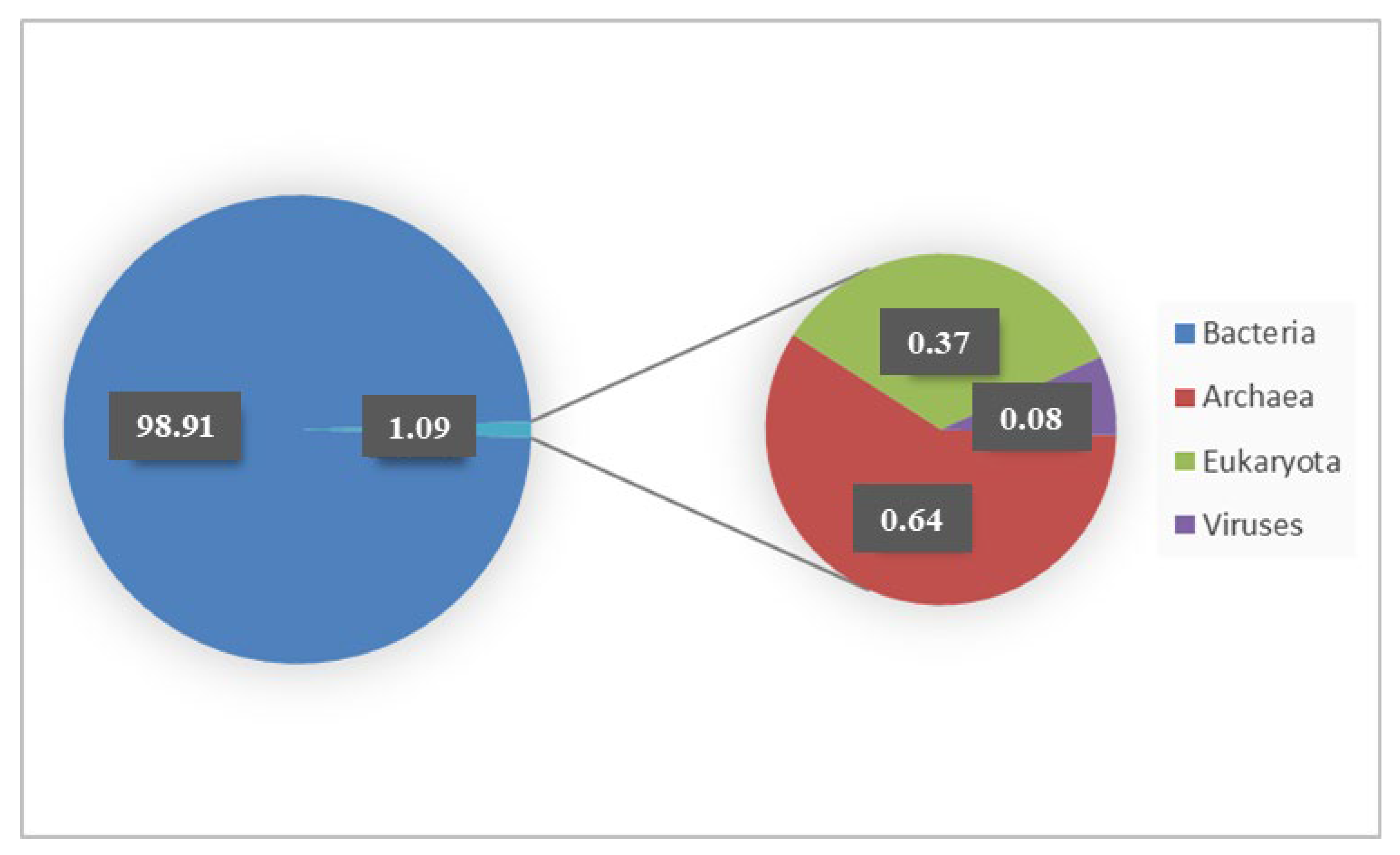
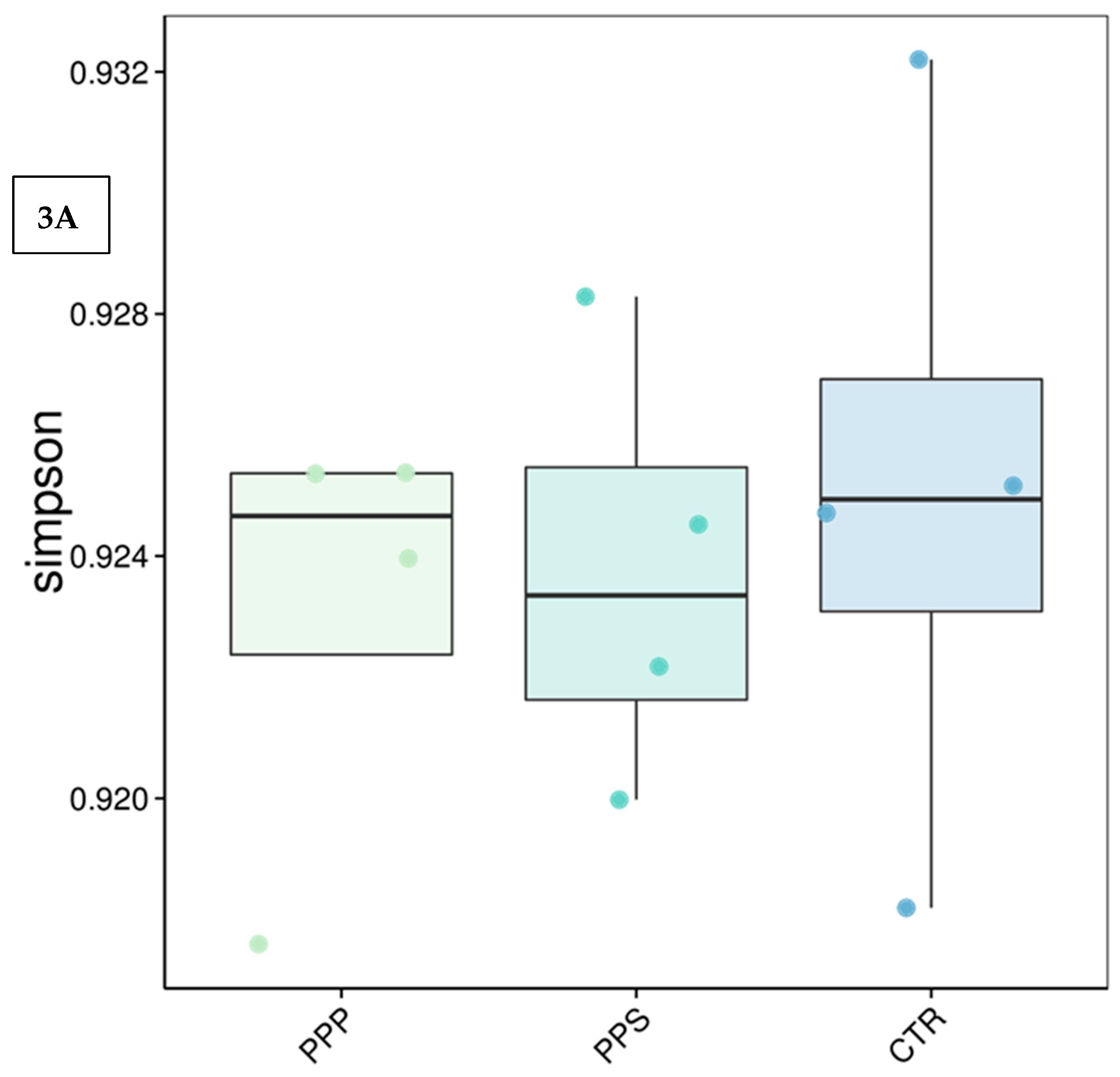
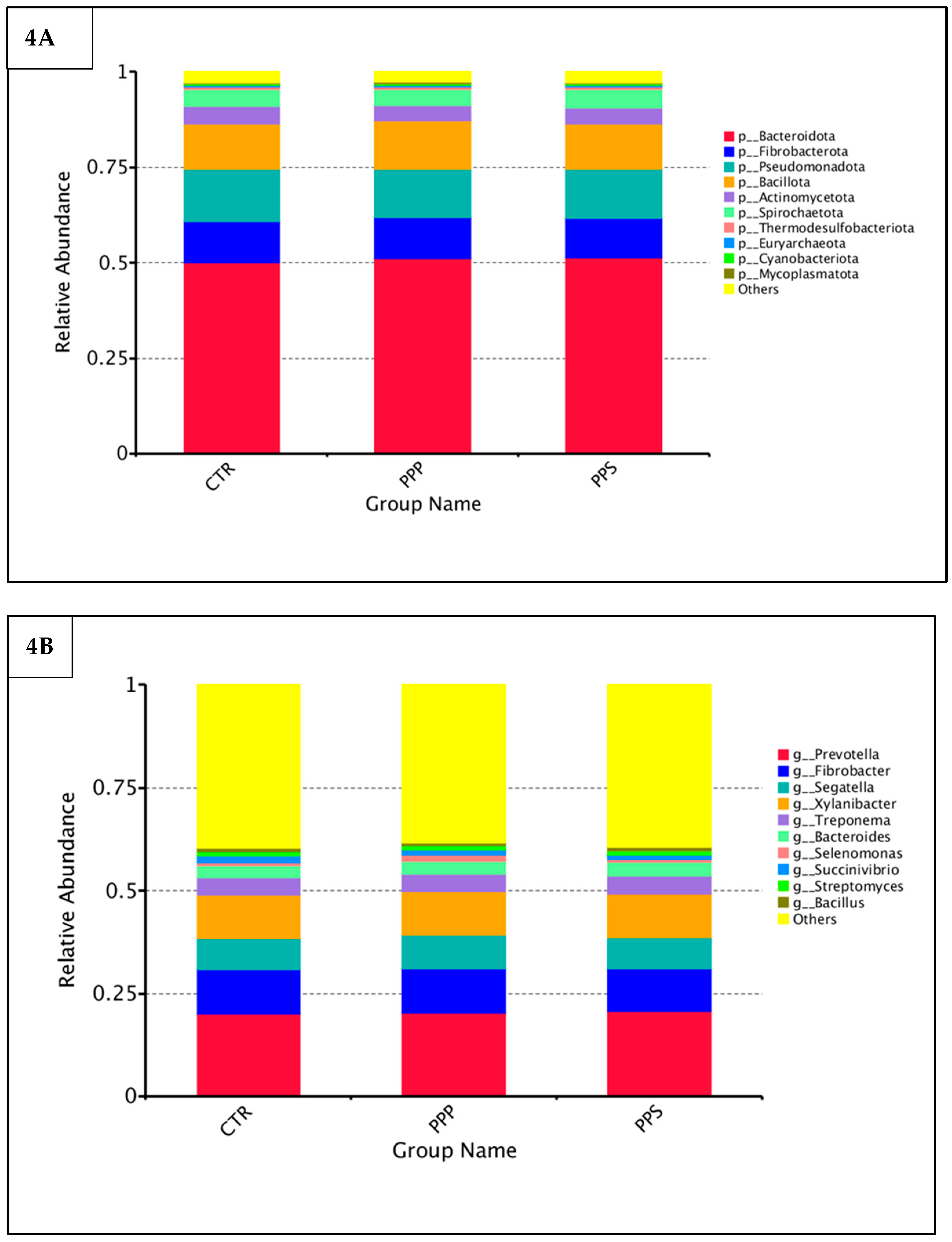
3.3. Microbiota Analyses of Rumen Fluid
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PPP | Prickly pear peels |
| PPS | Prickly pear peels + pulp + seeds |
| RL | Rumen liquor |
| FL | Fermented rumen liquor |
| OMd | Disappearance of organic matter |
| NDFd | Disappearance of neutral detergent fibre |
| GES | Gas Endeavour System |
| GRR | Combined gage the repeatability and reproducibility |
| GP | Gas production |
| MGP | Methane gas production |
References
- Gannuscio, R. Study of New Agro-Industrial by-Products from Prickly Pear to Increase the Sustainability of Livestock Farming. Ph.D. Thesis, University of Palermo, Piazza Marina, Palermo, Italy, 2025. [Google Scholar]
- El-Beltagi, H.S.; Ahmed, A.R.; Mohamed, H.I.; Al-Otaibi, H.H.; Ramadan, K.M.; Elkatry, H.O. Utilization of prickly pear peels flour as a natural source of minerals, dietary fiber and antioxidants: Effect on cakes production. Agronomy 2023, 13, 439. [Google Scholar] [CrossRef]
- Todaro, M.; Alabiso, M.; Di Grigoli, A.; Scatassa, M.L.; Cardamone, C.; Mancuso, I.; Mazza, F.; Bonanno, A. Prickly pear by-product in the feeding of livestock ruminants: Preliminary investigation. Animals 2020, 10, 949. [Google Scholar] [CrossRef]
- Vastolo, A.; Calabrò, S.; Cutrignelli, M.I.; Raso, G.; Todaro, M. Silage of prickly pears (Opuntia spp.) juice by-products. Animals 2020, 10, 1716. [Google Scholar] [CrossRef]
- Gannuscio, R.; Cardamone, C.; Vastolo, A.; Lucia, C.; D’Amico, A.; Maniaci, G.; Todaro, M. Ensiling as a Conservation Technique for Opuntia ficus indica (L.) By-Products: Peel and Pastazzo. Animals 2024, 14, 3196. [Google Scholar] [CrossRef]
- Gannuscio, R.; Vastolo, A.; Maniaci, G.; Lucia, C.; Calabrò, S.; Todaro, M.; Cutrignelli, M.I. Improve nutritive value of silage based on prickly pear peel by-products. Ital. J. Anim. Sci. 2024, 23, 492–503. [Google Scholar] [CrossRef]
- Hassan, M.U.; Vastolo, A.; Gannuscio, R.; Maniaci, G.; Mancuso, I.; Calabrò, S.; Gallo, A.; Todaro, M.; Cutrignelli, M.I. Effects of feeding prickly pear by-product silage as a partial replacement of concentrate on dairy ewes: Milk characteristics, nutrient utilisation and in vitro ruminal fermentation. Anim. Feed Sci. Technol. 2025, 324, 116330. [Google Scholar] [CrossRef]
- Amaya-Cruz, D.M.; Perez-Ramirez, I.F.; Delgado-Garcia, J.; Mondragon-Jacobo, C.; Dector-Espinoza, A.; Reynoso-Camacho, R. An integral profile of bioactive compounds and functional properties of prickly pear (Opuntia ficus indica L.) peel with different tonalities. Food Chem. 2019, 278, 568–578. [Google Scholar] [CrossRef]
- Vastolo, A.; Mora, B.; Kiatti, D.D.; Nocerino, M.; Haroutounian, S.; Baka, R.D.; Ligda, P.; Cutrignelli, M.I.; Niderkorn, V.; Calabrò, S. Assessment of the effect of agro-industrial by-products rich in polyphenols on in vitro fermentation and methane reduction in sheep. Front. Vet. Sci. 2025, 12, 1530419. [Google Scholar] [CrossRef]
- Elghandour, M.M.; Rodríguez-Ocampo, I.; Parra-Garcia, A.; Salem, A.Z.; Greiner, R.; Márquez-Molina, O.; Barros-Rodríguez, M.; Barbabosa-Pilego, A. Biogas production from prickly pear cactus containing diets supplemented with Moringa oleifera leaf extract for a cleaner environmental livestock production. J. Clean. Prod. 2018, 185, 547–553. [Google Scholar] [CrossRef]
- Ku-Vera, J.C.; Jiménez-Ocampo, R.; Valencia-Salazar, S.S.; Montoya-Flores, M.D.; Molina-Botero, I.C.; Arango, J.; Gómez-Bravo, C.A.; Aguilar-Pérez, C.F.; Solorio-Sánchez, F.J. Role of secondary plant metabolites on enteric methane mitigation in ruminants. Front. Vet. Sci. 2020, 7, 584. [Google Scholar] [CrossRef] [PubMed]
- Cardador-Martínez, A.; Jiménez-Martínez, C.; Sandoval, G. Revalorization of cactus pear (Opuntia spp.) wastes as a source of antioxidants. Food Sci. Technol. 2011, 31, 782–788. [Google Scholar] [CrossRef]
- Besharati, M.; Maggiolino, A.; Palangi, V.; Kaya, A.; Jabbar, M.; Eseceli, H.; De Palo, P.; Lorenzo, J.M. Tannin in ruminant nutrition. Molecules 2022, 27, 8273. [Google Scholar] [CrossRef]
- Vasta, V.; Daghio, M.; Cappucci, A.; Buccioni, A.; Serra, A.; Viti, C.; Mele, M. Invited review: Plant polyphenols and rumen microbiota responsible for fatty acid biohydrogenation, fiber digestion, and methane emission: Experimental evidence and methodological approaches. J. Dairy Sci. 2019, 102, 3781–3804. [Google Scholar] [CrossRef] [PubMed]
- Jalal, H.; Sucu, E.; Cavallini, D.; Giammarco, M.; Akram, M.Z.; Karkar, B.; Gao, M.; Pompei, L.; Lopez, J.E.A.; Prasinou, P.; et al. Rumen fermentation profile and anti-methanogenic properties of Mango and Avocado byproducts as feed ingredients and supplements. Sci. Rep. 2024, 15, 16164. [Google Scholar]
- Scicutella, F.; Foggi, G.; Daghio, M.; Mannelli, F.; Viti, C.; Mele, M.; Buccioni, A. A review of in vitro approaches as tools for studying rumen fermentation and ecology: Effectiveness compared to in vivo outcomes. Ital. J. Anim. Sci. 2025, 24, 589–608. [Google Scholar] [CrossRef]
- Uveges, Z.; Damak, M.; Klatyik, S.; Ramay, M.W.; Fekete, G.; Varga, Z.; Gyuricza, C.; Szekacs, A.; Aleksza, L. Biomethane Potential in Anaerobic Biodegradation of Commercial Bioplastic Materials. Fermentation 2023, 9, 261. [Google Scholar] [CrossRef]
- Iqbal, R.; Arango, S.; Tagliapietra, F.; Bailoni, L. Gas Endeavour: An Innovative Equipment for Estimating Methane Kinetics During In Vitro Rumen Fermentation. Animals 2025, 15, 1331. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; van Gorp, R.; Nistor, M. The new Gas Endeavour system from Bioprocess Control AB for in vitro assessment of animal feeds. In Proceedings of the 9th Nordic Feed Science Conference, Uppsala, Sweden, 12–13 June 2018; Swedish University of Agricultural Sciences: Uppsala, Sweden, 2018; pp. 135–142. [Google Scholar]
- Rota Graziosi, A.; Colombini, S.; Crovetto, G.M.; Galassi, G.; Chiaravalli, M.; Battelli, M.; Reginelli, D.; Petrera, F.; Rapetti, L. Partial replacement of soybean meal with soybean silage in lactating dairy cows diet: Part 1, milk production, digestibility, and N balance. Ital. J. Anim. Sci. 2022, 21, 634–644. [Google Scholar] [CrossRef]
- Ramos-Morales, E.; Arco-Pérez, A.; Martín-García, A.I.; Yáñez-Ruiz, D.R.; Frutos, P.; Hervás, G. Use of stomach tubing as an alternative to rumen cannulation to study ruminal fermentation and microbiota in sheep and goats. Anim. Feed Sci. Technol. 2014, 198, 57–66. [Google Scholar] [CrossRef]
- Yáñez-Ruiz, D.R.; Bannink, A.; Dijkstra, J.; Kebreab, E.; Morgavi, D.P.; O’Kiely, P.; Reynolds, C.K.; Schwarm, A.; Shingfield, K.J.; Yu, Z.; et al. Design, Implementation and Interpretation of in Vitro Batch Culture Experiments to Assess Enteric Methane Mitigation in Ruminants. Anim. Feed Sci. Technol. 2016, 216, 1–18. [Google Scholar] [CrossRef]
- BioProcess Instruments. Available online: https://bpcinstruments.com/ge-in-vitro-animal-nutrition-2024/ (accessed on 16 July 2025).
- Sahay, A.; Belt, M.B. Measurement System Analysis Gage Repeatability & Reproducibility (Gage R&R) Study. In Six Sigma Quality: Concepts & Cases, 1; Belt, M.B., Ed.; Real Lean Publishing: Salt Lake City, UT, USA, 2010; Chapter 7; pp. 1–34. Available online: https://www.realleansixsigmaquality.com/lss/wp-content/uploads/2014/06/Chapter7-Sample-Volume-1_2014.pdf (accessed on 5 July 2025).
- AIAG. Automotive Industry Action Group. Measurement Systems Analysis: Reference Manual, 4th ed.; Automotive Industry Action Group: Southfield, MI, USA, 2010; pp. 1–10. [Google Scholar]
- Cano, E.L.; Moguerza, J.M.; Redchuk, A. Six Sigma with R: Statistical Engineering for Process Improvement; Springer SBM: Heidelberg, Germany, 2012; p. 36. Available online: https://books.google.it/books?hl=it&lr=&id=58z6BISdTswC&oi=fnd&pg=PR3&dq=26.%09Cano,+E.L.%3B+Moguerza,+J.M.%3B+Redchuk,+A.+Six+sigma+with+R:+Statistical+engineering+for+process+improvement%3B+Springer+SBM:+Hei-delberg,+Germany++,+2012%3B+3&ots=Zn4QN60SAs&sig=dpsoyBWhT5DKqHxx-WSKWO4hcow&redir_esc=y#v=onepage&q&f=false (accessed on 8 May 2025).
- Soltan, Y.A.; Morsy, A.S.; Sallam, S.M.A.; Louvandini, H.; Abdalla, A.L. Comparative in vitro evaluation of forage legumes (prosopis, acacia, atriplex, and leucaena) on ruminal fermentation and methanogenesis. J. Anim. Feed Sci. 2012, 21, 759–772. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis; AOAC International, 18th ed.; AOAC International: Gaithersburg, MD, USA, 2005; pp. 25–30. [Google Scholar]
- van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for dietary fibre, neutral detergent fibre, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Groot, J.C.; Cone, J.W.; Williams, B.A.; Debersaques, F.M.; Lantinga, E.A. Multiphasic analysis of gas production kinetics for in vitro fermentation of ruminant feeds. J. Anim. Feed Sci. Tech. 1996, 64, 77–89. [Google Scholar] [CrossRef]
- Li, M.; Gong, J.; Cottrill, M.; Yu, H.; de Lange, C.; Burton, J.; Topp, E. Evaluation of QIAamp® DNA Stool Mini Kit for ecological studies of gut microbiota. J. Microbiol. Methods. 2003, 54, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Morgavi, D.P.; Rathahao-Paris, E.; Popova, M.; Boccard, J.; Nielsen, K.F.; Boudra, H. Rumen microbial communities influence metabolic phenotypes in lambs. Front. Vet. Sci. 2015, 6, 1060. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.K.; Zhang, X.X.; Li, F.D.; Li, C.; Li, G.Z.; Zhang, D.Y.; Song, Q.Z.; Li, X.L.; Zhao, Y.; Wang, W.M. Characterization of the rumen microbiota and its relationship with residual feed intake in sheep. Animal 2021, 15, 100161. [Google Scholar] [CrossRef]
- Spanghero, M.; Chiaravalli, M.; Colombini, S.; Fabro, C.; Froldi, F.; Mason, F.; Moschini, M.; Sarnataro, C.; Schiavon, S.; Tagliapietra, F. Rumen Inoculum Collected from Cows at Slaughter or from a Continuous Fermenter and Preserved in Warm Refrigerated, Chilled or Freeze-Dried Environments for In Vitro Tests. Animals 2019, 9, 815. [Google Scholar] [CrossRef] [PubMed]
- Chaudhry, A.S.; Khan, M.M.H. Impacts of different spices on in vitro rumen dry matter disappearance, fermentation and methane of wheat or ryegrass hay based substrates. Livest. Sci. 2012, 146, 84–90. [Google Scholar] [CrossRef]
- Kaewpila, C.; Gunun, P.; Kesorn, P.; Subepang, S.; Thip-Uten, S.; Cai, Y.; Pholsen, S.; Cherdthong, A.; Khota, W. Improving ensiling characteristics by adding lactic acid bacteria modifies in vitro digestibility and methane production of forage-sorghum mixture silage. Sci. Rep. 2021, 11, 1968. [Google Scholar] [CrossRef]
- Theodorou, M.K.; Williams, B.A.; Dhanoa, M.S.; McAllan, A.B.; France, J. A simple gas production method using a pressure transducer to determine the fermentation kinetics of ruminant feeds. Anim. Feed Sci. Technol. 1994, 48, 185–197. [Google Scholar] [CrossRef]
- Albores-Moreno, S.; Alayón-Gamboa, J.A.; Miranda-Romero, L.A.; Alarcón-Zúñiga, B.; Jiménez-Ferrer, G.; Ku-Vera, J.C.; Piñeiro-Vázquez, A.T. Effect of tree foliage supplementation of tropical grass diet on in vitro digestibility and fermentation, microbial biomass synthesis and enteric methane production in ruminants. Trop. Anim. Health Prod. 2019, 51, 893–904. [Google Scholar] [CrossRef]
- Ben Salem, H.; Nefzaoui, A.; Ben Salem, L.; Tisserand, J.L. Intake, digestibility, urinary excretion of purine derivatives and growth by sheep given fresh, air-dried or polyethylene glycol-treated foliage of Acacia cyanophylla Lindl. Anim. Feed Sci. Technol. 1999, 78, 297–311. [Google Scholar] [CrossRef]
- McSweeney, C.S.; Palmer, B.; McNeill, D.M.; Krause, D.O. Microbial interactions with tannins: Nutritional consequences for ruminants. Anim. Feed Sci. Technol. 2001, 91, 83–93. [Google Scholar] [CrossRef]
- Hervás, G.; Frutos, P.; Javier Giráldez, F.; Mantecón, Á.R.; Álvarez Del Pino, M.a.C. Effect of different doses of quebracho tannins extract on rumen fermentation in ewes. Anim. Feed Sci. Technol. 2003, 109, 65–78. [Google Scholar] [CrossRef]
- Navarro-Villa, A.; OBriena, M.; López, S.; Bolandb, T.M.; OKiely, P. Modifications of a gas production technique for assessing in vitro rumen methaneproduction from feedstuffs. Anim. Feed Sci. Technol. 2011, 166, 163–174. [Google Scholar] [CrossRef]
- Wang, L.; Li, Y.; Zhang, Y.; Wang, L. The effects of different concentrate-to-forage ratio diets on rumen bacterial microbiota and the structures of Holstein cows during the feeding cycle. Animals 2020, 10, 957. [Google Scholar] [CrossRef]
- Rey, M.; Enjalbert, F.; Combes, S.; Cauquil, L.; Bouchez, O.; Monteils, V. Establishment of ruminal bacterial community in dairy calves from birth to weaning is sequential. J. Appl. Microbiol. 2014, 116, 245–257. [Google Scholar] [CrossRef]
- Huws, S.A.; Edwards, J.E.; Creevey, C.J.; Rees Stevens, P.; Lin, W.; Girdwood, S.E.; Pachebat, J.A.; Kingston-Smith, A.H. Temporal dynamics of the metabolically active rumen bacteria colonizing fresh perennial ryegrass. FEMS Microbiol. Ecol. 2016, 92, 137. [Google Scholar] [CrossRef] [PubMed]
- Henderson, G.; Cox, F.; Ganesh, S.; Jonker, A.; Young, W. Rumen microbial community composition varies with diet and host, but a core microbiome is found a wide geographical range. Sci. Rep. 2015, 5, 14567. [Google Scholar] [CrossRef]
- Plaizier, J.C.; Li, S.; Danscher, A.M.; Derakshani, H.; Andersen, P.H.; Khafipour, E. Changes in microbiota in rumen digesta and feces due to a grain-based subacute ruminal acidosis (SARA) challenge. Microb. Ecol. 2017, 74, 485–495. [Google Scholar] [CrossRef]
- Denman, S.E.; Fernandez, G.M.; Shinkai, T.; Mitsumori, M.; McSweeney, C.S. Metagenomic analysis of the rumen microbial community following inhibition of methane formation by a halogenated methane analog. Front. Microbiol. 2015, 6, 1087. [Google Scholar] [CrossRef]
- Wang, Y.; Cao, P.; Wang, L.; Zhao, Z.; Chen, Y.; Yang, Y. Bacterial community diversity associated with different levels of dietary nutrition in the rumen of sheep. Appl. Microbiol. Biotechnol. 2017, 101, 3717–3728. [Google Scholar] [CrossRef]
- Thauer, R.K.; Kaster, A.-K.; Seedorf, H.; Buckel, W.; Hedderich, R. Methanogenic archaea: Ecologically relevant differences in energy conservation. Nat. Rev. Microbiol. 2008, 6, 579–591. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Wang, K.; Nan, X.; Cai, M.; Yang, L.; Xiong, B.; Zhao, Y. Synergistic Effects of 3-Nitrooxypropanol with Fumarate in the Regulation of Propionate Formation and Methanogenesis in Dairy Cows In Vitro. Appl. Environ. Microbiol. 2022, 88, e01908-21. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Sun, Y.; Guo, T.; Liu, W.; Tong, X.; Zhang, Z.; Sun, J.; Yang, Y.; Yang, S.; Li, D.; et al. Sargassum mcclurei Mitigating Methane Emissions and Affecting Rumen Microbial Community in In Vitro Rumen Fermentation. Animals 2024, 14, 2057. [Google Scholar] [CrossRef] [PubMed]
- Danielsson, R.; Dicksved, J.; Sun, L.; Gonda, H.; Müller, B.; Schnürer, A.; Bertilsson, J. Methane Production in Dairy Cows Correlates with Rumen Methanogenic and Bacterial Community Structure. Front. Microbiol. 2017, 8, 226. [Google Scholar] [CrossRef]


| Ingredients | Diets | ||
|---|---|---|---|
| CTR | PPP | PPS | |
| Offered (g/d/head) | |||
| Hay | 3000 | 2700 | 2300 |
| Silage | - | 1500 | 1000 |
| Concentrate | 900 | 500 | 500 |
| Feed Intake (g of DM/d/head) | |||
| Hay | 1499 | 1269 | 1403 |
| Silage | - | 307 | 410 |
| Concentrate | 795 | 442 | 442 |
| Nutrients Intake (g/d/head) | |||
| DM | 2410 | 2285 | 2471 |
| CP | 328 | 274 | 283 |
| EE | 57.70 | 61.10 | 59.10 |
| aNDFom | 1477 | 1393 | 1647 |
| ADFom | 856 | 821 | 1030 |
| ADL | 128 | 126 | 236 |
| NFC | 398 | 406 | 324 |
| NEL (MJ intake/d/head) | 11.46 | 11.04 | 10.39 |
| Source | DF | SS | MS | F | p |
|---|---|---|---|---|---|
| Time | 71 | 1,676,726 | 23,616 | 460.4 | <0.001 |
| Run | 1 | 1896 | 1896 | 36.9 | <0.001 |
| Repeatability | 215 | 11,029 | 51 | ||
| Total | 287 | 1,689,651 |
| Source | DF | SS | MS | F | p |
|---|---|---|---|---|---|
| Time | 71 | 146,939 | 2070 | 50.66 | <0.001 |
| Run | 1 | 495 | 495 | 12.12 | <0.001 |
| Run | 71 | 2900 | 41 | 6.24 | <0.001 |
| Repeatability | 144 | 943 | 6.6 | ||
| Total | 287 | 121,577 |
| Source | Standard Deviation (σ) | Study Variation (SV = 6σ) | % Study Variation (%SV) |
|---|---|---|---|
| Total Gage R&R | 8.01 | 48.04 | 10.38 |
| Repeatability | 7.16 | 42.97 | 9.28 |
| Reproducibility | 3.58 | 21.47 | 4.64 |
| Run | 3.58 | 21.47 | 4.64 |
| Part-To-Part | 76.75 | 160.52 | 99.46 |
| Total variation | 77.17 | 463.02 | 100.00 |
| ndc value | 13.51 |
| Source | Standard Deviation (σ) | Study Variation (6σ) | % Study Variation |
|---|---|---|---|
| Total Gage R&R | 5.18 | 31.09 | 22.42 |
| Repeatability | 2.56 | 15.36 | 11.08 |
| Reproducibility | 4.51 | 27.04 | 19.50 |
| Run | 1.78 | 10.66 | 7.69 |
| Run | 4.14 | 24.85 | 17.92 |
| Part-To-Part | 22.52 | 135.12 | 97.45 |
| Total variation | 23.11 | 138.65 | 100.00 |
| ndc value | 6.13 |
| Parameters | Diet | SEM | p | ||
|---|---|---|---|---|---|
| CTR | PPP | PPS | |||
| Gas production | |||||
| A | 2.53 | 8.89 | 7.12 | 3.87 | 0.514 |
| B | 11.86 Aa | 11.00 Ab | 9.69 B | 0.23 | 0.001 |
| Methane production | |||||
| A | 127 | 123 | 121 | 3 | 0.366 |
| B | 13.50 B | 11.88 C | 15.63 A | 0.57 | 0.004 |
| C | 1.14 | 1.18 | 1.14 | 0.09 | 0.939 |
| Items | Diet | SEM | p | ||
|---|---|---|---|---|---|
| CTR | PPP | PPS | |||
| pH | 6.86 B | 6.85 B | 6.90 A | 0.01 | 0.006 |
| Organic matter degradability (%) | 42.89 A | 37.33 B | 36.11 C | 0.16 | 0.001 |
| NDF degradability (%) | 40.05 | 42.30 | 39.62 | 0.94 | 0.160 |
| Gas production 24 h (mL/3g feed) | 272 Aa | 257 Ab | 227 B | 4.65 | 0.001 |
| Methane production 24 h (mL/3g feed) | 83.70 A | 85.70 A | 75.00 B | 1.89 | 0.009 |
| Methane/gas ratio (%) | 30.80 | 33.30 | 33.00 | 1.01 | 0.220 |
| Gas production 24 h (mL/g OMi) | 118 Aa | 110 Ab | 88 B | 2.66 | 0.001 |
| Methane production 24 h (mL/g OMi) | 36.20 A | 36.60 A | 29.10 B | 0.68 | 0.001 |
| Gas production 24 h (mL/g OMd) | 223 B | 294 A | 291 A | 15.07 | 0.013 |
| Methane production 24 h (ml/g OMd) | 73.30 B | 98.00 A | 94.10 A | 4.55 | 0.010 |
| Group | Phylum | Genera | ||
|---|---|---|---|---|
| R-Value | p-Value | R-Value | p-Value | |
| CTR-PPP | −0.08333 | 0.541 | −0.10417 | 0.587 |
| CTR-PPS | −0.14583 | 0.708 | −0.07292 | 0.503 |
| PPP-PPS | −0.10417 | 0.672 | 0.04167 | 0.437 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gannuscio, R.; Maniaci, G.; Todaro, M. Gas Endeavour Device for the Real-Time In Vitro Measurement of Carbon Dioxide and Methane Emissions Associated with Sheep Diets with Prickly Pear By-Products. Fermentation 2025, 11, 543. https://doi.org/10.3390/fermentation11090543
Gannuscio R, Maniaci G, Todaro M. Gas Endeavour Device for the Real-Time In Vitro Measurement of Carbon Dioxide and Methane Emissions Associated with Sheep Diets with Prickly Pear By-Products. Fermentation. 2025; 11(9):543. https://doi.org/10.3390/fermentation11090543
Chicago/Turabian StyleGannuscio, Riccardo, Giuseppe Maniaci, and Massimo Todaro. 2025. "Gas Endeavour Device for the Real-Time In Vitro Measurement of Carbon Dioxide and Methane Emissions Associated with Sheep Diets with Prickly Pear By-Products" Fermentation 11, no. 9: 543. https://doi.org/10.3390/fermentation11090543
APA StyleGannuscio, R., Maniaci, G., & Todaro, M. (2025). Gas Endeavour Device for the Real-Time In Vitro Measurement of Carbon Dioxide and Methane Emissions Associated with Sheep Diets with Prickly Pear By-Products. Fermentation, 11(9), 543. https://doi.org/10.3390/fermentation11090543








