Chitosan-Based Edible Films as Innovative Preservation Tools for Fermented and Dairy Products
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Edible Film Preparation
Combination of Chia Seed and Essential Oils
Production of Edible Films with Chitosan
Combination of Chitosan and Essential Oils (Rosemary Oil, Thyme Oil)
Combination of Chitosan, Chia Seed, and Essential Oils (Rosemary Oil and Thyme Oil)
2.2.2. Production of Cecil Cheese
Traditional Production
Industrial Production
Preparation of Edible Film Solutions for Coating Cecil Cheese
Coating of Cecil Cheeses with Edible Films
Storage and Analytical Procedure
2.3. Analyses and Methods
2.3.1. Antimicrobial Activity Analyses of Edible Films
Detection of Salmonella spp.
Determination of Bacillus subtilis
Analyses of Cecil Cheese Coated with Edible Films
2.3.2. Physical Analyses of Cecil Cheese
Color Analysis
Water Activity (aw) Analysis
2.3.3. Chemical Analyses of Cecil Cheese
pH Analysis
Titratable Acidity Analysis
Dry Matter Analysis
2.3.4. Microbiological Analyses of Cecil Cheese
Total Aerobic Mesophilic Bacteria Count
Yeast and Mold Count
Coliform Bacteria Count
Lactic Acid Bacteria Count
2.3.5. Sensory Analysis of Cecil Cheese
2.3.6. Texture Profile Analysis (TPA)
2.3.7. Statistical Analysis
3. Results and Discussions
3.1. Evaluation of Antimicrobial Activity Results of Edible Films
3.1.1. Evaluation of the Physicochemical Analysis Results of Cecil Cheese Samples
3.1.2. Measurement of the Color Characteristics of Cecil Cheese Samples
3.1.3. Evaluation of the Microbiological Data of Cecil Cheese Samples
3.1.4. Evaluation of the Sensory Analysis of Cecil Cheese Samples
3.1.5. Evaluation of Texture Analysis Results of Cecil Cheeses
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fox, P.F.; Guinee, T.P.; Cogan, T.M.; McSweeney, P.L.H. Fundamentals of Cheese Science, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Karagul-Yuceer, Y.; Hayaloglu, A.A. Traditional Cheeses: A Rich Heritage of Global Gastronomy. Foods 2021, 10, 1625. [Google Scholar]
- Hayaloglu, A.A.; Karagul-Yuceer, Y. Cheese: Pasta-Filata Cheeses. In Encyclopedia of Dairy Sciences, 3rd ed.; Academic Press: Cambridge, MA, USA, 2021; pp. 457–465. [Google Scholar]
- Kesenkas, H.; Akbulut, N.; Yerlikaya, O. Civil (Cecil) cheese: A traditional Turkish pasta-filata type cheese. J. Ethn. Foods 2020, 7, 28. [Google Scholar]
- Ozturk, H.I.; Oner, Z. The effect of starter culture on the characteristics of Civil cheese. Int. J. Dairy Technol. 2019, 72, 563–571. [Google Scholar]
- Cetinkaya, F.; Soyutemiz, G.E. Microbiological and chemical properties of Civil cheese, a traditional Turkish cheese. J. Food Sci. Technol. 2018, 55, 1187–1194. [Google Scholar]
- Gok, V.; Akkaya, L. Prevalence and antimicrobial resistance of Listeria monocytogenes and Staphylococcus aureus in traditional Turkish cheeses. Food Control 2020, 111, 107075. [Google Scholar]
- Bintsis, T. Microbial spoilage of dairy products: A review. Dairy Sci. Technol. 2018, 98, 457–474. [Google Scholar]
- Realini, C.E.; Marcos, B. Active and intelligent packaging systems for a modern society. Meat Sci. 2014, 98, 404–419. [Google Scholar] [CrossRef] [PubMed]
- Shankar, S.; Rhim, J.W. Preparation of antimicrobial and functional poly(lactide) composite films and their applications for active food packaging. Int. J. Biol. Macromol. 2021, 189, 696–705. [Google Scholar]
- Atarés, L.; Chiralt, A. Essential oils as additives in biodegradable films and coatings for active food packaging. Trends Food Sci. Technol. 2016, 48, 51–62. [Google Scholar] [CrossRef]
- Hassan, B.; Chatha, S.A.S.; Hussain, A.I.; Zia, K.M.; Akhtar, N. Recent advances on polysaccharides, lipids and protein based edible films and coatings: A review. Int. J. Biol. Macromol. 2018, 109, 1095–1107. [Google Scholar] [CrossRef]
- Galus, S.; Kadzińska, J. Food applications of emulsion-based edible films and coatings. Trends Food Sci. Technol. 2015, 45, 273–283. [Google Scholar] [CrossRef]
- Costa, M.J.; Maciel, C.; Teixeira, J.A.; Vicente, A.A. Use of edible coatings and films in the protection of cheese. In Edible Food Packaging; CRC Press: Boca Raton, FL, USA, 2019; pp. 245–270. [Google Scholar]
- Ke, C.L.; Deng, F.S. A review on the applications of chitosan and its derivatives in the food industry. J. Food Drug Anal. 2019, 27, 819–831. [Google Scholar]
- Youssef, A.M.; El-Sayed, S.M. Bionanocomposites for active and smart food packaging. In Nanomaterials in Food Packaging; Elsevier: Amsterdam, The Netherlands, 2021; pp. 1–31. [Google Scholar]
- Ribeiro-Santos, R.; Andrade, M.; de Melo, N.R.; Sanches-Silva, A. Use of essential oils in active food packaging: A review. Food Bioprocess Technol. 2017, 10, 1–15. [Google Scholar]
- Leyva-López, N.; Gutiérrez-Grijalva, E.P.; Heredia, J.B. Essential oils of oregano and rosemary: A review of their antimicrobial activity and potential use in food preservation. Food Bioprocess Technol. 2017, 10, 1745–1761. [Google Scholar]
- Kavas, N.; Kavas, G.; Gök, V. Effect of sodium alginate coating enriched with thyme and rosemary essential oils on the quality of Feta cheese. J. Food Process. Preserv. 2020, 44, e14663. [Google Scholar]
- Mehdizadeh, T.; Langroodi, A.M.; Ramezani, S. The effect of edible coatings made of whey protein concentrate, cress seed gum, and cinnamon essential oil on the quality of low-fat fresh cheese. J. Food Sci. 2021, 86, 205–215. [Google Scholar]
- Artiga-Artigas, M.; Acevedo-Fani, A.; Martín-Belloso, O. Improving the shelf life of low-fat cut cheese using nanoemulsion-based edible coatings containing oregano essential oil and mandarin fiber. Food Control 2017, 76, 1–12. [Google Scholar] [CrossRef]
- Conte, A.; Scrocco, C.; Sinigaglia, M.; Del Nobile, M.A. Innovative active packaging systems to prolong the shelf life of mozzarella cheese. J. Dairy Sci. 2007, 90, 2126–2131. [Google Scholar] [CrossRef]
- Çelik, S. Preservation of traditional methods in Civil cheese production. J. Turk. Culin. Cult. Res. 2010, 8, 55–62. [Google Scholar]
- Akat, Z. Characterization and Antimicrobial Activity of Nanocomposite Films Produced with Chitosan/Quince Seed Mucilage. Master’s Thesis, Firat University, Elazig, Türkiye, 2023. [Google Scholar]
- Zhang, Y.; Pan, T.; Yang, Z. Flexible polyethylene terephthalate/polyaniline composite paper with bending durability and effective electromagnetic shielding performance. Chem. Eng. J. 2020, 389, 124433. [Google Scholar] [CrossRef]
- Koca, N.; Metin, M. Technology and main quality criteria of Civil cheese. J. Dairy Technol. 2004, 18, 27–32. [Google Scholar]
- Ekinci, R.; Çağlar, A. Erzurum civil peynirlerinin mikrobiyolojik ve bazı kimyasal özellikleri. Gıda 2004, 29, 43–47. [Google Scholar]
- Koca, N.; Metin, M. Civil peynirinin teknolojisi ve başlıca kalite kriterleri. Süt Teknol. Derg. 2004, 18, 27–32. [Google Scholar]
- Bostan, K.; Tayar, M. Cecil peynirinde raf ömrü boyunca mikrobiyolojik kalite değişimi. Gıda 2012, 37, 95–101. [Google Scholar]
- ISO 6579-1:2017; Microbiology of the Food Chain—Horizontal Method for the Detection, Enumeration and Serotyping of Salmonella—Part 1: Detection of Salmonella spp. ISO: Geneva, Switzerland, 2017.
- Claus, D.; Berkeley, R.C.W. Genus Bacillus Cohn 1872. In Bergey’s Manual of Systematic Bacteriology; Williams & Wilkins: Philadelphia, PA, USA, 1986; Volume 2, pp. 1105–1139. [Google Scholar]
- Kurt, A.; Çakmakçı, S.; Durmuş, S. Analytical Methods in Milk and Dairy Products; Atatürk University Publications: Erzurum, Türkiye, 2012. [Google Scholar]
- Özdemir, S.; Sert, S. Microbiological Analysis Methods in Foods; Atatürk University Publications: Erzurum, Türkiye, 2001. [Google Scholar]
- ISO 21527-1; Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Enumeration of Yeasts and Moulds—Part 1: Colony Count Technique in Products with Water Activity Greater than 0.95. ISO: Geneva, Switzerland, 2008.
- Sengül, M.; Degirmenci, M.; Erkaya, T. Compositional and Microbiological Characteristics During Ripening of Cecil Cheese, a Traditional Turkish Cheese. Asian J. Chem. 2009, 21, 3087. [Google Scholar]
- Sıçramaz, H.; Güven, O.T.; Can, A.; Ayar, A.; Gül, Y. Impact of different starter cultures and Lactobacillus helveticus on volatile components, chemical and sensory properties of pasta filata cheese. Curr. Res. Food Sci. 2022, 5, 1009–1016. [Google Scholar] [CrossRef]
- Eroglu, A.; Dogan, M.; Toker, O.S.; Yilmaz, M.T. Classification of Kashar cheeses based on their chemical, color and instrumental textural characteristics using principal component and hierarchical cluster analysis. Int. J. Food Prop. 2015, 18, 909–921. [Google Scholar] [CrossRef]
- Coma, V. Bioactive packaging technologies for extended shelf life of meat-based products. Meat Sci. 2008, 78, 90–103. [Google Scholar] [CrossRef]
- Kong, M.; Chen, X.G.; Xing, K.; Park, H.J. Antimicrobial properties of chitosan and mode of action: A state of the art review. Int. J. Food Microbiol. 2010, 144, 51–63. [Google Scholar] [CrossRef]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef]
- Tavassoli-Kafrani, E.; Shekarchizadeh, H.; Masoudpour-Behabadi, M. Development of edible films and coatings from alginates and carrageenans. Carbohydr. Polym. 2016, 137, 360–374. [Google Scholar] [CrossRef]
- Liu, H.; Jin, Z.; An, L.; Wang, D.; Meng, Y.; Yang, A.; Zhang, H.; Zhang, Y. A reproducible and self-repairable ionic skin with robust performance retention enabled by modulating the noncovalent interactions. ACS Appl. Polym. Mater. 2025, 7, 1459–1470. [Google Scholar] [CrossRef]
- Santoyo, S.; Cavero, S.; Jaime, L.; Ibáñez, E.; Señoráns, F.J.; Reglero, G. Chemical composition and antimicrobial activity of Rosmarinus officinalis L. essential oil obtained via supercritical fluid extraction. J. Food Prot. 2005, 68, 790–795. [Google Scholar] [CrossRef]
- Yüceer, M. The Effect of Chitosan Coating Containing Thyme Oil on the Microbial Quality of Kashar Cheese. Master’s Thesis, Atatürk University, Erzurum, Türkiye, 2017. [Google Scholar]
- Karakuş, K. The Effect of an Edible Film Containing Chitosan and Thyme oil on Tulum Cheese. Master’s Thesis, Ege University, Izmir, Türkiye, 2021. [Google Scholar]
- Özkan, M. The Effect of Chitosan and Some Essential Oils on the Shelf Life of White Cheese. Master’s Thesis, Akdeniz University, Antalya, Türkiye, 2018. [Google Scholar]
- Karagöz, Ş.; Demirdöven, A. Effect of chitosan coatings with and without Stevia rebaudiana and modified atmosphere packaging on quality of cold stored fresh-cut apples. LWT 2019, 108, 332–337. [Google Scholar] [CrossRef]
- Kavas, G.; Kavas, N.; Saygili, D. The effects of thyme and clove essential oil fortified edible films on the physical, chemical and microbiological characteristics of kashar cheese. J. Food Qual. 2015, 38, 405–412. [Google Scholar] [CrossRef]
- Dikbaş, N.; Senguel, M.; Ertugay, M. Microbiological and Compositional Characteristics of Cecil Cheese Collected from Households in Erzurum, Turkey. Asian J. Chem. 2010, 22, 483–491. [Google Scholar]
- Cagri, A.; Ustunol, Z.; Ryser, E.T. Antimicrobial edible films and coatings. J. Food Prot. 2004, 67, 833–848. [Google Scholar] [CrossRef]
- Iqbal, M.W.; Riaz, T.; Yasmin, I.; Leghari, A.A.; Amin, S.; Bilal, M.; Qi, X. Chitosan-based materials as edible coating of cheese: A review. Starch-Stärke 2021, 73, 2100088. [Google Scholar] [CrossRef]
- Ressutte, J.B.; da Silva Saranti, T.F.; de Moura, M.R.; dos Santos Pozza, M.S.; da Silva Scapim, M.R.; Stafussa, A.P.; Madrona, G.S. Citric acid incorporated in a chitosan film as an active packaging material to improve the quality and duration of matured cheese shelf life. J. Dairy Res. 2022, 89, 201–207. [Google Scholar] [CrossRef]
- Casalini, R.; Ghisoni, F.; Bonetti, L.; Fiorati, A.; De Nardo, L. Development of acid-free chitosan films in food coating applications: Provolone cheese as a case study. Carbohydr. Polym. 2024, 331, 121842. [Google Scholar] [CrossRef]
- Arfat, Y.A.; Benjakul, S.; Vongkamjan, K. Development and characterization of gelatin-based active nanocomposite films incorporated with essential oils. Food Hydrocoll. 2015, 52, 220–229. [Google Scholar] [CrossRef]
- Bleoancă, I.; Enachi, E.; Borda, D. Thyme antimicrobial effect in edible films with high pressure thermally treated whey protein concentrate. Foods 2020, 9, 855. [Google Scholar] [CrossRef]
- Molina-Hernández, J.B.; Echeverri-Castro, A.; Martínez-Correa, H.A.; Andrade-Mahecha, M.M. Edible coating based on achira starch containing garlic/oregano oils to extend the shelf life of double cream cheese. Rev. Fac. Nac. De Agron. Medellín 2020, 73, 9099–9108. [Google Scholar] [CrossRef]
- Pires, A.; Pietruszka, H.; Bożek, A.; Szkolnicka, K.; Gomes, D.; Díaz, O.; Cobos, A.; Pereira, C. Sheep’s Second Cheese Whey Edible Coatings with Oregano and Clary Sage Essential Oils Used as Sustainable Packaging Material in Cheese. Foods 2024, 13, 674. [Google Scholar] [CrossRef]
- Pathare, P.B.; Opara, U.L.; Al-Said, F.A.-J. Color measurement and analysis in fresh and processed foods: A review. Food Bioprocess Technol. 2013, 6, 36–60. [Google Scholar] [CrossRef]
- Casalini, S.; Giacinti Baschetti, M. The use of essential oils in chitosan or cellulose-based materials for the production of active food packaging solutions: A review. J. Sci. Food Agric. 2023, 103, 1021–1041. [Google Scholar] [CrossRef]
- Vega, E.N.; Ciudad-Mulero, M.; Fernández-Ruiz, V.; Barros, L.; Morales, P. Natural sources of food colorants as potential substitutes for artificial additives. Foods 2023, 12, 4102. [Google Scholar] [CrossRef]
- Martillanes, S.; Rocha-Pimienta, J.; Cabrera-Bañegil, M.; Martín-Vertedor, D.; Delgado-Adámez, J. Application of phenolic compounds for food preservation: Food additive and active packaging. In Phenolic Compounds-Biological Activity; IntechOpen: London, UK, 2017. [Google Scholar]
- Yangılar, F. Chitosan/whey protein (CWP) Edible films efficiency for controlling mould growth and on microbiological, chemical and sensory properties during storage of Göbek Kashar cheese. Korean J. Food Sci. Anim. Resour. 2015, 35, 216. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Yang, Y.; Aziz, T.; Al-Asmari, F.; Sameeh, M.Y.; Lin, L. Exploring the potential of chlorogenic acid/chitosan nanoparticle-loaded edible films with photodynamic technology for Mongolian cheese application. Int. J. Biol. Macromol. 2024, 279, 135091. [Google Scholar] [CrossRef] [PubMed]
- Fajardo, P.; Martins, J.T.; Fuciños, C.; Pastrana, L.; Teixeira, J.A.; Vicente, A.A. Evaluation of a chitosan-based edible film as carrier of natamycin to control the growth of Aspergillus niger in cheese. J. Food Eng. 2010, 101, 349–356. [Google Scholar] [CrossRef]
- Kavas, G.; Kesenkas, H. Kekik yağı içeren yenilebilir filmin beyaz peynirin mikrobiyel kalitesi üzerine etkisi. Gıda 2018, 43, 345–352. [Google Scholar]
- Perdones, Á.; Vargas, M.; Atarés, L.; Chiralt, A. Physical, antioxidant and antimicrobial properties of chitosan–cinnamon leaf oil films as affected by oleic acid. Food Hydrocoll. 2014, 36, 256–264. [Google Scholar] [CrossRef]
- Gammariello, D.; Conte, A.; Buonocore, G.G.; Del Nobile, M.A. Bio-based nanocomposite coating to preserve quality of Fior di latte cheese. J. Dairy Sci. 2011, 94, 5298–5304. [Google Scholar] [CrossRef]
- Uzunsoy, I. Recent Advances in Antimicrobial Food Packaging for Cheese Preservation. In Proceedings of the Agribalkan 2023 V. Balkan Agricultural Congress, Edirne, Türkiye, 20–23 September 2023; p. 653. [Google Scholar]
- Fadlıoğlu, S.; Ertan, R. Gıda tüketiminde alışkanlıkların etkisi. Gıda 2013, 38, 309–315. [Google Scholar]
- Lawless, H.T.; Heymann, H. Sensory Evaluation of Food: Principles and Practices; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- Tomičić, R.M.; Čabarkapa, I.S.; Varga, A.O.; Tomičić, Z.M. Antimicrobial activity of essential oils against Listeria monocytogenes. Food Feed Res. 2018, 45, 37–44. [Google Scholar] [CrossRef]
- Vargas-Ramella, M.; Echegaray, N.; Campagnol, P.C.B.; Lorenzo, J.M. Natural Polymer-Based Coatings for Animal-Derived Products: A Review of Applications, Functionality, Characterization, and Challenges. Foods 2025, 14, 2255. [Google Scholar] [CrossRef]
- Kıngır, S.; Kardeş, N. The effect of media on healthy eating behavior. Safran J. Cult. Tour. Res. 2019, 2, 163–176. [Google Scholar]
- El-Sayed, S.M.; Youssef, A.M. Emergence of cheese packaging by edible coatings for enhancing its shelf-life. J. Food Meas. Charact. 2024, 18, 5265–5280. [Google Scholar] [CrossRef]
- Bourne, M. Food Texture and Viscosity: Concept and Measurement; Elsevier: Amsterdam, The Netherlands, 2002. [Google Scholar]
- Gao, X.; Zheng, Y.; Zhong, Y.; Zhou, R.; Li, B.; Ma, M. Preparation and characterization of novel chitosan coatings to reduce changes in quality attributes and physiochemical and water characteristics of Mongolian cheese during cold storage. Foods 2023, 12, 2731. [Google Scholar] [CrossRef]
- Ghasemian, S.O.; Ahmadi-Dastgerdi, A.; Abdollahi, A.; Tirtashi, F.E.; Zokaei, M.; Fallah, N.; Najafabadi, P.I.; Dolatyari, F. The effect of active packaging film based on chitosan containing rosemary (Rosmarinus officinalis L.) extract on cheese shelf life. J. Food Biochem. 2024, 2024, 2108707. [Google Scholar] [CrossRef]







| Group Code | Material Composition |
|---|---|
| A | Chia seed |
| C | Chitosan |
| A1 | Chia seed + Rosemary oil |
| C1 | Chitosan + Rosemary oil |
| A2 | Chia seed + Thyme oil |
| C2 | Chitosan + Thyme oil |
| AC1 | Chia seed + Chitosan + Rosemary oil |
| AC2 | Chia seed + Chitosan + Thyme oil |
| Edible Film | Salmonella | Bacillus subtilis |
|---|---|---|
| Chia seed | – | – |
| Chitosan | 23 mm | 21 mm |
| Chia seed + Rosemary oil | – | – |
| Chia seed + Thyme oil | – | – |
| Chitosan + Rosemary oil | 16 mm | – |
| Chitosan + Thyme oil | 32 mm | 33 mm |
| Chia seed + Chitosan + Rosemary oil | 20 mm | 16 mm |
| Chia seed + Chitosan + Thyme oil | 22 mm | 23 mm |
| Parameter | Sample Code | Storage Days | |||
|---|---|---|---|---|---|
| Day 1 | Day 15 | Day 30 | Day 45 | ||
| Water activity (aw) | GK | 0.88 Db ± 0.01 | 0.92 Ba ± 0.02 | 0.84 Fc ± 0.01 | 0.82 Ec ± 0.01 |
| GKİ | 0.88 Db ± 0.01 | 0.93 Aa ± 0.01 | 0.87 Db ± 0.01 | 0.80 Dc ± 0.01 | |
| GBİ | 0.88 Cb ± 0.01 | 0.90 CDa ± 0.01 | 0.87 Dc ± 0.01 | 0.87 Ac ± 0.01 | |
| GKE | 0.89 Cab ± 0.01 | 0.93 Ca ± 0.05 | 0.88 Cab ± 0.01 | 0.87 Ab ± 0.01 | |
| EK | 0.87 Da ± 0.01 | 0.87 Da ± 0.01 | 0.86 Eb ± 0.01 | 0.86 Cb ± 0.01 | |
| EKİ | 0.93 Ba ± 0.01 | 0.89 CDb ± 0.01 | 0.88 Bc ± 0.01 | 0.87 ABd ± 0.01 | |
| EBİ | 0.94 Aa ± 0.01 | 0.88 Dc ± 0.01 | 0.89 Ab ± 0.01 | 0.86 BCd ± 0.04 | |
| EKE | 0.94 Aa ± 0.01 | 0.89 CDb ± 0.01 | 0.89 Ab ± 0.01 | 0.86 BCc ± 0.04 | |
| pH | G K | 5.78 Ea ± 0.02 | 5.67 Fa ± 0.04 | 5.43 Cb ± 0.10 | 5.26 CDc ± 0.06 |
| GKİ | 6.31 Aa ± 0.02 | 5.66 Fb ± 0.04 | 5.47 Cc ± 0.01 | 5.20 Dd ± 0.05 | |
| GBİ | 6.30 Aa ± 0.01 | 5.88 Db ± 0.03 | 5.64 ABc ± 0.04 | 5.37 Ad ± 0.03 | |
| GKE | 5.96 Da ± 0.02 | 5.73 Eb ± 0.03 | 5.64 ABc ± 0.01 | 5.34 ABd ± 0.01 | |
| EK | 6.01 Ca ± 0.03 | 5.94 Cb ± 0.01 | 5.57 Bc ± 0.01 | 5.30 ABCd ± 0.04 | |
| EKİ | 6.16 Ba ± 0.05 | 6.09 Bb ± 0.01 | 5.58 ABc ± 0.01 | 5.28 BCd ± 0.01 | |
| EBİ | 6.30 Aa ± 0.01 | 6.04 Bb ± 0.01 | 5.61 ABc ± 0.01 | 5.32 ABCd ± 0.02 | |
| EKE | 6.32 Aa ± 0.01 | 6.19 Ab ± 0.01 | 5.65 Ac ± 0.01 | 5.36 Ad ± 0.04 | |
| Titratable acidity (% lactic acid) | G K | 1.14 Bd ± 0.02 | 1.23 Dc ± 0.01 | 1.39 BCb ± 0.05 | 1.66 Ba ± 0.03 |
| GKİ | 1.09 CDd ± 0.01 | 1.25 BCDc ± 0.02 | 1.37 Cb ± 0.01 | 1.57 Ca ± 0.01 | |
| GBİ | 1.16 ABd ± 0.02 | 1.32 Ac ± 0.02 | 1.44 ABCb ± 0.02 | 1.66 Ba ± 0.01 | |
| GKE | 1.18 Ac ± 0.01 | 1.29 ABCb ± 0.06 | 1.37 Cb ± 0.07 | 1.67 Ba ± 0.03 | |
| EK | 1.09 CDd ± 0.01 | 1.27 ABCDc ± 0.01 | 1.39 BCb ± 0.04 | 1.61 Ca ± 0.02 | |
| EKİ | 1.10 Cd ± 0.02 | 1.30 ABc ± 0.02 | 1.50 Ab ± 0.03 | 1.78 Aa ± 0.01 | |
| EBİ | 1.06 DEd ± 0.01 | 1.27 ABCDc ± 0.01 | 1.47 ABb ± 0.06 | 1.74 Aa ± 0.01 | |
| EKE | 1.05 Ed ± 0.02 | 1.24 CDc ± 0.03 | 1.45 ABCb ± 0.03 | 1.74 Aa ± 0.04 | |
| Dry matter (%) | GK | 51.59 BCd ± 0.53 | 52.80 Dc ± 0.30 | 55.27 Bb ± 0.76 | 56.64 Aa ± 0.29 |
| GKİ | 49.95 Dd ± 0.05 | 51.46 Ec ± 0.29 | 53.57 Cb ± 0.34 | 55.44 Ca ± 0.43 | |
| GBİ | 50.81 CDc ± 1.70 | 53.13 CDb ± 0.12 | 55.70 ABa ± 0.21 | 56.65 Aa ± 0.15 | |
| GKE | 51.88 BCc ± 0.61 | 53.39 Cb ± 0.36 | 55.88 ABa ± 0.15 | 56.41 Aa ± 0.03 | |
| EK | 52.48 ABc ± 0.14 | 54.81 Bb ± 0.17 | 56.17 Aa ± 0.21 | 56.37 ABa ± 0.51 | |
| EKİ | 53.29 Ad ± 0.18 | 54.61 Bc ± 0.27 | 55.23 Bb ± 0.12 | 55.90 BCa ± 0.11 | |
| EBİ | 53.54 Ac ± 0.23 | 55.73 Ab ± 0.28 | 56.37 Aa ± 0.40 | 56.89 Aa ± 0.13 | |
| EKE | 52.80 ABd ± 0.09 | 54.62 Bc ± 0.43 | 55.86 ABb ± 0.14 | 56.48 Aa ± 0.18 | |
| Parameter | Sample Code | Storage Days | |||
|---|---|---|---|---|---|
| Day 1 | Day 15 | Day 30 | Day 45 | ||
| G K | 81.09 BCa ± 1.32 | 72.03 Ab ± 1.36 | 80.60 Aa ± 1.19 | 79.24 Aa ± 0.77 | |
| GKİ | 80.69 Ca ± 1.12 | 74.00 Ab ± 1.42 | 70.71 Dc ± 2.07 | 78.36 ABa ± 1.36 | |
| GBİ | 82.07 BCa ± 1.11 | 79.32 Ab ± 0.22 | 72.36 CDc ± 1.02 | 73.36 Cc ± 1.02 | |
| L* | GKE | 81.43 BCa ± 0.69 | 79.80 Aa ± 0.06 | 73.10 Cb ± 1.45 | 74.10 Cb ± 0.86 |
| EK | 79.01 Da ± 0.98 | 76.24 Aa ± 0.91 | 80.45 Aa ± 0.16 | 79.38 Aa ± 1.39 | |
| EKİ | 82.43 ABa ± 0.17 | 76.06 Ac ± 1.23 | 79.55 ABb ± 0.76 | 73.72 Cd ± 0.39 | |
| EBİ | 82.22 ABCa ± 0.24 | 74.73 Ab ± 1.62 | 78.54 ABab ± 0.52 | 69.12 Dc ± 0.78 | |
| EKE | 83.70 Aa ± 0.05 | 80.52 Ab ± 1.47 | 77.87 Bc ± 0.66 | 77.19 Bc ± 0.49 | |
| G K | 7.21 BCDa ± 0.71 | 6.68 Aa ± 1.61 | 7.16 ABCa ± 0.64 | 6.71 BCDa ± 0.16 | |
| GKİ | 7.73 ABa ± 0.43 | 7.45 Aa ± 0.24 | 7.93 ABa ± 0.26 | 6.57 CDb ± 0.37 | |
| a* | GBİ | 8.10 Aa ± 0.60 | 7.02 Ab ± 0.10 | 6.79 Cb ± 0.74 | 6.32 Db ± 0.24 |
| GKE | 7.37 ABa ± 0.38 | 6.72 Aa ± 0.09 | 7.03 BCa ± 0.70 | 7.54 ABa ± 0.34 | |
| EK | 6.53 Da ± 0.10 | 6.98 Aa ± 0.09 | 7.62 ABCa ± 0.23 | 7.27 ABCa ± 0.53 | |
| EKİ | 7.50 ABa ± 0.09 | 8.01 Aa ± 0.14 | 8.10 Aa ± 0.36 | 7.78 Aa ± 0.93 | |
| EBİ | 6.60 CDbc ± 0.06 | 7.00 Aab ± 0.37 | 7.57 ABCa ± 0.49 | 6.29 Dc ± 0.05 | |
| EKE | 7.27 BCa ± 0.09 | 7.05 Aa ± 0.42 | 6.95 Ca ± 0.02 | 6.77 BCDa ± 0.51 | |
| G K | 26.48 ABa ± 0.59 | 23.74 Ab ± 2.02 | 26.50 Aa ± 0.24 | 26.61 ABa ± 0.58 | |
| GKİ | 26.12 ABa ± 0.55 | 26.25 Aa ± 0.61 | 25.36 Aa ± 1.18 | 26.12 Ba ± 0.94 | |
| GBİ | 26.85 Aa ± 0.13 | 25.78 Ab ± 0.06 | 26.08 Ab ± 0.50 | 26.08 Bb ± 0.45 | |
| b* | GKE | 25.90 Bab ± 0.36 | 24.69 Ab ± 0.14 | 25.84 Aab ± 1.87 | 27.27 ABa ± 1.24 |
| EK | 26.12 ABa ± 0.58 | 27.99 Aa ± 0.45 | 27.09 Aa ± 0.71 | 28.32 Aa ± 2.45 | |
| EKİ | 26.87 Aab ± 0.11 | 24.78 Ab ± 1.20 | 25.80 Ab ± 1.63 | 28.33 Aa ± 0.95 | |
| EBİ | 26.48 ABa ± 0.62 | 24.88 Ab ± 0.31 | 26.66 Aa ± 0.70 | 27.18 ABa ± 0.08 | |
| EKE | 26.53 ABb ± 0.10 | 25.74 Ac ± 0.36 | 27.31 Aa ± 0.21 | 27.47 ABa ± 0.13 | |
| G K | 74.77 ABa ± 1.27 | 63.53 Db ± 1.18 | 74.88 ABa ± 1.17 | 75.84 ABCa ± 0.41 | |
| GKİ | 73.51 BCa ± 0.71 | 65.44 Cb ± 0.63 | 72.63 Ca ± 0.67 | 74.10 Ca ± 1.82 | |
| GBİ | 73.22 Ca ± 1.13 | 63.77 Db ± 0.22 | 75.23 Aa ± 1.31 | 75.23 ABCa ± 1.31 | |
| Δη | GKE | 74.12 BCa ± 0.62 | 64.12 Db ± 0.62 | 74.78 ABa ± 0.92 | 74.54 BCa ± 0.17 |
| EK | 75.95 Aa ± 0.52 | 76.21 Aa ± 0.11 | 73.77 ABCb ± 0.14 | 75.57 ABCa ± 0.47 | |
| EKİ | 74.40 BCab ± 0.13 | 74.23 Bab ± 0.15 | 73.00 BCb ± 1.51 | 75.65 ABCa ± 0.43 | |
| EBİ | 75.99 Aa ± 0.22 | 75.93 Aa ± 0.21 | 74.16 ABCb ± 0.83 | 76.93 Aa ± 0.86 | |
| EKE | 74.66 ABab ± 0.14 | 74.55 Bb ± 0.14 | 74.97 ABab ± 1.44 | 76.34 ABa ± 0.89 | |
| Parameter | Sample Code | Storage Days | |||
|---|---|---|---|---|---|
| Day 1 | Day 15 | Day 30 | Day 45 | ||
| Total mesophilic aerobic bacteria (log CFU/g) | G K | 4.25 Aa ± 0.02 | 3.32 Ac ± 0.25 | 3.22 Ad ± 0.02 | 3.43 Ab ± 0.05 |
| GKİ | 4.11 Ca ± 0.01 | 3.22 Bc ± 0.25 | 3.20 Ac ± 0.15 | 3.30 Bb ± 0.01 | |
| GBİ | 4.05 Da ± 0.01 | 3.11 Dc ± 0.15 | 3.11 Cc ± 0.01 | 3.28 Bb ± 0.01 | |
| GKE | 4.06 Da ± 0.03 | 3.09 Dd ± 0.05 | 3.18 Ac ± 0.15 | 3.26 Cb ± 0.01 | |
| EK | 3.14 Ec ± 0.02 | 3.09 Dd ± 0.01 | 3.16 Bb ± 0.01 | 3.24 Ca ± 0.57 | |
| EKİ | 4.20 Ba ± 0.01 | 3.20 BCb ± 0.11 | 2.94 Dc ± 0.01 | 3.21 Db ± 0.15 | |
| EBİ | 3.07 Fc ± 0.01 | 3.20 BCa ± 0.05 | 2.86 Ed ± 0.05 | 3.13 Eb ± 0.15 | |
| EKE | 3.05 Fc ± 0.03 | 3.19 Cb ± 0.05 | 2.79 Fd ± 0.01 | 3.21 Da ± 0.57 | |
| Total yeast and mold count (log CFU/g) | G K | 3.12 Ab ± 0.05 | 3.41 Aa ± 0.15 | 2.21 Fd ± 0.01 | 2.85 Ac ± 0.57 |
| GKİ | 2.84 Cc ± 0.15 | 3.11 Da ± 0.01 | 2.91 Db ± 0.02 | 2.73 Cd ± 0.11 | |
| GBİ | 3.00 Ba ± 0.57 | 2.82 Fb ± 0.34 | 2.81 Eb ± 0.02 | 2.62 Dc ± 0.04 | |
| GKE | 2.82 Db ± 0.02 | 2.71 Gc ± 0.15 | 2.90 Da ± 0.01 | 2.73 Cc ± 0.57 | |
| EK | 2.55 Ed ± 0.02 | 3.31 Ba ± 0.15 | 3.06 Bb ± 0.05 | 2.71 Cc ± 0.17 | |
| EKİ | 1.20 Hc ± 0.01 | 3.23 Ca ± 0.02 | 3.25 Aa ± 0.05 | 2.71 Cb ± 0.57 | |
| EBİ | 2.05 Fc ± 0.01 | 2.92 Ea ± 0.02 | 2.94 Ca ± 0.01 | 2.70 Cb ± 0.11 | |
| EKE | 1.62 Gd ± 0.02 | 2.70 Gc ± 0.20 | 2.90 Da ± 0.03 | 2.81 Bb ± 0.17 | |
| Total coliform count (log CFU/g) | G K | <1 | <1 | <1 | <1 |
| GKİ | <1 | <1 | <1 | <1 | |
| GBİ | <1 | <1 | <1 | <1 | |
| GKE | <1 | <1 | <1 | <1 | |
| EK | <1 | <1 | <1 | <1 | |
| EKİ | <1 | <1 | <1 | <1 | |
| EBİ | <1 | <1 | <1 | <1 | |
| EKE | <1 | <1 | <1 | <1 | |
| Total lactic acid bacteria count (log CFU/g) | G K | 4.13 Ba ± 0.15 | 2.74 Fd ± 0.05 | 3.00 Cc ± 0.57 | 3.16 DEb ± 0.57 |
| GKİ | 3.26 Ca ± 0.01 | 3.11 Db ± 0.05 | 3.01 Cc ± 0.02 | 3.25 Ba ± 0.05 | |
| GBİ | 4.16 Aa ± 0.57 | 2.86 Ed ± 0.01 | 3.01 Cc ± 0.01 | 3.20 Cb ± 0.05 | |
| GKE | 4.16 Aa ± 0.57 | 2.58 Gd ± 0.11 | 3.02 Cc ± 0.02 | 3.16 Eb ± 0.07 | |
| EK | 2.74 Ed ± 0.57 | 3.26 Ca ± 0.05 | 3.00 Cc ± 0.05 | 3.17 Db ± 0.17 | |
| EKİ | 2.62 Gb ± 0.05 | 3.56 Aa ± 0.01 | 3.56 Aa ± 0.01 | 3.55 Aa ± 0.05 | |
| EBİ | 2.77 Db ± 0.51 | 3.54 Ba ± 0.01 | 3.56 Aa ± 0.11 | 3.56 Aa ± 0.50 | |
| EKE | 2.72 Fd ± 0.57 | 3.27 Cc ± 0.05 | 3.43 Bb ± 0.57 | 3.56 Aa ± 0.18 | |
| Parameter | Sample Code | Storage Days | |||
|---|---|---|---|---|---|
| Day 1 | Day 15 | Day 30 | Day 45 | ||
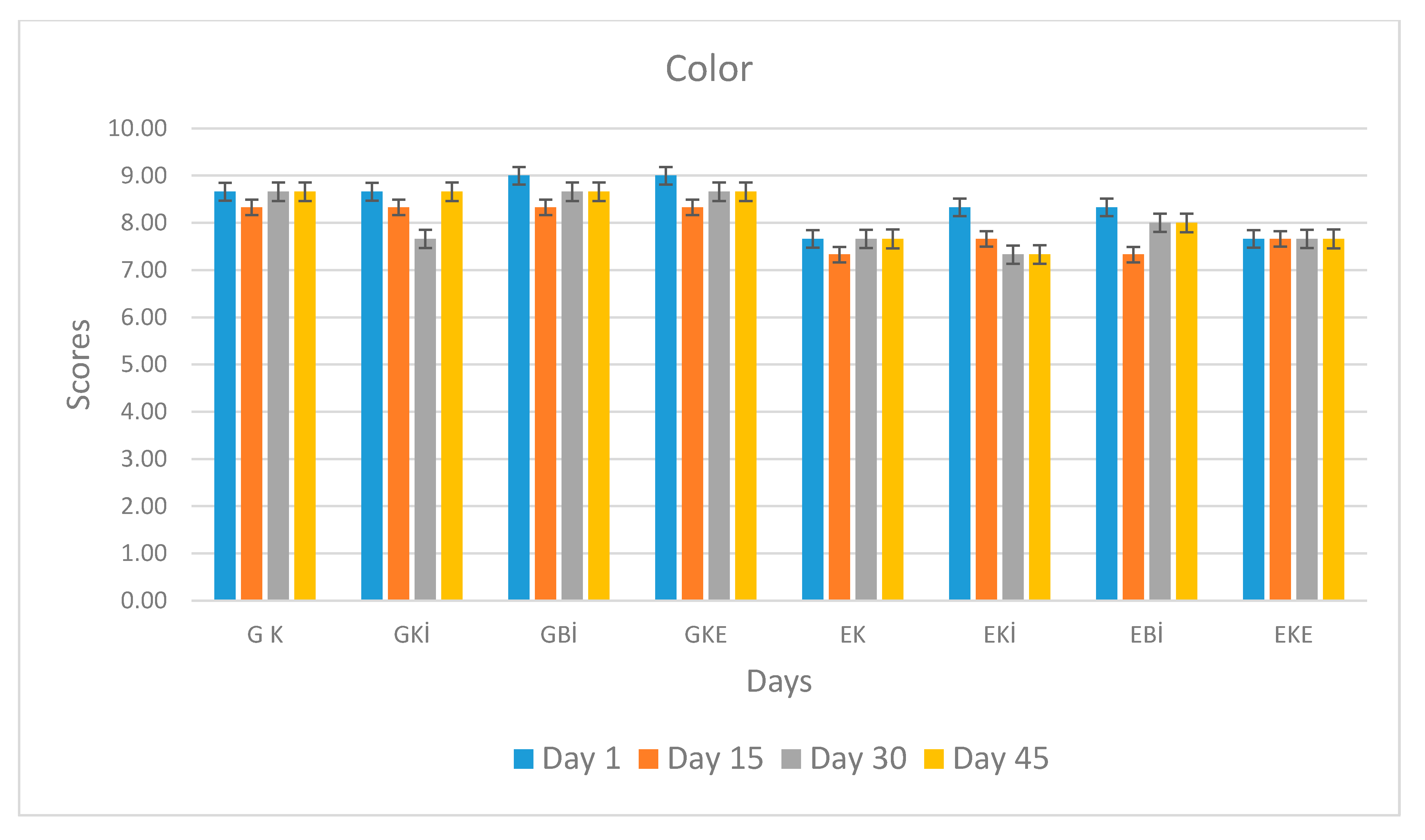
| Color | G K | 8.66 Aa ± 0.27 | 8.33 Aa ± 0.57 | 8.66 Aa ± 0.15 | 8.66 Aa ± 0.05 |
| GKİ | 8.66 Aa ± 0.27 | 8.33 Aa ± 0.57 | 7.66 ABa ± 0.57 | 8.66 Aa ± 0.19 | |
| GBİ | 8.78 Aa ± 0.03 | 8.33 Aa ± 0.57 | 8.66 Aa ± 0.12 | 8.66 Aa ± 0.21 | |
| GKE | 8.15 Aa ± 0.05 | 8.33 Aa ± 0.57 | 8.66 Aa ± 0.24 | 8.66 Aa ± 0.06 | |
| EK | 7.66 Ba ± 0.57 | 7.33 Aa ± 0.57 | 7.66 ABa ± 0.57 | 7.66 ABa ± 0.57 | |
| EKİ | 8.33 ABa ± 0.57 | 7.66 Aa ± 0.57 | 7.33 Ba ± 0.57 | 7.33 Ba ± 0.57 | |
| EBİ | 8.33 ABa ± 0.57 | 7.33 Ab ± 0.57 | 8.00 ABab ± 0.50 | 8.00 ABab ± 0.50 | |
| EKE | 7.66 Ba ± 0.57 | 7.66 Aa ± 0.57 | 7.66 ABa ± 0.57 | 7.66 ABa ± 0.57 | |
| Texture | G K | 8.52 Aa ± 0.05 | 8.33 Aa ± 0.18 | 8.48 Aa ± 0.27 | 8.30 Aa ± 0.57 |
| GKİ | 8.23 ABa ± 0.37 | 8.40 Aa ± 0.15 | 7.33 Ba ± 0.57 | 8.00 ABa ± 0.50 | |
| GBİ | 7.33 CDb ± 0.57 | 8.00 Aab ± 0.35 | 8.36 Aa ± 0.27 | 8.36 Aa ± 0.25 | |
| GKE | 7.00 Db ± 0.15 | 7.66 Ab ± 0.57 | 8.56 Aa ± 0.37 | 8.46 Aa ± 0.07 | |
| EK | 7.66 BCDa ± 0.57 | 7.33 Aa ± 0.57 | 7.33 Ba ± 0.50 | 7.33 Ba ± 0.57 | |
| EKİ | 8.33 ABa ± 0.57 | 7.33 Aa ± 0.57 | 7.66 ABa ± 0.57 | 7.66 ABa ± 0.57 | |
| EBİ | 8.00 BCa ± 0.27 | 7.33 Aa ± 0.57 | 7.66 ABa ± 0.57 | 7.66 ABa ± 0.57 | |
| EKE | 7.66 BCDa ± 0.57 | 7.66 Aa ± 0.57 | 7.66 ABa ± 0.57 | 7.66 ABa ± 0.57 | |
| Odor | G K | 8.61 Aa ± 0.15 | 8.66 Aa ± 0.22 | 8.46 Aa ± 0.13 | 8.46 Aa ± 0.27 |
| GKİ | 8.53 Aa ± 0.17 | 8.66 Aa ± 0.57 | 8.72 Aa ± 0.01 | 8.33 ABa ± 0.57 | |
| GBİ | 6.00 Ba ± 1.00 | 6.66 Ba ± 0.57 | 7.33 BCa ± 0.57 | 7.33 BCDa ± 0.57 | |
| GKE | 6.66 Ba ± 0.57 | 5.33 Cb ± 0.57 | 6.66 BCa ± 0.57 | 6.66 CDa ± 0.57 | |
| EK | 7.00 Ba ± 0.17 | 7.00 Ba ± 1.00 | 7.66 Ba ± 0.57 | 7.66 ABCa ± 0.57 | |
| EKİ | 8.33 Aa ± 0.37 | 7.33 Ba ± 0.57 | 7.66 Ba ± 0.57 | 7.66 ABCa ± 0.57 | |
| EBİ | 6.33 Ba ± 1.52 | 6.33 BCa ± 0.57 | 6.66 BCa ± 0.57 | 6.66 CDa ± 0.57 | |
| EKE | 6.66 Ba ± 0.57 | 6.33 Ba ± 0.57 | 6.33 Ca ± 0.57 | 6.33 Da ± 0.57 | |
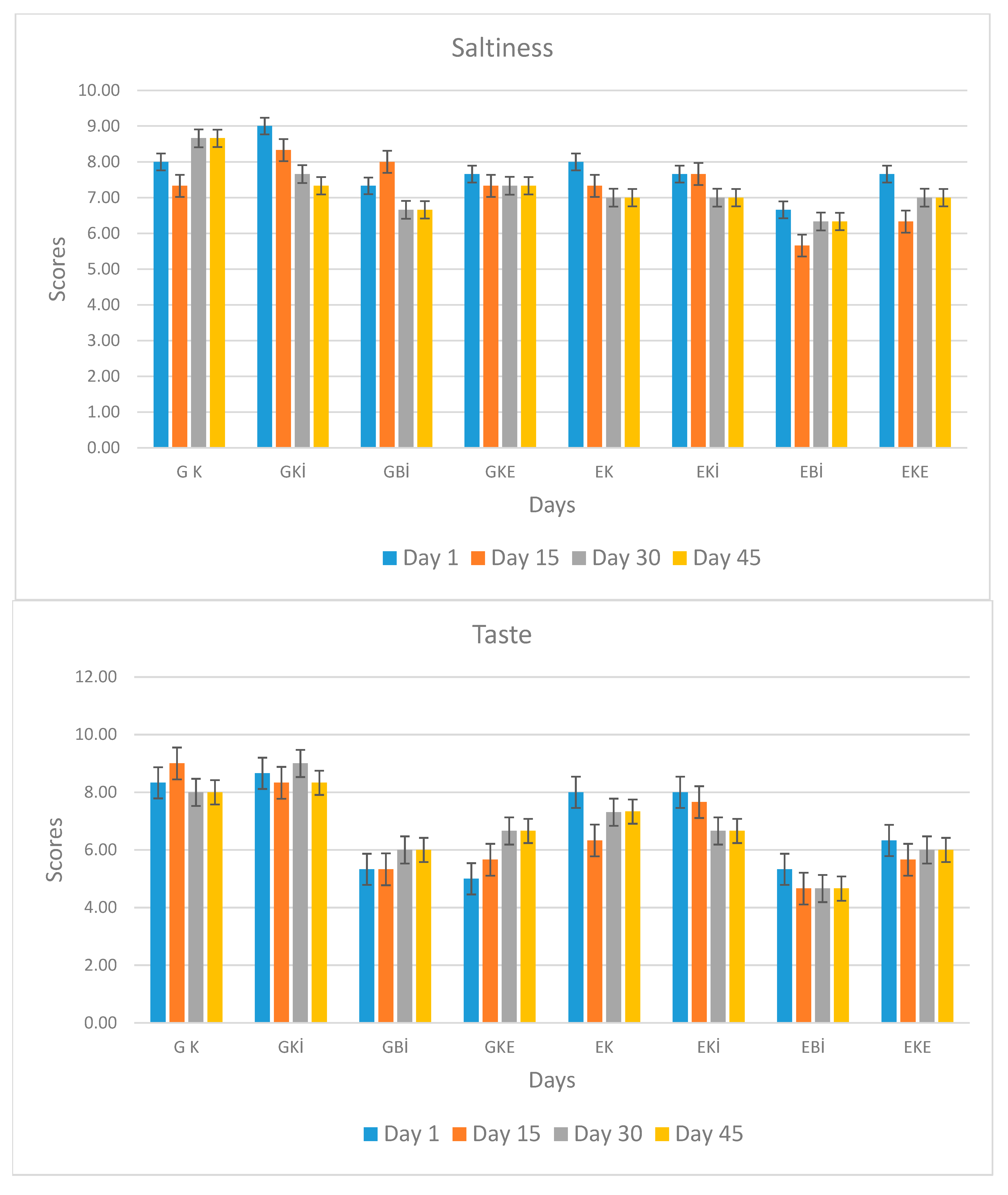
| Taste | G K | 8.33 Ab ± 0.23 | 8.71 Aa ± 0.35 | 8.00 Bb ± 0.15 | 8.00 Ab ± 0.50 |
| GKİ | 8.60 Aa ± 0.27 | 8.33 ABa ± 0.57 | 8.72 Aa ± 0.17 | 8.33 Aa ± 0.57 | |
| GBİ | 5.33 Ba ± 1.15 | 5.33 CDa ± 0.57 | 6.00 Da ± 1.00 | 6.00 Ca ± 1.00 | |
| GKE | 5.00 Bb ± 1.00 | 5.66 CDab ± 0.57 | 6.66 CDa ± 0.57 | 6.66 BCa ± 0.57 | |
| EK | 8.00 Aa ± 0.50 | 6.33 Cb ± 0.57 | 7.33 BCa ± 0.57 | 7.33 ABa ± 0.57 | |
| EKİ | 8.00 Aa ± 1.00 | 7.66 Ba ± 0.57 | 6.66 CDa ± 0.57 | 6.66 BCa ± 0.57 | |
| EBİ | 5.33 Ba ± 0.57 | 4.66 Da ± 0.57 | 4.66 Ea ± 0.57 | 4.66 Da ± 0.57 | |
| EKE | 6.33 Ba ± 0.57 | 5.66 CDa ± 0.57 | 6.00 Da ± 0.17 | 6.00 Ca ± 0.35 | |
| Saltiness | G K | 8.00 ABa ± 0.56 | 7.33 ABa ± 0.57 | 8.46 Aa ± 0.14 | 8.50 Aa ± 0.17 |
| GKİ | 8.42 Aa ± 0.35 | 8.33 Aab ± 0.18 | 7.66 Bbc ± 0.57 | 7.33 Bc ± 0.57 | |
| GBİ | 7.33 BCab ± 0.57 | 8.00 Aa ± 0.37 | 6.66 BCb ± 0.57 | 6.66 Bb ± 0.57 | |
| GKE | 7.66 BCa ± 0.57 | 7.33 ABa ± 0.57 | 7.33 BCa ± 0.57 | 7.33 Ba ± 0.57 | |
| EK | 8.00 ABa ± 0.35 | 7.33 ABb ± 0.57 | 7.00 BCb ± 0.17 | 7.00 Bb ± 0.37 | |
| EKİ | 7.66 BCa ± 0.57 | 7.66 Aa ± 0.57 | 7.00 BCa ± 0.50 | 7.00 Ba ± 0.17 | |
| EBİ | 6.66 Ca ± 0.57 | 5.66 Ca ± 0.57 | 6.33 Ca ± 0.57 | 6.33 Ba ± 0.57 | |
| EKE | 7.66 BCa ± 0.57 | 6.33 BCa ± 1.15 | 7.00 BCa ± 1.00 | 7.00 Ba ± 1.00 | |
| Overall acceptability | G K | 8.60 Aa ± 0.15 | 8.33 Aa ± 0.51 | 8.50 Aa ± 0.32 | 8.45 Aa ± 0.15 |
| GKİ | 8.53 Aa ± 0.15 | 8.53 Aa ± 0.27 | 8.33 ABa ± 0.32 | 8.73 Aa ± 0.25 | |
| GBİ | 5.66 Ca ± 0.57 | 6.00 Ca ± 0.17 | 6.00 Ca ± 1.00 | 6.00 Ca ± 1.00 | |
| GKE | 6.66 Ba ± 0.57 | 6.66 BCa ± 0.57 | 7.33 Ba ± 0.57 | 7.33 Ba ± 0.57 | |
| EK | 7.33 Ba ± 0.57 | 7.33 ABa ± 0.57 | 8.00 ABa ± 0.50 | 8.00 ABa ± 0.17 | |
| EKİ | 8.56 Aa ± 0.11 | 7.33 ABb ± 0.57 | 7.33 Bb ± 0.57 | 7.33 Bb ± 0.57 | |
| EBİ | 6.66 Ba ± 0.57 | 6.66 BCa ± 0.57 | 5.66 Ca ± 1.15 | 5.66 Ca ± 1.15 | |
| EKE | 7.00 Ba ± 0.50 | 7.00 BCa ± 1.00 | 7.33 Ba ± 0.57 | 7.33 Ba ± 0.57 | |
| Purchase Intention | G K | 1.00 Ca ± 0.15 | 1.00 Ba ± 0.25 | 1.00 Ca ± 0.15 | 1.00 Da ± 0.25 |
| GKİ | 1.00 Ca ± 0.32 | 1.00 Ba ± 0.55 | 1.00 Ca ± 0.25 | 1.00 Da ± 0.15 | |
| GBİ | 2.33 Ba ± 0.57 | 2.66 Aa ± 0.57 | 2.66 Aa ± 0.57 | 2.66 ABa ± 0.57 | |
| GKE | 2.00 Ba ± 0.15 | 2.33 Aa ± 0.57 | 2.66 Aa ± 0.57 | 2.66 ABa ± 0.57 | |
| EK | 1.33 Ca ± 0.43 | 1.33 Ba ± 0.57 | 1.66 Ba ± 0.57 | 1.66 CDa ± 0.57 | |
| EKİ | 1.00 Cb ± 0.15 | 1.33 Bb ± 0.57 | 2.00 Ba ± 0.15 | 2.00 BCa ± 0.17 | |
| EBİ | 3.00 Aa ± 0.50 | 3.00 Aa ± 0.50 | 3.00 Aa ± 0.25 | 3.00 Aa ± 0.15 | |
| EKE | 3.00 Aa ± 0.50 | 3.00 Aa ± 0.50 | 3.00 Aa ± 0.25 | 2.66 ABa ± 0.57 | |
| Parameter | Sample Code | Storage Days | |
|---|---|---|---|
| Day 1 | Day 45 | ||
| G K | 1256.14 Da ± 64.88 | 543.27 Fb ± 16.64 | |
| GKİ | 680.60 Eb ± 58.84 | 1145.96 Da ± 62.81 | |
| GBİ | 734.14 Ea ± 63.53 | 755.53 Ea ± 32.21 | |
| Hardness | GKE | 251.03 Fb ± 15.31 | 1103.32 Da ± 76.51 |
| EK | 3411.68 Aa ± 200.82 | 1469.79 BCb ± 12.02 | |
| EKİ | 2396.27 Ba ± 77.68 | 2178.78 Ab ± 68.83 | |
| EBİ | 1681.39 Ca ± 40.30 | 1565.94 Bb ± 38.27 | |
| EKE | 1185.54 Db ± 21.33 | 1405.90 Ca ± 13.13 | |
| G K | −0.20 Aa ± 0.05 | −0.66 Cb ± 0.06 | |
| GKİ | −0.52 ABa ± 0.10 | −0.56 BCa ± 0.02 | |
| Adhesiveness | GBİ | −2.89 Db ± 0.12 | −1.31 Da ± 0.05 |
| GKE | −2.33 Cb ± 0.09 | −1.47 Da ± 0.20 | |
| EK | −8.72 Eb ± 0.55 | −1.56 Da ± 0.21 | |
| EKİ | −0.15 Aa ± 0.01 | −0.21 ABa ± 0.04 | |
| EBİ | −0.29 Ab ± 0.07 | −0.10 Aa ± 0.04 | |
| EKE | −0.78 Ba ± 0.07 | −1.27 Db ± 0.10 | |
| G K | 0.99 Ca ± 0.05 | 0.97 ABa ± 0.03 | |
| GKİ | 0.87 Cb ± 0.01 | 1.01 Aa ± 0.02 | |
| GBİ | 0.88 Cb ± 0.03 | 0.96 ABCa ± 0.03 | |
| Elasticity | GKE | 4.57 Aa ± 0.74 | 1.05 Ab ± 0.05 |
| EK | 0.83 Cb ± 0.04 | 0.95 ABCa ± 0.01 | |
| EKİ | 4.39 Aa ± 0.39 | 0.88 BCb ± 0.01 | |
| EBİ | 0.99 Ca ± 0.02 | 0.98 ABa ± 0.03 | |
| EKE | 2.60 Ba ± 0.22 | 0.85 Cb ± 0.02 | |
| G K | 0.73 Da ± 0.02 | 0.76 BCa ± 0.02 | |
| GKİ | 0.74 CDb ± 0.02 | 0.82 ABa ± 0.01 | |
| GBİ | 0.76 CDb ± 0.01 | 0.85 Aa ± 0.01 | |
| Consistency | GKE | 0.78 BCa ± 0.02 | 0.78 BCa ± 0.01 |
| EK | 0.79 Ba ± 0.01 | 0.78 BCa ± 0.01 | |
| EKİ | 0.84 Aa ± 0.01 | 0.79 BCb ± 0.03 | |
| EBİ | 0.74 Db ± 0.01 | 0.79 BCa ± 0.03 | |
| EKE | 0.73 Da ± 0.02 | 0.72 Ca ± 0.03 | |
| G K | 1052.82 Da ± 45.84 | 428.97 Eb ± 12.87 | |
| GKİ | 500.26 Eb ± 27.98 | 595.14 Da ± 40.93 | |
| Chewiness | GBİ | 1401.94 Ca ± 147.07 | 631.05 Db ± 23.47 |
| GKE | 345.04 Eb ± 16.93 | 791.37 Ca ± 10.39 | |
| EK | 2790.34 Aa ± 207.14 | 1177.59 Bb ± 9.86 | |
| EKİ | 1927.28 Ba ± 34.16 | 1461.04 Ab ± 29.35 | |
| EBİ | 1279.77 CDa ± 80.54 | 758.65 Cb ± 37.77 | |
| EKE | 1142.79 CDb ± 21.04 | 1420.52 Aa ± 53.00 | |
| G K | 1330.24 Ca ± 124.10 | 416.96 Fb ± 5.59 | |
| GKİ | 466.75 Db ± 10.29 | 1246.50 CDa ± 125.66 | |
| GBİ | 1412.71 Ca ± 75.04 | 798.02 Eb ± 12.63 | |
| Gumminess | GKE | 1854.55 Ba ± 25.45 | 808.82 Eb ± 30.01 |
| EK | 3695.65 Aa ± 229.11 | 1140.50 Db ± 29.43 | |
| EKİ | 3851.00 Aa ± 68.57 | 1619.71 Bb ± 29.57 | |
| EBİ | 1367.44 Ca ± 38.37 | 1323.69 Ca ± 32.41 | |
| EKE | 1543.39 Cb ± 17.38 | 1813.42 Aa ± 25.22 | |
| G K | 0.52 BCa ± 0.02 | 0.48 Db ± 0.01 | |
| GKİ | 0.48 CDb ± 0.03 | 0.57 Aa ± 0.01 | |
| GBİ | 0.46 DEa ± 0.01 | 0.46 DEa ± 0.03 | |
| Resilience ratio | GKE | 0.44 Eb ± 0.02 | 0.46 Ea ± 0.03 |
| EK | 0.51 BCa ± 0.01 | 0.48 Db ± 0.03 | |
| EKİ | 0.56 Aa ± 0.01 | 0.48 DEb ± 0.02 | |
| EBİ | 0.52 Ba ± 0.02 | 0.53 Ba ± 0.03 | |
| EKE | 0.46 DEb ± 0.03 | 0.51 Ca ± 0.07 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seyrekoğlu, F.; Efdal, E. Chitosan-Based Edible Films as Innovative Preservation Tools for Fermented and Dairy Products. Fermentation 2025, 11, 542. https://doi.org/10.3390/fermentation11090542
Seyrekoğlu F, Efdal E. Chitosan-Based Edible Films as Innovative Preservation Tools for Fermented and Dairy Products. Fermentation. 2025; 11(9):542. https://doi.org/10.3390/fermentation11090542
Chicago/Turabian StyleSeyrekoğlu, Fadime, and Esra Efdal. 2025. "Chitosan-Based Edible Films as Innovative Preservation Tools for Fermented and Dairy Products" Fermentation 11, no. 9: 542. https://doi.org/10.3390/fermentation11090542
APA StyleSeyrekoğlu, F., & Efdal, E. (2025). Chitosan-Based Edible Films as Innovative Preservation Tools for Fermented and Dairy Products. Fermentation, 11(9), 542. https://doi.org/10.3390/fermentation11090542






