Use of Lachancea thermotolerans and Metschnikowia pulcherrima to Improve Acidity and Sensory Profile of Verdejo Wines from Different Vine Management Systems
Abstract
1. Introduction
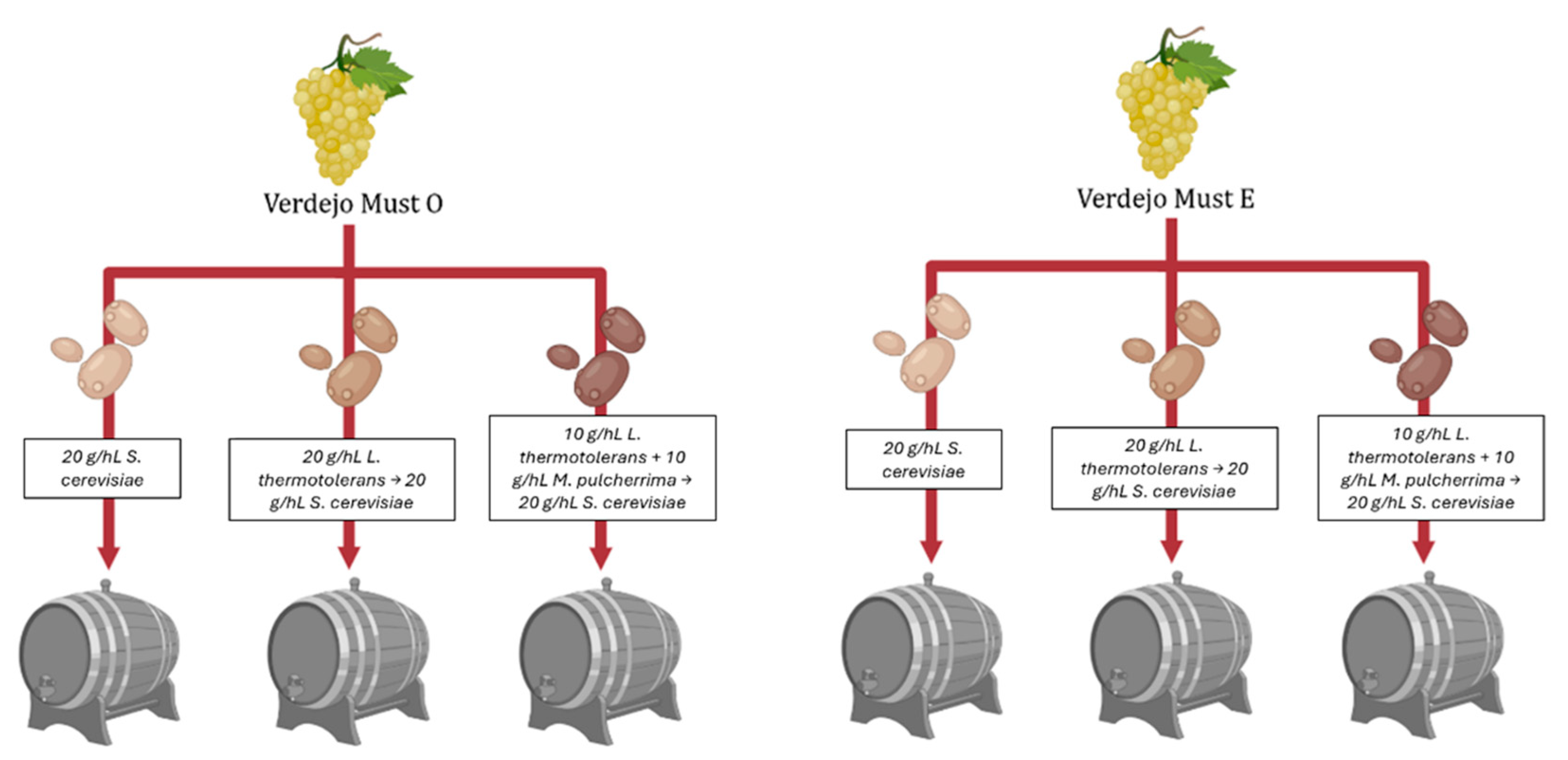
2. Materials and Methods
2.1. Verdejo White Must
2.2. Yeast Strains Used for Fermentation
2.3. Must Fermentation
2.4. Analysis of General Oenological Parameters
2.5. Enzyme Analysis
2.6. Colour Analysis
2.7. Analysis of Fermentative Volatile Compounds
2.8. Sensory Analysis
2.9. Statistical Analysis
3. Results and Discussion
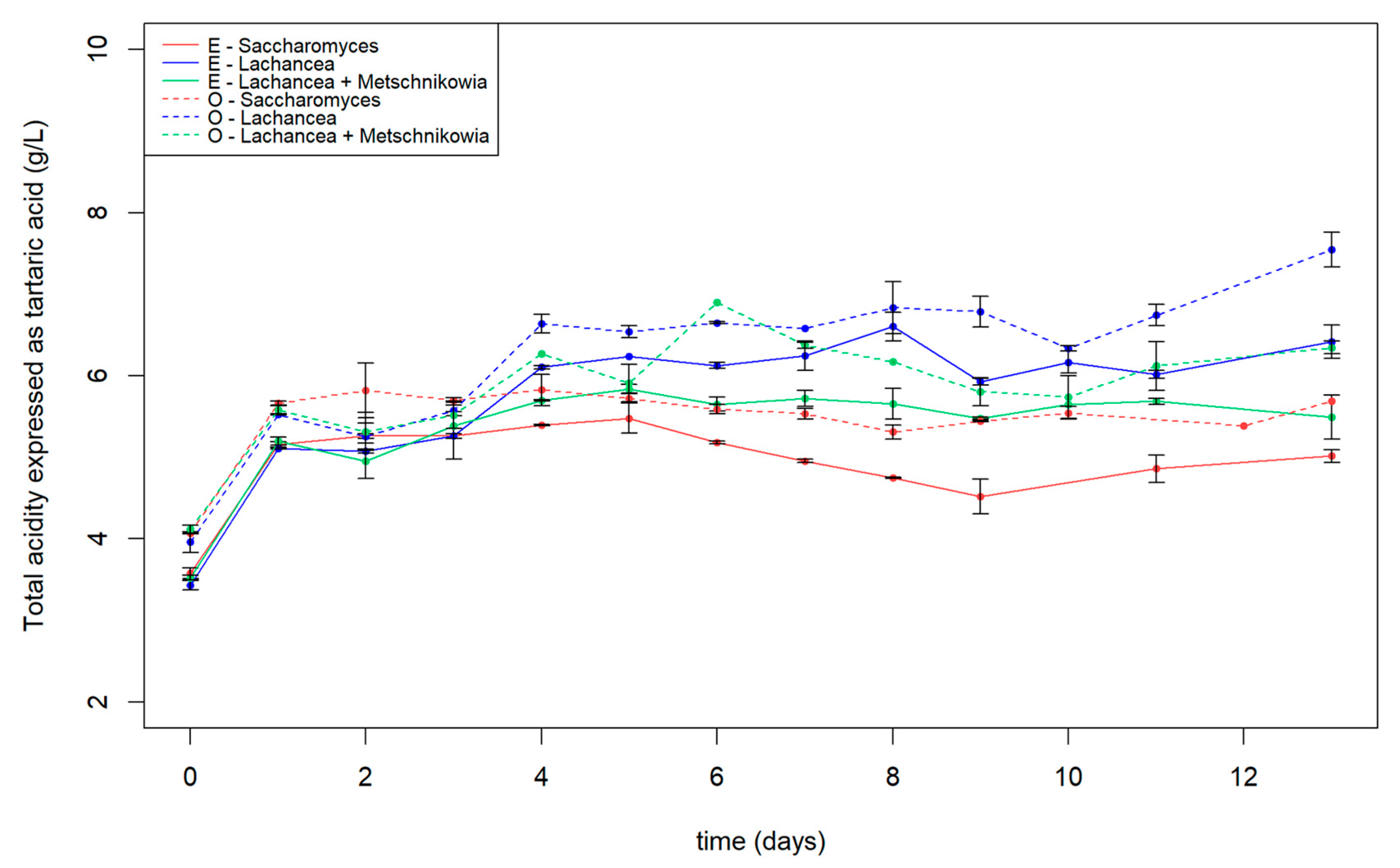
3.1. General Oenological Parameters
3.2. Enzymatic Analysis
3.3. Colour Analysis
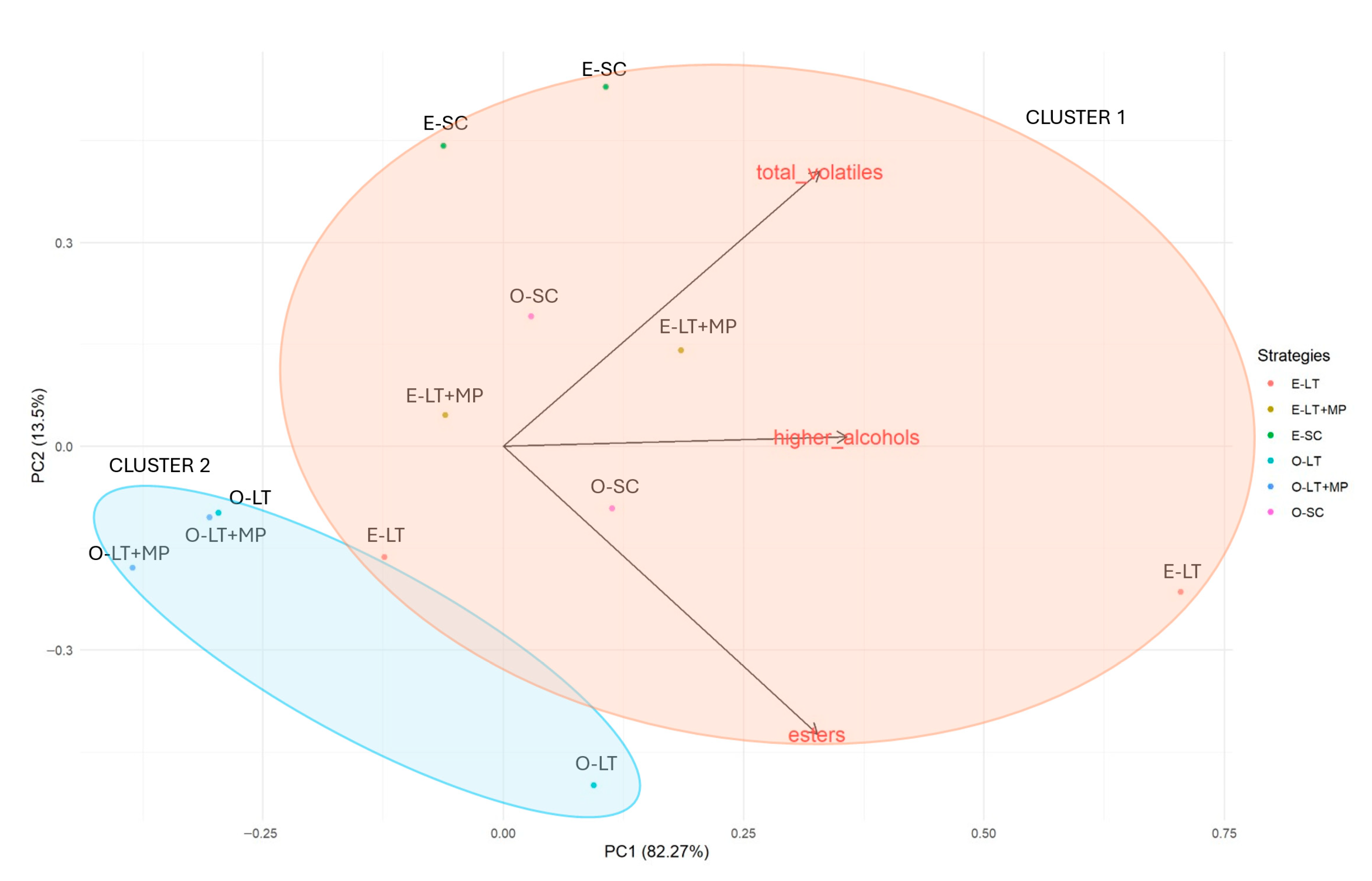
3.4. Volatile Analysis
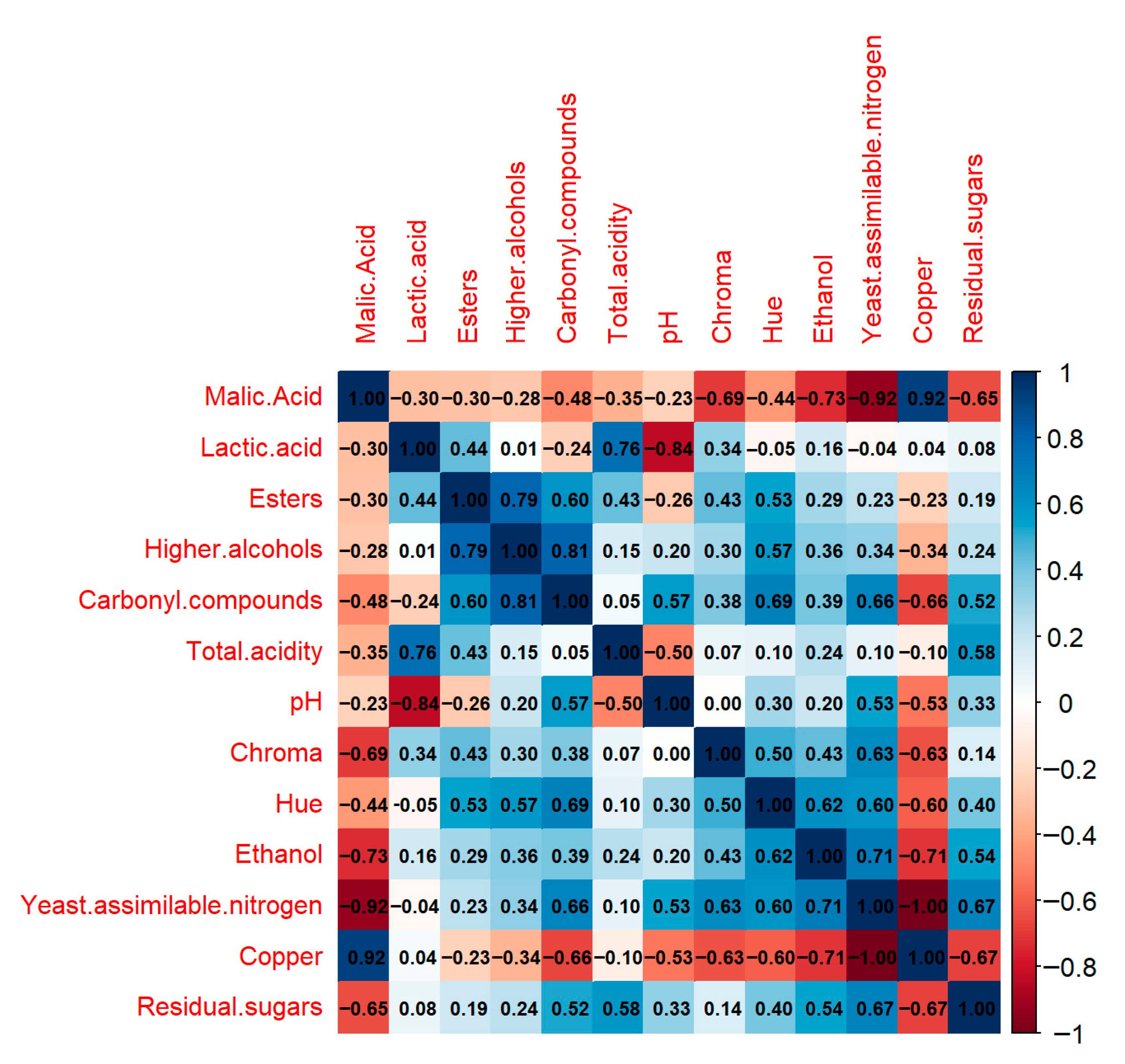
3.5. Variable Correlation
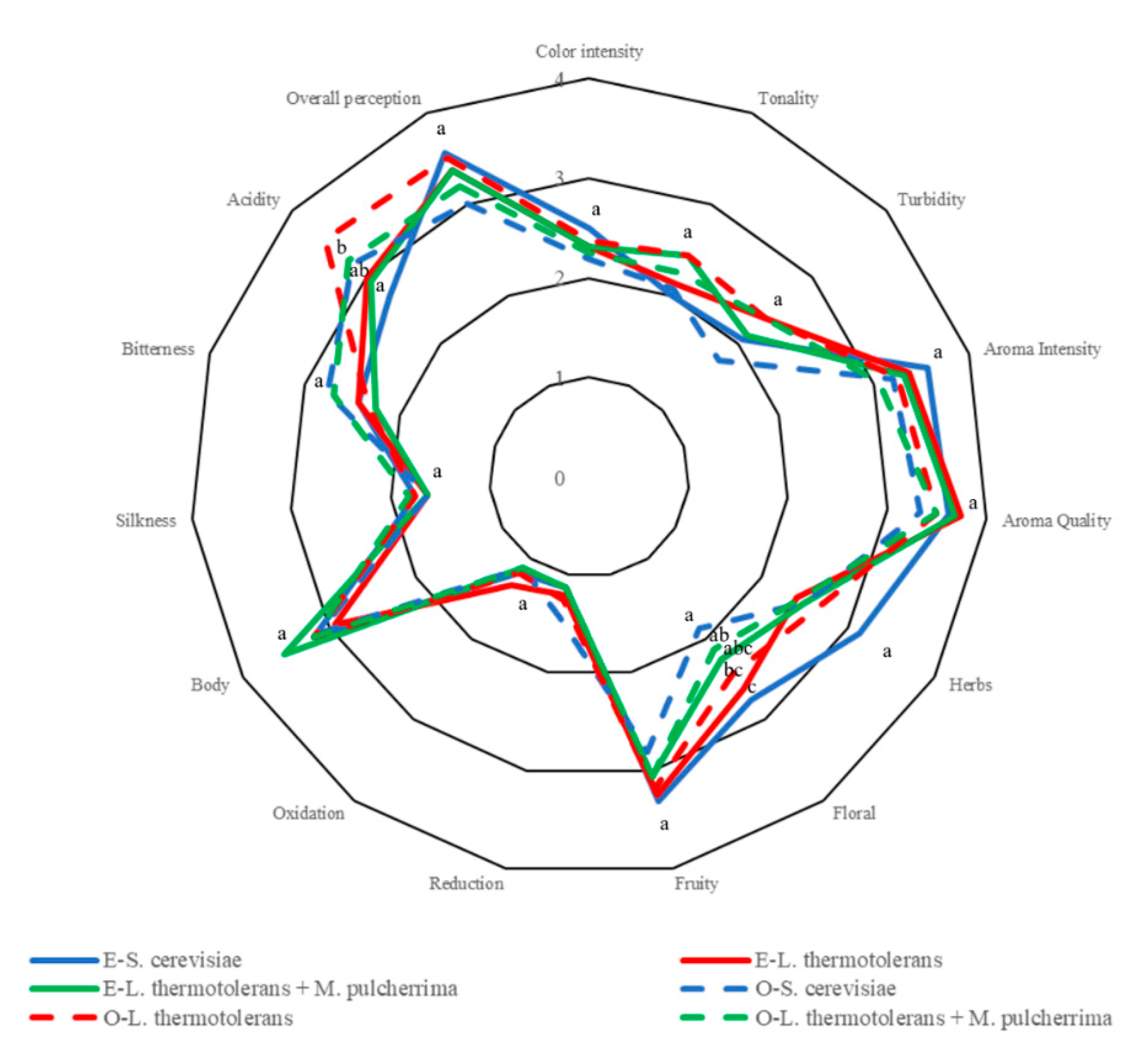
3.6. Sensory Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Lt | Lachancea thermotolerans |
| Mp | Metschnikowia pulcherrima |
References
- Morata, A.; Escott, C.; Bañuelos, M.A.; Loira, I.; del Fresno, J.M.; González, C.; Suárez-Lepe, J.A. Contribution of Non-Saccharomyces Yeasts to Wine Freshness. A Review. Biomolecules 2019, 10, 34. [Google Scholar] [CrossRef]
- Ciliberti, N.; Fermaud, M.; Roudet, J.; Rossi, V. Environmental Conditions Affect Botrytis cinerea Infection of Mature Grape Berries More Than the Strain or Transposon Genotype. Phytopathology 2015, 105, 1090–1096. [Google Scholar]
- Loureiro, V. Spoilage yeasts in the wine industry. Int. J. Food Microbiol. 2003, 86, 23–50. [Google Scholar] [CrossRef]
- García-Muñoz, S.; Muñoz-Organero, G.; Fernández-Fernández, E.; Cabello, F. Sensory characterisation and factors influencing quality of wines made from 18 minor varieties (Vitis vinifera L.). Food Qual. Prefer. 2014, 32, 241–252. [Google Scholar]
- Jolly, N.P.; Varela, C.; Pretorius, I.S. Not your ordinary yeast: Non-Saccharomyces yeasts in wine production uncovered. FEMS Yeast Res. 2014, 14, 215–237. [Google Scholar]
- Morata, A.; Loira, I.; González, C.; Escott, C. Non-Saccharomyces as Biotools to Control the Production of Off-Flavors in Wines. Molecules 2021, 26, 4571. [Google Scholar] [PubMed]
- Lachance, M.A.; Kurtzman, C.P. Lachancea. In The Yeasts; Elsevier: Amsterdam, The Netherlands, 2011; pp. 511–519. [Google Scholar]
- Ciani, M.; Beco, L.; Comitini, F. Fermentation behaviour and metabolic interactions of multistarter wine yeast fermentations. Int. J. Food Microbiol. 2006, 108, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Comitini, F.; Gobbi, M.; Domizio, P.; Romani, C.; Lencioni, L.; Mannazzu, I.; Ciani, M. Selected non-Saccharomyces wine yeasts in controlled multistarter fermentations with Saccharomyces cerevisiae. Food Microbiol. 2011, 28, 873–882. [Google Scholar] [CrossRef] [PubMed]
- Morata, A.; Bañuelos, M.A.; Vaquero, C.; Loira, I.; Cuerda, R.; Palomero, F.; González, C.; Suárez-Lepe, J.A.; Wang, J.; Han, S.; et al. Lachancea thermotolerans as a tool to improve pH in red wines from warm regions. Eur. Food Res. Technol. 2019, 245, 885–894. [Google Scholar] [CrossRef]
- Porter, T.J.; Divol, B.; Setati, M.E. Lachancea yeast species: Origin, biochemical characteristics and oenological significance. Food Res. Int. 2019, 119, 378–389. [Google Scholar] [CrossRef]
- Hranilovic, A.; Albertin, W.; Capone, D.L.; Gallo, A.; Grbin, P.R.; Danner, L.; Bastian, S.E.; Masneuf-Pomarede, I.; Coulon, J.; Bely, M.; et al. Impact of Lachancea thermotolerans on chemical composition and sensory profiles of Merlot wines. Food Chem. 2021, 349, 129015. [Google Scholar] [CrossRef]
- Morata, A.; Loira, I.; González, C.; Bañuelos, M.A.; Cuerda, R.; Heras, J.M.; Vaquero, C.; Suárez-Lepe, J.A. Biological acidification by Lachancea thermotolerans. In White Wine Technology; Elsevier: Amsterdam, The Netherlands, 2022; pp. 131–142. [Google Scholar]
- Vaquero, C.; Loira, I.; Bañuelos, M.A.; Heras, J.M.; Cuerda, R.; Morata, A. Industrial Performance of Several Lachancea thermotolerans Strains for pH Control in White Wines from Warm Areas. Microorganisms 2020, 8, 830. [Google Scholar] [CrossRef]
- Sgouros, G.; Mallouchos, A.; Filippousi, M.E.; Banilas, G.; Nisiotou, A. Molecular Characterization and Enological Potential of A High Lactic Acid-Producing Lachancea thermotolerans Vineyard Strain. Foods 2020, 9, 595. [Google Scholar] [CrossRef] [PubMed]
- Escott, C.; Vaquero, C.; Loira, I.; López, C.; González, C.; Morata, A. Synergetic Effect of Metschnikowia pulcherrima and Lachancea thermotolerans in Acidification and Aroma Compounds in Airén Wines. Foods 2022, 11, 3734. [Google Scholar] [CrossRef] [PubMed]
- Martin, V.; Valera, M.J.; Medina, K.; Boido, E.; Carrau, F. Oenological Impact of the Hanseniaspora/Kloeckera Yeast Genus on Wines—A Review. Fermentation 2018, 4, 76. [Google Scholar]
- Escott, C.; Vaquero, C.; del Fresno, J.M.; Topo, A.; Comuzzo, P.; Gonzalez, C.; Morata, A. Effect of processing Verdejo grape must by UHPH using non-Saccharomyces yeasts in the absence of SO2. Sustain. Food Technol. 2024, 2, 437–446. [Google Scholar] [CrossRef]
- Izquierdo-Cañas, P.M.; del Fresno, J.M.; Malfeito-Ferreira, M.; Mena-Morales, A.; García-Romero, E.; Heras, J.M.; Loira, I.; González, C.; Morata, A. Wine bioacidification: Fermenting Airén grape juices with Lachancea thermotolerans and Metschnikovia pulcherrima followed by sequential Saccharomyces cerevisiae inoculation. Int. J. Food Microbiol. 2025, 427, 110977. [Google Scholar]
- Sipiczki, M. Taxonomic Revision of the pulcherrima Clade of Metschnikowia (Fungi): Merger of Species. Taxonomy 2022, 2, 107–123. [Google Scholar] [CrossRef]
- Morata, A.; Loira, I.; Escott, C.; del Fresno, J.M.; Bañuelos, M.A.; Suárez-Lepe, J.A. Applications of Metschnikowia pulcherrima in Wine Biotechnology. Fermentation 2019, 5, 63. [Google Scholar] [CrossRef]
- Puyo, M.; Simonin, S.; Bach, B.; Klein, G.; Alexandre, H.; Tourdot-Maréchal, R. Bio-protection in oenology by Metschnikowia pulcherrima: From field results to scientific inquiry. Front. Microbiol. 2023, 14, 1252973. [Google Scholar] [CrossRef]
- Canonico, L.; Agarbati, A.; Galli, E.; Comitini, F.; Ciani, M. Metschnikowia pulcherrima as biocontrol agent and wine aroma enhancer in combination with a native Saccharomyces cerevisiae. LWT 2023, 181, 114758. [Google Scholar] [CrossRef]
- Türkel, S.; Korukluoğlu, M.; Yavuz, M. Biocontrol Activity of the Local Strain of Metschnikowia pulcherrima on Different Postharvest Pathogens. Biotechnol. Res. Int. 2014, 2014, 397167. [Google Scholar] [CrossRef]
- Fernández-González, M.; Di Stefano, R.; Briones, A. Hydrolysis and transformation of terpene glycosides from muscat must by different yeast species. Food Microbiol. 2003, 20, 35–41. [Google Scholar] [CrossRef]
- Zott, K.; Thibon, C.; Bely, M.; Lonvaud-Funel, A.; Dubourdieu, D.; Masneuf-Pomarede, I. The grape must non-Saccharomyces microbial community: Impact on volatile thiol release. Int. J. Food Microbiol. 2011, 151, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, C.; Lage, P.; Esteves, M.; Chambel, L.; Mendes-Faia, A.; Mendes-Ferreira, A. Molecular and Phenotypic Characterization of Metschnikowia pulcherrima Strains from Douro Wine Region. Fermentation 2018, 4, 8. [Google Scholar] [CrossRef]
- Ruiz, J.; Belda, I.; Beisert, B.; Navascués, E.; Marquina, D.; Calderón, F.; Rauhut, D.; Santos, A.; Benito, S. Analytical impact of Metschnikowia pulcherrima in the volatile profile of Verdejo white wines. Appl. Microbiol. Biotechnol. 2018, 102, 8501–8509. [Google Scholar] [CrossRef]
- Hranilovic, A.; Gambetta, J.M.; Jeffery, D.W.; Grbin, P.R.; Jiranek, V. Lower-alcohol wines produced by Metschnikowia pulcherrima and Saccharomyces cerevisiae co-fermentations: The effect of sequential inoculation timing. Int. J. Food Microbiol. 2020, 329, 108651. [Google Scholar]
- Morales, P.; Rojas, V.; Quirós, M.; Gonzalez, R. The impact of oxygen on the final alcohol content of wine fermented by a mixed starter culture. Appl. Microbiol. Biotechnol. 2015, 99, 3993–4003. [Google Scholar] [CrossRef]
- Organisation Internationale de la Vigne et du Vin (OIV). Métodos OIV-MA-AS323-04A y OIV-MA-AS323-04B para la Determinación de Dióxido de Azufre Libre y Total. In Compendio de Métodos In-ternacionales de Análisis de los Vinos y de los Mostos; OIV: Paris, Francia, 2016. [Google Scholar]
- Abalos, D.; Vejarano, R.; Morata, A.; González, C.; Suárez-Lepe, J.A. The use of furfural as a metabolic inhibitor for reducing the alcohol content of model wines. Eur. Food Res. Technol. 2011, 232, 663–669. [Google Scholar] [CrossRef]
- International Organization for Standardization. Sensory Analysis: General Guidance for the Design of Test Rooms; International Organization for Standardization: Geneva, Switzerland, 2010. [Google Scholar]
- International Organization for Standardization. Sensory Analysis: Vocabulary; International Organization for Standardization: Geneva, Switzerland, 2010. [Google Scholar]
- Errichiello, F.; Picariello, L.; Forino, M.; Blaiotta, G.; Petruzziello, E.; Moio, L.; Gambuti, A. Copper (II) Level in Musts Affects Acetaldehyde Concentration, Phenolic Composition, and Chromatic Characteristics of Red and White Wines. Molecules 2024, 29, 2907. [Google Scholar] [CrossRef]
- Bell, S.J.; Henschke, P.A. Implications of nitrogen nutrition for grapes, fermentation and wine. Aust. J. Grape Wine Res. 2005, 11, 242–295. [Google Scholar] [CrossRef]
- Lakso, A.N.; Kliewer, W.M. The Influence of Temperature on Malic Acid Metabolism in Grape Berries. II. Temperature Responses of Net Dark CO2 Fixation and Malic Acid Pools. Am. J. Enol. Vitic. 1978, 29, 145–149. [Google Scholar]
- Ferreira, A.M.; Barbosa, C.; Lage, P.; Mendes-Faia, A. The impact of nitrogen on yeast fermentation and wine quality. Ciência Técnica Vitivinícola 2011, 26, 17–32. [Google Scholar]
- Gobert, A.; Tourdot-Maréchal, R.; Sparrow, C.; Morge, C.; Alexandre, H. Influence of nitrogen status in wine alcoholic fermentation. Food Microbiol. 2019, 83, 71–85. [Google Scholar] [CrossRef]
- Jackson, R.S. Wine Science; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Rapp, A.; Versini, G. Influence of nitrogen compounds in grapes on aroma compounds of wines. In Developments in Food Science; Elsevier: Amsterdam, The Netherlands, 1995; pp. 1659–1694. [Google Scholar]
- Hazelwood, L.A.; Daran, J.M.; van Maris, A.J.A.; Pronk, J.T.; Dickinson, J.R. The Ehrlich pathway for fusel alcohol production: A century of research on Saccharomyces cerevisiae metabolism. Appl. Environ. Microbiol. 2008, 74, 2259–2266. [Google Scholar] [PubMed]
- Martínez-García, R.; García-Martínez, T.; Puig-Pujol, A.; Mauricio, J.C.; Moreno, J. Changes in sparkling wine aroma during the second fermentation under CO2 pressure in sealed bottle. Food Chem. 2017, 237, 1030–1040. [Google Scholar] [CrossRef] [PubMed]
- Vilanova, M.; Martínez, C. First study of determination of aromatic compounds of red wine from Vitis vinifera cv. Castañal grown in Galicia (NW Spain). Eur. Food Res. Technol. 2007, 224, 431–436. [Google Scholar]
- Peinado, R.A.; Moreno, J.; Bueno, J.E.; Moreno, J.A.; Mauricio, J.C. Comparative study of aromatic compounds in two young white wines subjected to pre-fermentative cryomaceration. Food Chem. 2004, 84, 585–590. [Google Scholar] [CrossRef]
- Moreno-Arribas, M.V.; Polo, M.C. Wine Chemistry and Biochemistry; Springer: New York, NY, USA, 2009. [Google Scholar]
- Cosme, F.; Filipe-Ribeiro, L.; Nunes, F. Wine Stabilisation: An Overview of Defects and Treatments. In Chemistry and Biochemistry of Winemaking, Wine Stabilization and Aging, 1st ed.; IntechOpen: London, UK, 2021; pp. 173–204. [Google Scholar]
- Lorrain, B.; Tempere, S.; Iturmendi, N.; Moine, V.; de Revel, G.; Teissedre, P.L. Influence of phenolic compounds on the sensorial perception and volatility of red wine esters in model solution: An insight at the molecular level. Food Chem. 2013, 140, 76–82. [Google Scholar] [CrossRef]
- Bartowsky, E.J.; Pretorius, I.S. Microbial Formation and Modification of Flavor and Off-Flavor Compounds in Wine. In Biology of Microorganisms on Grapes, in Must and in Wine; Springer: Berlin/Heidelberg, Germany, 2009; pp. 209–231. [Google Scholar]
- Binati, R.L.; Lemos Junior, W.J.F.; Luzzini, G.; Slaghenaufi, D.; Ugliano, M.; Torriani, S. Contribution of non-Saccharomyces yeasts to wine volatile and sensory diversity: A study on Lachancea thermotolerans, Metschnikowia spp. and Starmerella bacillaris strains isolated in Italy. Int. J. Food Microbiol. 2020, 318, 108470. [Google Scholar] [CrossRef]
- Balikci, E.K.; Tanguler, H.; Jolly, N.P.; Erten, H. Influence of Lachancea thermotolerans on cv. Emir wine fermentation. Yeast 2016, 33, 313–321. [Google Scholar] [CrossRef] [PubMed]

| Must O | Must E | |
|---|---|---|
| Density (g/L) | 1095 | 1096 |
| Brix degree (°) | 22.8 | 23 |
| pH | 3.64 | 3.71 |
| Free SO2 (mg/L) | 10 | 0 |
| Total SO2 (mg/L) | 40 | 0 |
| Cu2+ (mg/L) | 1.6 | 1.2 |
| Total acidity expressed as tartaric acid (g/L) | 5.76 | 5.03 |
| Malic acid (g/L) | 2.3 | 1.6 |
| α-NH2 nitrogen (mg/L) | 238 | 333 |
| NH4 nitrogen (mg/L) | 83 | 92 |
| Strategy | Ethanol (%vol) | pH | Glucose Plus Fructose (g/L) | Volatile Acidity (g/L Acetic Acid) | Malic Acid (g/L) | Lactic Acid (g/L) |
|---|---|---|---|---|---|---|
| O-S. cerevisiae | 12.8 ± 0.0 a | 3.66 ± 0.03 c | 5.9 ± 0.1 b | 0.420 ± 0.009 a | 2.48 ± 0.05 b | 0.000 ± 0.000 a |
| O-L. thermotolerans | 12.8 ± 0.0 a | 3.49 ± 0.00 a | 3.8 ± 0.4 ab | 0.425 ± 0.009 a | 2.16 ± 0.08 b | 2.510 ± 0.028 c |
| O-L. thermotolerans + M. pulcherrima | 12.8 ± 0.2 a | 3.56 ± 0.01 b | 3.7 ± 0.6 ab | 0.380 ± 0.009 a | 2.23 ± 0.04 b | 1.150 ± 0.028 b |
| E-S. cerevisiae | 13.3 ± 0.4 b | 3.79 ± 0.00 d | 2.7 ± 0.1 a | 0.555 ± 0.009 b | 1.805 ± 0.021 a | 0.000 ± 0.000 a |
| E-L. thermotolerans | 13.5 ± 0.1 b | 3.57 ± 0.00 b | 5.3 ± 1.3 ab | 0.510 ± 0.009 b | 1.62 ± 0.05 a | 2.255 ± 0.078 c |
| E-L. thermotolerans + M. pulcherrima | 13.3 ± 0.6 b | 3.65 ± 0.01 c | 3.5 ± 0.4 ab | 0.395 ± 0.009 a | 1.74 ± 0.10 a | 1.19 ± 0.10 b |
| Wine | Colour Intensity (Absorbance Units) | Tonality (Adimensional) | Chroma | Hue (°) | L | a | b |
|---|---|---|---|---|---|---|---|
| O-S. cerevisiae | 0.54 ± 0.08 a | 1.82 ± 0.10 abc | 11.5 ± 1.7 a | 93.4 ± 0.3 ab | 87.9 ± 1.9 ab | −0.6 ± 0.6 ab | 11.45 ± 1.8 ab |
| O-L. thermotolerans | 0.604 ± 0.007 a | 1.528 ± 0.011 ab | 10.69 ± 0.18 a | 89.4 ± 1.1 ab | 85.6 ± 0.6 ab | 0.11 ± 0.24 b | 10.69 ± 0.18 a |
| O-L. thermotolerans + M. pulcherrima | 0.71 ± 0.07 a | 1.37 ± 0.03 a | 10.40 ± 0.13 a | 86.3 ± 0.3 a | 82.4 ± 1.7 a | 0.70 ± 0.05 b | 10.38 ± 0.13 a |
| E-S. cerevisiae | 0.36 ± 0.03 a | 2.13 ± 0.23 bcd | 10.7 ± 0.7 a | 92.5 ± 0.3 ab | 92.6 ± 1.0 b | −0.48 ± 0.13 ab | 10.7 ± 0.7 a |
| E-L. thermotolerans | 0.37 ± 0.11 a | 2.86 ± 0.00 d | 16.4 ± 0.9 b | 96.3 ± 0.6 b | 94 ± 3 b | −1.79 ± 0.05 a | 16.3 ± 0.9 b |
| E-L. thermotolerans + M. pulcherrima | 0.51 ± 0.04 a | 2.37 ± 0.05 cd | 15.4 ± 1.1 b | 92.8 ± 0.6 ab | 90.0 ± 0.8 ab | −0.8 ± 0.3 ab | 15.4 ± 1.0 ab |
| Compounds (mg/L) | O-SC | O-LT | O-LT + MP | E-SC | E-LT | E-LT + MP | |
|---|---|---|---|---|---|---|---|
| Carbonyl compounds | Acetaldehyde | 42.6 ± 2.1 a | 37.9 ± 2.0 a | 26.0 ± 3.0 a | 43.2 ± 1.4 a | 40.0 ± 8.0 a | 37.0 ± 2.0 a |
| Diacetyl (butan-2,3-dione) | 1.67 ± 0.04 a | 0.00 ± 0.00 a | 1.76 ± 0.03 a | 0.90 ± 0.90 a | 0.00 ± 0.00 a | 1.91 ± 0.07 a | |
| Acetoin (3-hydroxybutan-2-one) | 15.5 ± 2.3 a | 20.0 ± 4.0 a | 16.7 ± 1.0 a | 14.1 ± 0.9 a | 14.4 ± 1.0 a | 15.0 ± 0.5 a | |
| 2,3-Butanediol (butane-2,3-diol) | 1280 ± 70 c | 870 ± 4 ab | 707 ± 7 a | 1499 ± 70 c | 1120 ± 120 bc | 1260 ± 14 c | |
| Higher alcohols | 1-Propanol (propan-1-ol) | 102 ± 5 bc | 60 ± 10 ab | 50.3 ± 1.1 a | 110 ± 8 c | 72 ± 8 abc | 74 ± 12 abc |
| Isobutanol (2-methylpropan-1-ol) | 35.0 ± 0.7 a | 57.0 ± 9.0 a | 50.5 ± 1.1 a | 30.9 ± 1.2 a | 68.0 ± 17.0 a | 62.0 ± 7.0 a | |
| 3-Methyl-1-butanol (2-methylbutan-1-ol) | 160.0 ± 3.0 a | 158.0 ± 22.0 a | 132.0 ± 1.6 a | 157.0 ± 14.0 a | 190.0 ± 40.0 a | 162.0 ± 18.0 a | |
| 2-Methyl-1-butanol (3-methylbutan-1-ol) | 53.0 ± 3.0 a | 67.0 ± 8.0 a | 57.3 ± 0.9 a | 37.0 ± 2.0 a | 72.0 ± 14.0 a | 68.0 ± 13.0 a | |
| Hexanol (hexan-1-ol) | 4.30 ± 0.20 a | 4.27 ± 0.18 a | 4.44 ± 0.08 a | 3.82 ± 0.01 a | 4.80 ± 0.50 a | 4.42 ± 0.27 a | |
| 2-Phenyl ethanol (2-phenylethanol) | 12.3 ± 0.4 a | 13.0 ± 1.8 a | 12.6 ± 0.3 a | 11.7 ± 0.6 a | 12.6 ± 1.8 a | 12.8 ± 1.3 a | |
| Total higher alcohols | 332 ± 16 a | 300 ± 60 a | 257 ± 2 a | 320 ± 40 a | 350 ± 100 a | 320 ± 60 a | |
| Esters | Ethyl acetate | 106 ± 3 a | 127 ± 20 a | 92 ± 7 a | 101 ± 3 a | 190 ± 50 a | 135 ± 7 a |
| Isobutyl acetate | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.0 ± 0.00 a | 3.30 ± 1.30 a | 2.20 ± 2.20 a | 1.65 ± 0.08 a | |
| Ethyl butyrate | 1.82 ± 0.19 a | 0.90 ± 0.90 a | 1.98 ± 0.06 a | 0.80 ± 0.80 a | 1.20 ± 1.20 a | 2.12 ± 0.19 a | |
| Ethyl lactate (2-hydroxypropanoate) | 52 ± 11 a | 32 ± 16 a | 23 ± 15 a | 21 ± 15 a | 22 ± 3 a | 18 ± 10 a | |
| Isoamyl acetate | 8.10 ± 0.70 a | 7.80 ± 0.90 a | 6.47 ± 0.23 a | 6.50 ± 0.30 a | 9.80 ± 1.30 a | 9.70 ± 0.60 a | |
| 2-Phenylethyl acetate | 6.14 ± 0.00 a | 7.50 ± 0.90 a | 8.01 ± 0.06 a | 5.57 ± 0.08 a | 6.20 ± 0.40 a | 6.40 ± 0.40 a | |
| Total esters | 175 ± 20 a | 180 ± 50 a | 132 ± 10 a | 138 ± 5 a | 230 ± 80 a | 175 ± 3 a | |
| Methanol | 75.8 ± 0.2 a | 81.0 ± 24.0 a | 70.4 ± 1.5 a | 68.0 ± 3.0 a | 110.0 ± 30.0 a | 90.0 ± 13.0 a | |
| Total volatile | 1960 ± 50 b | 1540 ± 80 ab | 1263 ± 7 a | 2020 ± 100 b | 1900 ± 300 b | 1970 ± 50 b | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soler, M.; Del Fresno, J.M.; Bañuelos, M.A.; Morata, A.; Loira, I. Use of Lachancea thermotolerans and Metschnikowia pulcherrima to Improve Acidity and Sensory Profile of Verdejo Wines from Different Vine Management Systems. Fermentation 2025, 11, 541. https://doi.org/10.3390/fermentation11090541
Soler M, Del Fresno JM, Bañuelos MA, Morata A, Loira I. Use of Lachancea thermotolerans and Metschnikowia pulcherrima to Improve Acidity and Sensory Profile of Verdejo Wines from Different Vine Management Systems. Fermentation. 2025; 11(9):541. https://doi.org/10.3390/fermentation11090541
Chicago/Turabian StyleSoler, María, Juan Manuel Del Fresno, María Antonia Bañuelos, Antonio Morata, and Iris Loira. 2025. "Use of Lachancea thermotolerans and Metschnikowia pulcherrima to Improve Acidity and Sensory Profile of Verdejo Wines from Different Vine Management Systems" Fermentation 11, no. 9: 541. https://doi.org/10.3390/fermentation11090541
APA StyleSoler, M., Del Fresno, J. M., Bañuelos, M. A., Morata, A., & Loira, I. (2025). Use of Lachancea thermotolerans and Metschnikowia pulcherrima to Improve Acidity and Sensory Profile of Verdejo Wines from Different Vine Management Systems. Fermentation, 11(9), 541. https://doi.org/10.3390/fermentation11090541








