Smart and Functional Probiotic Microorganisms: Emerging Roles in Health-Oriented Fermentation
Abstract
1. Introduction
2. Probiotic Microorganisms in Functional Fermentation
2.1. Defining Probiotics and Their Role in Health-Oriented Foods
2.2. Functional Properties of Conventional Probiotic Strains
2.3. The Interaction Between Probiotic Microorganisms and Prebiotic Compounds in Fermentation Processes
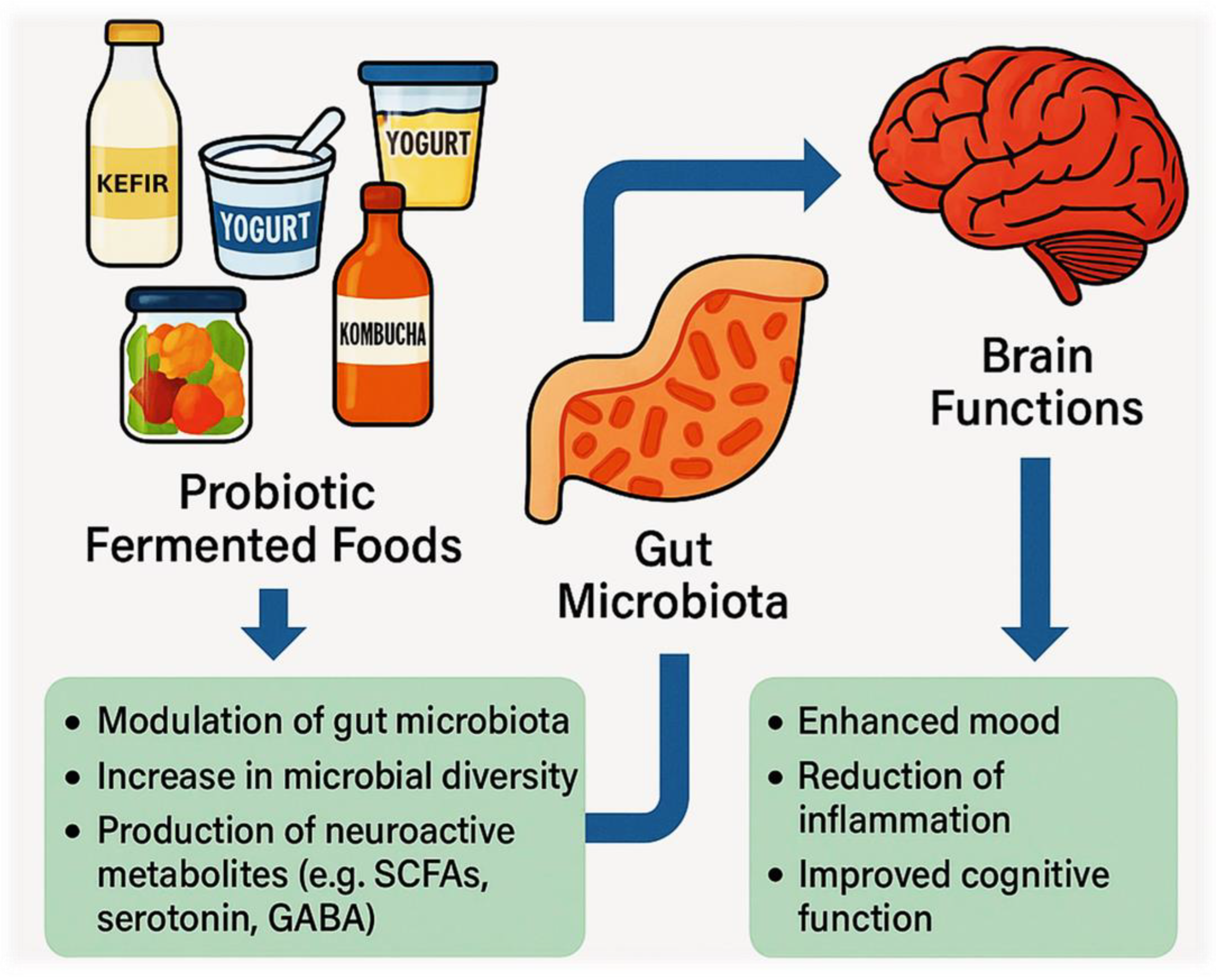
2.4. Consumption of Probiotic Fermented Foods and the Regulatory Benefits on the Gut–Brain Axis
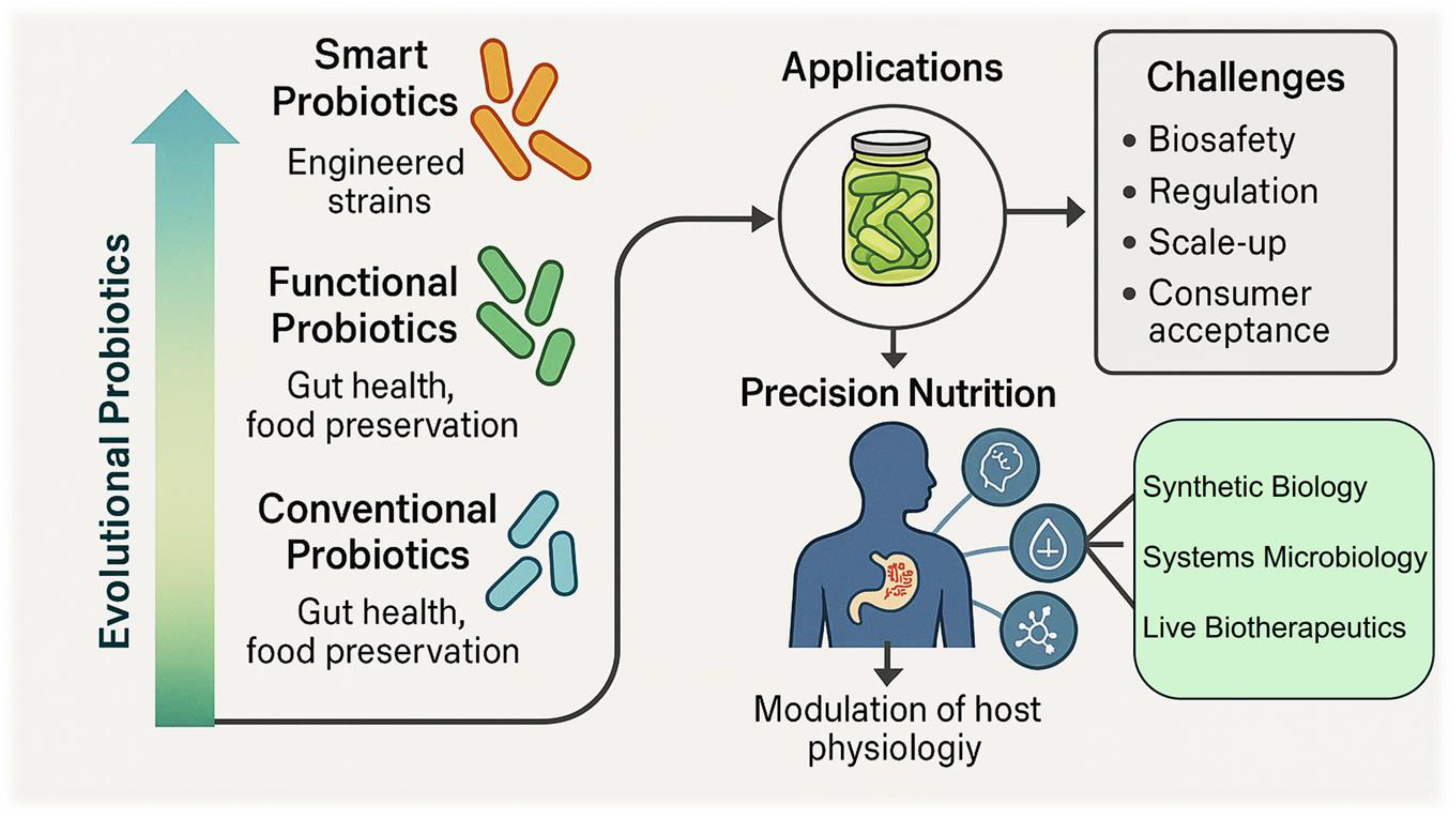
3. Smart Probiotics: Concepts and Innovations in Fermentation
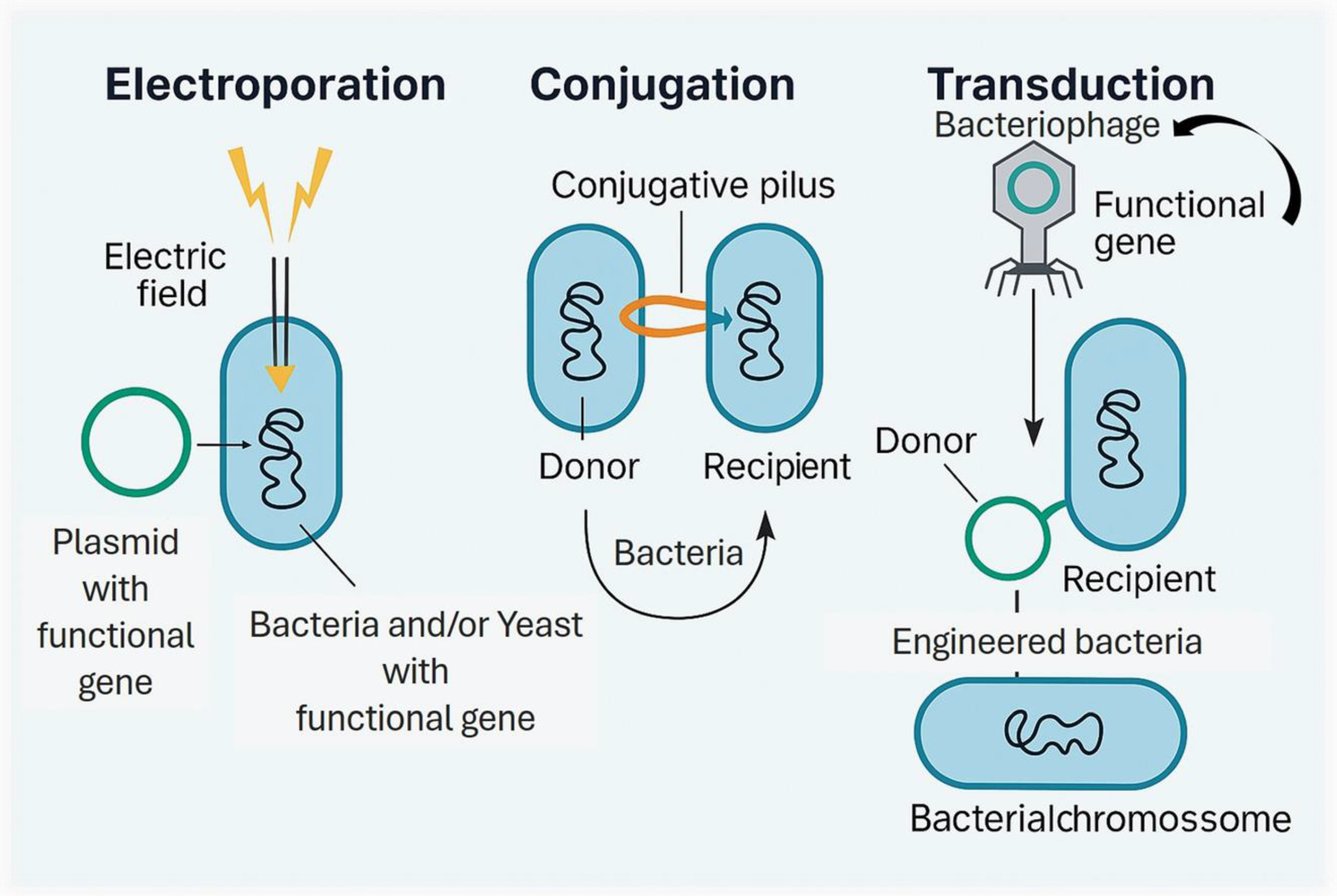
3.1. From Conventional to Smart Probiotics
3.2. Design and Engineering of Smart Probiotic Strains: Functional Outcomes and Health Applications
3.3. Smart Probiotic Strains Applied to Fermentation Processes and the Preparation of Fermented Foods
3.3.1. Applications in Fermentation Processes
3.3.2. Functional Food Development and Health-Promoting Potential
- Blood pressure regulation, as seen in fermented milk enriched with ACE-inhibitory peptides [6];
4. Perspectives and Future Directions
4.1. Toward Personalized and Precision Fermentation
4.2. Research Gaps and Clinical Validation Needs
4.3. Industrial and Regulatory Challenges
4.4. Biosafety, Regulatory Approval, and Consumer Acceptance of Smart Probiotics
- Biosafety remains a primary consideration, as genetic modifications may alter microbial metabolism, host interactions, and ecological persistence. Potential risks include unintended metabolic pathways, horizontal gene transfer, and disruption of native gut microbiota [12]. Comprehensive risk assessment should be integrated in in vitro and in vivo studies, focusing on genomic stability, metabolic profiling, and long-term host safety [9]. Advances in synthetic biology and metabolic engineering have enabled the development of controlled genetic circuits and self-limiting systems to minimize environmental and host-related risks [14,15,59].
- Regulatory approval pathways for smart probiotics are evolving and vary across jurisdictions. In the United States, the Food and Drug Administration (FDA) typically regulates these products under the live biotherapeutic products (LBP) framework, requiring pre-market evidence of safety, stability, and efficacy [16]. In the European Union, the European Food Safety Authority (EFSA) applies the Qualified Presumption of Safety (QPS) approach in conjunction with novel food regulations [23]. Products intended for medical applications face stricter clinical validation requirements, as demonstrated by engineered yeast probiotics for inflammatory bowel disease undergoing rigorous preclinical and clinical testing [60].
- Consumer acceptance is equally critical for market adoption. Public perception may be influenced by awareness of genetic engineering, trust in regulatory oversight, and a clear understanding of health benefits versus perceived risks [19]. Transparent labeling, accessible scientific communication, and educational outreach can improve acceptance, particularly when innovation aligns with sensory quality and cultural preferences [1,3]. Integrating consumer feedback early in the development process can guide both product formulation and dissemination strategies.
- Addressing biosafety, regulatory, and consumer dimensions from the earliest stages of research ensures not only scientific advancement but also ethical responsibility and societal readiness for the integration of smart probiotics into health-oriented foods and therapeutics.
4.5. The Future of Smart Probiotics
5. Conclusions and Limitations of This Study
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yee, C.S.; Zahia-Azizan, N.A.; Abd Rahim, M.H.; Mohd Zaini, N.A.; Raja-Razali, R.B.; Ushidee-Radzi, M.A.; Ilham, Z.; Wan-Mohtar, W.A.A.Q.I. Smart Fermentation Technologies: Microbial Process Control in Traditional Fermented Foods. Fermentation 2025, 11, 323. [Google Scholar] [CrossRef]
- Marco, M.L.; Sanders, M.E.; Gänzle, M.; Arrieta, M.C.; Cotter, P.D.; De Vuyst, L.; Hill, C.; Holzapfel, W.; Lebeer, S.; Merenstein, D.; et al. The International Scientific Association for Probiotics and Prebiotics (ISAPP) Consensus Statement on Fermented Foods. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 196–208. [Google Scholar] [CrossRef]
- Ibrahim, S.A.; Yeboah, P.J.; Ayivi, R.D.; Eddin, A.S.; Wijemanna, N.D.; Paidari, S.; Bakhshayesh, R.V. A Review and Comparative Perspective on Health Benefits of Probiotic and Fermented Foods. Int. J. Food Sci. Technol. 2023, 58, 4948–4964. [Google Scholar] [CrossRef]
- da Anunciação, T.A.; Guedes, J.D.S.; Tavares, P.P.L.G.; de Melo Borges, F.E.; Ferreira, D.D.; Costa, J.A.V.; Umsza-Guez, M.A.; Magalhães-Guedes, K.T. Biological Significance of Probiotic Microorganisms from Kefir and Kombucha: A Review. Microorganisms 2024, 12, 1127. [Google Scholar] [CrossRef]
- do Nascimento, A.S.M.; da Silva, R.N.A.; Tavares, P.P.L.G.; Borges, A.S.; Cardoso, M.P.S.; Lobato, A.K.C.L.; Almeida, R.C.C.; Magalhães-Guedes, K.T. Kefir Probiotic-Enriched Non-Alcoholic Beers: Microbial, Genetic, and Sensory-Chemical Assessment. Beverages 2025, 11, 75. [Google Scholar] [CrossRef]
- Beltrán-Barrientos, L.M.; Hernández-Mendoza, A.; Torres-Llanez, M.J.; González-Córdova, A.F.; Vallejo-Córdoba, B. Invited Review: Fermented Milk as Antihypertensive Functional Food. J. Dairy Sci. 2016, 99, 4099–4110. [Google Scholar] [CrossRef] [PubMed]
- Bourrie, B.C.T.; Willing, B.P.; Cotter, P.D. The Microbiota and Health Promoting Characteristics of the Fermented Beverage Kefir. Front. Microbiol. 2016, 7, 647. [Google Scholar] [CrossRef]
- Dahiya, D.; Nigam, P.S. The Gut Microbiota Influenced by the Intake of Probiotics and Functional Foods with Prebiotics Can Sustain Wellness and Alleviate Certain Ailments like Gut-Inflammation and Colon-Cancer. Microorganisms 2022, 10, 665. [Google Scholar] [CrossRef]
- El Hage, R.; Hernandez-Sanabria, E.; Van de Wiele, T. Emerging Trends in “Smart Probiotics”: Functional Consideration for the Development of Novel Health and Industrial Applications. Front. Microbiol. 2017, 8, 1889. [Google Scholar] [CrossRef]
- Yan, X.; Liu, X.Y.; Zhang, D.; Zhang, Y.D.; Li, Z.H.; Liu, X.; Wu, F.; Chen, G.-Q. Construction of a Sustainable 3-Hydroxybutyrate-Producing Probiotic Escherichia coli for Treatment of Colitis. Cell. Mol. Immunol. 2021, 18, 2344–2357. [Google Scholar] [CrossRef]
- Han, M.; Li, S.; Wang, W.; Li, J.; Luo, J.; Zhou, Z.; Li, J.; Wang, D.; Yang, J. Engineered sonosensitive probiotics extracellular vesicles coating for ultrasound-driven anti-infection and immunoregulation in implant infection treatment. Chem. Eng. J. 2025, 504, 158946. [Google Scholar] [CrossRef]
- Virolle, C.; Goldlust, K.; Djermoun, S.; Bigot, S.; Lesterlin, C. Plasmid Transfer by Conjugation in Gram-Negative Bacteria: From the Cellular to the Community Level. Genes 2020, 11, 1239. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.; Yu, L.; Li, Y.Z.; Cai, M.; He, J.Z.; Liu, J.Y.; Hao, L.; Xu, H.; Qiao, M. Developing Multi-Copy Chromosomal Integration Strategies for Heterologous Biosynthesis of Caffeic Acid in Saccharomyces cerevisiae. Front. Microbiol. 2022, 13, 851706. [Google Scholar] [CrossRef]
- Ma, J.; Lyu, Y.; Liu, X.; Jia, X.; Cui, F.; Wu, X.; Deng, S.; Yue, C. Engineered Probiotics. Microb. Cell Fact. 2022, 21, 72. [Google Scholar] [CrossRef]
- Loucif, L.; Chelaghma, W.; Bendjama, E.; Cherak, Z.; Khellaf, M.; Khemri, A.; Rolain, J.-M. Detection of blaOXA-48 and mcr-1 Genes in Escherichia coli Isolates from Pigeon (Columba livia) in Algeria. Microorganisms 2022, 10, 975. [Google Scholar] [CrossRef]
- Murali, S.K.; Mansell, T.J. Next Generation Probiotics: Engineering Live Biotherapeutics. Biotechnol. Adv. 2024, 72, 108336. [Google Scholar] [CrossRef] [PubMed]
- Magalhães-Guedes, K.T.; Borges, A.S.; Almeida da Silva, R.N. A Conceptual Framework for Understanding the Interaction Between Smart Probiotics and the Gut–Brain Axis in Mood Regulation: An Integrative Approach. Int. J. Appl. Sci. Res. 2025, 8, 3. [Google Scholar] [CrossRef]
- Han, K.; Park, J.S.; Kim, Y.-W.; Lee, W.; Park, K.; Kim, S.-K. Efficient surface display of single-chain variable fragments against tumor necrosis factor α on engineered probiotic Saccharomyces boulardii and its application in alleviating intestinal inflammation in vivo. New Biotechnol. 2025, 86, 107–114. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, Y.; Deng, Y.; Yi, C.; Wang, Y.; Ding, M.; Liu, J.; Jin, X.; Shen, L.; He, Y.; et al. Probiotic Supplements: Hope or Hype? Front. Microbiol. 2020, 11, 160. [Google Scholar] [CrossRef]
- Fidélix, M.; Milenkovic, D.; Sivieri, K.; Cesar, T. Microbiota Modulation and Effects on Metabolic Biomarkers by Orange Juice: A Controlled Clinical Trial. Food Funct. 2020, 11, 1599–1610. [Google Scholar] [CrossRef] [PubMed]
- Dore, M.P.; Rocchi, C.; Longo, N.P.; Scanu, A.M.; Vidili, G.; Padedda, F.; Pes, G.M. Effect of Probiotic Use on Adverse Events in Adult Patients with Inflammatory Bowel Disease: A Retrospective Cohort Study. Probiotics Antimicrob. Proteins 2020, 12, 152–159. [Google Scholar] [CrossRef]
- Santos, E.N.; Magalhães-Guedes, K.T.; Borges, F.E.M.; Ferreira, D.D.; da Silva, D.F.; Conceição, P.C.G.; Lima, A.K.C.; Cardoso, L.G.; Umsza-Guez, M.A.; Ramos, C.L. Probiotic Microorganisms in Inflammatory Bowel Diseases: Live Biotherapeutics as Food. Foods 2024, 13, 4097. [Google Scholar] [CrossRef]
- Manzoor, R.; Ahmed, W.; Afify, N.; Memon, M.; Yasin, M.; Memon, H.; Rustom, M.; Al Akeel, M.; Alhajri, N. Trust Your Gut: The Association of Gut Microbiota and Liver Disease. Microorganisms 2022, 10, 1045. [Google Scholar] [CrossRef] [PubMed]
- Bravo, J.A.; Forsythe, P.; Chew, M.V.; Escaravage, E.; Savignac, H.M.; Dinan, T.G.; Bienenstock, J.; Cryan, J.F. Ingestion of Lactobacillus Strain Regulates Emotional Behavior and Central GABA Receptor Expression in a Mouse via the Vagus Nerve. Proc. Natl. Acad. Sci. USA 2011, 108, 16050–16055. [Google Scholar] [CrossRef] [PubMed]
- Foster, J.A.; Neufeld, K.A.M. Gut–Brain Axis: How the Microbiome Influences Anxiety and Depression. Trends Neurosci. 2013, 36, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Sabit, H.; Patel, H.; Alwan, H.; Riaz, O.; Khan, M. The Effect of Probiotic Supplementation on the Gut–Brain Axis in Psychiatric Patients. Curr. Issues Mol. Biol. 2023, 45, 4080–4099. [Google Scholar] [CrossRef]
- Magalhães-Guedes, K.T. Psychobiotic Therapy: Method to Reinforce the Immune System. Clin. Psychopharmacol. Neurosci. 2022, 20, 17–25. [Google Scholar] [CrossRef]
- Liu, W.; Yang, H.; Li, T.; Qiu, C.; Zhao, J. Gamma-Aminobutyric Acid as a Potential Postbiotic Mediator in the Gut–Brain Axis. NPJ Sci. Food 2024, 8, 10. [Google Scholar] [CrossRef]
- Cryan, J.F.; Dinan, T.G. Mind-Altering Microorganisms: The Impact of the Gut Microbiota on Brain and Behaviour. Nat. Rev. Neurosci. 2012, 13, 701–712. [Google Scholar] [CrossRef]
- Sarkar, A.; Lehto, S.M.; Harty, S.; Dinan, T.G.; Dunbar, R.I.M.; Cryan, J.F. Psychobiotics and the Manipulation of Bacteria–Gut–Brain Signals. Trends Neurosci. 2016, 39, 763–781. [Google Scholar] [CrossRef]
- Magalhães, K.T.; Pereira, M.A.; Dragone, G.; Nicolau, A.; Domingues, L.; Teixeira, J.A.; Silva, J.B.A.; Schwan, R.F. Production of Fermented Cheese Whey-Based Beverage Using Kefir Grains as Starter Culture: Evaluation of Morphological and Microbial Variations. Bioresour. Technol. 2010, 101, 8843–8850. [Google Scholar] [CrossRef]
- De la Rosa González, A.; Guerra-Ojeda, S.; Camacho-Villa, M.A.; Valls, A.; Alegre, E.; Quintero-Bernal, R.; Martorell, P.; Chenoll, E.; Serna-García, M.; Mauricio, M.D.; et al. Effect of Probiotics on Gastrointestinal Health through the Aryl Hydrocarbon Receptor Pathway: A Systematic Review. Foods 2024, 13, 3479. [Google Scholar] [CrossRef]
- Staniszewski, A.; Staniszewska, P.; Komoń-Janczara, E.; Kordowska-Wiater, M. Probiotic Yeast and How to Use Them—Combining Traditions and New Waves in Fermented Beverages. Foods 2025, 14, 2921. [Google Scholar] [CrossRef]
- Zhang, J.-Y.; Xiao, X.; Dong, Y.; Wu, J.; Yao, F.; Zhou, X.-H. Effect of Fermented Wheat Germ Extract with Lactobacillus plantarum DY-1 on HT-29 Cell Proliferation and Apoptosis. J. Agric. Food Chem. 2015, 63, 2449–2457. [Google Scholar] [CrossRef]
- Dey, G. Non-Dairy Probiotic Foods: Innovations and Market Trends. In Innovations in Technologies for Fermented Food and Beverage Industries; Gupta, R., Ed.; Springer: New York, NY, USA, 2018; pp. 159–173. [Google Scholar]
- Dimidi, E.; Cox, S.R.; Rossi, M.; Whelan, K. Fermented Foods: Definitions and Characteristics, Impact on the Gut Microbiota and Effects on Gastrointestinal Health and Disease. Nutrients 2019, 11, 1806. [Google Scholar] [CrossRef] [PubMed]
- Qu, Z.; Wu, Y.; Jiang, Y.; Fan, J.; Cao, L.; Dong, Y.; Fang, S.; Gu, S. Effects of Two Compound Probiotic Formulations on Gastrointestinal Symptoms and Gut Microbiota: A 4-Week Randomized, Double-Blind Intervention Trial. Nutrients 2025, 17, 2886. [Google Scholar] [CrossRef]
- Falah, F.; Vasiee, A.; Yazdi, F.T.; Behbahani, B.A. Preparation and Functional Properties of Synbiotic Yogurt Fermented with Lactobacillus brevis PML1 Derived from a Fermented Cereal-Dairy Product. Biomed Res. Int. 2021, 2021, 1057531. [Google Scholar] [CrossRef] [PubMed]
- Fazilah, N.F.; Hamidon, N.H.; Ariff, A.B.; Khayat, M.E.; Wasoh, H.; Halim, M. Microencapsulation of Lactococcus lactis Gh1 with Gum Arabic and Synsepalum dulcificum via Spray Drying for Potential Inclusion in Functional Yogurt. Molecules 2019, 24, 1422. [Google Scholar] [CrossRef] [PubMed]
- Batista, A.; Silva, R.; Cappato, L.P.; Ferreira, M.V.S.; Nogueira, M.C.L.; Freitas, M.Q.; Silva, M.C.; Raices, R.S.L.; Esmerino, E.A.; Cruz, A.G. Developing A Synbiotic Fermented Milk Using Probiotic Bacteria and Organic Green Banana Flour. J. Funct. Foods 2017, 38, 242–250. [Google Scholar] [CrossRef]
- Kareb, O.; Aïder, M. Whey and Its Derivatives for Probiotics, Prebiotics, Synbiotics, and Functional Foods: A Critical Review. Probiotics Antimicrob. Proteins 2019, 11, 348–369. [Google Scholar] [CrossRef]
- Budhwar, S.; Sethi, K.; Chakraborty, M. Efficacy of Germination and Probiotic Fermentation on Underutilized Cereal and Millet Grains. Food Prod. Process. Nutr. 2020, 2, 12. [Google Scholar] [CrossRef]
- Erdem, Ö.; Gültekin-Özgüven, M.; Berktaş, İ.; Turan, S.; Özçelik, B. Development of a Novel Synbiotic Dark Chocolate Enriched with Bacillus indicus HU36, Maltodextrin and Lemon Fiber: Optimization by Response Surface Methodology. LWT–Food Sci. Technol. 2014, 56, 187–193. [Google Scholar] [CrossRef]
- Akram, N.; Faisal, Z.; Irfan, R.; Shah, Y.A.; Batool, S.A.; Zahid, T.; Zulfiqar, A.; Fatima, A.; Jahan, Q.; Tariq, H.; et al. Exploring the serotonin-probiotics-gut health axis: A review of current evidence and potential mechanisms. Food Sci. Nutr. 2023, 12, 694–706. [Google Scholar] [CrossRef]
- Balasubramanian, R.; Schneider, E.; Gunnigle, E.; Cotter, P.D.; Cryan, J.F. Fermented foods: Harnessing their potential to modulate the microbiota-gut-brain axis for mental health. Neurosci. Biobehav. Rev. 2024, 158, 105562. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, E.Y.; McBride, S.W.; Hsien, S.; Sharon, G.; Hyde, E.R.; McCue, T.; Codelli, J.A.; Chow, J.; Reisman, S.E.; Petrosino, J.F.; et al. Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell 2013, 155, 1451–1463. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Fu, L.; Chen, Y.; Yang, H.; Xiao, Y.; Ren, Y.; Wang, C. Integrated Metabolome and Microbiome Analysis Reveals the Regulatory Effects of Fermented Soybean Meal on the Gut Microbiota of Late Gestation. Fermentation 2025, 11, 315. [Google Scholar] [CrossRef]
- Mazzantini, D.; Calvigioni, M.; Celandroni, F.; Saba, A.; Ghelardi, E. In Vitro Analysis of an Alkalihalobacillus clausii Spore-Based Probiotic Formulation Clarifies the Mechanisms Underlying Its Beneficial Properties. Biomolecules 2025, 15, 1294. [Google Scholar] [CrossRef]
- Kelly, J.R.; Borre, Y.; Aidy, S.E.; Deane, J.; Patterson, E.; Kennedy, P.J.; Beers, S.; Scott, K.; Moloney, G.; Scott, L.; et al. Transferring the blues: Depression-associated gut microbiota induces neurobehavioural changes in the rat. Eur. Neuropsychopharmacol. 2016, 26 (Suppl. S1), S85–S86. [Google Scholar] [CrossRef]
- Li, J.; Li, D.; Chen, Y.; Chen, W.; Xu, J.; Gao, L. Gut microbiota and aging: Traditional Chinese medicine and modern medicine. Clin. Interv. Aging 2023, 18, 963–986. [Google Scholar] [CrossRef]
- Li, Z.; Liao, Y.; Zhou, Q.; Qu, Q.; Sheng, M.; Lv, L.; Yang, J.; Shi, Y.; Shi, X. Changes of gut microbiota in autism spectrum disorders and common probiotics & Chinese herbal medicine therapeutic mechanisms: A review. Adv. Neurodev. Disord. 2022, 6, 290–303. [Google Scholar] [CrossRef]
- Li, Y.; Peng, Y.; Shen, Y.; Zhang, Y.; Liu, L.; Yang, X. Dietary polyphenols: Regulate the advanced glycation end products-RAGE axis and the microbiota-gut-brain axis to prevent neurodegenerative diseases. Crit. Rev. Food Sci. Nutr. 2022, 63, 9816–9842. [Google Scholar] [CrossRef]
- Wu, T.; Hu, F.; Tang, S.; Xu, X.; Li, D. Enhancing Antioxidant Activity and Modulating Gut Microbiota Through Lactiplantibacillus plantarum-Fermented Processing Wastewater of Yuba (FPWY). Fermentation 2025, 11, 212. [Google Scholar] [CrossRef]
- Park, M.-K.; Hwang, T.-K.; Kim, W.; Jo, Y.; Park, Y.-J.; Kim, M.-C.; Son, H.; Seo, D.; Shin, J.-H. Probiotic Feed Additives Mitigate Odor Emission in Cattle Farms through Microbial Community Changes. Fermentation 2024, 10, 473. [Google Scholar] [CrossRef]
- Hung, Y.-P.; Lee, C.-C.; Lee, J.-C.; Tsai, P.-J.; Hsueh, P.-R.; Ko, W.-C. The Potential of Probiotics to Eradicate Gut Carriage of Pathogenic or Antimicrobial-Resistant Enterobacterales. Antibiotics 2021, 10, 1086. [Google Scholar] [CrossRef]
- Baba, T.; Ara, T.; Hasegawa, M.; Takai, Y.; Okumura, Y.; Baba, M.; Datsenko, K.A.; Tomita, M.; Wanner, B.L.; Mori, H. Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: The Keio collection. Mol. Syst. Biol. 2006, 2, 2006.0008. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Song, Y.; Yan, Y.; Chen, W.; Ren, T.; Ma, A.; Li, S.; Jia, Y. Characterization of an epilactose-producing cellobiose 2-epimerase from Clostridium sp. TW13 and reutilization of waste milk. Food Chem. 2025, 480, 143948. [Google Scholar] [CrossRef]
- Pandhal, J.; Woodruff, L.B.A.; Jaffe, S.; Desai, P.; Ow, S.Y.; Noirel, J.; Gill, R.T.; Wright, P.C. Inverse metabolic engineering to improve Escherichia coli as an N-glycosylation host. Biotechnol. Bioeng. 2013, 110, 2482–2493. [Google Scholar] [CrossRef]
- Silver, A.P.; Riglar, T.D. Engineering bacteria for diagnostic and therapeutic applications. Nat. Rev. Microbiol. 2018, 16, 214–225. [Google Scholar] [CrossRef]
- Scott, B.M.; Gutiérrez-Vázquez, C.; Sanmarco, L.M.; Pereira, J.A.D.S.; Quintana, F.J. Self-tunable engineered yeast probiotics for the treatment of inflammatory bowel disease. Nat. Med. 2021, 27, 1212–1222. [Google Scholar] [CrossRef]
- Riglar, D.T.; Giessen, T.W.; Baym, M.; Kerns, S.J.; Niederhuber, M.J.; Bronson, R.T.; Kotula, J.W.; Gerber, G.K.; Way, J.C.; Silver, P.A. Engineered bacteria can function in the mammalian gut long-term as live diagnostics of inflammation. Nat. Biotechnol. 2017, 35, 653–658. [Google Scholar] [CrossRef]
- Loong, C.; Qing, H.; Chua, K.J.; Kang, A.; Hon, K.; Ling, K.; Lin, W.S. Engineered commensal microbes for diet-mediated colorectal-cancer chemoprevention. Nat. Biomed. Eng. 2018, 2, 27–37. [Google Scholar] [CrossRef]
- Zahid, H.F.; Ranadheera, C.S.; Fang, Z.; Ajlouni, S. Functional and Healthy Yogurts Fortified with Probiotics and Fruit Peel Powders. Fermentation 2022, 8, 469. [Google Scholar] [CrossRef]
- López, D.N.; Forastieri, P.S.; Calvo, N.L.; Cossia, M.B.; Tedaldi, C.; Llopart, E.E.; Steffolani, M.E.; Boeris, V. Yellow Pea Flour Fermented with Kefir as a Valuable Ingredient for the Techno-Functional and Sensory Improvement of Gluten-Free Bread. Fermentation 2025, 11, 521. [Google Scholar] [CrossRef]
- Kumalawati, D.A.; Dewi, R.S.; Fitriani, N.R.; Muchtar, S.Z.; Leonardo, J.; Astuti Taslim, N.; Romano, R.; Santini, A.; Nurkolis, F. Sea Grape (Caulerpa racemosa) Kombucha: A Comprehensive Study of Metagenomic and Metabolomic Profiling, Its Molecular Mechanism of Action as an Antioxidative Agent, and the Impact of Fermentation Time. Beverages 2025, 11, 134. [Google Scholar] [CrossRef]
- Ambrose, L.; Dinu, C.A.; Gurau, G.; Maftei, N.-M.; Matei, M.N.; Hincu, M.-A.; Radu, M.; Mehedinti, M.-C. The Role of Probiotics in Healing Burns and Skin Wounds; An Integrative Approach in the Context of Regenerative Medicine. Life 2025, 15, 1434. [Google Scholar] [CrossRef]
- Zhong, F.; Liu, X.; Wang, X.; Hou, M.; Guo, L.; Luo, X. An AI-Designed Antibody-Engineered Probiotic Therapy Targeting Urease to Combat Helicobacter pylori Infection in Mice. Microorganisms 2025, 13, 2043. [Google Scholar] [CrossRef]
- Fang, H.; Wang, Y.; Li, L.; Qin, X.; Zhu, D.; Liu, P.; Yang, Q.; Gao, Y.; Shi, Z.; Ma, X.; et al. Microenvironment-responsive living hydrogel containing engineered probiotic for treatment of massive bone defects. Bioact. Mater. 2025, 50, 556–570. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, N.; Chen, H.; Tang, C.; Wang, J.; Wang, Y.; Zhang, Y.; Guo, H.; Yuan, J. Recent advances of engineered probiotics for therapeutic applications. BioDes. Res. 2025, 7, 100039. [Google Scholar] [CrossRef]
- Saleh, M.; Heydari, R.; Ghanbari Boroujeni, M.R.; Abiri, R. Engineered probiotics that produce antibiotic binding sites: A potential strategy to protect gut microbiome and prevent antibiotic resistance. Med. Hypotheses 2025, 195, 111558. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Magalhães, K.T.; da Silva, R.N.A.; Borges, A.S.; Siqueira, A.E.B.; Puerari, C.; Bento, J.A.C. Smart and Functional Probiotic Microorganisms: Emerging Roles in Health-Oriented Fermentation. Fermentation 2025, 11, 537. https://doi.org/10.3390/fermentation11090537
Magalhães KT, da Silva RNA, Borges AS, Siqueira AEB, Puerari C, Bento JAC. Smart and Functional Probiotic Microorganisms: Emerging Roles in Health-Oriented Fermentation. Fermentation. 2025; 11(9):537. https://doi.org/10.3390/fermentation11090537
Chicago/Turabian StyleMagalhães, Karina Teixeira, Raquel Nunes Almeida da Silva, Adriana Silva Borges, Ana Elisa Barbosa Siqueira, Claudia Puerari, and Juliana Aparecida Correia Bento. 2025. "Smart and Functional Probiotic Microorganisms: Emerging Roles in Health-Oriented Fermentation" Fermentation 11, no. 9: 537. https://doi.org/10.3390/fermentation11090537
APA StyleMagalhães, K. T., da Silva, R. N. A., Borges, A. S., Siqueira, A. E. B., Puerari, C., & Bento, J. A. C. (2025). Smart and Functional Probiotic Microorganisms: Emerging Roles in Health-Oriented Fermentation. Fermentation, 11(9), 537. https://doi.org/10.3390/fermentation11090537








