The Construction of Corynebacterium glutamicum for Producing γ-Aminobutyric Acid and Analysis of the Fermentation Process
Abstract
1. Introduction
2. Materials and Methods
2.1. Heterologous Expression
2.2. Culture Condition
2.3. Fed-Batch Fermentation
2.3.1. Culture Conditions
2.3.2. Effect of Initial Glucose Concentration on the Fermentation Process
2.3.3. Glucose-Controlled Culture
2.3.4. Effect of Different pH Control Stages on the Fermentation Process
2.3.5. Analytical Methods
2.4. Empirical Correlation Equations of Fermentation Process
2.4.1. Empirical Correlation Equation of Cell Growth
2.4.2. Empirical Correlation Equation of Glucose Consumption
2.4.3. Empirical Correlation Equation of GAD Activity
2.4.4. Empirical Correlation Equation of GABA Production
2.5. Statistical Analysis
3. Results and Discussion
3.1. Construction of C. glutamicum for Producing GABA
3.2. Production of GABA by Recombinant C. glutamicum in Fed-Batch Fermentation
3.2.1. Effect of Different Initial Glucose Concentrations on the Fermentation Process
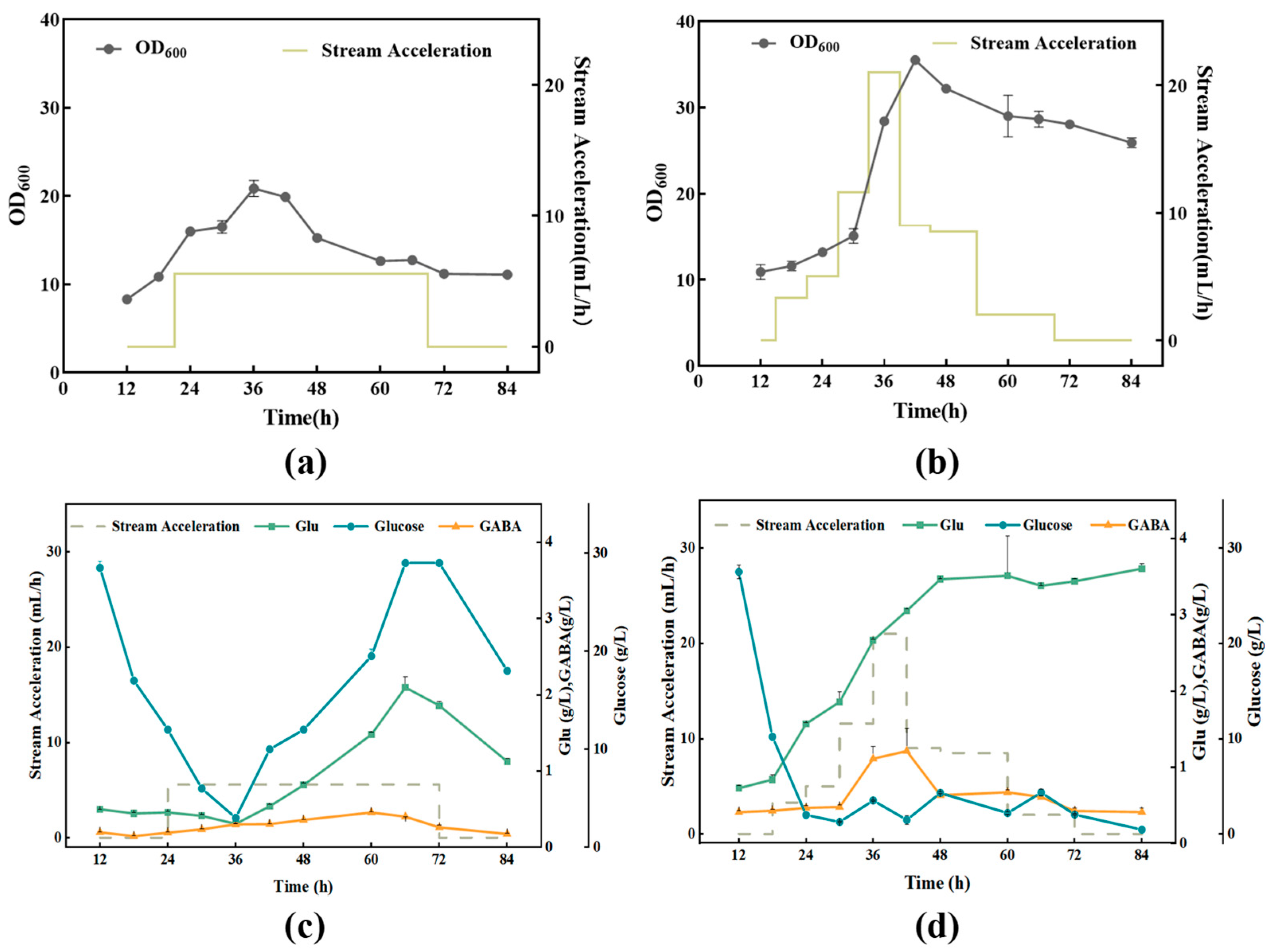
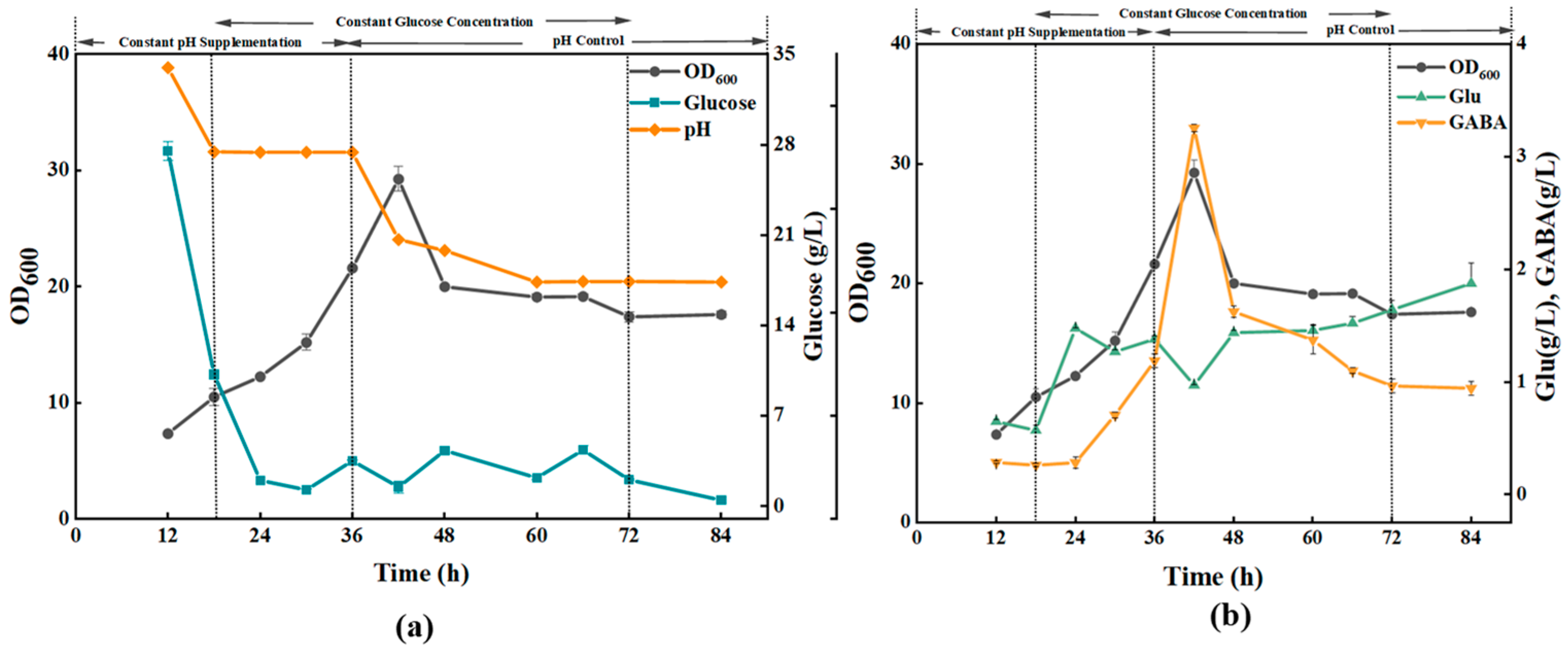
3.2.2. Effect of Different Glucose Supplementation Methods on the Fermentation Process
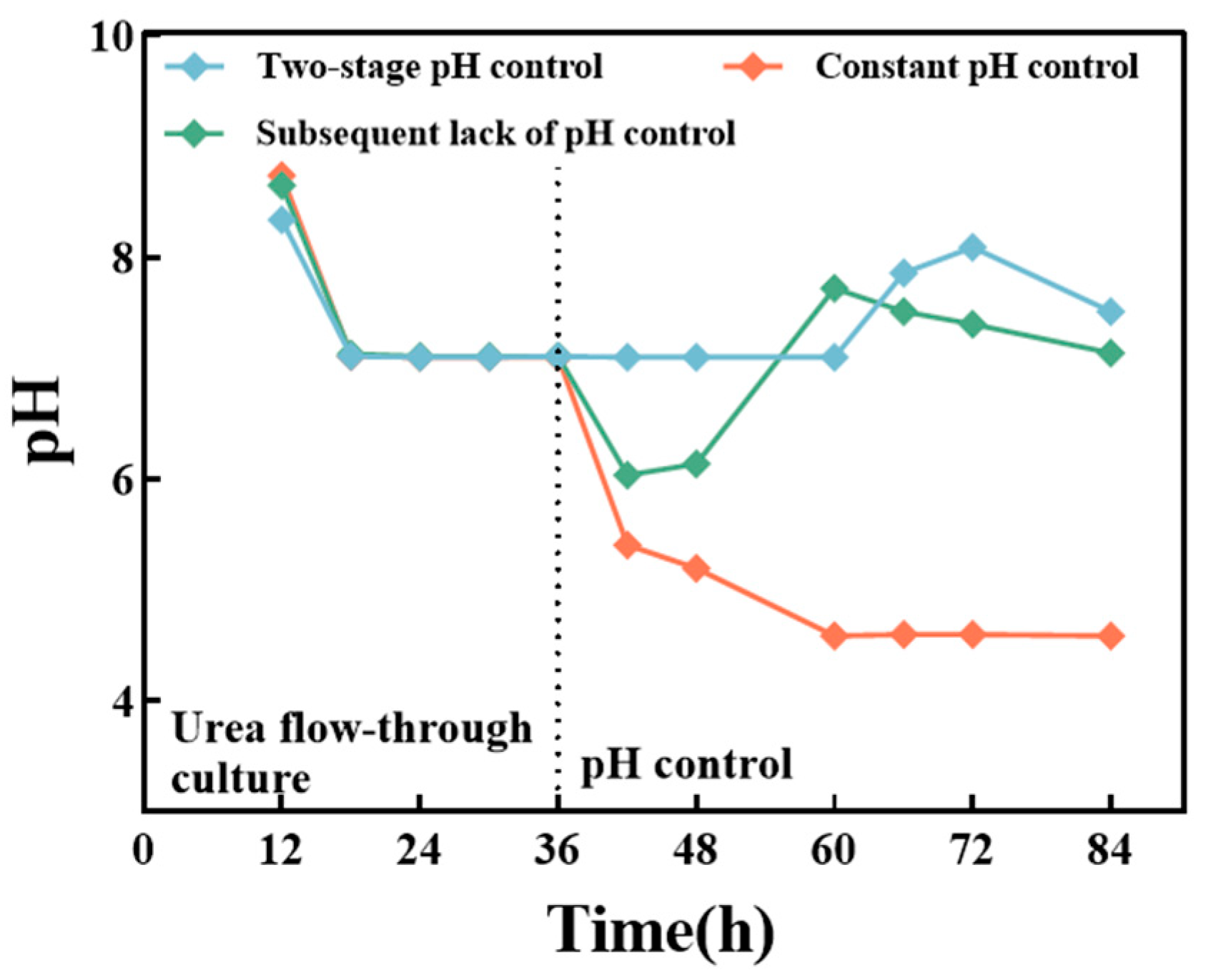
3.2.3. Effects of Different pH on the Fermentation Process
3.2.4. Variable Speed Glucose Replenishment Fermentation Results and Each Parameter Empirical Correlation Equation of the Two-Stage pH Control Strategy
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| GABA | γ-aminobutyric acid |
| Glu | Glutamate acid |
| GAD | Glutamate acid decarboxylase |
References
- Milon, R.B.; Hu, P.; Zhang, X.; Hu, X.; Ren, L. Recent advances in the biosynthesis and industrial biotechnology of Gamma-amino butyric acid. Bioresour. Bioprocess. 2024, 11, 32. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ma, W.; Zhou, J.; Wang, X.; Zhao, L. Microbial chassis design and engineering for production of gamma-aminobutyric acid. World J. Microbiol. Biotechnol. 2024, 40, 159. [Google Scholar] [CrossRef]
- Kwon, M.J.; Kim, J.E.; Lee, S.P. Functional kimchi beverage enhanced with γ-aminobutyric acid (GABA) through serial co-fermentation using Leuconostoc citreum S5 and Lactiplantibacillus plantarum KS2020. Fermentation 2025, 11, 44. [Google Scholar] [CrossRef]
- Grewal, J.; Khare, S.K. 2-Pyrrolidone synthesis from γ-aminobutyric acid produced by Lactobacillus brevis under solid-state fermentation utilizing toxic deoiled cottonseed cake. Bioprocess. Biosyst. Eng. 2017, 40, 145–152. [Google Scholar] [CrossRef]
- Wu, L.T.; Huang, Y.H.; Hsieh, L.S. Production of γ-aminobutyric acid by immobilization of two Yarrowia lipolytica glutamate decarboxylases on electrospun nanofibrous membrane. Int. J. Biol. Macromol. 2024, 278 Pt 4, 135046. [Google Scholar] [CrossRef]
- Sabna, B.S.; Mahendran, R.; Balakrishnan, J.; Huang, C.Y.; Jerimon, P.J.; Thomas, A.; Eswaran, R.; Naveen, P.; Jayaraman, A. Purification and characterization of gamma-aminobutyric acid (GABA) from E. faecium BS5 and its antidiabetic efficacy in streptozotocin-Induced diabetic rats. Probiotics Antimicrob. Proteins 2025. [Google Scholar] [CrossRef] [PubMed]
- Barakat, H.; Aljutaily, T. Role of γ-Aminobutyric acid (GABA) as an inhibitory neurotransmitter in diabetes management: Mechanisms and therapeutic implications. Biomolecules 2025, 15, 399. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhu, L.; Li, H.; Chen, Q.; Li, N.; Li, J.; Zhao, Z.; Xiao, D.; Tang, T.; Bi, C.; et al. Insights and progress on the biosynthesis, metabolism, and physiological functions of gamma-aminobutyric acid (GABA): A review. PeerJ 2024, 12, e18712. [Google Scholar] [CrossRef]
- Ahmad, R.; Pandit, C.; Yu, Y.H.; Chen, W.J.; Cheng, Y.C.; Ali, I.; Cheng, Y.H. The impact of fermented gamma-aminobutyric acid on poultry growth performance through insulin-like growth factor-1 activation. Fermentation 2025, 11, 84. [Google Scholar] [CrossRef]
- Wang, Y.L.; Zhang, Z.H.; Wang, W.K.; Wu, Q.C.; Zhang, F.; Li, W.J.; Li, S.L.; Wang, W.; Cao, Z.J.; Yang, H.J. The effect of γ-aminobutyric acid addition on in vitro ruminal fermentation characteristics and methane production of diets differing in forage-to-concentrate ratio. Fermentation 2023, 9, 105. [Google Scholar] [CrossRef]
- Altaib, H.; El-Nouby, M.A.; Badr, Y. An Overview of GABA Production by Microorganisms. Microb. Nutraceuticals Prod. Process. 2025, 17, 365–398. [Google Scholar] [CrossRef]
- Hu, Y.; Huang, X.; Xiao, Q.; Wu, X.; Tian, Q.; Ma, W.; Shoaib, N.; Liu, Y.; Zhao, H.; Feng, Z. Advances in plant GABA research: Biological functions, synthesis mechanisms and regulatory pathways. Plants 2024, 13, 2891. [Google Scholar] [CrossRef]
- Zhu, N.; Wang, T.; Ge, L.; Li, Y.; Zhang, X.; Bao, H. γ-amino butyric acid (GABA) synthesis enabled by copper-catalyzed carboamination of alkenes. Org. Lett. 2017, 19, 4718–4721. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Dou, N.; Zhang, H.; Wu, C. The versatile GABA in plants. Plant Signal Behav. 2021, 16, 1862565. [Google Scholar] [CrossRef] [PubMed]
- Hou, D.; Tang, J.; Feng, Q.; Niu, Z.; Shen, Q.; Wang, L.; Zhou, S. Gamma-aminobutyric acid (GABA): A comprehensive review of dietary sources, enrichment technologies, processing effects, health benefits, and its applications. Crit. Rev. Food Sci. Nutr. 2024, 64, 8852–8874. [Google Scholar] [CrossRef] [PubMed]
- Pannerchelvan, S.; Rios-Solis, L.; Wong, F.W.F.; Zaidan, U.H.; Wasoh, H.; Mohamed, M.S.; Tan, J.S.; Mohamad, R.; Halim, M. Strategies for improvement of gamma-aminobutyric acid (GABA) biosynthesis via lactic acid bacteria (LAB) fermentation. Food Funct. 2023, 14, 3929–3948. [Google Scholar] [CrossRef]
- Lyu, J.F.; Lyu, C.J.; Cao, J.R.; Mei, J.Q.; Hu, S.; Zhao, W.R.; Xu, T.Y.; Wang, Y.T.; Wang, D.L.; Hang, J.; et al. High level production of γ-aminobutyric acid in engineered Escherichia coli by refactoring the glutamate decarboxylase. Process Biochem. 2022, 118, 243–251. [Google Scholar] [CrossRef]
- Jia, M.; Zhu, Y.; Wang, L.; Sun, T.; Pan, H.; Li, H. pH auto-sustain-based fermentation supports efficient gamma-aminobutyric acid production by Lactobacillus brevis CD0817. Fermentation 2022, 8, 208. [Google Scholar] [CrossRef]
- Abdel-motaal, H.; Abdelazez, A.; Wang, P.; Abady, G.; Abozaed, S.; Ye, B.; Xu, L.; Zhao, Y.; Niu, J.; Alshehry, J.; et al. Exploring phenotype, genotype, and the production of promising GABA postbiotics by Lactiplantibacillus plantarum: A comprehensive investigation. Fermentation 2024, 10, 309. [Google Scholar] [CrossRef]
- Nakatani, Y.; Fukaya, T.; Kishino, S.; Ogawa, J. Production of GABA-enriched tomato juice by Lactiplantibacillus plantarum KB1253. J. Biosci. Bioeng. 2022, 134, 424–431. [Google Scholar] [CrossRef]
- Sheng, Q.; Wu, X.Y.; Xu, X.; Tan, X.; Li, Z.; Zhang, B. Production of L-glutamate family amino acids in Corynebacterium glutamicum: Physiological mechanism, genetic modulation, and prospects. Synth. Syst. Biotechnol. 2021, 6, 302–325. [Google Scholar] [CrossRef]
- Shi, F.; Jiang, J.; Li, Y.; Li, Y.; Xie, Y. Enhancement of γ-aminobutyric acid production in recombinant Corynebacterium glutamicum by co-expressing two glutamate decarboxylase genes from Lactobacillus brevis. J. Ind. Microbiol. Biotechnol. 2013, 40, 1285–1296. [Google Scholar] [CrossRef]
- Son, J.; Baritugo, K.A.; Sohn, Y.J.; Kang, K.H.; Kim, H.T.; Joo, J.C.; Park, S.J. Production of γ-aminobutyrate (GABA) in recombinant Corynebacterium glutamicum by expression of glutamate decarboxylase active at neutral pH. ACS Omega 2022, 7, 29106–29115. [Google Scholar] [CrossRef]
- Wen, J.; Sun, W.; Leng, G.; Li, D.; Feng, C.; Tian, Z.; Wang, X.J. Enhanced fermentative γ-aminobutyric acid production by a metabolic engineered Corynebacterium glutamicum. Biotechnol. Bioprocess. Eng. 2024, 29, 129–140. [Google Scholar] [CrossRef]
- Litsanov, B.; Kabus, A.; Brocker, M.; Bott, M. Efficient aerobic succinate production from glucose in minimal medium with Corynebacterium glutamicum. Microb. Biotechnol. 2012, 5, 116–128. [Google Scholar] [CrossRef]
- Wang, Y.; Cao, G.; Xu, D.; Fan, L.; Wu, X.; Ni, X.; Zhao, S.; Zheng, P.; Sun, J.; Ma, Y. A novel Corynebacterium glutamicum L-glutamate exporter. Appl. Environ. Microbiol. 2018, 84, e02691-17. [Google Scholar] [CrossRef] [PubMed]
- Alharbi, N.S.; Kadaikunnan, S.; Khaled, J.M.; Almanaa, T.N.; Innasimuthu, G.M.; Rajoo, B.; Alanzi, K.F.; Rajaram, S.K. Optimization of glutamic acid production by Corynebacterium glutamicum using response surface methodology. J. King Saud. Univ. Sci. 2020, 32, 1403–1408. [Google Scholar] [CrossRef]
- Fernández-Castané, A.; Vine, C.E.; Caminal, G.; López-Santín, J. Evidencing the role of lactose permease in IPTG uptake by Escherichia coli in fed-batch high cell density cultures. J. Biotechnol. 2012, 157, 391–398. [Google Scholar] [CrossRef]
- Nakayama, Y.; Hashimoto, K.I.; Sawada, Y.; Sokabe, M.; Kawasaki, H.; Martinac, B. Corynebacterium glutamicum mechanosensitive channels: Towards unpuzzling “glutamate efflux” for amino acid production. Biophys. Rev. 2018, 10, 1359–1369. [Google Scholar] [CrossRef] [PubMed]
- Yogeswara, I.B.A.; Maneerat, S.; Haltrich, D. A brief review on glutamate decarboxylase from lactic acid bacteria. Preprints 2020, 8, 1923. [Google Scholar] [CrossRef]
- Kim, Y.H.; Kim, H.J.; Shin, J.H.; Bhatia, S.K.; Seo, H.M.; Kim, Y.G.; Lee, Y.K.; Yang, Y.H.; Park, K. Application of diethyl ethoxymethylenemalonate (DEEMM) derivatization for monitoring of lysine decarboxylase activity. J. Mol. Catal. B Enzym. 2015, 115, 151–154. [Google Scholar] [CrossRef]
- Yun, S.; Kim, Y.C.; Ghosh, R.; Song, D.M.; Joo, S.; Khobragade, T.; Giri, P.; Cho, S.; Lee, C.H.; Yun, H. Elucidation of broad substrate specificity of a novel γ-glutamyl transferase from Bacillus atrophaeus and rational design for enhanced substrate selectivity. J. Agric. Food Chem. 2025, 73, 15825–15834. [Google Scholar] [CrossRef] [PubMed]
- Ai, S.; Liang, L.; Liu, M.; Grierson, D.; Chen, K.; Xu, C. PpERF17 alleviates peach fruit postharvest chilling injury under elevated CO2 by activating jasmonic acid and γ-aminobutyric acid biosynthesis. Hortic. Res. 2025, 12, uhaf014. [Google Scholar] [CrossRef]
- Ruan, H.; Yu, H.; Xu, J. The glucose uptake systems in Corynebacterium glutamicum: A review. World J. Microbiol. Biotechnol. 2020, 36, 126. [Google Scholar] [CrossRef]
- Luo, W.; Yang, L.; Ding, Y.; Zhuang, Y.; Sun, L.; Gu, Y.; Fan, X. Research progress regarding physiological functions of probiotics and techniques to enhance their stress resistance: A review. Crit. Rev. Food Sci. Nutr. 2025, 1–29. [Google Scholar] [CrossRef]
- McLoone, P.; Warnock, M.; Fyfe, L. Honey: A realistic antimicrobial for disorders of the skin. J. Microbiol. Immunol. Infect. 2016, 49, 161–167. [Google Scholar] [CrossRef]
- Esser, D.S.; Leveau, J.H.; Meyer, K.M. Modeling microbial growth and dynamics. Appl. Microbiol. Biotechnol. 2015, 99, 8831–8846. [Google Scholar] [CrossRef]
- Simpson-Lavy, K.; Kupiec, M. Carbon catabolite repression: Not only for glucose. Curr. Genet. 2019, 65, 1321–1323. [Google Scholar] [CrossRef]
- Liu, J.; Qiao, Z.; Zhao, Y.; Xu, M.; Zhang, X.; Yang, T.; Rao, Z. Rational metabolic engineering of Corynebacterium glutamicum for efficient synthesis of L-glutamate. Chin. J. Biotechnol. 2023, 39, 3273–3289. [Google Scholar] [CrossRef]
- Nakayama, Y.; Hashimoto, K.I.; Kawasaki, H.; Martinac, B. “Force-from-lipids” mechanosensation in Corynebacterium glutamicum. Biophys. Rev. 2019, 11, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Noda, M.; Ozaki, M.; Ogura, S.; Danshiitsoodol, N.; Nakashima, E.; Sugiyama, M. Characterization of the gamma-aminobutyric acid (GABA) biosynthetic gene cluster in high GABA-producing Enterococcus avium G-15. Fermentation 2024, 10, 379. [Google Scholar] [CrossRef]
- Gourdon, P.; Raherimandimby, M.; Dominguez, H.; Cocaign-Bousquet, M.; Lindley, N.D. Osmotic stress, glucose transport capacity and consequences for glutamate overproduction in Corynebacterium glutamicum. J. Biotechnol. 2003, 104, 77–85. [Google Scholar] [CrossRef]
- Ikeda, M. Sugar transport systems in Corynebacterium glutamicum: Features and applications to strain development. Appl. Microbiol. Biotechnol. 2012, 96, 1191–1200. [Google Scholar] [CrossRef]
- Zhao, Z.; Ding, J.Y.; Ma, W.H.; Zhou, N.Y.; Liu, S.J. Identification and characterization of γ-aminobutyric acid uptake system GabP Cg (NCgl0464) in Corynebacterium glutamicum. Appl. Environ. Microbiol. 2012, 78, 2596–2601. [Google Scholar] [CrossRef]
- Shi, F.; Si, H.; Ni, Y.; Zhang, L.; Li, Y. Transaminase encoded by NCgl2515 gene of Corynebacterium glutamicum ATCC13032 is involved in γ-aminobutyric acid decomposition. Process Biochem. 2017, 55, 55–60. [Google Scholar] [CrossRef]
- Nie, Y.; Tian, Y.; Ren, X.; Liang, J.; Li, B.; Xiong, Z.Q. Microbial production of L-threonine using metabolically engineered Escherichia coli. J. Agric. Food Chem. 2025, 73, 13125–13141. [Google Scholar] [CrossRef]
- Wu, Q.; Tun, H.M.; Law, Y.S.; Khafipour, E.; Shah, N.P. Common distribution of gad operon in Lactobacillus brevis and its GadA contributes to efficient GABA synthesis toward cytosolic near-neutral pH. Front. Microbiol. 2017, 8, 206. [Google Scholar] [CrossRef]
- Liu, L.; Chen, G.; Liu, J.; Bao, W.; Li, X.; Yang, K.; Shi, S.; Zhao, B.; Wang, Q.; Cao, X. Sequential production of secondary metabolites by one operon affects interspecies interactions in Enterobacter sp. CGMCC 5087. Innov. Life 2023, 1, 100023. [Google Scholar] [CrossRef]
- Xiao, J.; Han, J.; Qiao, Z.; Zhang, G.; Huang, W.; Qian, K.; Xu, M.; Zhang, X.; Yang, T.; Rao, Z. Efficient biosynthesis of γ-aminobutyric acid by rationally engineering the catalytic pH range of a glutamate decarboxylase from Lactobacillus plantarum. Chin. J. Biotechnol. 2023, 39, 2108–2125. [Google Scholar] [CrossRef]
- Espeso, E.A.; Arst Jr, H.N. On the mechanism by which alkaline pH prevents expression of an acid-expressed gene. Mol. Cell Biol. 2000, 20, 3355–3363. [Google Scholar] [CrossRef] [PubMed]
- Gourdon, P.; Lindley, N.D. Metabolic analysis of glutamate production by Corynebacterium glutamicum. Metab. Eng. 1999, 1, 224–231. [Google Scholar] [CrossRef]
- Monteagudo-Mera, A.; Fanti, V.; Rodriguez-Sobstel, C.; Gibson, G.; Wijeyesekera, A.; Karatzas, K.A.; Chakrabarti, B. Gamma aminobutyric acid production by commercially available probiotic strains. J. Appl. Microbiol. 2023, 134, lxac066. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, M.; Ghasemi, Y.; Sharifan, A.; Bakhoda, H. Gamma-aminobutyric acid (GABA) biosynthesis from Lactobacillus plantarum subsp. plantarum IBRC10817 optimized and modeled in response to heat and ultrasonic shock. Probiotics Antimicrob. Proteins 2024, 16, 1304–1312. [Google Scholar] [CrossRef] [PubMed]
- Ni, Y.; Shi, F.; Wang, N. Specific γ-aminobutyric acid decomposition by gabP and gabT under neutral pH in recombinant Corynebacterium glutamicum. Biotechnol. Lett. 2015, 37, 2219–2227. [Google Scholar] [CrossRef]
- Lv, Q.; Hu, M.; Tian, L.; Liu, F.; Wang, Q.; Xu, M.; Rao, Z. Enhancing L-glutamine production in Corynebacterium glutamicum by rational metabolic engineering combined with a two-stage pH control strategy. Bioresour. Technol. 2021, 341, 125799. [Google Scholar] [CrossRef] [PubMed]
- Seifi, H.S.; Curvers, K.; De Vleesschauwer, D.; Delaere, I.; Aziz, A.; Höfte, M. Concurrent overactivation of the cytosolic glutamine synthetase and the GABA shunt in the GABA-deficient sitiens mutant of tomato leads to resistance against otrytis cinerea. New Phytol. 2013, 199, 490–504. [Google Scholar] [CrossRef]
- Sharafi, Y.; Aghdam, M.S.; Luo, Z.; Jannatizadeh, A.; Razavi, F.; Razavi Fard, J.; Farmani, B. Melatonin treatment promotes endogenous melatonin accumulation and triggers GABA shunt pathway activity in tomato fruits during cold storage. Sci. Hortic. 2019, 254, 222–227. [Google Scholar] [CrossRef]
- Guo, J.; Ma, Z.; Gao, J.; Zhao, J.; Wei, L.; Liu, J.; Xu, N. Recent advances of pH homeostasis mechanisms in Corynebacterium glutamicum. World J. Microbiol. Biotechnol. 2019, 35, 192. [Google Scholar] [CrossRef]
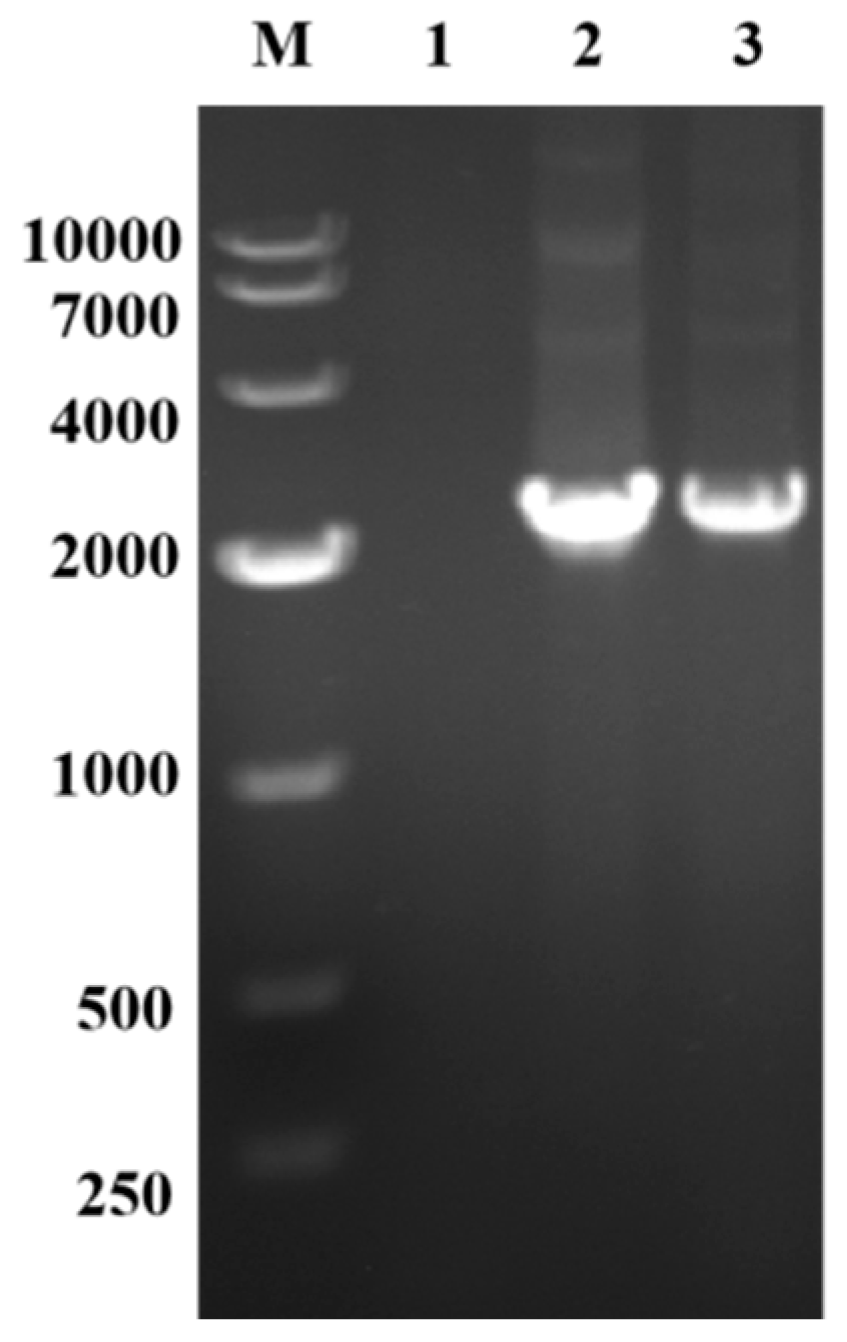

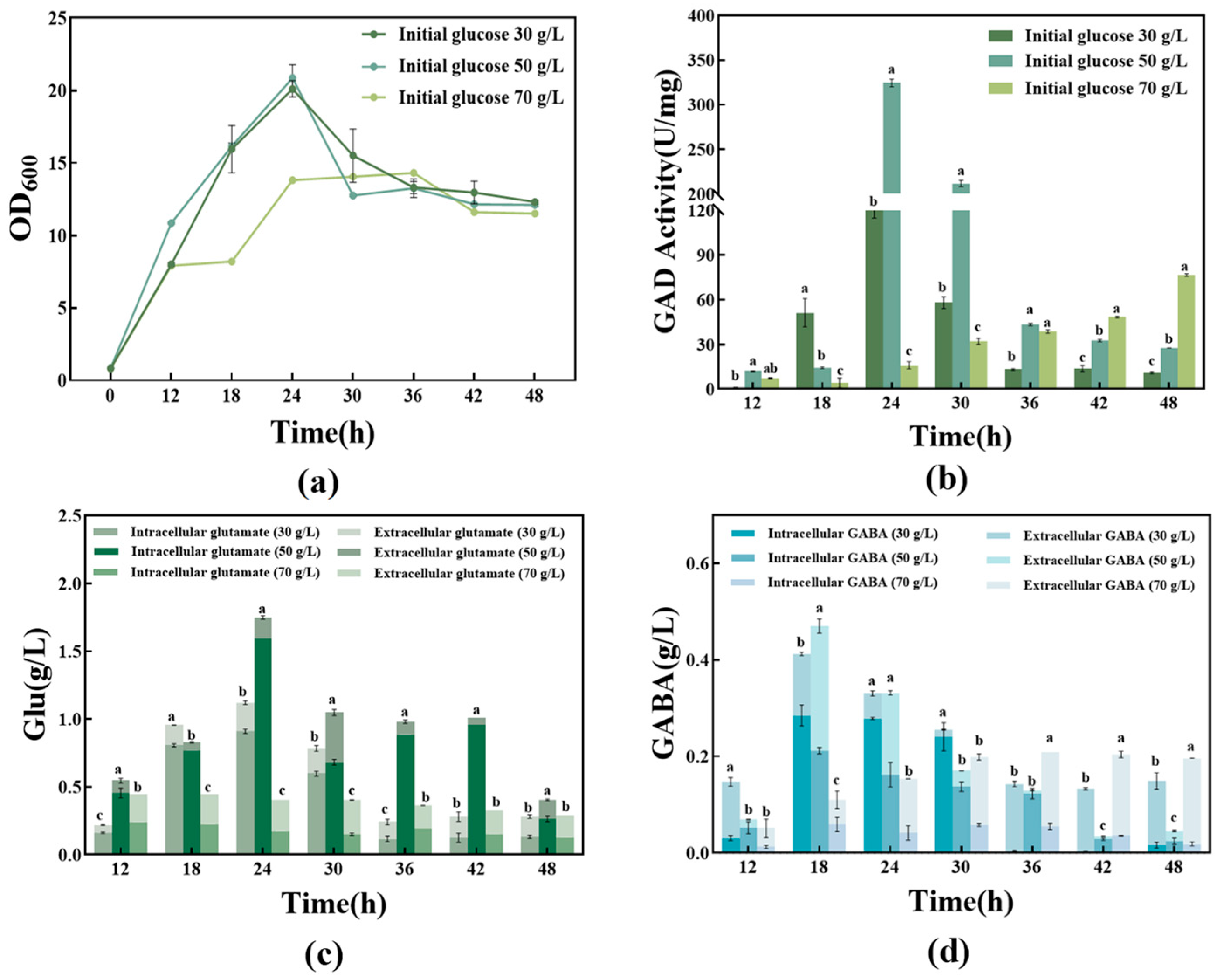


| Initial Glucose 30 g/L | Initial Glucose 50 g/L | Initial Glucose 70 g/L | |
|---|---|---|---|
| Glucose utilization ratio | 99.3% | 99.8% | 80% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, Q.; Wen, Y.; Zhang, R.; Cai, J. The Construction of Corynebacterium glutamicum for Producing γ-Aminobutyric Acid and Analysis of the Fermentation Process. Fermentation 2025, 11, 534. https://doi.org/10.3390/fermentation11090534
Deng Q, Wen Y, Zhang R, Cai J. The Construction of Corynebacterium glutamicum for Producing γ-Aminobutyric Acid and Analysis of the Fermentation Process. Fermentation. 2025; 11(9):534. https://doi.org/10.3390/fermentation11090534
Chicago/Turabian StyleDeng, Qijie, Ying Wen, Runmei Zhang, and Jun Cai. 2025. "The Construction of Corynebacterium glutamicum for Producing γ-Aminobutyric Acid and Analysis of the Fermentation Process" Fermentation 11, no. 9: 534. https://doi.org/10.3390/fermentation11090534
APA StyleDeng, Q., Wen, Y., Zhang, R., & Cai, J. (2025). The Construction of Corynebacterium glutamicum for Producing γ-Aminobutyric Acid and Analysis of the Fermentation Process. Fermentation, 11(9), 534. https://doi.org/10.3390/fermentation11090534






