Seasonal Temperature Effects on EPS Composition and Sludge Settling Performance in Full-Scale Wastewater Treatment Plant: Mechanisms and Mitigation Strategies
Abstract
1. Introduction
2. Methodology
2.1. Full-Scale and Lab-Scale Wastewater Treatment Process
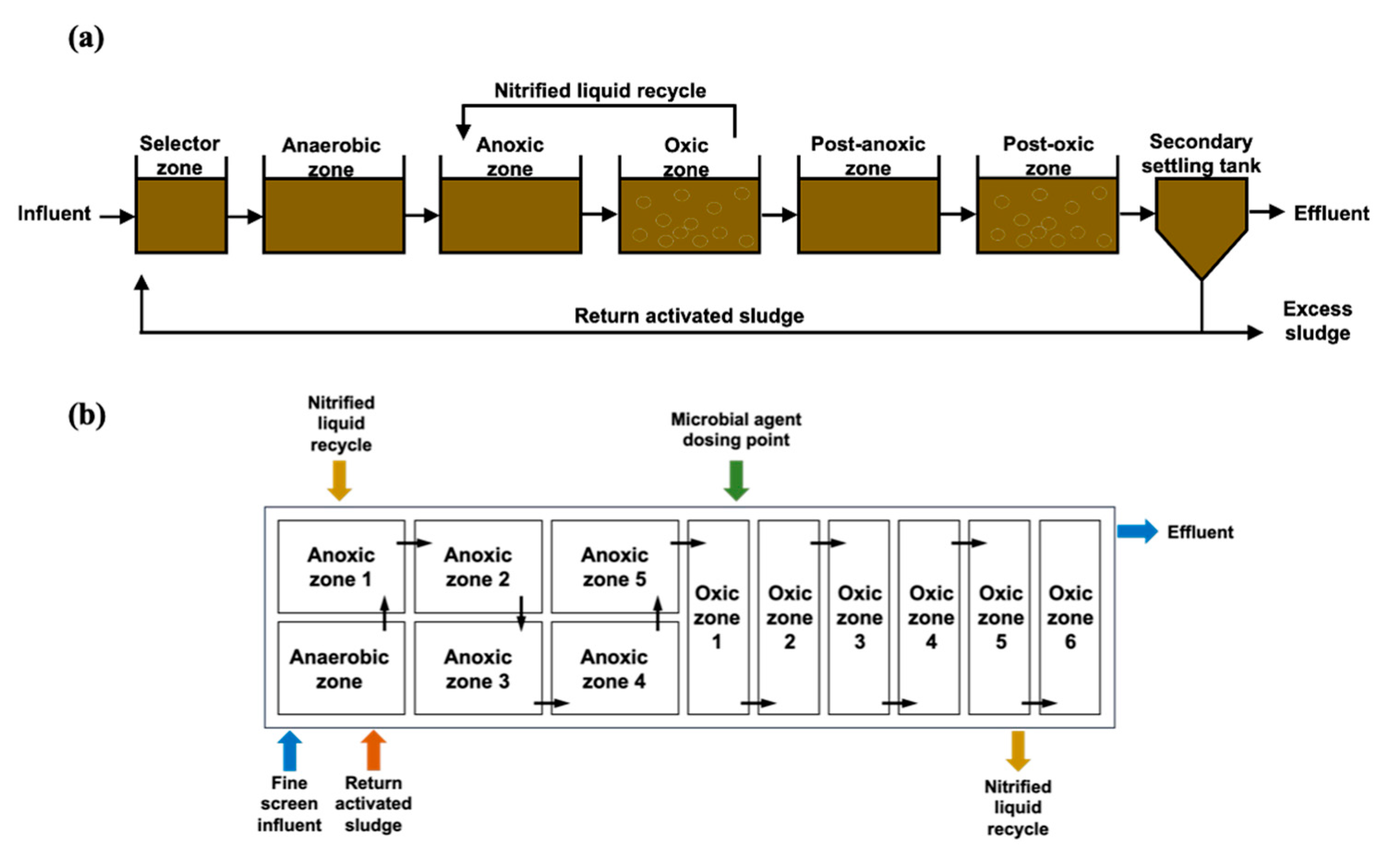
2.1.1. Full-Scale Wastewater Treatment Process
2.1.2. Lab-Scale Wastewater Treatment Process
2.2. Chemical Analysis
2.3. Metagenomic Sequencing
3. Results and Discussion
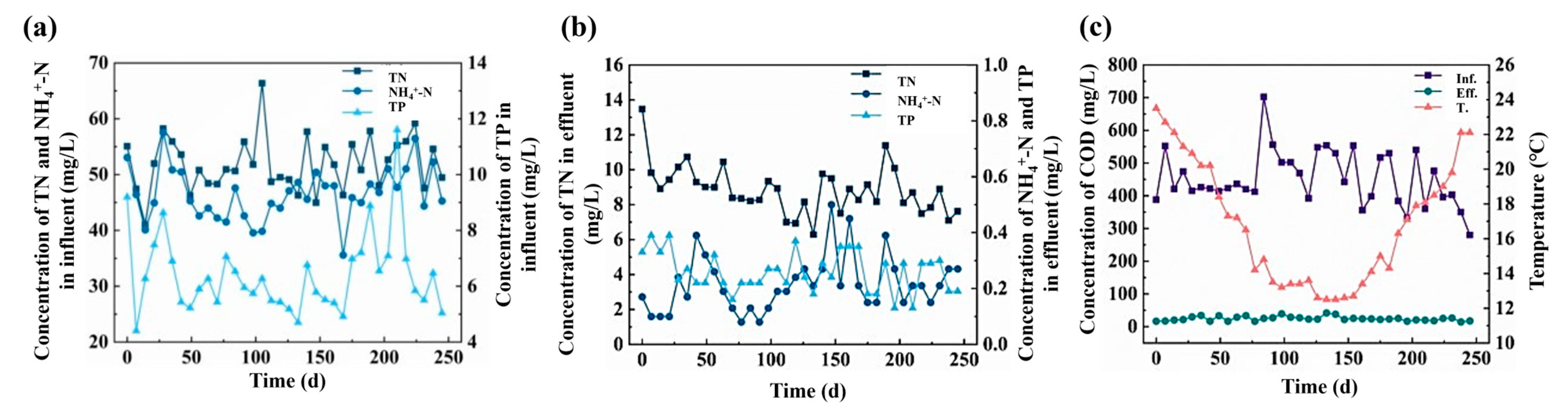
3.1. Removal Efficiency of Nutrients
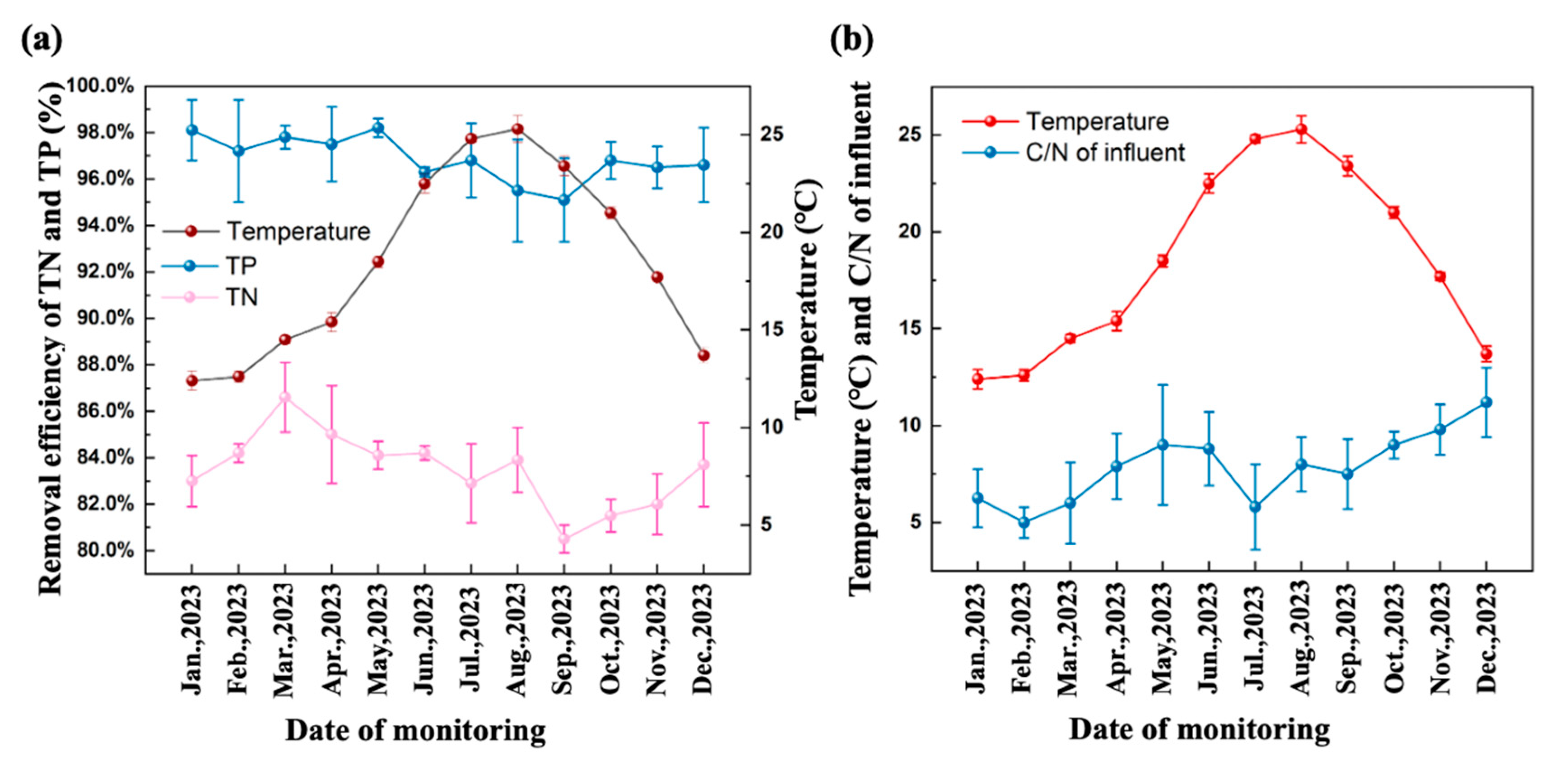
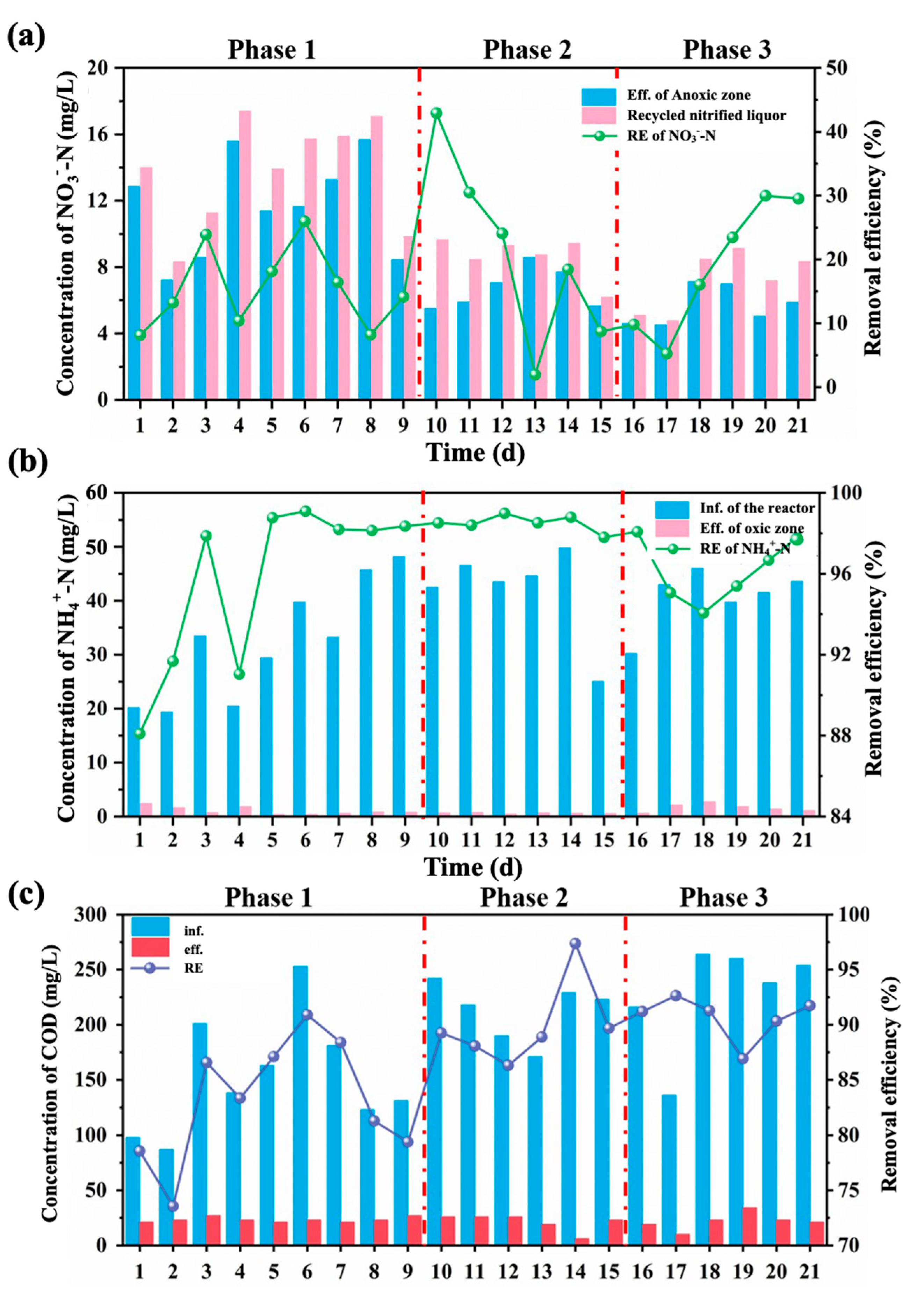
3.1.1. Seasonal Nutrients Removal Performance
3.1.2. Seasonal Effects of Nutrients Removal Performance
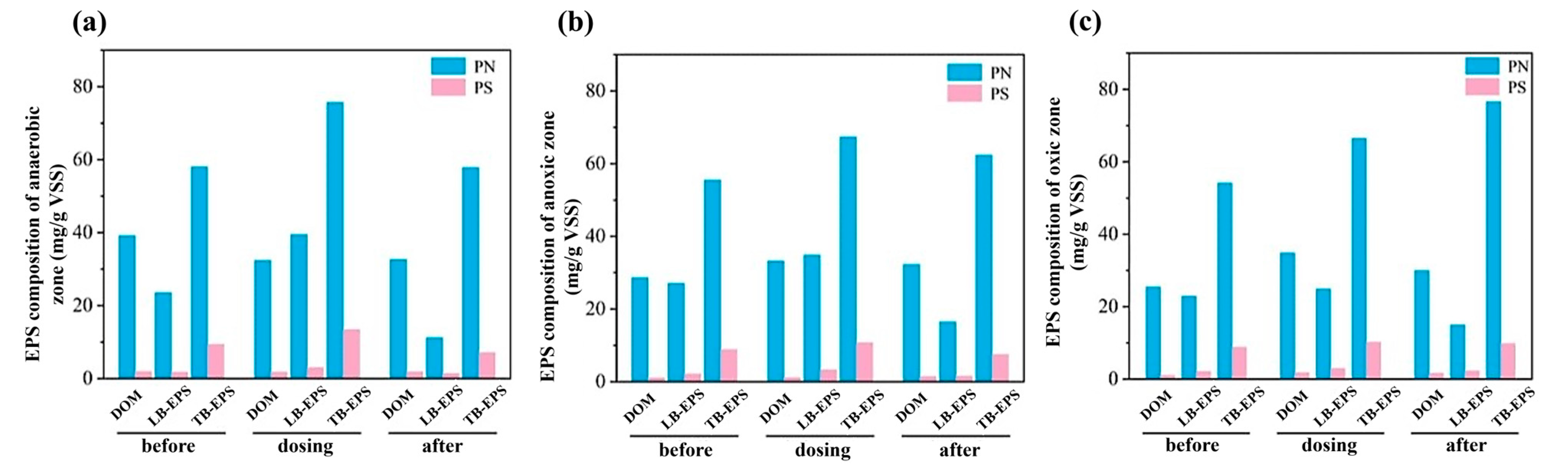
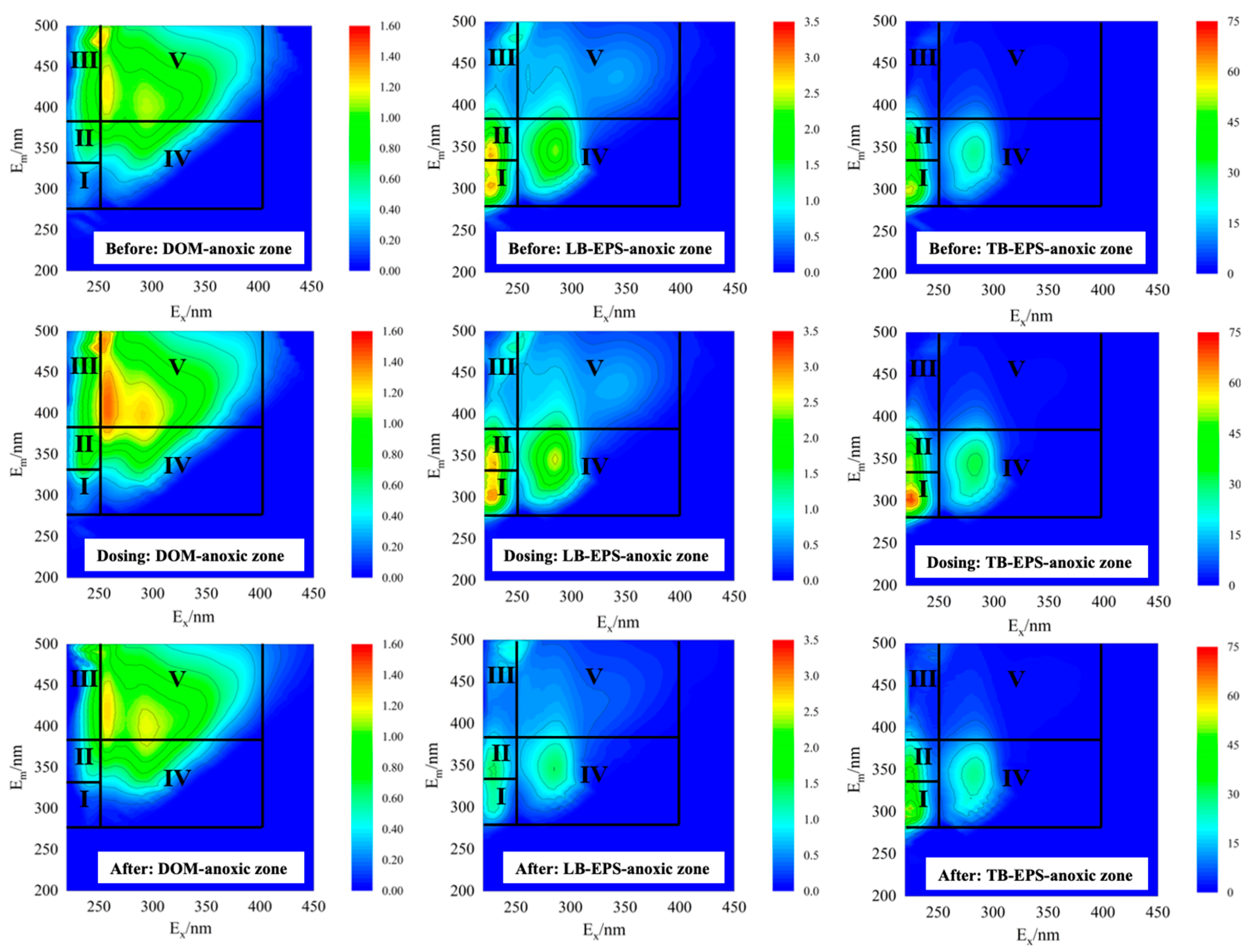
3.2. Components and Characteristics of EPS
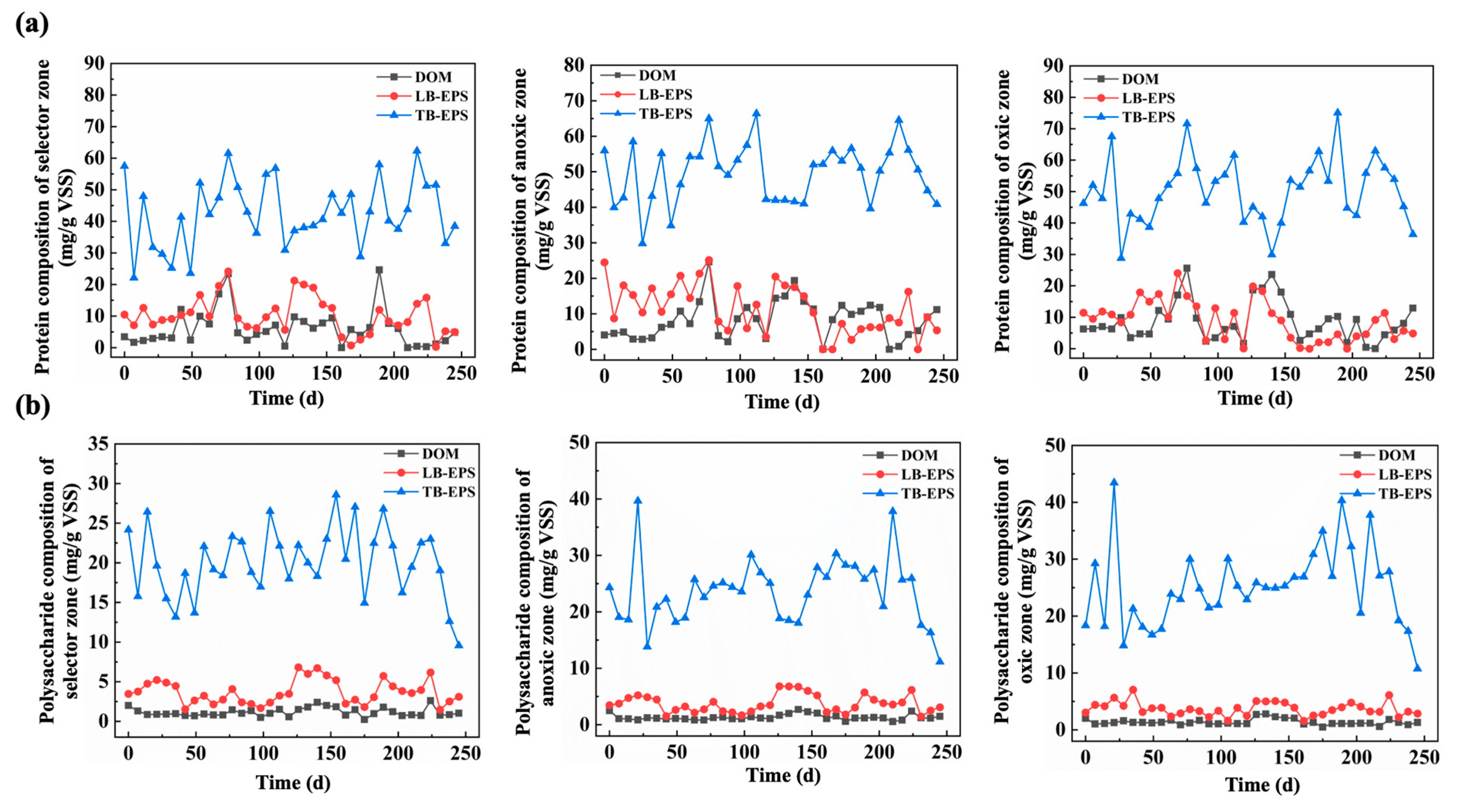
3.2.1. Changes in EPS Components During Temperature Fluctuations
3.2.2. Effect of PS on Nutrients Removal Efficiency
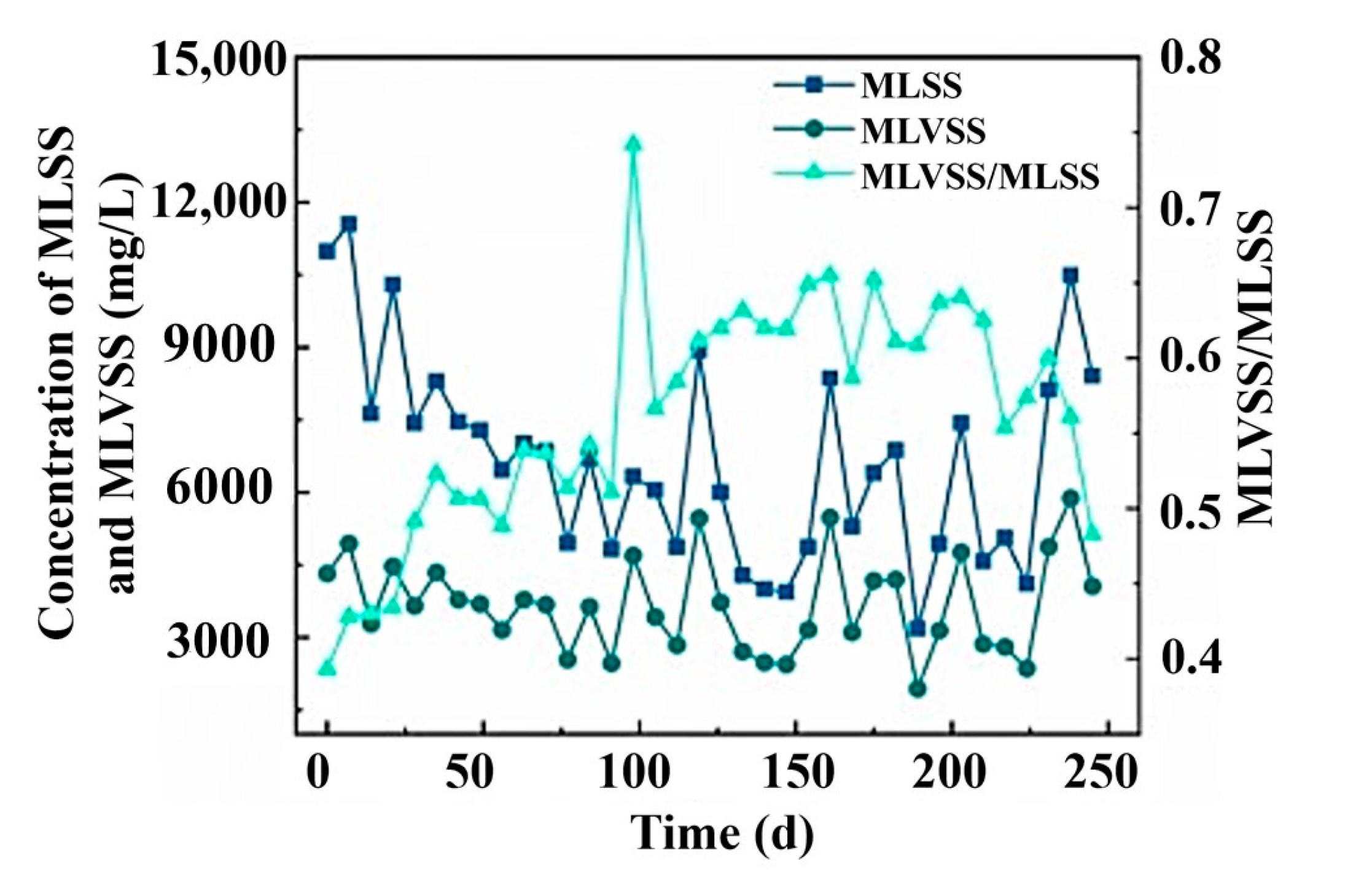
3.2.3. EPS and Sludge Settleability
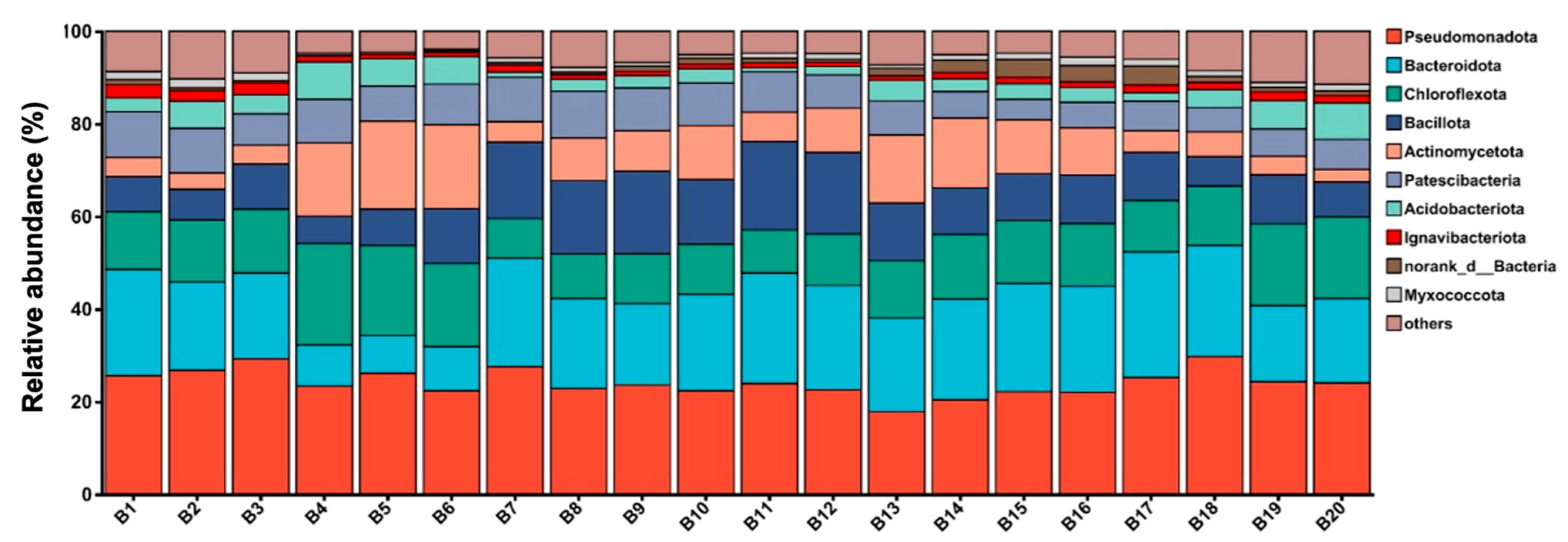
3.3. Structure of Microbial Community
3.3.1. Overall Analysis of Illumina Sequence Data
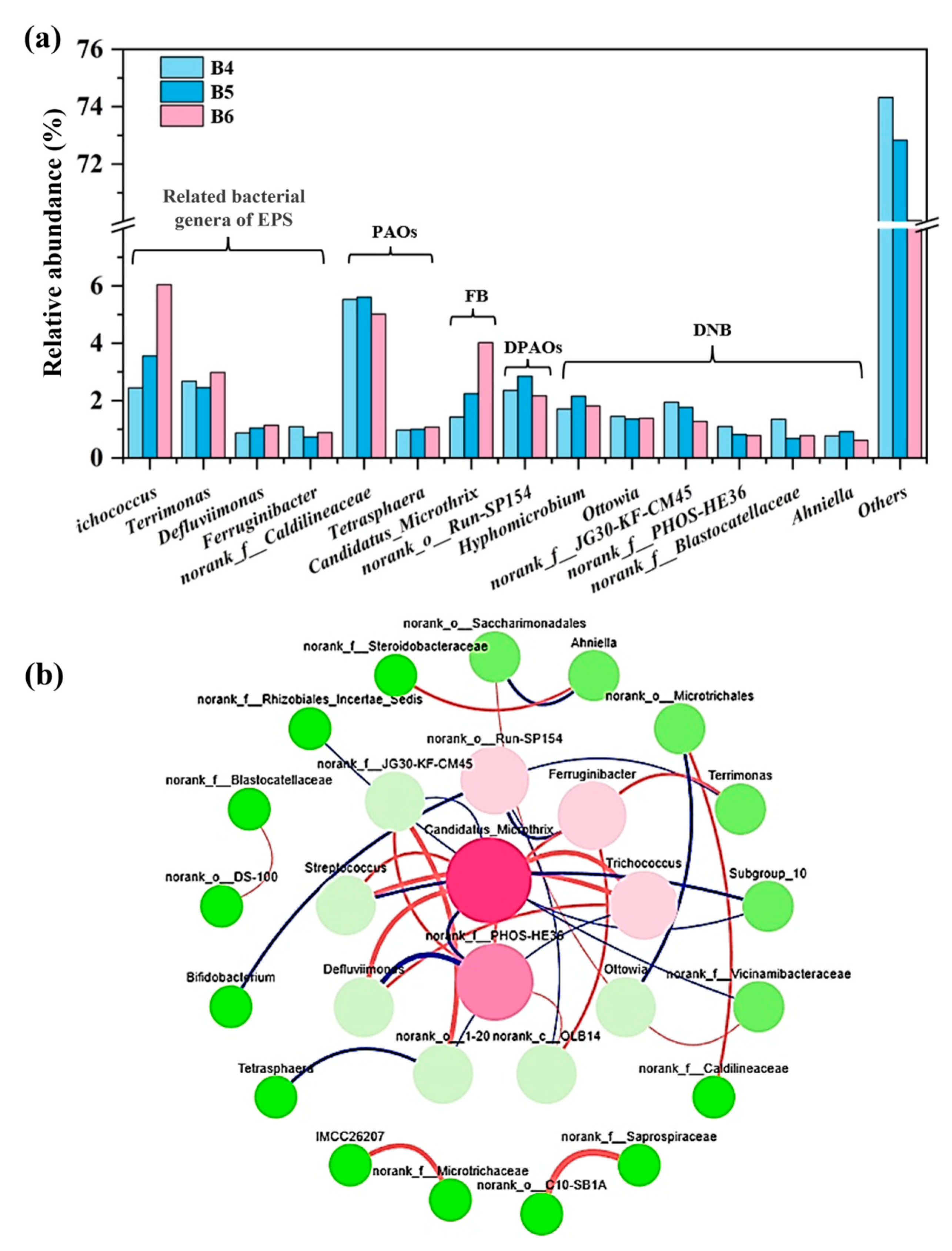
3.3.2. Microbial Structure at the Genus Level Under Cold Waves
3.4. Effect of the EPS-Optimizing Microbial Agents on Temperature Shock Resistance
3.4.1. Effect of the Microbial Agents on Nutrients Removal Efficiency
3.4.2. Variation of EPS Components
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, G.; van Loosdrecht, M.C.M.; Ekama, G.A.; Brdjanovic, D. Biological Wastewater Treatment: Principles, Modelling and Design; IWA Publishing: London, UK, 2023. [Google Scholar] [CrossRef]
- Li, W.; Sangeetha, T.; Han, X.; Yan, W.-M.; Yang, L.; Zhao, J.; Cai, W.; Yao, H. Tracking the diversity and interaction of methanogens in the energy recovery process of a full-scale wastewater treatment plant. Environ. Res. 2022, 211, 113010. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, B.; Li, R.; Cui, Y.; Xie, F.; Zhou, A.; Li, J.; Yue, X. Study on the feasibility of carbon source recovery by upflow anaerobic sludge blanket in simulated municipal wastewater. Sci. Total Environ. 2023, 878, 163157. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, X.; Zhang, S.; Qi, H.; Hou, Y.; Gao, M.; Wang, J.; Zhang, A.; Chen, Y.; Liu, Y. A comparison between exogenous carriers enhanced aerobic granulation under low organic loading in the aspect of sludge characteristics, extracellular polymeric substances and microbial communities. Bioresour. Technol. 2022, 346, 126567. [Google Scholar] [CrossRef] [PubMed]
- Revollar, S.; Meneses, M.; Vilanova, R.; Francisco, M.; Vega, P. Adapting WWTP performance to seasonality and climate change: Temperature-driven strategies for enhanced nitrogen removal. Process Saf. Environ. Prot. 2025, 195, 106792. [Google Scholar] [CrossRef]
- Wang, P.; Yu, Z.; Qi, R.; Zhang, H. Detailed comparison of bacterial communities during seasonal sludge bulking in a municipal wastewater treatment plant. Water Res. 2016, 105, 157–166. [Google Scholar] [CrossRef]
- Yuan, F.; Liu, M.; Sun, H.; Feng, Z.; Liu, X.; Dong, X.; Zhao, S.; Ding, J.; Chen, Y. Low-temperature sludge bulking in wastewater treatment plants: Causal analysis and mechanistic investigation. J. Water Process Eng. 2025, 76, 108233. [Google Scholar] [CrossRef]
- Cong, S.; Yu, G.; Xia, S.; Yu, H.; Sun, Y.; Gao, Y.; Liu, Y.; Zou, D. Pilot–scale vertical continuous–flow reactors applied to treat rural domestic wastewater at low temperature via aerobic granular sludge. J. Water Process Eng. 2024, 60, 105220. [Google Scholar] [CrossRef]
- Peng, S.; Hu, A.; Ai, J.; Zhang, W.; Wang, D. Changes in molecular structure of extracellular polymeric substances (EPS) with temperature in relation to sludge macro-physical properties. Water Res. 2021, 201, 117316. [Google Scholar] [CrossRef]
- Sam, S.B.; Ward, B.J.; Niederdorfer, R.; Morgenroth, E.; Strande, L. Elucidating the role of extracellular polymeric substances (EPS) in dewaterability of fecal sludge from onsite sanitation systems, and changes during anaerobic storage. Water Res. 2022, 222, 118915. [Google Scholar] [CrossRef]
- Jin, B.; Wilén, B.-M.; Lant, P. Impacts of morphological, physical and chemical properties of sludge flocs on dewaterability of activated sludge. Chem. Eng. J. 2004, 98, 115–126. [Google Scholar] [CrossRef]
- Mikkelsen, L.H.; Keiding, K. Physico-chemical characteristics of full scale sewage sludges with implications to dewatering. Water Res. 2002, 36, 2451–2462. [Google Scholar] [CrossRef]
- Wingender, J.; Neu, T.R.; Flemming, H.-C. What are Bacterial Extracellular Polymeric Substances? In Microbial Extracellular Polymeric Substances: Characterization, Structure and Function; Wingender, J., Neu, T.R., Flemming, H.-C., Eds.; Springer: Berlin/Heidelberg, Germany, 1999; pp. 1–19. [Google Scholar]
- Liang, S.; Liu, C.; Song, L. Soluble microbial products in membrane bioreactor operation: Behaviors, characteristics, and fouling potential. Water Res. 2007, 41, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Aquino, S.F.; Stuckey, D.C. Soluble microbial products formation in anaerobic chemostats in the presence of toxic compounds. Water Res. 2004, 38, 255–266. [Google Scholar] [CrossRef]
- Ni, B.-J.; Rittmann, B.E.; Yu, H.-Q. Soluble microbial products and their implications in mixed culture biotechnology. Trends Biotechnol. 2011, 29, 454–463. [Google Scholar] [CrossRef]
- Raszka, A.; Chorvatova, M.; Wanner, J. The role and significance of extracellular polymers in activated sludge. Part I: Literature review. Acta Hydroch. Hydrob. 2006, 34, 411–424. [Google Scholar] [CrossRef]
- Ben Hamed, H.; Mainardis, M.; Moretti, A.; Toye, D.; Léonard, A. Extracellular polymeric substances (EPS) in sewage sludge management: A call for methodological standardization. J. Environ. Manag. 2025, 376, 124407. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Liu, Y.; Lv, J.; Wu, D.; Jiang, L.; Lv, W. Short-time aerobic digestion treatment of waste activated sludge to enhance EPS production and sludge dewatering performance by changing microbial communities: The impact of temperature. Water Cycle 2024, 5, 146–155. [Google Scholar] [CrossRef]
- Pérez-Sancho, M.; Vela, A.I.; Wiklund, T.; Kostrzewa, M.; Domínguez, L.; Fernández-Garayzábal, J.F. Differentiation of Flavobacterium psychrophilum from Flavobacterium psychrophilum-like species by MALDI-TOF mass spectrometry. Res. Vet. Sci. 2017, 115, 345–352. [Google Scholar] [CrossRef]
- Sun, H.-J.; Zhao, X.; Ding, J.; Wang, Y.-Q.; Wang, W.-S.; Feng, Z.-H.; Zhao, S.; Wang, L.; Ren, N.-Q.; Yang, S.-S. Unveiling dynamics of microbial communities, species interactions, and ecological assembly during low-temperature-induced sludge bulking in full-scale wastewater treatment systems. Bioresour. Technol. 2025, 435, 132950. [Google Scholar] [CrossRef]
- Ranieri, E.; D’Onghia, G.; Lopopolo, L.; Gikas, P.; Ranieri, F.; Gika, E.; Spagnolo, V.; Herrera, J.A.; Ranieri, A.C. Influence of climate change on wastewater treatment plants performances and energy costs in Apulia, south Italy. Chemosphere 2024, 350, 141087. [Google Scholar] [CrossRef]
- Zhao, B.; Xie, F.; Zhou, A.; Liu, Z.; Ji, L.; Zhang, G.; Yue, X. Analysis of energy recovery and microbial community in an amalgamated CSTR-UASBs reactor for a three-stage anaerobic fermentation process of cornstalks. Water Sci. Technol. 2022, 86, 1848–1857. [Google Scholar] [CrossRef]
- Walter, W. APHA Standard Methods for the Examination of Water and Wastewater. Am. J. Public Health Nations Health 1961, 51, 940. [Google Scholar] [CrossRef]
- Bian, X.; Wu, Y.; Li, J.; Yin, M.; Li, D.; Pei, H.; Chang, S.; Guo, W. Effect of dissolved oxygen on high C/N wastewater treatment in moving bed biofilm reactors based on heterotrophic nitrification and aerobic denitrification: Nitrogen removal performance and potential mechanisms. Bioresour. Technol. 2022, 365, 128147. [Google Scholar] [CrossRef]
- SEPA. State Environmental Protection Administration, the People’s Republic of China. Discharge Standard of Pollutants for Municipal Wastewater Treatment Plant (GB 18918–2002). 2002. Available online: https://www.mee.gov.cn/ywgz/fgbz/bz/bzwb/shjbh/swrwpfbz/200307/t20030701_66529.shtml (accessed on 26 July 2025).
- Tkach, O.; Sangeetha, T.; Maria, S.; Wang, A. Performance of low temperature Microbial Fuel Cells (MFCs) catalyzed by mixed bacterial consortia. J. Environ. Sci. 2017, 52, 284–292. [Google Scholar] [CrossRef]
- Xie, Y.; Zhang, Q.; Wu, Q.; Zhang, J.; Dzakpasu, M.; Wang, X.C. Advancing nitrogen removal in low-temperature sewage treatment: Role of the adaptive activated sludge process. Chem. Eng. J. 2025, 504, 158887. [Google Scholar] [CrossRef]
- Li, B.; Godfrey, B.J.; RedCorn, R.; Candry, P.; Abrahamson, B.; Wang, Z.; Goel, R.; Winkler, M.-K.H. Mainstream nitrogen removal from low temperature and low ammonium strength municipal wastewater using hydrogel-encapsulated comammox and anammox. Water Res. 2023, 242, 120303. [Google Scholar] [CrossRef]
- Xiong, X.; Yang, N.; Liu, M.; Jiang, X.; Luo, D.; Lei, Y. Resilience of electroactive pollutant removal and power generation to long-term temperature shifts in domestic wastewater treatment during the autumn-winter-spring period. J. Environ. Chem. Eng. 2025, 13, 117386. [Google Scholar] [CrossRef]
- Wang, L.; Yuan, L.; Li, Z.-H.; Zhang, X.; Leung, K.M.Y.; Sheng, G.-P. Extracellular polymeric substances (EPS) associated extracellular antibiotic resistance genes in activated sludge along the AAO process: Distribution and microbial secretors. Sci. Total Environ. 2022, 816, 151575. [Google Scholar] [CrossRef] [PubMed]
- More, T.T.; Yadav, J.S.S.; Yan, S.; Tyagi, R.D.; Surampalli, R.Y. Extracellular polymeric substances of bacteria and their potential environmental applications. J. Environ. Manag. 2014, 144, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Melo, A.; Costa, J.; Quintelas, C.; Amaral, A.L.; Ferreira, E.C.; Mesquita, D.P. Quantitative image analysis for assessing extracellular polymeric substances in activated sludge under atrazine exposure. Sep. Purif. Technol. 2024, 349, 127831. [Google Scholar] [CrossRef]
- Ma, X.; Zhao, B.; Zhang, X.; Xie, F.; Cui, Y.; Li, H.; Yue, X. Effect of periodic temperature shock on nitrogen removal performance and microbial community structure in plug-flow microaerobic sludge blanket. Chemosphere 2020, 241, 124934. [Google Scholar] [CrossRef]
- Cao, S.; Du, R.; Li, B.; Ren, N.; Peng, Y. High-throughput profiling of microbial community structures in an ANAMMOX-UASB reactor treating high-strength wastewater. Appl. Microbiol. Biotechnol. 2016, 100, 6457–6467. [Google Scholar] [CrossRef]
- Kono, M.; Haruta, S. Coaggregation Occurs between a Piliated Unicellular Cyanobacterium, Thermosynechococcus, and a Filamentous Bacterium, Chloroflexus aggregans. Microorganisms 2024, 12, 1904. [Google Scholar] [CrossRef]
- Zhao, Y.; Jiang, B.; Tang, X.; Liu, S. Metagenomic insights into functional traits variation and coupling effects on the anammox community during reactor start-up. Sci. Total Environ. 2019, 687, 50–60. [Google Scholar] [CrossRef]
- Zhao, J.-Y.; Geng, S.; Xu, L.; Hu, B.; Sun, J.-Q.; Nie, Y.; Tang, Y.-Q.; Wu, X.-L. Complete genome sequence of Defluviimonas alba cai42T, a microbial exopolysaccharides producer. J. Biotechnol. 2016, 239, 9–12. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Qiao, Y.; Fu, Z. Shifts of bacterial community and predictive functional profiling of denitrifying phosphorus removal—Partial nitrification—Anammox three-stage nitrogen and phosphorus removal before and after coupling for treating simulated wastewater with low C/N. Chem. Eng. J. 2023, 451, 138601. [Google Scholar] [CrossRef]
- Liu, H.; Zeng, W.; Meng, Q.; Fan, Z.; Peng, Y. Phosphorus removal performance, intracellular metabolites and clade-level community structure of Tetrasphaera-dominated polyphosphate accumulating organisms at different temperatures. Sci. Total Environ. 2022, 842, 156913. [Google Scholar] [CrossRef]
- Liu, R.; Chen, Y.; Li, S.-Y.; Chen, Y.-P.; Guo, J.-S.; Liu, S.-Y.; Yan, P. Filamentous bacteria in activated sludge: Geographic distribution and impact of treatment processes. J. Environ. Manag. 2025, 379, 124859. [Google Scholar] [CrossRef]
- Wang, K.; Zhou, C.; Zhou, H.; Jiang, M.; Chen, G.; Wang, C.; Zhang, Z.; Zhao, X.; Jiang, L.-M.; Zhou, Z. Comparison on biological nutrient removal and microbial community between full-scale anaerobic/anoxic/aerobic process and its upgrading processes. Bioresour. Technol. 2023, 374, 128757. [Google Scholar] [CrossRef]
- Shi, W.; He, Z.; Lu, J.; Wang, L.; Guo, J.; Qiu, S.; Ge, S. Response of nitrifiers to gradually increasing pH conditions in a membrane nitrification bioreactor: Microbial dynamics and alkali-resistant mechanism. Water Res. 2025, 268, 122567. [Google Scholar] [CrossRef]
- Liu, Q.; Liao, X.; Li, Y.; Zhang, M.; Li, J. Reduction of chromate and nitrate by type II aerobic methanotrophs under micro-aerobic conditions. Chem. Eng. J. 2025, 505, 159286. [Google Scholar] [CrossRef]
- Liu, Z.; Cui, Z.; Guo, Z.; Li, D.; He, Z.; Liu, W.; Yue, X.; Zhou, A. Insights into the effect of nitrate photolysis on short-chain fatty acids production from waste activated sludge in anaerobic fermentation system: Performance and mechanisms. Water Res. 2024, 258, 121772. [Google Scholar] [CrossRef]
- Sun, J.; Pan, F.; Zhu, H.; Wu, Q.; Pan, C.; Lu, F. Enhancing low-temperature anaerobic digestion of low-strength organic wastewater through bio-electrochemical technology. Int. J. Hydrogen Energy 2024, 58, 1062–1074. [Google Scholar] [CrossRef]
- Xu, L.; Li, L.; Liu, J.; Wang, X.; Zhao, Y.; Zhao, J.; Gu, L.; He, Q.; Wang, X.; Zhang, J. Ferrihydrite optimizing Feammox inoculum to enhance ammonia removal from concentrate wastewater through continuous upflow anaerobic sludge blanket (UASB). J. Environ. Chem. Eng. 2024, 12, 114469. [Google Scholar] [CrossRef]
- Gao, L.; Wang, S.; Xu, X.; Zheng, J.; Cai, T.; Jia, S. Metagenomic analysis reveals the distribution, function, and bacterial hosts of degradation genes in activated sludge from industrial wastewater treatment plants. Environ. Pollut. 2024, 340, 122802. [Google Scholar] [CrossRef]
- Zhang, S.; Chen, J.; Sang, W.; Li, M.; Prodanovic, V.; Zhang, K. Metagenomic insights into the explanation of biofilter performance distinction induced by dissolved oxygen increment. Process Saf. Environ. Prot. 2021, 153, 329–338. [Google Scholar] [CrossRef]
- Tian, W.; Li, L.; Liu, F.; Zhang, Z.; Yu, G.; Shen, Q.; Shen, B. Assessment of the maturity and biological parameters of compost produced from dairy manure and rice chaff by excitation–emission matrix fluorescence spectroscopy. Bioresour. Technol. 2012, 110, 330–337. [Google Scholar] [CrossRef]
- Zhen, G.; Lu, X.; Wang, B.; Zhao, Y.; Chai, X.; Niu, D.; Zhao, A.; Li, Y.; Song, Y.; Cao, X. Synergetic pretreatment of waste activated sludge by Fe(II)–activated persulfate oxidation under mild temperature for enhanced dewaterability. Bioresour. Technol. 2012, 124, 29–36. [Google Scholar] [CrossRef]
| Parameter | Full-Scale WWTP | Laboratory Reactor |
|---|---|---|
| Total volume | ~15,000 m3 | 180.3 L |
| Treatment capacity | 47,275–110,899 m3/d | 7.2 L/d |
| Anaerobic HRT (h) | 4.5 | 2.0 |
| Anoxic HRT (h) | 8.0 | 10.6 |
| Oxic HRT (h) | 12.0 | 12.9 |
| SRT (h) | ~16 | ~15 |
| Internal reflux ratio (%) | 250–300 | 300 |
| External reflux ratio (%) | 80–100 | 100 |
| Temperature (°C) | 12–25 | 18–22 |
| Indicators | pH | SS | COD | BOD5 | NH4+-N | TN | TP |
|---|---|---|---|---|---|---|---|
| Max (mg/L) 1 | 7.6 | 8.0 | 46.0 | 6.1 | 4.39 | 14.24 | 0.59 |
| Min (mg/L) 1 | 6.3 | 2.0 | 12.0 | 1.1 | 0.02 | 2.30 | 0.02 |
| Effluent limit 2 (mg/L) | 6–9 | 10 | 50 | 10 | 5 | 15 | 0.5 |
| Compliance rate 3 (%) | 100 | 100 | 100 | 100 | 100 | 98 | 99 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, F.; Tian, C.; Ma, X.; Ji, L.; Zhao, B.; Danish, M.E.; Gao, F.; Yang, Z. Seasonal Temperature Effects on EPS Composition and Sludge Settling Performance in Full-Scale Wastewater Treatment Plant: Mechanisms and Mitigation Strategies. Fermentation 2025, 11, 532. https://doi.org/10.3390/fermentation11090532
Xie F, Tian C, Ma X, Ji L, Zhao B, Danish ME, Gao F, Yang Z. Seasonal Temperature Effects on EPS Composition and Sludge Settling Performance in Full-Scale Wastewater Treatment Plant: Mechanisms and Mitigation Strategies. Fermentation. 2025; 11(9):532. https://doi.org/10.3390/fermentation11090532
Chicago/Turabian StyleXie, Fei, Chenzhe Tian, Xiao Ma, Li Ji, Bowei Zhao, Muhammad Ehsan Danish, Feng Gao, and Zhihong Yang. 2025. "Seasonal Temperature Effects on EPS Composition and Sludge Settling Performance in Full-Scale Wastewater Treatment Plant: Mechanisms and Mitigation Strategies" Fermentation 11, no. 9: 532. https://doi.org/10.3390/fermentation11090532
APA StyleXie, F., Tian, C., Ma, X., Ji, L., Zhao, B., Danish, M. E., Gao, F., & Yang, Z. (2025). Seasonal Temperature Effects on EPS Composition and Sludge Settling Performance in Full-Scale Wastewater Treatment Plant: Mechanisms and Mitigation Strategies. Fermentation, 11(9), 532. https://doi.org/10.3390/fermentation11090532






