A Potential of Agro-Industrial Biowaste as Low-Cost Substrates for Carotenoid Production by Rhodotorula mucilaginosa
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection of Secondary Raw Materials
2.2. Yeast and Inoculum Preparation
2.3. Cultivation Conditions
2.4. Carotenoid Extraction
2.5. Quantification of Carotenoids and Yeast Biomass Concentration
2.6. Bioreactor Validation of Carotenoid Production on Best-Performing Substrate
2.7. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Astudillo, Á.; Rubilar, O.; Briceño, G.; Diez, M.C.; Schalchli, H. Advances in Agroindustrial Waste as a Substrate for Obtaining Eco-Friendly Microbial Products. Sustainability 2023, 15, 3467. [Google Scholar] [CrossRef]
- Mishra, B.; Mohanta, Y.K.; Reddy, C.N.; Reddy, S.D.M.; Mandal, S.K.; Yadavalli, R.; Sarma, H. Valorization of agro-industrial biowaste to biomaterials: An innovative circular bioeconomy approach. Circ. Econ. 2023, 2, 100050. [Google Scholar] [CrossRef]
- Barcelos, M.C.; Ramos, C.L.; Kuddus, M.; Rodriguez-Couto, S.; Srivastava, N.; Ramteke, P.W.; Mishra, P.K.; Molina, G. Enzymatic potential for the valorization of agro-industrial by-products. Biotechnol. Lett. 2020, 42, 1799–1827. [Google Scholar] [CrossRef] [PubMed]
- Pal, P.; Singh, A.K.; Srivastava, R.K.; Rathore, S.S.; Sahoo, U.K.; Subudhi, S.; Sarangi, P.K.; Prus, P. Circular Bioeconomy in Action: Transforming Food Wastes into Renewable Food Resources. Foods 2024, 13, 3007. [Google Scholar] [CrossRef]
- Ramchuran, S.O.; O’Brien, F.; Dube, N.; Ramdas, V. An overview of green processes and technologies, biobased chemicals and products for industrial applications. Curr. Opin. Green Sustain. Chem. 2023, 41, 100832. [Google Scholar] [CrossRef]
- Šovljanski, O.; Beronja, M.; Saveljić, A.; Travičić, V.; Tomić, A. Microorganisms as Biotechnological Source of Carotenoids. In Natural Products; Ramawat, K.G., Mérillon, J.-M., Eds.; Springer: Berlin/Heidelberg, Germany, 2025. [Google Scholar]
- Šeregelj, V.; Škrobot, D.; Kojić, J.; Pezo, L.; Šovljanski, O.; Tumbas Šaponjac, V.; Vulić, J.; Hidalgo, A.; Brandolini, A.; Čanadanović-Brunet, J.; et al. Quality and Sensory Profile of Durum Wheat Pasta Enriched with Carrot Waste Encapsulates. Foods 2022, 11, 1130. [Google Scholar] [CrossRef]
- Mannazzu, I.; Landolfo, S.; da Silva, T.L.; Buzzini, P. Red yeasts and carotenoid production: Outlining a future for non-conventional yeasts of biotechnological interest. World J. Microbiol. Biotechnol. 2015, 11, 1665–1673. [Google Scholar] [CrossRef]
- Ambati, R.R.; Gogisetty, D.; Aswathanarayana, R.G.; Ravi, S.; Bikkina, P.N.; Bo, L.; Yuepeng, S. Industrial potential of carotenoid pigments from microalgae: Current trends and future prospects. Crit. Rev. Food Sci. Nutr. 2019, 59, 1880–1902. [Google Scholar] [CrossRef]
- Kulczyk-Małysa, E.; Bogusławska-Wąs, E. Carotenoid Yeasts and Their Application Potential. Foods 2025, 14, 1866. [Google Scholar] [CrossRef]
- Li, Z.; Li, C.; Cheng, P.; Yu, G. Rhodotorula mucilaginosa—Alternative sources of natural carotenoids, lipids, and enzymes for industrial use. Heliyon 2022, 8, e11505. [Google Scholar] [CrossRef]
- Bonadio, M.D.P.; Freita, L.A.D.; Mutton, M.J.R. Carotenoid production in sugarcane juice and synthetic media supplemented with nutrients by Rhodotorula rubra l02. Braz. J. Microbiol. 2018, 49, 872–878. [Google Scholar] [CrossRef] [PubMed]
- Ochoa-Viñals, N.; Alonso-Estrada, D.; Pacios-Michelena, S.; García-Cruz, A.; Ramos-González, R.; Faife-Pérez, E.; Michelena-Álvarez, L.G.; Martínez-Hernández, J.L.; Iliná, A. Current Advances in Carotenoid Production by Rhodotorula sp. Fermentation 2024, 10, 190. [Google Scholar] [CrossRef]
- Mata-Gómez, L.C.; Montañez, J.C.; Méndez-Zavala, A.; Aguilar, C.N. Biotechnological production of carotenoids by yeasts: An overview. Microb. Cell Fact. 2014, 13, 12. [Google Scholar] [CrossRef] [PubMed]
- Nelson, D.W.; Sommers, L.E. Total carbon, organic carbon, and organic matter. In Methods of Soil Analysis, Part 3—Chemical Methods; Sparks, D.L., Ed.; Soil Science Society of America: Madison, WI, USA, 1996; pp. 961–1010. [Google Scholar]
- AOAC International. Official Methods of Analysis of AOAC International, 18th ed.; AOAC International: Gaithersburg, MD, USA, 2005. [Google Scholar]
- Buzzini, P.; Innocenti, M.; Turchetti, B.; Libkind, D.; van Broock, M.; Mulinacci, N. Carotenoid profiles of yeasts belonging to the genera Rhodotorula, Rhodotoropsis, Sporobolomyces, and Sporidiobolus. Can. J. Microbiol. 2007, 53, 1024–1031. [Google Scholar] [CrossRef]
- Kot, A.M.; Błażejak, S.; Kurcz, A.; Gientka, I.; Kieliszek, M. Rhodotorula glutinis—Potential source of lipids, carotenoids, and enzymes for use in industries. App. Microbiol. Biotechnol. 2019, 103, 371–382. [Google Scholar] [CrossRef]
- Kot, A.M.; Błażejak, S.; Gientka, I.; Kieliszek, M.; Bryś, J. Torulene and torularhodin: “New” fungal carotenoids for industry? Microb. Cell Fact. 2018, 17, 49. [Google Scholar] [CrossRef]
- Šovljanski, O.; Saveljić, A.; Tomić, A.; Šeregelj, V.; Lončar, B.; Cvetković, D.; Ranitović, A.; Pezo, L.; Ćetković, G.; Markov, S.; et al. Carotenoid-Producing Yeasts: Selection of the Best-Performing Strain and the Total Carotenoid Extraction Procedure. Processes 2022, 10, 1699. [Google Scholar] [CrossRef]
- Saini, R.K.; Keum, Y.-S. Carotenoid extraction methods: A review of recent developments. Food Chem. 2018, 240, 90–103. [Google Scholar] [CrossRef]
- Areniello, M. Microbial Protein Production from Sulfide-Rich Biogas Through Methane- and Sulfur-Oxidizing Consortia. Ph.D. Thesis, University of Galway Research Repository, Galway, Ireland, 2024. [Google Scholar]
- Šovljanski, O.; Pezo, L.; Stanojev, J.; Bajac, B.; Kovač, S.; Tóth, E.; Ristić, I.; Tomić, A.; Ranitović, A.; Cvetković, D.; et al. Comprehensive Profiling of Microbiologically Induced CaCO3 Precipitation by Ureolytic Bacillus Isolates from Alkaline Soils. Microorganisms 2021, 9, 1691. [Google Scholar] [CrossRef]
- Ross, T. Indices for performance evaluation of predictive models in food microbiology. J. Appl. Bacteriol. 1996, 81, 501–508. [Google Scholar] [CrossRef]
- Zwietering, M.H.; Jongenburger, I.; Rombouts, F.M.; van ’t Riet, K. Modeling of the bacterial growth curve. Appl. Environ. Microbiol. 1990, 56, 1875–1881. [Google Scholar] [CrossRef]
- Šovljanski, O.; Pezo, L.; Grahovac, J.; Tomić, A.; Ranitović, A.; Cvetković, D.; Markov, S. Best-performing Bacillus strains for microbiologically induced CaCO3 precipitation: Screening of relative influence of operational and environmental factors. J. Biotechnol. 2022, 350, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Šelo, G.; Planinić, M.; Tišma, M.; Tomas, S.; Koceva Komlenić, D.; Bucić-Kojić, A. A Comprehensive Review on Valorization of Agro-Food Industrial Residues by Solid-State Fermentation. Foods 2021, 10, 927. [Google Scholar] [CrossRef] [PubMed]
- Kot, A.M.; Błażejak, S.; Kieliszek, M.; Gientka, I.; Piwowarek, K.; Brzezińska, R. Production of lipids and carotenoids by Rhodotorula gracilis ATCC 10788 yeast in a bioreactor using low-cost wastes. Biocatal. Agric. Biotechnol. 2020, 26, 101634. [Google Scholar] [CrossRef]
- Ijaz, N.; Bashir, S.; Ikram, A.; Zafar, A.; Ul Ain, H.B.; Ambreen, S.; Ahmad, M.; Almalki, R.S.; Khalid, M.Z.; Khalid, W.; et al. Valorization of potato peel: A sustainable eco-friendly approach. CyTA J. Food 2024, 22, 2306951. [Google Scholar] [CrossRef]
- Saenge, C.; Cheirsilp, B.; Suksaroge, T.T.; Bourtoom, T. Potential use of oleaginous red yeast Rhodotorula glutinis for the bioconversion of crude glycerol from biodiesel plant to lipids and carotenoids. Process Biochem. 2011, 46, 210–218. [Google Scholar] [CrossRef]
- da Costa Cardoso, L.A.; Kanno, K.Y.F.; Karp, S.G. Microbial production of carotenoids A review. Afr. J. Biotechnol. 2017, 16, 139–146. [Google Scholar] [CrossRef]
- Karastergiou, A.; Gancel, A.L.; Jourdes, M.; Teissedre, P.L. Transforming winemaking waste: Grape pomace as a sustainable source of bioactive compounds. OENO One 2025, 59. [Google Scholar] [CrossRef]
- Abreu, T.; Sousa, P.; Gonçalves, J.; Hontman, N.; Teixeira, J.; Câmara, J.S.; Perestrelo, R. Grape Pomace as a Renewable Natural Biosource of Value-Added Compounds with Potential Food Industrial Applications. Beverages 2024, 10, 45. [Google Scholar] [CrossRef]
- Marova, I.; Carnecka, M.; Halienova, A.; Certik, M.; Dvorakova, T.; Haronikova, A. Use of several waste substrates for carotenoid-rich yeast biomass production. J. Environ. Manag. 2012, 95, S338–S342. [Google Scholar] [CrossRef]
- Suman, G.; Nupur, M.; Anuradha, S.; Pradeep, B. Characterization of dairy waste and its utilisation as substrate for production of single cell protein. J. Biotechnol. Biochem. 2017, 3, 73–78. [Google Scholar]
- Vescovo, D.; Manetti, C.; Ruggieri, R.; Spizzirri, U.G.; Aiello, F.; Martuscelli, M.; Restuccia, D. The Valorization of Potato Peels as a Functional Ingredient in the Food Industry: A Comprehensive Review. Foods 2025, 14, 1333. [Google Scholar] [CrossRef] [PubMed]
- Ngwenya, T.T.; Shukla, P.; Baboolal, N.; Permaul, K.; Singh, S. An industrial perspective of factors affecting molasses fermentation by Saccharomyces cerevisiae. J. Brew. Distill. 2012, 3, 23–28. [Google Scholar] [CrossRef]
- García-Lomillo, J.; González-SanJosé, M.L. Applications of wine pomace in the food industry: Approaches and functions. Compr. Rev. Food Sci. Food Saf. 2017, 16, 3–22. [Google Scholar] [CrossRef] [PubMed]
- Dulf, F.V.; Vodnar, D.C.; Toşa, M.I.; Dulf, E.H. Simultaneous enrichment of grape pomace with γ-linolenic acid and carotenoids by solid-state fermentation with Zygomycetes fungi and antioxidant potential of the bioprocessed substrates. Food Chem. 2020, 310, 125927. [Google Scholar] [CrossRef]
- Yu, J.; Ahmedna, M. Functional components of grape pomace: Their composition, biological properties and potential applications. Int. J. Food Sci. Technol. 2013, 48, 221–237. [Google Scholar] [CrossRef]
- Mussatto, S.I.; Teixeira, J.A. Lignocellulose as raw material in fermentation processes. In Current Research, Technology and Education Topics in Applied Microbiology and Microbial Biotechnology; Formatex Research Center: Badajoz, Spain, 2010; pp. 897–907. [Google Scholar]
- Watcharawipas, A.; Kocharin, K.; Runguphan, W. Engineered yeasts for high-value carotenoid production. Adv. Yeast Biotechnol. Biofuels Sustain. 2023, 331–352. [Google Scholar] [CrossRef]
- Kaur, P.; Singh, S.; Ghoshal, G.; Ramamurthy, P.C.; Parihar, P.; Singh, J.; Singh, A. Valorization of agri-food industry waste for the production of microbial pigments: An eco-friendly approach. In Advances in Agricultural and Industrial; Volume 1: Microbial Diversity and Application in Agroindustry; Springer: Singapore, 2022; pp. 137–167. [Google Scholar]
- Poddar, N.; Elahee Doomun, S.N.; Callahan, D.L.; Kowalski, G.M.; Martin, G.J. The assimilation of glycerol into lipid acyl chains and associated carbon backbones of Nannochloropsis salina varies under nitrogen replete and deplete conditions. Biotechnol. Bioeng. 2020, 117, 3299–3309. [Google Scholar] [CrossRef]
- Oliveira, A.S.; Ferreira, C.; Pereira, J.O.; Pintado, M.E.; Carvalho, A.P. Valorisation of protein-rich extracts from spent brewer’s yeast (Saccharomyces cerevisiae): An overview. Biomass Convers. Biorefinery 2025, 15, 1771–1793. [Google Scholar] [CrossRef]
- Cebrián, M.; García-Roldán, A.; Ibarruri, J.; Zufía, J.; Jauregi, P. Biorefinery approach for an integrated valorisation of grape pomace in distilleries. Biomass Convers. Biorefinery 2024, 1–12. [Google Scholar] [CrossRef]
- Rodrigues, R.P.; Gando-Ferreira, L.M.; Quina, M.J. Increasing value of winery residues through integrated biorefinery processes: A review. Molecules 2022, 27, 4709. [Google Scholar] [CrossRef]
- Kokkinomagoulos, E.; Kandylis, P. Grape pomace, an undervalued by-product: Industrial reutilization within a circular economy vision. Rev. Environ. Sci. Bio/Technol. 2023, 22, 739–773. [Google Scholar] [CrossRef]
- Palmqvist, E.; Hahn-Hägerdal, B. Fermentation of lignocellulosic hydrolysates. I: Inhibition and detoxification. Bioresour. Technol. 2000, 74, 17–24. [Google Scholar] [CrossRef]
- Palmqvist, E.; Hahn-Hägerdal, B. Fermentation of lignocellulosic hydrolysates. II: Inhibitors and mechanisms of inhibition. Bioresour. Technol. 2000, 74, 25–33. [Google Scholar] [CrossRef]
- Mir, S.A.; Rizwan, D.; Bakshi, R.A.; Wani, S.M.; Masoodi, F.A. Extraction of carotenoids from agro-industrial waste. In Extraction of Natural Products from Agro-Industrial Wastes; Elsevier: Amsterdam, The Netherlands, 2023; pp. 157–178. [Google Scholar]
- Pirt, S.J. Principles of Microbe and Cell Cultivation; Wiley: London, UK, 1975; pp. 1–274. [Google Scholar]
- Ratkowsky, D.A. Model fitting and uncertainty. In Modeling Microbial Responses in Food; McKellar, R.C., Lu, X., Eds.; CRC Press: Boca Raton, FL, USA, 2003; Chapter 4. [Google Scholar] [CrossRef]
- Kosseva, M.R. Processing of food wastes. Adv. Food Nutr. Res. 2009, 58, 57–136. [Google Scholar]
- Baranyi, J.; Roberts, T.A. A dynamic approach to predicting bacterial growth in food. Int. J. Food Microbiol. 1994, 23, 277–294. [Google Scholar] [CrossRef]
- Muniz, E.D.N.; Montenegro, R.T.D.Q.; da Silva, D.N.; D’Almeida, A.P.; Gonçalves, L.R.B.; de Albuquerque, T.L. Advances in biotechnological strategies for sustainable production of non-animal proteins: Challenges, innovations, and applications. Fermentation 2024, 10, 638. [Google Scholar] [CrossRef]
- Koutinas, A.A.; Chatzifragkou, A.; Kopsahelis, N.; Papanikolaou, S.; Kookos, I.K. Design and techno-economic evaluation of microbial oil production as a renewable resource for biodiesel and oleochemical production. Fuel 2014, 116, 566–577. [Google Scholar] [CrossRef]
- Fudala-Ksiazek, S.; Pierpaoli, M.; Kulbat, E.; Luczkiewicz, A. A modern solid waste management strategy–the generation of new by-products. Waste Manag. 2016, 49, 516–529. [Google Scholar] [CrossRef]
- Sharma, R.; Ghoshal, G. Optimization of carotenoids production by Rhodotorula mucilaginosa (MTCC-1403) using agro-industrial waste in bioreactor: A statistical approach. Biotechnol. Rep. 2020, 25, e00407. [Google Scholar] [CrossRef]

| Biowaste | Physical Pretreatment 1 | Chemical Pretreatment | C:N Ratio (Before Supplementation) | Additional C-Source (g/L) *** | Additional N-Source (g/L) *** |
|---|---|---|---|---|---|
| Crude glycerol | Filtration, dilution | Sterilization * | ~45:1 | — | 3 |
| Treated 2 white grape pomace | Milling, decantation, filtration | pH adjustment | ~30:1 | 15 | 2 |
| Untreated 3 white grape pomace | Milling, decantation, filtration | pH adjustment | ~28:1 | 15 | 2 |
| Treated 2 red grape pomace | Milling, decantation, filtration | pH adjustment | ~27:1 | 15 | 2 |
| Untreated 3 red grape pomace | Milling, decantation, filtration | pH adjustment | ~26:1 | 15 | 2 |
| Pea protein isolate | Milling | Sterilization ** | ~7:1 | 10 | - |
| Sugar beet juice | Filtration | Sterilization * | ~40:1 | — | 3 |
| Whey | Filtration | Sterilization * | ~18:1 | 15 | 2 |
| Molasses | Dilution, filtration | Sterilization * | ~38:1 | — | 3 |
| Hydrolyzed corn waste flour | Milling | Sterilization ** | ~25:1 | 15 | 2 |
| Chicken feathers | Milling | Alkaline hydrolysis 4, neutralization | ~5:1 | 20 | - |
| Potato peels | Milling | Sterilization * | ~22:1 | 15 | 2 |
| Sweet potato peels | Milling | Sterilization * | ~23:1 | 15 | 2 |
| Biowaste | Kinetic Parameters | Verification of Kinetic Models | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| a | d | c | b | R2 | RMSE | χ2 | MBE | MPE | Skew. | |
| Crude glycerol | 4.06 | 8.35 | 55.52 | 5.30 | 0.9996 | 0.028 | 0.362 | 0.458 | 5.703 | 0.572 |
| Treated white grape pomace | 3.26 | 3.94 | 99.00 | 10.00 | 0.941 | 0.077 | 0.259 | −0.274 | −8.373 | −0.400 |
| Untreated white grape pomace | 4.68 | 10.00 | 113.22 | 1.48 | 0.991 | 0.125 | 0.090 | 0.105 | 1.946 | 0.235 |
| Treated red grape pomace | 3.86 | 7.15 | 95.52 | 4.09 | 0.996 | 0.079 | 0.078 | 0.058 | 0.520 | −0.669 |
| Untreated red grape pomace | 4.15 | 5.37 | 39.73 | 5.32 | 0.996 | 0.036 | 0.050 | 0.122 | 2.176 | 0.452 |
| Pea protein isolate | 4.99 | 7.84 | 37.87 | 3.21 | 0.987 | 0.138 | 0.216 | 0.353 | 5.037 | 1.038 |
| Sugar beet juice | 4.30 | 10.00 | 200 * | 1.09 | 0.947 | 0.226 | 0.183 | 0.283 | 4.964 | −0.351 |
| Hydrolyzed corn waste flour | 3.57 | 4.18 | 92.21 | 4.84 | 0.984 | 0.031 | 0.171 | −0.29 | −7.79 | −0.279 |
| Chicken feathers | 4.12 | 6.07 | 114.72 | 8.60 | 0.991 | 0.073 | 0.160 | −0.108 | −3.366 | −0.043 |
| Sweet potato peels | 4.31 | 6.62 | 58.83 | 1.34 | 0.935 | 0.185 | 0.140 | 0.284 | 5.135 | −0.087 |
| Rank | Biowaste | Biomass (log CFU/mL) | Dry Biomass (g/L) | Carotenoid Yield (mg/g DW) | Productivity (mg/L) |
|---|---|---|---|---|---|
| 1 | Pea protein isolate | 8.00 ± 0.12 a | 4.5 ± 0.20 a | 3.33 ± 0.05 a | 14.98 ± 0.42 a |
| 2 | Untreated white grape pomace | 8.26 ± 0.10 a | 4.6 ± 0.15 a | 3.06 ± 0.04 ab | 14.09 ± 0.38 a |
| 3 | Crude glycerol | 8.30 ± 0.11 a | 4.7 ± 0.18 a | 2.95 ± 0.03 ab | 13.87 ± 0.36 a |
| 4 | Chicken feathers | 6.00 ± 0.09 b | 3.0 ± 0.12 b | 2.84 ± 0.02 b | 8.53 ± 0.28 b |
| 5 | Sugar beet juice | 7.15 ± 0.13 ab | 3.3 ± 0.14 b | 2.31 ± 0.02 d | 7.65 ± 0.26 bc |
| 6 | Sweet potato peels | 6.00 ± 0.08 b | 2.2 ± 0.10 c | 2.54 ± 0.02 bc | 5.59 ± 0.19 cd |
| 7 | Hydrolyzed corn waste flour | 3.60 ± 0.07 c | 1.9 ± 0.08 c | 1.67 ± 0.02 e | 3.17 ± 0.12 de |
| 8 | Treated red grape pomace | 6.85 ± 0.10 b | 1.6 ± 0.07 cd | 2.44 ± 0.0 c | 3.91 ± 0.14 de |
| 9 | Untreated red grape pomace | 5.32 ± 0.09 c | 1.2 ± 0.05 d | 2.125 ± 0.01 f | 2.55 ± 0.10 e |
| 10 | Treated white grape pomace | 3.30 ± 0.06 d | 0.4 ± 0.02 e | 1.775 ± 0.01 f | 0.71 ± 0.05 f |
| Parameter | Shake Flask | Fed-Batch Bioreactor | Improvement (%) |
|---|---|---|---|
| Maximum biomass (g/L) | 4.6 | 6.2 | +35% |
| Maximum biomass (log CFU/mL) | 8.26 | 8.5 | +3% |
| Carotenoid yield (mg/100 g DW) | 170.7 | 195.4 | +14% |
| Volumetric productivity (mg/L) | 14.1 | 20.1 | +43% |
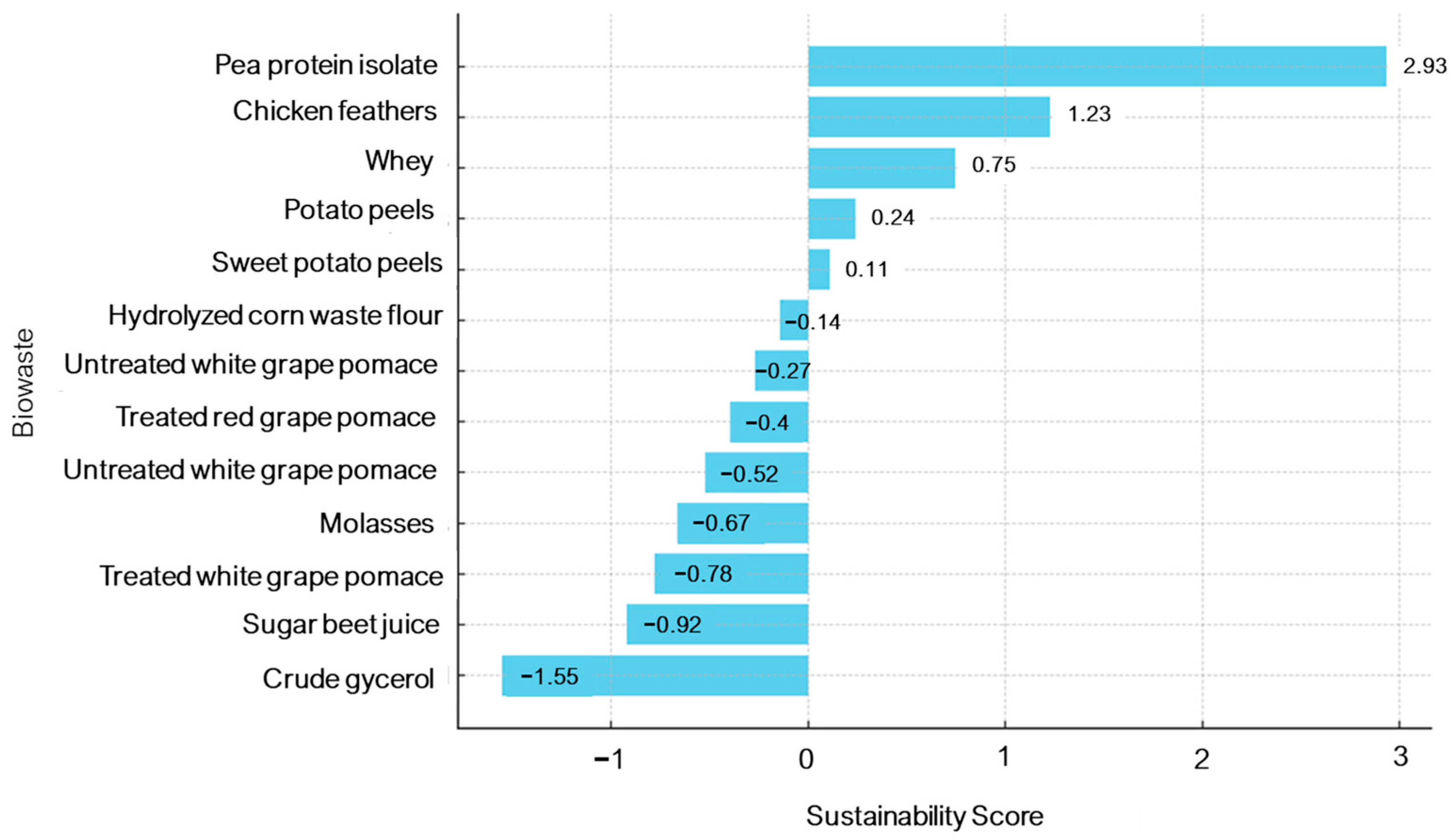
| Substrate | Estimated Raw Material Cost (€/ton) * | Productivity (mg/L) | Sustainability Score | Relative Feasibility ** |
|---|---|---|---|---|
| Pea protein isolate | ~1800–2000 | 14.98 | 2.93 | Low (too costly despite high yield) |
| Crude glycerol | ~100–200 | 13.87 | −1.56 | High (cheap, abundant, scalable) |
| Untreated grape pomace | ~0–50 (disposal cost avoided) | 14.09 | −0.78 | High (zero-cost waste, valorization benefits) |
| Chicken feathers | ~50–100 | 8.53 | 1.23 | Moderate (low cost, but processing required) |
| Sugar beet juice | ~200–300 | 7.65 | −0.92 | Moderate (regional availability, requires supplementation) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Šovljanski, O.; Cvetković, D.; Budimac, T.; Vučetić, A.; Tomić, A.; Marić, T.; Ranitović, A. A Potential of Agro-Industrial Biowaste as Low-Cost Substrates for Carotenoid Production by Rhodotorula mucilaginosa. Fermentation 2025, 11, 531. https://doi.org/10.3390/fermentation11090531
Šovljanski O, Cvetković D, Budimac T, Vučetić A, Tomić A, Marić T, Ranitović A. A Potential of Agro-Industrial Biowaste as Low-Cost Substrates for Carotenoid Production by Rhodotorula mucilaginosa. Fermentation. 2025; 11(9):531. https://doi.org/10.3390/fermentation11090531
Chicago/Turabian StyleŠovljanski, Olja, Dragoljub Cvetković, Tara Budimac, Anja Vučetić, Ana Tomić, Teodora Marić, and Aleksandra Ranitović. 2025. "A Potential of Agro-Industrial Biowaste as Low-Cost Substrates for Carotenoid Production by Rhodotorula mucilaginosa" Fermentation 11, no. 9: 531. https://doi.org/10.3390/fermentation11090531
APA StyleŠovljanski, O., Cvetković, D., Budimac, T., Vučetić, A., Tomić, A., Marić, T., & Ranitović, A. (2025). A Potential of Agro-Industrial Biowaste as Low-Cost Substrates for Carotenoid Production by Rhodotorula mucilaginosa. Fermentation, 11(9), 531. https://doi.org/10.3390/fermentation11090531







