Evaluation of Biomass, Lipid and Chlorophyll Production of a Microalgal Consortium Cultured in Dairy Wastewater
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection of Biological Material and Wastewater
2.2. Description of the Experimentation Stage, Production of Biomass, Lipids, and Chlorophyll
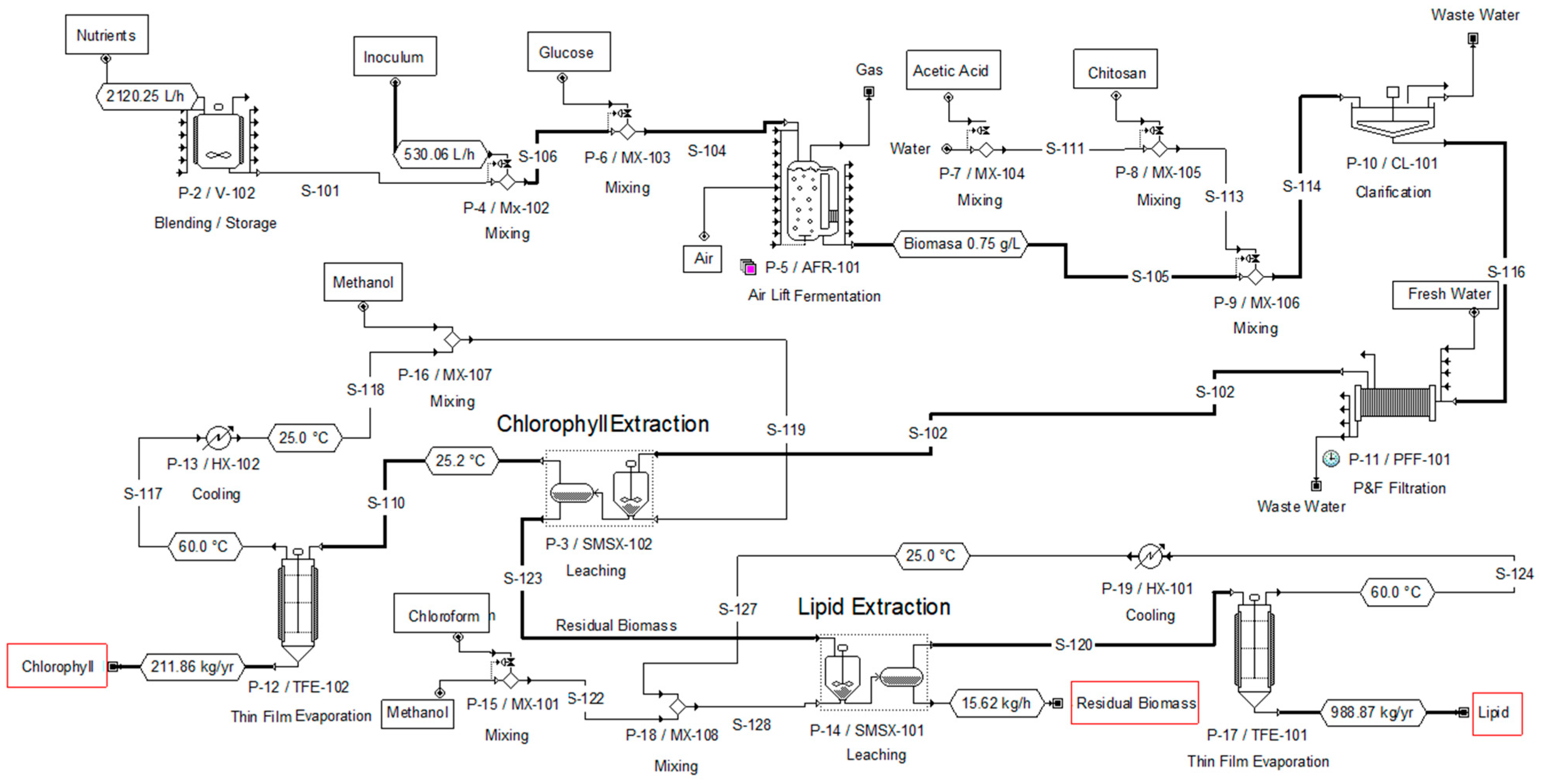
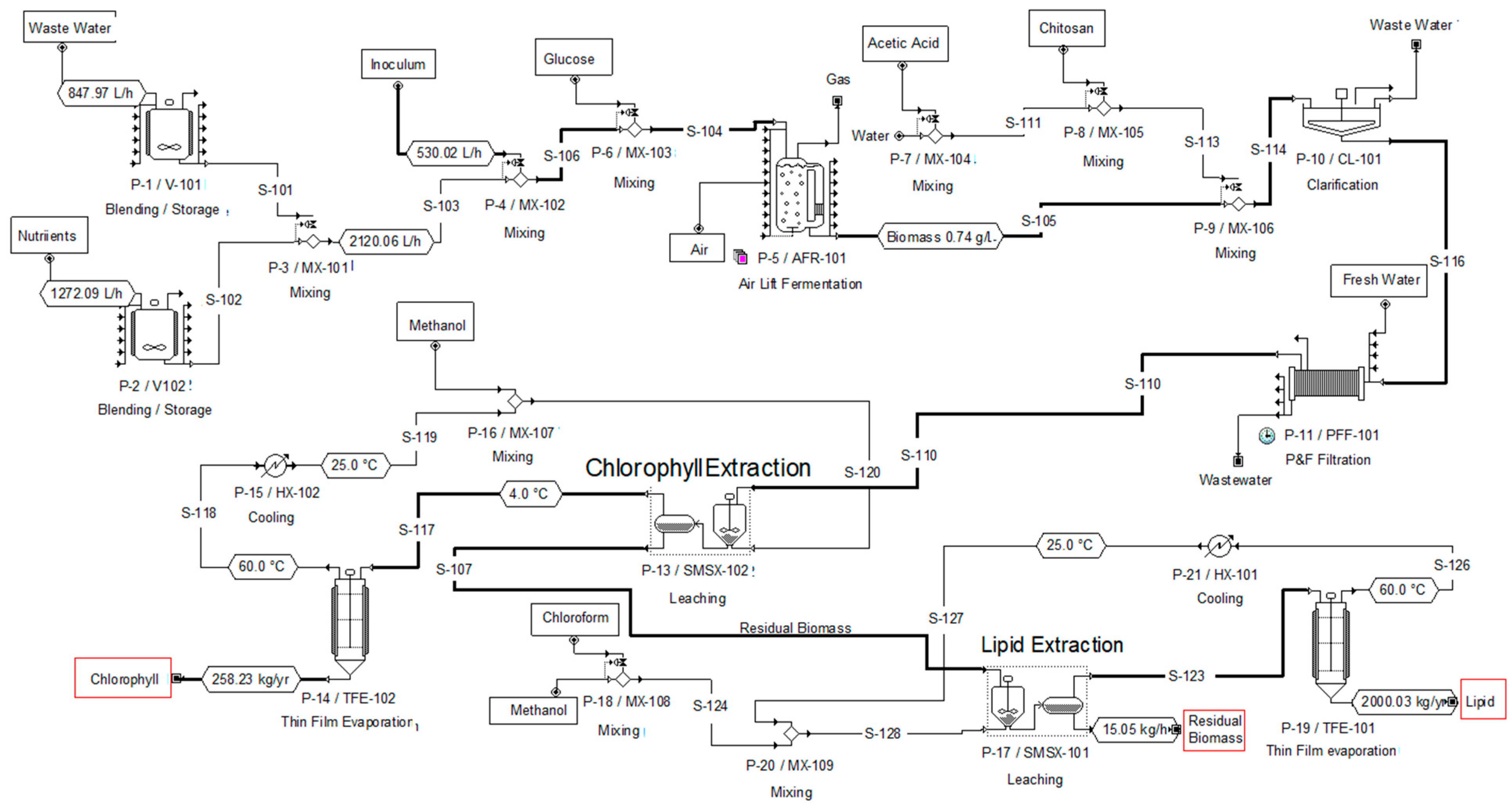
2.3. Process Simulation and Economic Projection of Biomass, Lipid and Chlorophyll Production
3. Results and Discussion
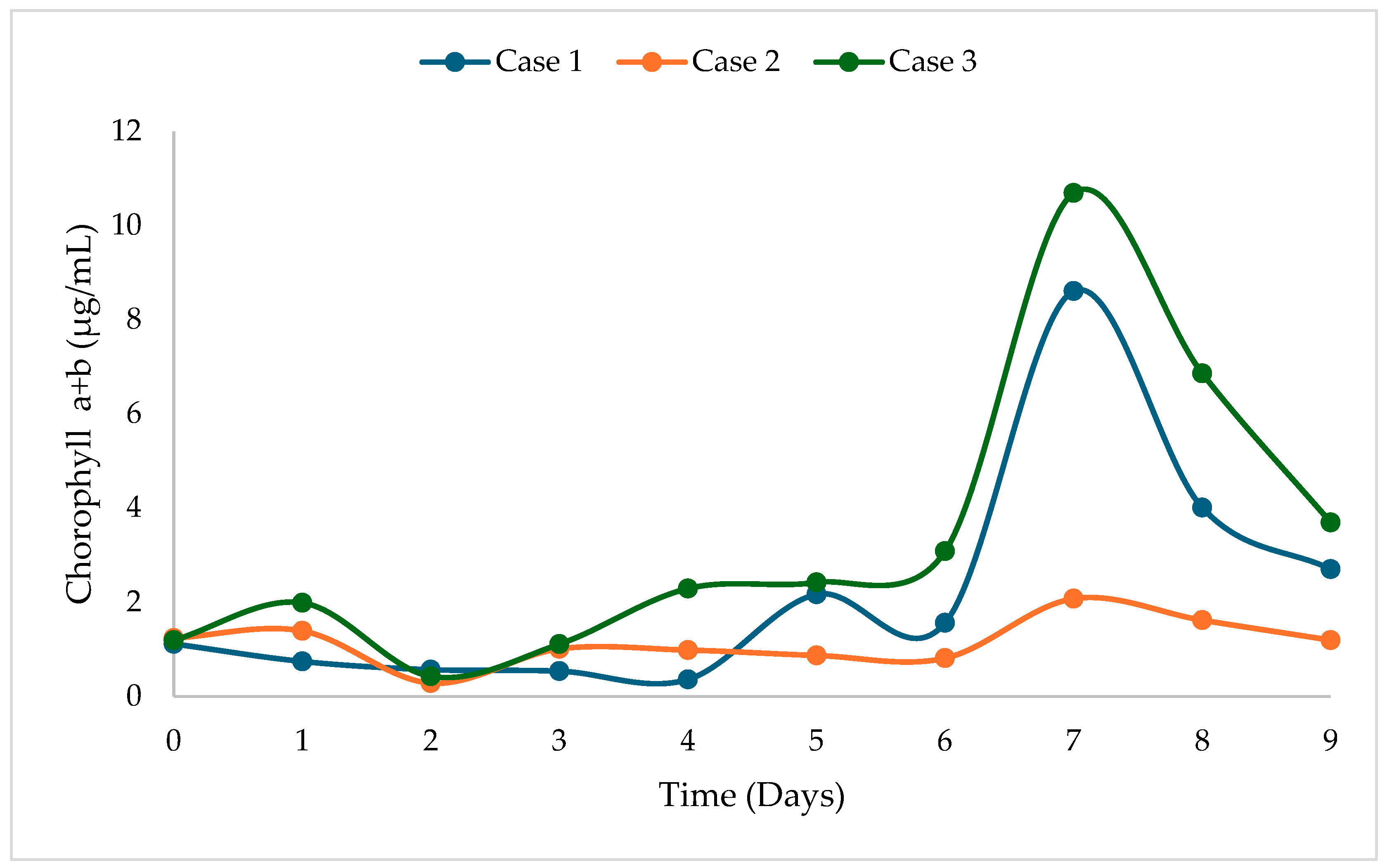
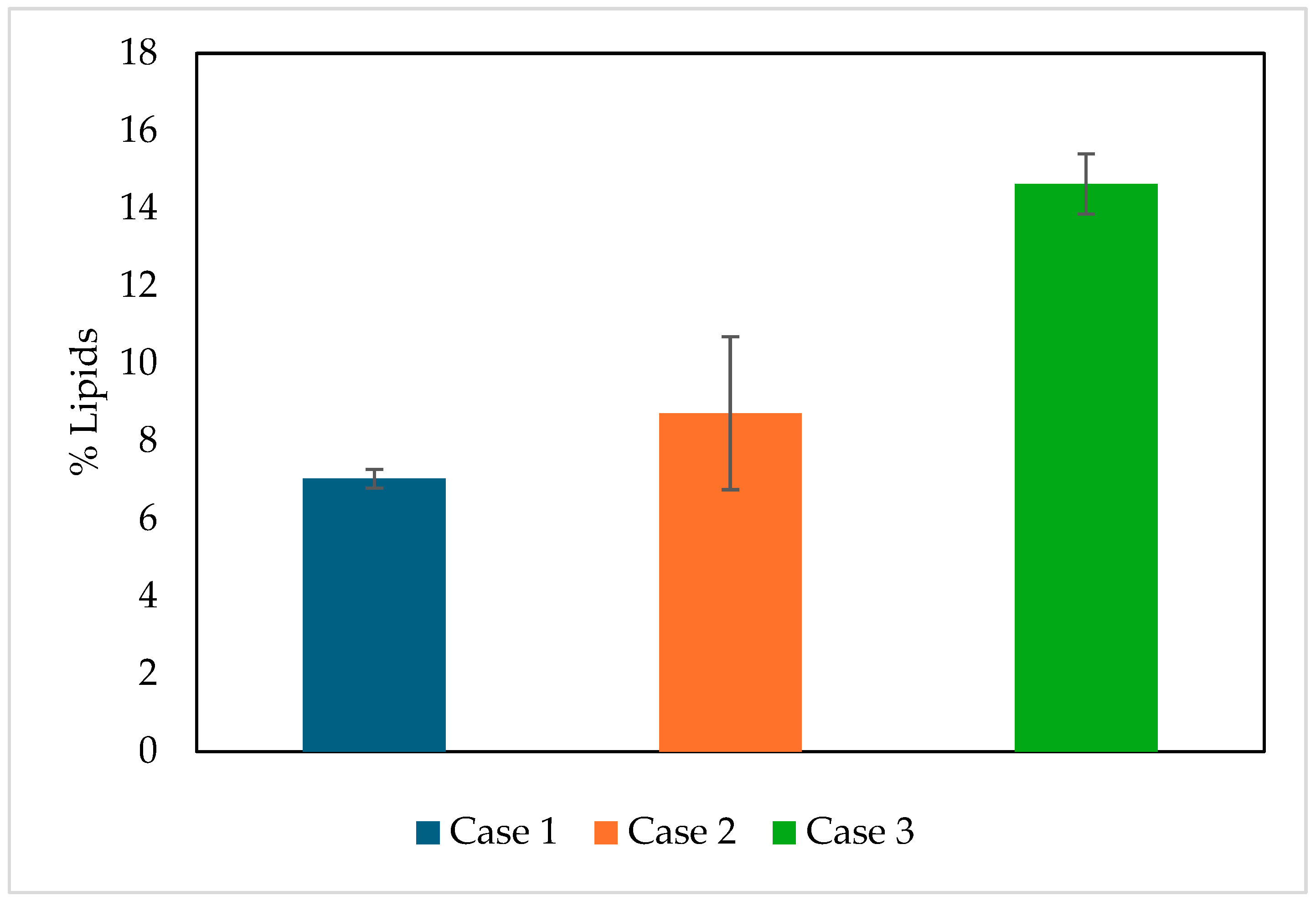
3.1. Experimental Evaluation of Biomass, Lipid, and Chlorophyll Production
3.2. Results of the Techno-Economic Evaluation for the Production of Chlorophyll and Lipids from a Microalgal Consortium
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BBM-3N | Bold Basal Mineral Medium-3 Nitrogen |
| WWDI | Wastewater Dairy Industry |
| COD | Chemistry Oxygen Demand |
| NO3 | Nitrate |
| PO4 | Phosphorus |
| NH4 | Ammonium |
| SD | Standard Deviation |
| MT | Megatons |
References
- Secretaría de Agricultura y Desarrollo Rural. 2023. Available online: https://www.gob.mx/agricultura/prensa/al-alza-produccion-lechera-en-mexico-crece-9-en-los-ultimos-cinco-anos (accessed on 30 July 2025).
- Baldev, E.; Ali, D.M.; Pugazhendhi, A.; Thajuddin, N. Wastewater as an economical and ecofriendly green medium for microalgal biofuel production. Fuel 2021, 294, 120484. [Google Scholar] [CrossRef]
- Handayani, T.; Mulyanto, A.; Priyanto, F.E.; Nugroho, R. Utilization of Dairy Industry Wastewater for Nutrition of Microalgae Chlorella vulgaris. J. Phys. Conf. Ser. 2020, 1655, 012123. [Google Scholar] [CrossRef]
- Daneshvar, E.; Zarrinmehr, M.J.; Koutra, E.; Kornaros, M.; Farhadian, O.; Bhatnagar, A. Sequential cultivation of microalgae in raw and recycled dairy wastewater: Microalgal growth, wastewater treatment and biochemical composition. Bioresour. Technol. 2019, 273, 556–564. [Google Scholar] [CrossRef]
- Gramegna, G.; Scortica, A.; Scafati, V.; Ferella, F.; Gurrieri, L.; Giovannoni, M.; Bassi, R.; Sparla, F.; Mattei, B.; Benedetti, M. Exploring the potential of microalgae in the recycling of dairy wastes. Bioresour. Technol. Rep. 2020, 12, 100604. [Google Scholar] [CrossRef]
- Pahazri, N.F.; Mohamed, R.M.; Al-Gheethi, A.A.; Mohd, A.H. Production and harvesting of microalgae biomass from wastewater: A critical review. Environ. Technol. Rev. 2016, 5, 39–56. [Google Scholar] [CrossRef]
- Eida, M.F.; Darwesh, O.M.; Matter, I.A. Cultivation of oleaginous microalgae Scenedesmus obliquus on secondary treated municipal wastewater as growth medium for biodiesel production. J. Ecol. Eng. 2018, 19, 38–51. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.K.; Wang, X.; Miao, J.; Tian, Y.T. Tofu whey wastewater is a promising basal medium for microalgae culture. Bioresour. Technol. 2018, 253, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Vieira-Costa, J.A.; Gonzales-Cruz, C.; Centeno-Rosa, P. Insights into the technology utilized to cultivate microalgae in dairy effluents. Biocatal. Agric. Biotechnol. 2021, 35, 102106. [Google Scholar] [CrossRef]
- Carvalho, J.C.; Sydney, E.B.; Assu-Tessari, L.F.; Soccol, C.R. Chapter 2: Culture media for mass production of microalgae. In Biofuels from Algae, 2nd ed.; Elsevier: Burlington, MA, USA, 2019; pp. 33–50. [Google Scholar] [CrossRef]
- Gomez-Serrano, C.; Morales-Amaral, M.M.; Acien, F.G.; Escudero, R.; Fernandez-Sevilla, J.M.; Molina-Grima, E. Utilization of secundary-treated wastewater for the production of freshwater microalgae. Appl. Microbiol. Biotechnol. 2015, 99, 6931–6944. [Google Scholar] [CrossRef]
- Fernández-Linares, L.C.; Guerrero-Barajas, C.; Durán-Páramo, E.; Badillo-Corona, J.A. Assessment of Chlorella vulgaris and indigenous microalgae biomass with treated wastewater as growth culture medium. Bioresour. Technol. 2017, 244, 400–406. [Google Scholar] [CrossRef]
- Chandra, R.; Pradhan, S.; Patel, A.; Ghosh, U.K. An approach for dairy wastewater remediation using mixture of microalgae and biodiesel production for sustainable transportation. J. Environ. Manag. 2021, 297, 113210. [Google Scholar] [CrossRef]
- Biswas, T.; Bhushan, S.; Prajapati, S.K.; Chaudhuri, S.R. An eco-friendly strategy for dairy wastewater remediation with high lipid microalgae-bacterial biomass production. J. Environ. Manag. 2021, 286, 112196. [Google Scholar] [CrossRef]
- Acosta-Ferreira, S.; Castillo, O.S.; Madera-Santana, J.T.; Mendoza-García, D.A.; Nuñez-Colín, C.A.; Grijalva-Verdugo, C.; Villa-Lerema, A.G.; Morales-Vargas, A.T.; Rodríguez-Nuñez, J.R. Production and physicochemical characterization of chitosan for the harvesting of wild microalgae consortia. Biotechnol. Rep. 2020, 28, e00554. [Google Scholar] [CrossRef] [PubMed]
- Liang, F.; Wen, X.; Geng, Y.; Ouyang, Z.; Luo, L.; Li, Y. Growth rate and biomass productivity of Chlorella as affected by culture depth and cell density in an open circular photobioreactor. Food Microbiol. Biotechnol. 2013, 23, 539–544. [Google Scholar] [CrossRef] [PubMed]
- Porra, R.J. The chequered history of the development and use of simultaneous equations for the accurate determination of chlorophylls a and b. Photosynth. Res. 2002, 73, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Montero, E.; Olguín, E.J.; De Philippis, R. Mixotrophic cultivation of Chlorococcum sp. under non-controlled conditions using a digestate from pig manure within a biorefinery. J. Appl. Phycol. 2018, 30, 2847–2857. [Google Scholar] [CrossRef]
- Francisci, D.; Su, Y.; Iital, A.; Angelidaki, I. Evaluation of microalgae production coupled with wastewater treatment. Environ. Technol. 2017, 5, 581–592. [Google Scholar] [CrossRef]
- Valdovinos-García, E.M.; Petriz-Prieto, M.A.; Olán-Acosta, M.d.l.Á.; Barajas-Fernández, J.; Guzmán-López, A.; Bravo-Sánchez, M.G. Production of microalgal biomass in photobioreactors as feedstock for bioenergy and other uses: A techno-economic study of harvesting stage. Appl. Sci. 2021, 11, 4386. [Google Scholar] [CrossRef]
- Cabrera-Capetillo, C.A.; Castillo-Baltazar, O.S.; Petriz-Prieto, M.A.; Guzmán-López, A.; Valdovinos-García, E.M.; Bravo-Sánchez, M.G. Simulation and economic analysis of the biotechnological potential of biomass production from a microalgal consortium. Mar. Drugs 2023, 21, 321. [Google Scholar] [CrossRef]
- Valdovinos-García, E.M.; Bravo-Sánchez, M.G.; Olán-Acosta, M.d.l.Á.; Barajas-Fernández, J.; Guzmán-López, A.; Petriz-Prieto, M.A. Technoeconomic evaluation of microalgae oil production: Effect of cell disruption method. Fermentation 2022, 8, 301. [Google Scholar] [CrossRef]
- Rashd, J.A.; Kassim, M.A.; Allzrag, A.M.; Allafi, F.A.; Shaah, M.A.; Wijaya, D.; Lalung, J. Harnessing Scenedesmus parvus and Coccomyxa dispar microalgae for lake water remediation and biofuel potential. Energy Nexus 2025, 19, 100497. [Google Scholar] [CrossRef]
- Ooi, W.C.; Dominic, D.; Kassim, M.A.; Baidurah, S. Biomass fuel production through cultivation of microalgae Coccomyxa dispar and Scenedesmus parvus in palm oil mill effluent and simultaneous phycoremediation. Agriculture 2023, 13, 336. [Google Scholar] [CrossRef]
- Qin, L.; Wang, Z.; Sun, Y.; Shu, Q.; Feng, P.; Zhu, L.; Xu, J.; Yuan, Z. Microalgae consortia cultivation in dairy wastewater to improve the potential of nutrient removal and biodiesel feedstock production. Enviromental Sci. Pollut. Res. 2016, 23, 8379–8387. [Google Scholar] [CrossRef] [PubMed]
- Chinnasamy, S.; Bhatnagar, A.; Hunt, R.W.; Das, K. Microalgae cultivation in a wastewater dominated by carpet mill effluents for biofuel applications. Bioresour. Technol. 2010, 101, 3097–3105. [Google Scholar] [CrossRef]
- Taufikurahman, T.; Ardiansyah, M.A.; Astutiningsih, N.T.; Suyono, E.A. Cultivation of Chlorella vulgaris in anaerobically digested dairy manure wastewater (ADDMW) for protein and chlorophyll production. Sains Malays. 2020, 49, 2035–2042. [Google Scholar] [CrossRef]
- Talapatra, N.; Gautam, R.; Mittal, V.; Ghosh, U.K. A comparative study of the growth of microalgae-bacteria symbiotic consortium with the axenic culture of microalgae in dairy wastewater through extraction and quantification of chlorophyll. Mater. Today Proc. 2023, 80, 2268–2273. [Google Scholar] [CrossRef]
- Choi, Y.K.; Jang, H.M.; Kan, E. Microalgal biomass and lipid production on dairy effluent using a novel microalga, Chlorella sp. isolated from dairy wastewater. Biotechnol. Bioprocess Eng. 2018, 23, 333–340. [Google Scholar] [CrossRef]
- Mercado, I.; Álvarez, X.; Verduga, M.-E.; Cruz, A. Enhancement of biomass and lipid productivities of Scenedesmus sp. cultivated in the wastewater of the dairy industry. Processes 2020, 8, 1458. [Google Scholar] [CrossRef]
- Khalaji, M.; Hosseini, S.A.; Ghorbani, R.; Agh, N.; Rezaei, H.; Kornaros, M.; Koutra, E. Treatment of dairy wastewater by microalgae Chlorella vulgaris for biofuels production. Biomass Convers. Biorefin. 2021, 13, 3259–3265. [Google Scholar] [CrossRef]
- Shu, Q.; Qin, L.; Yuan, Z.; Zhu, S.; Xu, J.; Xu, Z.; Feng, P.; Wang, Z. Comparison of dairy wastewater and synthetic medium for biofuels production by microalgae cultivation. Energy Sources Part A Recovery Util. Environ. Eff. 2018, 40, 751–758. [Google Scholar] [CrossRef]
- Kumar, V.; Gururani, P.; Parveen, A.; Verma, M.; Kim, H.; Vlaskin, M.; Grigorenko, A.V.; Rindin, K.G. Dairy Industry wastewater and stormwater energy valorization: Effect of wastewater nutrients on microalgae-yeast biomass. Biomass Conv. Biorefin. 2023, 13, 13563–13572. [Google Scholar] [CrossRef]
- Valdovinos-García, E.M.; Cabrera-Capetillo, C.A.; Bravo-Sánchez, M.G.; Barajas-Fernández, J.; Olán-Acosta, M.d.l.Á.; Petriz-Prieto, M.A. Evaluation of technical and economic indicators for the production process of microalgae lipids considering CO2 capture of a thermoelectric plant and use of piggery wastewater. Energies 2024, 17, 92. [Google Scholar] [CrossRef]
- Gojkovic, Z.; Skrobonja, A.; Radojicic, V.; Mattei, B. The use of flocculation as a Preconcentration step in the Microalgae Harvesting Process. Physiol. Plant. 2025, 177, e70366. [Google Scholar] [CrossRef]
- D’Ascoli, M.; Langellotti, A.L.; Russo, G.L.; Sorrentino, A.; Pierro, P.D. Phycocyanin extraction from Limnospira spp.: Sustainable source of natural blue color for the food industry. Curr. Res. Food Sci. 2025, 11, 101141. [Google Scholar] [CrossRef]
- Kopp, G.; Lauritano, C. Greener Extraction solutions for microalgal compounds. Mar. Drugs 2025, 23, 269. [Google Scholar] [CrossRef] [PubMed]
- Fatima, I.; Munir, M.; Qureshi, R.; Hanif, U.; Gulzar, N.; Sheikh, A.A. Advanced methods of algal pigments extraction: A review. Crit. Rev. Food Sci. Nutr. 2023, 64, 9771–9788. [Google Scholar] [CrossRef]




| Case | Composition of the Medium | Biomass (g/L) | Total Chlorophyll (µg/mL) | % Lipids |
|---|---|---|---|---|
| 1 | 100% BBM-3N (control) | 0.7470 | 8.6062 | 7.03 |
| 2 | 60% Fresh water + 40% WWDI | 0.4493 | 2.0731 | 8.72 |
| 3 | 60% BBM-3N + 40% WWDI | 0.7543 | 10.6890 | 14.63 |
| Case | Medium Composition | Biomass (kg/yr) | Chlorophyll (kg/yr) | Lipids (kg/yr) |
|---|---|---|---|---|
| 1 | 100% BBM-3N (control) | 15949.97 | 211.86 | 988.87 |
| 2 | 60% Fresh water + 40% WWDI | 9130.33 | 49.17 | 701.34 |
| 3 | 60% BBM-3N + 40% WWDI | 15501.28 | 258.23 | 2000 |
| Case | Labor (USD/yr) | Material (USD/yr) | Utilities (USD/yr) | Facility- Dependent (USD/yr) | Operating (USD/yr) |
|---|---|---|---|---|---|
| 1 | 5,032,729 | 2,021,992 | 2,716,876 | 436,101,000 | 445,873,000 |
| 2 | 5,802,358 | 328,687 | 2,714,377 | 436,364,000 | 445,209,000 |
| 3 | 5,812,693 | 1,346,801 | 2,717,453 | 436,387,000 | 446,264,000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cabrera-Capetillo, C.A.; Castillo-Baltazar, O.S.; Peña-Caballero, V.; Petriz-Prieto, M.A.; Guzmán-López, A.; Valdovinos-García, E.M.; Bravo-Sánchez, M.G. Evaluation of Biomass, Lipid and Chlorophyll Production of a Microalgal Consortium Cultured in Dairy Wastewater. Fermentation 2025, 11, 506. https://doi.org/10.3390/fermentation11090506
Cabrera-Capetillo CA, Castillo-Baltazar OS, Peña-Caballero V, Petriz-Prieto MA, Guzmán-López A, Valdovinos-García EM, Bravo-Sánchez MG. Evaluation of Biomass, Lipid and Chlorophyll Production of a Microalgal Consortium Cultured in Dairy Wastewater. Fermentation. 2025; 11(9):506. https://doi.org/10.3390/fermentation11090506
Chicago/Turabian StyleCabrera-Capetillo, Christian Ariel, Omar Surisadai Castillo-Baltazar, Vicente Peña-Caballero, Moisés Abraham Petriz-Prieto, Adriana Guzmán-López, Esveidi Montserrat Valdovinos-García, and Micael Gerardo Bravo-Sánchez. 2025. "Evaluation of Biomass, Lipid and Chlorophyll Production of a Microalgal Consortium Cultured in Dairy Wastewater" Fermentation 11, no. 9: 506. https://doi.org/10.3390/fermentation11090506
APA StyleCabrera-Capetillo, C. A., Castillo-Baltazar, O. S., Peña-Caballero, V., Petriz-Prieto, M. A., Guzmán-López, A., Valdovinos-García, E. M., & Bravo-Sánchez, M. G. (2025). Evaluation of Biomass, Lipid and Chlorophyll Production of a Microalgal Consortium Cultured in Dairy Wastewater. Fermentation, 11(9), 506. https://doi.org/10.3390/fermentation11090506







