Plant-Based Fermented Foods: Classification, Biochemical Transformations, and Health Benefits
Abstract
1. Introduction
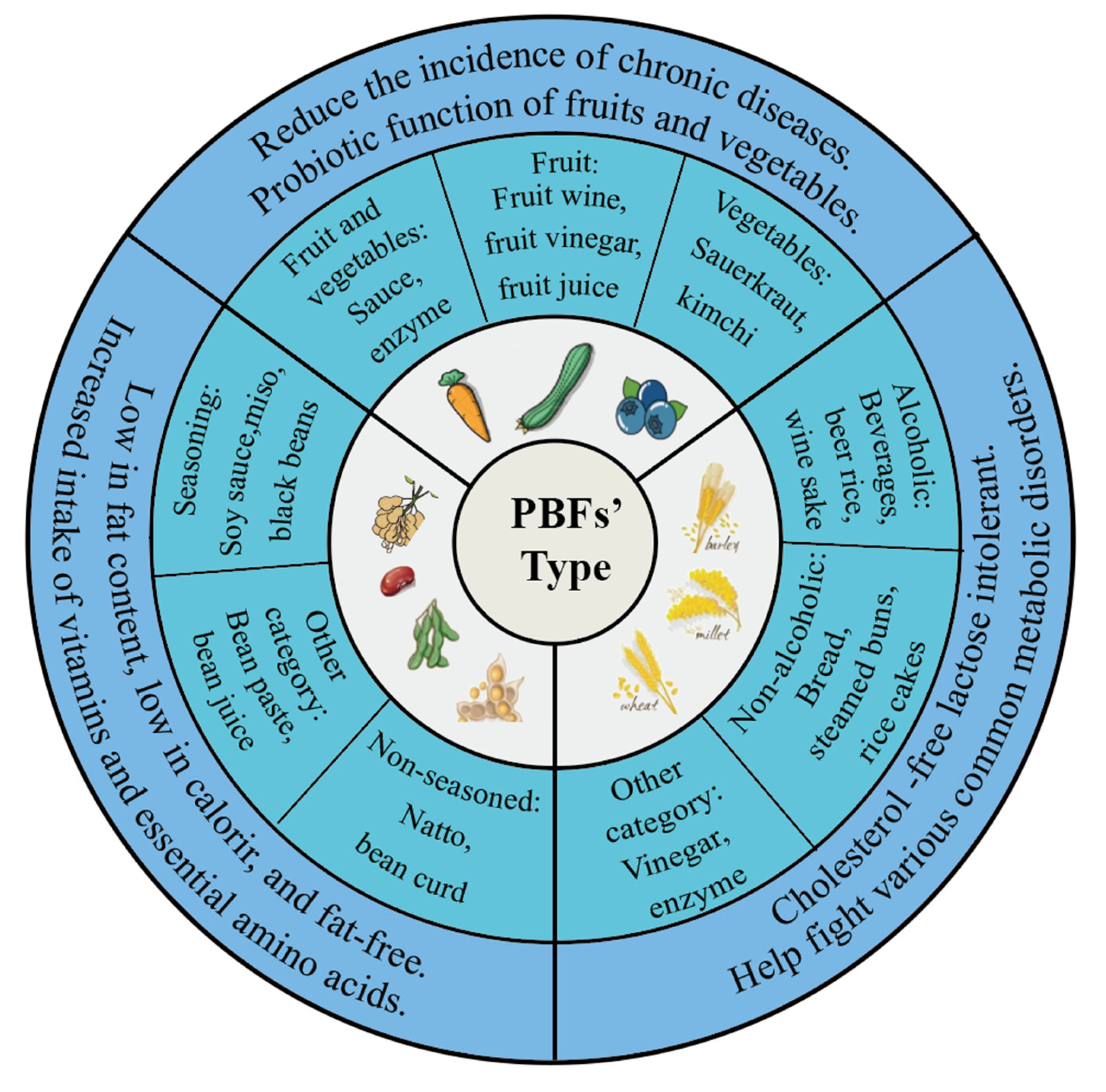
2. Reaction Mechanisms and Common Types of PBFs
2.1. Common Classification of PBFs
2.1.1. Fermented Fruits and Vegetables
2.1.2. Fermented Soy
2.1.3. Fermented Grains
2.2. Reaction Mechanisms of PBFs
2.2.1. Synergistic Effects of Microbial Communities
2.2.2. Generation and Function of Metabolites
2.2.3. Dynamic Regulation of Substrate Conversion
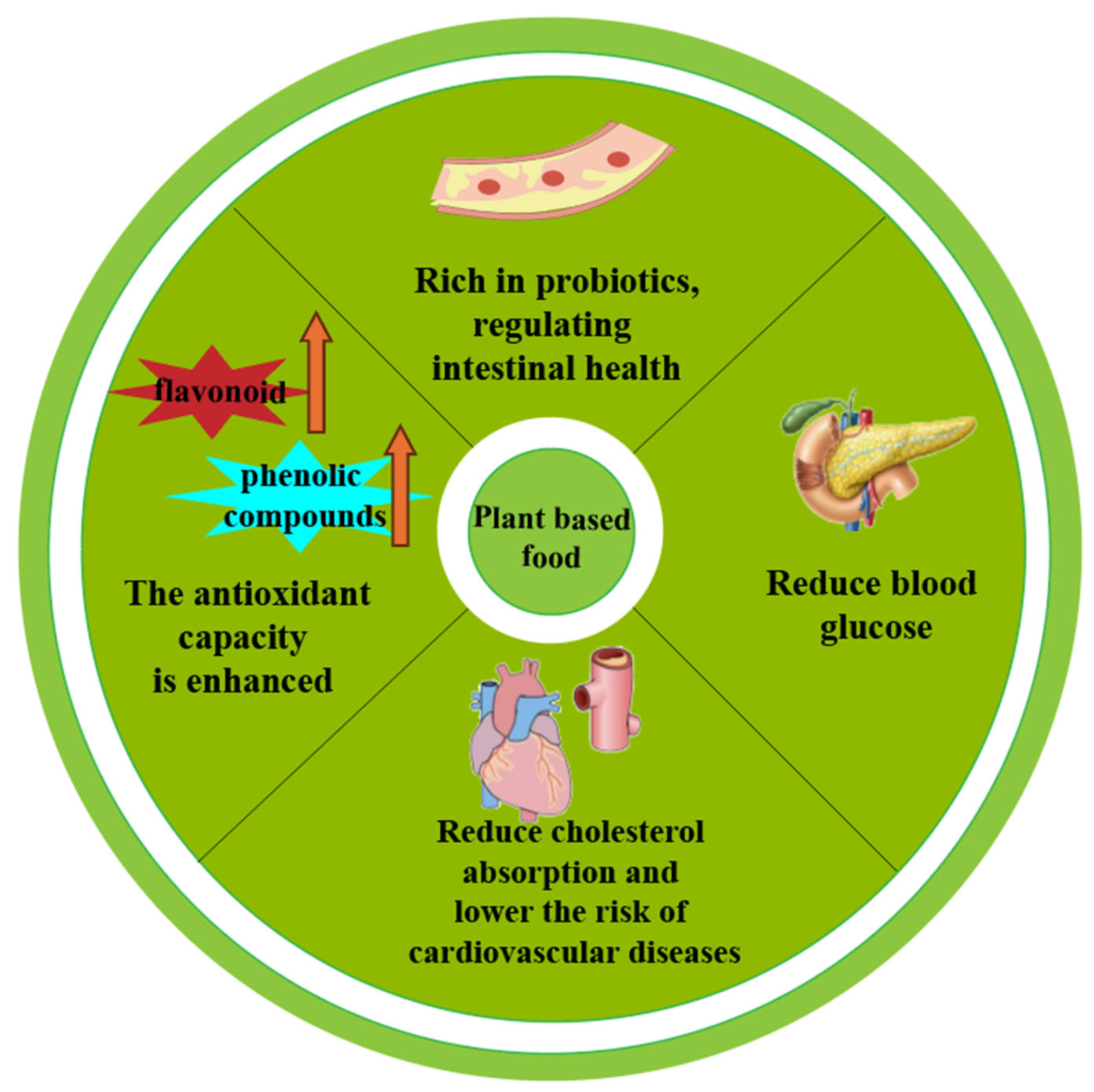
3. Benefits of PBFs
3.1. Beneficial Substance Produced by Metabolic Processes
3.1.1. Organic Acids
3.1.2. Active Substances
| Raw Material | Fermentation Strain | Organic Acid Content g/L | References | |
|---|---|---|---|---|
| Before Fermentation | After Fermentation | |||
| Papaya | Lactobacillus | 0.55 ± 0.05 (pyruvate) | 1.13 ± 0.15 (pyruvate) | [103] |
| 53.2 ± 2.8 (malic acid) | 77.6 ± 3.5 (malic acid) | |||
| Lactiplantibacillus plantarum | 0.55 ± 0.05 (pyruvate) | 1.20 ± 0.11 (pyruvate) | ||
| 53.2 ± 2.8 (malic acid) | 74.2 ± 2.0 (malic acid) | |||
| Blueberry | LacidophilusSaccharomyces cerevisiae RV002, Pichia fermentans JT-1-3, Saccharomyces cerevisiae NP-7-5 | 1.73±0.08 (citric acid | 0.98 ± 0.02 (citric acid) | [112] |
| 1.53 ± 0.10 (malic acid) | 0.99 ± 0.03 (malic acid) | |||
| 0.9 ± 0.04 (tartaric acid) | 0.52 ± 0.06 (tartaric acid) | |||
| Grape | Saccharomyces cerevisiae | 5.07 ± 0.02 (tartaric acid) | 3.97 ± 0.04 (tartaric acid) | [113] |
| 1.87 ± 0.02 (malic acid) | 1.43 ± 0.00 (malic acid) | |||
| Raw Material | Fermentation Strain | Biologically Active Ingredient mg/g | References | |||
|---|---|---|---|---|---|---|
| Before Fermentation | After Fermentation | |||||
| Total Phenol Content | Total Flavonoid Content | Total Phenol Content | Total Flavonoid Content | |||
| Papaya | Lactobacillus acidophilus | 0.032 ± 0.006 | 0.50 ± 0.06 | 0.027 ± 0.009 | 1.11 ± 0.17 | [103] |
| Lactiplantibacillus plantarum | 0.032 ± 0.008 | 0.029 ± 0.006 | 1.45 ± 0.13 | |||
| Wheat bran | Lactobacillus acidophilus KCTC 3164 | 1.61 ± 0.62 | 0.88 ± 0.11 | 2.80 ± 0.27 | 1.01 ± 0.08 | [114] |
| Lactobacillus helveticus KCTC 3545 | 1.98 ± 0.21 | 0.71 ± 0.07 | ||||
| Enterococcus faecalis KCTC 3206 | 2.33 ± 0.09 | 0.71 ± 0.08 | ||||
| Saccharin japonica | Monascus purpureus | 1.658 | 0.227 | 8.443 | 0.463 | [115] |
| White quinoa | Lactiplantibacillus plantarum 299v | 4.68 ± 0.05 | - | 7.78 ± 0.07 | - | [116] |
| Rice | Lactobacillus fermentum KKL1 | 11.8 | 0.04 | 63.42 | 45.36 | [117] |
3.2. Improve Food Sensory Properties
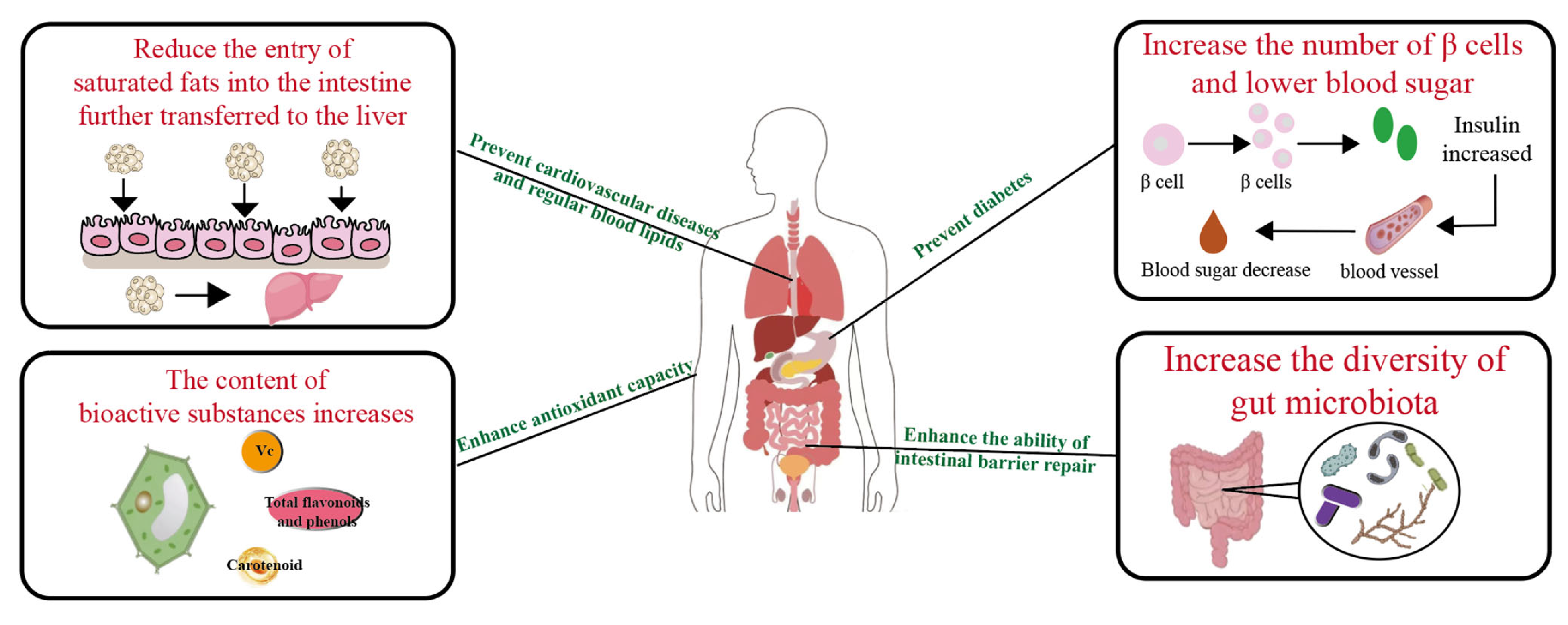
4. PBFs’s Potential Benefits to Human Health
4.1. Regulate Blood Sugar and Improve Insulin Resistance
4.2. Reduce Cholesterol and Triglyceride Absorption
4.3. Enhances Antioxidant Capacity
4.4. Regulation of Intestinal Microbiota
5. Industrialization Challenges of PBFs
5.1. Potential Safety Risks
5.1.1. Exogenous Pollution
5.1.2. Endogenous Contamination
5.2. Inconsistency of Product Efficacy
5.2.1. Fluctuation of Bioactive Components in Raw Materials
5.2.2. Process Sensitivity and Standardization Challenges
6. Summary and Prospect
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cordain, L.; Eaton, S.B.; Sebastian, A.; Mann, N.; Lindeberg, S.; Watkins, B.A.; O’Keefe, J.H.; Brand-Miller, J. Origins and Evolution of the Western Diet: Health Implications for the 21st Century1,2. Am. J. Clin. Nutr. 2005, 81, 341–354. [Google Scholar] [CrossRef] [PubMed]
- World Healthy Organization Noncommunicable Diseases. Available online: https://www.who.int/news-room/fact-sheets/detail/noncommunicable-diseases (accessed on 27 May 2025).
- Ng, M.; Gakidou, E.; Lo, J.; Abate, Y.H.; Abbafati, C.; Abbas, N.; Abbasian, M.; Abd ElHafeez, S.; Abdel-Rahman, W.M.; Abd-Elsalam, S.; et al. Global, Regional, and National Prevalence of Adult Overweight and Obesity, 1990–2021, with Forecasts to 2050: A Forecasting Study for the Global Burden of Disease Study 2021. Lancet 2025, 405, 813–838. [Google Scholar] [CrossRef] [PubMed]
- Kerr, J.A.; Patton, G.C.; Cini, K.I.; Abate, Y.H.; Abbas, N.; Abd Al Magied, A.H.A.; Abd ElHafeez, S.; Abd-Elsalam, S.; Abdollahi, A.; Abdoun, M.; et al. Global, Regional, and National Prevalence of Child and Adolescent Overweight and Obesity, 1990–2021, with Forecasts to 2050: A Forecasting Study for the Global Burden of Disease Study 2021. Lancet 2025, 405, 785–812. [Google Scholar] [CrossRef] [PubMed]
- Swinburn, B.; Sacks, G.; Ravussin, E. Increased Food Energy Supply Is More than Sufficient to Explain the US Epidemic of Obesity1. Am. J. Clin. Nutr. 2009, 90, 1453–1456. [Google Scholar] [CrossRef]
- Cena, H.; Calder, P.C. Defining a Healthy Diet: Evidence for the Role of Contemporary Dietary Patterns in Health and Disease. Nutrients 2020, 12, 334. [Google Scholar] [CrossRef]
- McClements, D.J.; Grossmann, L. The Science of Plant-Based Foods: Constructing next-Generation Meat, Fish, Milk, and Egg Analogs. Compr. Rev. Food Sci. Food Saf. 2021, 20, 4049–4100. [Google Scholar] [CrossRef]
- Zhong, V.W.; Allen, N.B.; Greenland, P.; Carnethon, M.R.; Ning, H.; Wilkins, J.T.; Lloyd-Jones, D.M.; Van Horn, L. Protein Foods from Animal Sources, Incident Cardiovascular Disease and All-Cause Mortality: A Substitution Analysis. Int. J. Epidemiol. 2021, 50, 223–233. [Google Scholar] [CrossRef]
- Reyes-Jurado, F.; Soto-Reyes, N.; Dávila-Rodríguez, M.; Lorenzo-Leal, A.C.; Jiménez-Munguía, M.T.; Mani-López, E.; López-Malo, A. Plant-Based Milk Alternatives: Types, Processes, Benefits, and Characteristics. Food Rev. Int. 2023, 39, 2320–2351. [Google Scholar] [CrossRef]
- Friedman, M.; Brandon, D.L. Nutritional and Health Benefits of Soy Proteins. J. Agric. Food Chem. 2001, 49, 1069–1086. [Google Scholar] [CrossRef]
- Krishnan, H.B. Engineering Soybean for Enhanced Sulfur Amino Acid Content. Crop Sci. 2005, 45, 454–461. [Google Scholar] [CrossRef]
- Sethi, S.; Tyagi, S.K.; Anurag, R.K. Plant-Based Milk Alternatives an Emerging Segment of Functional Beverages: A Review. J. Food Sci. Technol. 2016, 53, 3408–3423. [Google Scholar] [CrossRef] [PubMed]
- Tangyu, M.; Muller, J.; Bolten, C.J.; Wittmann, C. Fermentation of Plant-Based Milk Alternatives for Improved Flavour and Nutritional Value. Appl. Microbiol. Biotechnol. 2019, 103, 9263–9275. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Sedó Molina, G.E.; Tovar, M.; Minh Quoc, H.; Hansen, E.B.; Bang-Berthelsen, C.H. Isolation and Characterization of Plant-Based Lactic Acid Bacteria from Spontaneously Fermented Foods Using a New Modified Medium. LWT 2024, 192, 115695. [Google Scholar] [CrossRef]
- Şanlier, N.; Gökcen, B.B.; Sezgin, A.C. Health Benefits of Fermented Foods. Crit. Rev. Food Sci. Nutr. 2019, 59, 506–527. [Google Scholar] [CrossRef]
- Rul, F.; Béra-Maillet, C.; Champomier-Vergès, M.C.; El-Mecherfi, K.E.; Foligné, B.; Michalski, M.C.; Milenkovic, D.; Savary-Auzeloux, I. Underlying Evidence for the Health Benefits of Fermented Foods in Humans. Food Funct. 2022, 13, 4804–4824. [Google Scholar] [CrossRef]
- Beltrán-Barrientos, L.; Hernández-Mendoza, A.; González-Córdova, A.; Astiazarán-García, H.; Esparza-Romero, J.; Vallejo-Córdoba, B. Mechanistic Pathways Underlying the Antihypertensive Effect of Fermented Milk with Lactococcus Lactis NRRL B-50571 in Spontaneously Hypertensive Rats. Nutrients 2018, 10, 262. [Google Scholar] [CrossRef]
- Rocchetti, G.; Miragoli, F.; Zacconi, C.; Lucini, L.; Rebecchi, A. Impact of Cooking and Fermentation by Lactic Acid Bacteria on Phenolic Profile of Quinoa and Buckwheat Seeds. Food Res. Int. 2019, 119, 886–894. [Google Scholar] [CrossRef]
- Revuelta, J.L.; Serrano-Amatriain, C.; Ledesma-Amaro, R.; Jiménez, A. Formation of Folates by Microorganisms: Towards the Biotechnological Production of This Vitamin. Appl. Microbiol. Biotechnol. 2018, 102, 8613–8620. [Google Scholar] [CrossRef]
- Zhang, D.; Jian, Y.-P.; Zhang, Y.-N.; Li, Y.; Gu, L.-T.; Sun, H.-H.; Liu, M.-D.; Zhou, H.-L.; Wang, Y.-S.; Xu, Z.-X. Short-Chain Fatty Acids in Diseases. Cell Commun. Signal. 2023, 21, 212. [Google Scholar] [CrossRef]
- Marco, M.L.; Sanders, M.E.; Gänzle, M.; Arrieta, M.C.; Cotter, P.D.; De Vuyst, L.; Hill, C.; Holzapfel, W.; Lebeer, S.; Merenstein, D.; et al. The International Scientific Association for Probiotics and Prebiotics (ISAPP) Consensus Statement on Fermented Foods. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 196–208. [Google Scholar] [CrossRef]
- Zhou, Y.; Cui, Y.; Qu, X. Exopolysaccharides of Lactic Acid Bacteria: Structure, Bioactivity and Associations: A Review. Carbohydr. Polym. 2019, 207, 317–332. [Google Scholar] [CrossRef] [PubMed]
- Sam, Q.H.; Ling, H.; Yew, W.S.; Tan, Z.; Ravikumar, S.; Chang, M.W.; Chai, L.Y.A. The Divergent Immunomodulatory Effects of Short Chain Fatty Acids and Medium Chain Fatty Acids. Int. J. Mol. Sci. 2021, 22, 6453. [Google Scholar] [CrossRef] [PubMed]
- Gaggìa, F.; Baffoni, L.; Galiano, M.; Nielsen, D.S.; Jakobsen, R.R.; Castro-Mejía, J.L.; Bosi, S.; Truzzi, F.; Musumeci, F.; Dinelli, G.; et al. Kombucha Beverage from Green, Black and Rooibos Teas: A Comparative Study Looking at Microbiology, Chemistry and Antioxidant Activity. Nutrients 2018, 11, 1. [Google Scholar] [CrossRef] [PubMed]
- Bell, V.; Ferrão, J.; Pimentel, L.; Pintado, M.; Fernandes, T. One Health, Fermented Foods, and Gut Microbiota. Foods 2018, 7, 195. [Google Scholar] [CrossRef]
- Mikelsaar, M.; Sepp, E.; Štšepetova, J.; Hütt, P.; Zilmer, K.; Kullisaar, T.; Zilmer, M. Regulation of Plasma Lipid Profile by Lactobacillus fermentum (Probiotic Strain ME-3 DSM14241) in a Randomised Controlled Trial of Clinically Healthy Adults. BMC Nutr. 2015, 1, 27. [Google Scholar] [CrossRef]
- Orisakwe, O.E.; Amadi, C.N.; Frazzoli, C.; Dokubo, A. Nigerian Foods of Probiotics Relevance and Chronic Metal Exposure: A Systematic Review. Environ. Sci. Pollut. Res. 2020, 27, 19285–19297. [Google Scholar] [CrossRef]
- Chiou, S.-Y.; Sung, J.-M.; Huang, P.-W.; Lin, S.-D. Antioxidant, Antidiabetic, and Antihypertensive Properties of Echinacea Purpurea Flower Extract and Caffeic Acid Derivatives Using In Vitro Models. J. Med. Food 2017, 20, 171–179. [Google Scholar] [CrossRef]
- Yahia, E.M.; García-Solís, P.; Celis, M.E.M. Chapter 2—Contribution of Fruits and Vegetables to Human Nutrition and Health. In Postharvest Physiology and Biochemistry of Fruits and Vegetables; Yahia, E.M., Ed.; Woodhead Publishing: Sawston, UK, 2019; Volume 2, pp. 19–45. [Google Scholar]
- del Río-Celestino, M.; Font, R. The Health Benefits of Fruits and Vegetables. Foods 2020, 9, 369. [Google Scholar] [CrossRef]
- Elik, A.; Yanik, D.K.; Istanbullu, Y.; Guzelsoy, N.A.; Yavuz, A.; Gogus, F. Strategies to Reduce Post-Harvest Losses for Fruits and Vegetables. Int. J. Sci. Technol. Res. 2019, 5, 29–39. [Google Scholar]
- Yuan, X.; Wang, T.; Sun, L.; Qiao, Z.; Pan, H.; Zhong, Y.; Zhuang, Y. Recent Advances of Fermented Fruits: A Review on Strains, Fermentation Strategies, and Functional Activities. Food Chem. X 2024, 22, 101482. [Google Scholar] [CrossRef]
- Paramithiotis, S.; Das, G.; Shin, H.-S.; Patra, J.K. Fate of Bioactive Compounds during Lactic Acid Fermentation of Fruits and Vegetables. Foods 2022, 11, 733. [Google Scholar] [CrossRef] [PubMed]
- Rai, A.; Kumaraswamy, J. Health Benefits of Functional Proteins in Fermented Foods. In Health Benefits Fermented Foods Beverages; CRC Press: Boca Raton, FL, USA, 2015; Chapter 14; pp. 457–476. [Google Scholar]
- Park, Y.K.; Lee, J.H.; Mah, J.-H. Occurrence and Reduction of Biogenic Amines in Traditional Asian Fermented Soybean Foods: A Review. Food Chem. 2019, 278, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Cerdá-Bernad, D.; Valero-Cases, E.; Pastor, J.-J.; Frutos, M.J.; Pérez-Llamas, F. Probiotic Red Quinoa Drinks for Celiacs and Lactose Intolerant People: Study of Functional, Physicochemical and Probiotic Properties during Fermentation and Gastrointestinal Digestion. Int. J. Food Sci. Nutr. 2022, 73, 49–59. [Google Scholar] [CrossRef]
- Shi, H.; Li, J.; Zhang, Y.; Ding, K.; Zhao, G.; Duan, X.; Hadiatullah, H. Effect of Wheat Germination on Nutritional Properties and the Flavor of Soy Sauce. Food Biosci. 2022, 48, 101738. [Google Scholar] [CrossRef]
- Kumari, M.; Kokkiligadda, A.; Dasriya, V.; Naithani, H. Functional Relevance and Health Benefits of Soymilk Fermented by Lactic Acid Bacteria. J. Appl. Microbiol. 2022, 133, 104–119. [Google Scholar] [CrossRef]
- Sasaki, H.; Pham Thi Ngoc, D.; Nishikawa, M.; Kanauchi, M. Lipopolysaccharide Neutralizing Protein in Miso, Japanese Fermented Soybean Paste. J. Food Sci. 2020, 85, 2498–2505. [Google Scholar] [CrossRef]
- He, X.; Rong, P.; Liu, H.; Gan, B.; Wu, D.; Li, H.; Gan, R. Co-Fermentation of Edible Mushroom By-Products with Soybeans Enhances Nutritional Values, Isoflavone Aglycones, and Antioxidant Capacity of Douchi Koji. Foods 2022, 11, 2943. [Google Scholar] [CrossRef]
- Escamilla, D.M.; Rosso, M.L.; Holshouser, D.L.; Chen, P.; Zhang, B. Improvement of Soybean Cultivars for Natto Production through the Selection of Seed Morphological and Physiological Characteristics and Seed Compositions: A Review. Plant Breed. 2019, 138, 131–139. [Google Scholar] [CrossRef]
- Ferri, M.; Serrazanetti, D.I.; Tassoni, A.; Baldissarri, M.; Gianotti, A. Improving the Functional and Sensorial Profile of Cereal-Based Fermented Foods by Selecting Lactobacillus Plantarum Strains via a Metabolomics Approach. Food Res. Int. 2016, 89, 1095–1105. [Google Scholar] [CrossRef]
- Sakandar, H.A.; Hussain, R.; Kubow, S.; Sadiq, F.A.; Huang, W.; Imran, M. Sourdough Bread: A Contemporary Cereal Fermented Product. J. Food Process. Preserv. 2019, 43, e13883. [Google Scholar] [CrossRef]
- Rosa-Sibakov, N.; Poutanen, K.; Micard, V. How Does Wheat Grain, Bran and Aleurone Structure Impact Their Nutritional and Technological Properties? Trends Food Sci. Technol. 2015, 41, 118–134. [Google Scholar] [CrossRef]
- Giacco, R.; Della Pepa, G.; Luongo, D.; Riccardi, G. Whole Grain Intake in Relation to Body Weight: From Epidemiological Evidence to Clinical Trials. Nutr. Metab. Cardiovasc. Dis. 2011, 21, 901–908. [Google Scholar] [CrossRef] [PubMed]
- Nionelli, L.; Pontonio, E.; Gobbetti, M.; Rizzello, C.G. Use of Hop Extract as Antifungal Ingredient for Bread Making and Selection of Autochthonous Resistant Starters for Sourdough Fermentation. Int. J. Food Microbiol. 2018, 266, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Palla, M.; Agnolucci, M.; Calzone, A.; Giovannetti, M.; Di Cagno, R.; Gobbetti, M.; Rizzello, C.G.; Pontonio, E. Exploitation of Autochthonous Tuscan Sourdough Yeasts as Potential Starters. Int. J. Food Microbiol. 2019, 302, 59–68. [Google Scholar] [CrossRef]
- Menezes, A.G.T.; Ramos, C.L.; Dias, D.R.; Schwan, R.F. Combination of Probiotic Yeast and Lactic Acid Bacteria as Starter Culture to Produce Maize-Based Beverages. Food Res. Int. 2018, 111, 187–197. [Google Scholar] [CrossRef]
- Tamang, J.P.; Cotter, P.D.; Endo, A.; Han, N.S.; Kort, R.; Liu, S.Q.; Mayo, B.; Westerik, N.; Hutkins, R. Fermented Foods in a Global Age: East Meets West. Compr. Rev. Food Sci. Food Saf. 2020, 19, 184–217. [Google Scholar] [CrossRef]
- Liu, R.; Gao, G.; Bai, Y.; Hou, L. Fermentation of High-Salt Liquid–State Soy Sauce without Any Additives by Inoculation of Lactic Acid Bacteria and Yeast. Food Sci. Technol. Int. 2020, 26, 642–654. [Google Scholar] [CrossRef]
- Liang, Z.; Yi, M.; Sun, J.; Zhang, T.; Wen, R.; Li, C.; Reshetnik, E.I.; Gribanova, S.L.; Liu, L.; Zhang, G. Physicochemical Properties and Volatile Profile of Mung Bean Flour Fermented by Lacticaseibacillus Casei and Lactococcus Lactis. LWT 2022, 163, 113565. [Google Scholar] [CrossRef]
- Niamah, A.K.; Al-fekaiki, D.F.; Thyab Gddoa Al-Sahlany, S.; Verma, D.K.; Patel, A.R.; Singh, S. Investigating the Effect of Addition of Probiotic Microorganisms (Bacteria or Yeast) to Yoghurt on the Viability and Volatile Aromatic Profiles. J. Food Meas. Charact. 2023, 17, 5463–5473. [Google Scholar] [CrossRef]
- Díaz-Montaño, D.M.; Délia, M.-L.; Estarrón-Espinosa, M.; Strehaiano, P. Fermentative Capability and Aroma Compound Production by Yeast Strains Isolated from Agave Tequilana Weber Juice. Enzym. Microb. Technol. 2008, 42, 608–616. [Google Scholar] [CrossRef]
- Jiang, X.; Peng, D.; Zhang, W.; Duan, M.; Ruan, Z.; Huang, S.; Zhou, S.; Fang, Q. Effect of Aroma-Producing Yeasts in High-Salt Liquid-State Fermentation Soy Sauce and the Biosynthesis Pathways of the Dominant Esters. Food Chem. 2021, 344, 128681. [Google Scholar] [CrossRef] [PubMed]
- Sanjukta, S.; Rai, A.K. Production of Bioactive Peptides during Soybean Fermentation and Their Potential Health Benefits. Trends Food Sci. Technol. 2016, 50, 1–10. [Google Scholar] [CrossRef]
- Xu, D.; Pan, L.; Zhao, H.; Zhao, M.; Sun, J.; Liu, D. Breeding and Identification of Novel Koji Molds with High Activity of Acid Protease by Genome Recombination between Aspergillus Oryzae and Aspergillus Niger. J. Ind. Microbiol. Biotechnol. 2011, 38, 1255–1265. [Google Scholar] [CrossRef] [PubMed]
- Harada, R.; Yuzuki, M.; Ito, K.; Shiga, K.; Bamba, T.; Fukusaki, E. Influence of Yeast and Lactic Acid Bacterium on the Constituent Profile of Soy Sauce during Fermentation. J. Biosci. Bioeng. 2017, 123, 203–208. [Google Scholar] [CrossRef]
- Esfahani, B.N.; Kadivar, M.; Shahedi, M.; Soleimanian-Zad, S. Reduction of Acrylamide in Whole-Wheat Bread by Combining Lactobacilli and Yeast Fermentation. Food Addit. Contam. Part A 2017, 34, 1904–1914. [Google Scholar] [CrossRef]
- Rinaldi, M.; Paciulli, M.; Caligiani, A.; Scazzina, F.; Chiavaro, E. Sourdough Fermentation and Chestnut Flour in Gluten-Free Bread: A Shelf-Life Evaluation. Food Chem. 2016, 224, 144–152. [Google Scholar] [CrossRef]
- Maras, B.; Sweeney, G.; Barra, D.; Bossa, F.; John, R.A. The Amino Acid Sequence of Glutamate Decarboxylase from Escherichia Coli. Eur. J. Biochem. 1992, 204, 93–98. [Google Scholar] [CrossRef]
- Hao, R.; Schmit, J.C. Cloning of the Gene for Glutamate Decarboxylase and Its Expression during Conidiation in Neurospora Crassa. Biochem. J. 1993, 293, 735–738. [Google Scholar] [CrossRef]
- Santos, L.O.; Silva, P.G.P.; Lemos Junior, W.J.F.; de Oliveira, V.S.; Anschau, A. Glutathione Production by Saccharomyces Cerevisiae: Current State and Perspectives. Appl. Microbiol. Biotechnol. 2022, 106, 1879–1894. [Google Scholar] [CrossRef]
- Narayan, V.S.; Nair, P.M. Metabolism, Enzymology and Possible Roles of 4-Aminobutyrate in Higher Plants. Phytochemistry 1990, 29, 367–375. [Google Scholar] [CrossRef]
- Bertoldi, M.; Carbone, V.; Borri Voltattorni, C. Ornithine and Glutamate Decarboxylases Catalyse an Oxidative Deamination of Their Alpha-Methyl Substrates. Biochem. J. 1999, 342, 509–512. [Google Scholar] [CrossRef] [PubMed]
- Kato, Y.; Kato, Y.; Furukawa, K.; Hara, S. Cloning and Nucleotide Sequence of the Glutamate Decarboxylase-Encoding Gene gadA from Aspergillus Oryzae. Biosci. Biotechnol. Biochem. 2002, 66, 2600–2605. [Google Scholar] [CrossRef] [PubMed]
- Rice, E.W.; Johnson, C.H.; Dunnigan, M.E.; Reasoner, D.J. Rapid Glutamate Decarboxylase Assay for Detection of Escherichia Coli. Appl. Environ. Microbiol. 1993, 59, 4347–4349. [Google Scholar] [CrossRef]
- Diana, M.; Quílez, J.; Rafecas, M. Gamma-Aminobutyric Acid as a Bioactive Compound in Foods: A Review. J. Funct. Foods 2014, 10, 407–420. [Google Scholar] [CrossRef]
- Junior, W.J.F.L.; Treu, L.; Nadai, C.; Duarte, V.d.S.; Campanaro, S.; Fabrega-Prats, M.; Giacomini, A.; Corcih, V. Genomic Insights into the Glutathione Metabolism of the Non-Conventional Wine Yeast Starmerella bacillaris. OENO One 2021, 55, 105–117. [Google Scholar]
- Lavigne, V.; Pons, A.; Dubourdieu, D. Assay of Glutathione in Must and Wines Using Capillary Electrophoresis and Laser-Induced Fluorescence Detection: Changes in Concentration in Dry White Wines during Alcoholic Fermentation and Aging. J. Chromatogr. A 2007, 1139, 130–135. [Google Scholar] [CrossRef]
- Kiriyama, K.; Hara, K.Y.; Kondo, A. Oxidized Glutathione Fermentation Using Saccharomyces Cerevisiae Engineered for Glutathione Metabolism. Appl. Microbiol. Biotechnol. 2013, 97, 7399–7404. [Google Scholar] [CrossRef]
- Joshi, D.; Roy, S.; Banerjee, S. Chapter 19—Prebiotics: A Functional Food in Health and Disease. In Natural Products and Drug Discovery; Mandal, S.C., Mandal, V., Konishi, T., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 507–523. [Google Scholar]
- Muñoz-González, C.; Brule, M.; Martin, C.; Feron, G.; Canon, F. Influence of Prebiotic Fructans on Retronasal Aroma from Elderly Individuals. Molecules 2021, 26, 2906. [Google Scholar] [CrossRef]
- Brown, G.D.; Gordon, S. Fungal β-Glucans and Mammalian Immunity. Immunity 2003, 19, 311–315. [Google Scholar] [CrossRef]
- Kogan, G.; Šandula, J.; Korolenko, T.A.; Falameeva, O.V.; Poteryaeva, O.N.; Zhanaeva, S.Y.; Levina, O.A.; Filatova, T.G.; Kaledin, V.I. Increased Efficiency of Lewis Lung Carcinoma Chemotherapy with a Macrophage Stimulator—Yeast Carboxymethyl Glucan. Int. Immunopharmacol. 2002, 2, 775–781. [Google Scholar] [CrossRef]
- Ohno, N.; Furukawa, M.; Miura, N.N.; Adachi, Y.; Motoi, M.; Yadomae, T. Antitumor Beta Glucan from the Cultured Fruit Body of Agaricus Blazei. Biol. Pharm. Bull. 2001, 24, 820. [Google Scholar] [CrossRef] [PubMed]
- Cao, Q.-Z.; Lin, Z.-B. Antitumor and Anti-Angiogenic Activity of Ganoderma Lucidum Polysaccharides Peptide. Acta Pharmacol. Sin. 2004, 25, 833–838. [Google Scholar] [PubMed]
- Scalbert, A.; Mila, I.; Expert, D.; Marmolle, F.; Albrecht, A.M.; Hurrell, R.; Huneau, J.F.; Tomé, D. Polyphenols, Metal Ion Complexation and Biological Consequences. Basic Life Sci. 2000, 66, 545–554. [Google Scholar]
- Raihanatu, M.B.; Modu, S.; Falmata, A.S.; Shettima, Y.A.; Heman, M. Effect of Processing (Sprouting and Fermentation) of Five Local Varieties of Sorghum on Some Biochemical Parameters. Biokemistri 2011, 23, 91–96. [Google Scholar]
- Correia, I.; Nunes, A.; Duarte, I.F.; Barros, A.; Delgadillo, I. Sorghum Fermentation Followed by Spectroscopic Techniques. Food Chem. 2005, 90, 853–859. [Google Scholar] [CrossRef]
- Persson, H.; Türk, M.; Nyman, M.; Sandberg, A.-S. Binding of Cu2+, Zn2+, and Cd2+ to Inositol Tri-, Tetra-, Penta-, and Hexaphosphates. J. Agric. Food Chem. 1998, 46, 3194–3200. [Google Scholar] [CrossRef]
- Holm, P.B.; Kristiansen, K.N.; Pedersen, H.B. Transgenic Approaches in Commonly Consumed Cereals to Improve Iron and Zinc Content and Bioavailability. J. Nutr. 2002, 132, 514S–516S. [Google Scholar] [CrossRef]
- Dong, Q.; Saneoka, H. Physiological Characteristics, Phytase Activity, and Mineral Bioavailability of a Low-Phytate Soybean Line during Germination. Plant Foods Hum. Nutr. 2020, 75, 383–389. [Google Scholar] [CrossRef]
- Vats, P.; Bhattacharyya, M.S.; Banerjee, U.C. Use of Phytases (Myo-Inositolhexakisphosphate Phosphohydrolases) for Combatting Environmental Pollution: A Biological Approach. Crit. Rev. Environ. Sci. Technol. 2005, 35, 469–486. [Google Scholar] [CrossRef]
- Ali, N.; Paul, S.; Gayen, D.; Sarkar, S.N.; Datta, K.; Datta, S.K. Development of Low Phytate Rice by RNAi Mediated Seed-Specific Silencing of Inositol 1,3,4,5,6-Pentakisphosphate 2-Kinase Gene (IPK1). PLoS ONE 2013, 8, e68161. [Google Scholar] [CrossRef]
- Schlemmer, U.; Frølich, W.; Prieto, R.M.; Grases, F. Phytate in Foods and Significance for Humans: Food Sources, Intake, Processing, Bioavailability, Protective Role and Analysis. Mol. Nutr. Food Res. 2009, 53, S330–S375. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, S.M.B.; Gholamhosseinpour, A.; Mousavi Khaneghah, A. Fermentation of Acorn Dough by Lactobacilli Strains: Phytic Acid Degradation and Antioxidant Activity. LWT 2019, 100, 144–149. [Google Scholar] [CrossRef]
- Hurrell, R.; Juillerat, M.; Reddy, M.; Lynch, S.; Dassenko, S.; Cook, J. Soy Protein, Phytate, and Iron Absorption in Humans. Am. J. Clin. Nutr. 1992, 56, 573–578. [Google Scholar] [CrossRef]
- Martínez, T.F.; Moyano, F.J. Effect of Tannic Acid on in Vitro Enzymatic Hydrolysis of Some Protein Sources. J. Sci. Food Agric. 2003, 83, 456–464. [Google Scholar] [CrossRef]
- Makkar, H.P.; Francis, G.; Becker, K. Protein Concentrate from Jatropha Curcas Screw-Pressed Seed Cake and Toxic and Antinutritional Factors in Protein Concentrate. J. Sci. Food Agric. 2008, 88, 1542–1548. [Google Scholar] [CrossRef]
- Lajtha, L. Chemistry of Vegetable Tannins. Nature 1966, 212, 883. [Google Scholar]
- Ojha, P.; Adhikari, R.; Karki, R.; Mishra, A.; Subedi, U.; Karki, T.B. Malting and Fermentation Effects on Antinutritional Components and Functional Characteristics of Sorghum Flour. Food Sci. Nutr. 2018, 6, 47–53. [Google Scholar] [CrossRef]
- Kumar, R.A.; Gunasekaran, P.; Lakshmanan, M. Biodegradation of Tannic Acid by Citrobacter Freundii Isolated from a Tannery Effluent. J. Basic Microbiol. 1999, 39, 161–168. [Google Scholar] [CrossRef]
- Liu, L.; Chen, X.; Hao, L.; Zhang, G.; Jin, Z.; Li, C.; Yang, Y.; Rao, J.; Chen, B. Traditional Fermented Soybean Products: Processing, Flavor Formation, Nutritional and Biological Activities. Crit. Rev. Food Sci. Nutr. 2022, 62, 1971–1989. [Google Scholar] [CrossRef]
- Nielsen, S.D.; Jakobsen, L.M.A.; Geiker, N.R.W.; Bertram, H.C. Chemically Acidified, Live and Heat-Inactivated Fermented Dairy Yoghurt Show Distinct Bioactive Peptides, Free Amino Acids and Small Compounds Profiles. Food Chem. 2022, 376, 131919. [Google Scholar] [CrossRef]
- Hutchinson, A.N.; Tingö, L.; Brummer, R.J. The Potential Effects of Probiotics and ω-3 Fatty Acids on Chronic Low-Grade Inflammation. Nutrients 2020, 12, 2402. [Google Scholar] [CrossRef] [PubMed]
- Strazzullo, P.; D’Elia, L.; Kandala, N.-B.; Cappuccio, F.P. Salt Intake, Stroke, and Cardiovascular Disease: Meta-Analysis of Prospective Studies. BMJ 2009, 339, b4567. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Yu, F.; Kang, J.; Li, Q.; Warusawitharana, H.K.; Li, B. Quality Chemistry, Physiological Functions, and Health Benefits of Organic Acids from Tea (Camellia sinensis). Molecules 2023, 28, 2339. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Pu, D.; Zhou, X.; Zhang, Y. Recent Progress in the Study of Taste Characteristics and the Nutrition and Health Properties of Organic Acids in Foods. Foods 2022, 11, 3408. [Google Scholar] [CrossRef]
- Li, M.; Qin, J.; Zhong, B.; Hao, F.; Wu, Z. Improving Acidity and Flavors of Citrus Juice as Well as Its Antioxidant Activity by Cofermentation with Deacidification Bacteria Combination. Food Biosci. 2023, 53, 102592. [Google Scholar] [CrossRef]
- Zhong, W.; Chen, T.; Yang, H.; Li, E. Isolation and Selection of Non-Saccharomyces Yeasts Being Capable of Degrading Citric Acid and Evaluation Its Effect on Kiwifruit Wine Fermentation. Fermentation 2020, 6, 25. [Google Scholar] [CrossRef]
- Piard, J.C.; Desmazeaud, M. Inhibiting Factors Produced by Lactic Acid Bacteria. 1. Oxygen Metabolites and Catabolism End-Products. Lait 1991, 71, 525–541. [Google Scholar] [CrossRef]
- Camu, N.; De Winter, T.; Verbrugghe, K.; Cleenwerck, I.; Vandamme, P.; Takrama, J.S.; Vancanneyt, M.; De Vuyst, L. Dynamics and Biodiversity of Populations of Lactic Acid Bacteria and Acetic Acid Bacteria Involved in Spontaneous Heap Fermentation of Cocoa Beans in Ghana. Appl. Environ. Microbiol. 2007, 73, 1809–1824. [Google Scholar] [CrossRef]
- Chen, R.; Chen, W.; Chen, H.; Zhang, G.; Chen, W. Comparative Evaluation of the Antioxidant Capacities, Organic Acids, and Volatiles of Papaya Juices Fermented by Lactobacillus Acidophilus and Lactobacillus Plantarum. J. Food Qual. 2018, 2018, 9490435. [Google Scholar] [CrossRef]
- del Río, C.; Segura-Carretero, A. Neuroprotection with Bioactive Compounds. Nutrients 2023, 15, 4612. [Google Scholar] [CrossRef]
- Xu, L.; Du, B.; Xu, B. A Systematic, Comparative Study on the Beneficial Health Components and Antioxidant Activities of Commercially Fermented Soy Products Marketed in China. Food Chem. 2015, 174, 202–213. [Google Scholar] [CrossRef] [PubMed]
- Bursać Kovačević, D.; Brdar, D.; Fabečić, P.; Barba, F.J.; Lorenzo, J.M.; Putnik, P. Chapter 2—Strategies to Achieve a Healthy and Balanced Diet: Fruits and Vegetables as a Natural Source of Bioactive Compounds. In Agri-Food Industry Strategies for Healthy Diets and Sustainability; Barba, F.J., Putnik, P., Kovačević, D.B., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 51–88. [Google Scholar]
- Larsson, S.C.; Åkesson, A.; Gigante, B.; Wolk, A. Chocolate Consumption and Risk of Myocardial Infarction: A Prospective Study and Meta-Analysis. Heart 2016, 102, 1017–1022. [Google Scholar] [CrossRef] [PubMed]
- Fraga, C.G.; Actis-Goretta, L.; Ottaviani, J.I.; Carrasquedo, F.; Lotito, S.B.; Lazarus, S.; Schmitz, H.H.; Keen, C.L. Regular Consumption of a Flavanol-Rich Chocolate Can Improve Oxidant Stress in Young Soccer Players. J. Immunol. Res. 2005, 12, 606407. [Google Scholar] [CrossRef]
- Hu, Y.; He, Y.; Niu, Z.; Shen, T.; Zhang, J.; Wang, X.; Hu, W.; Cho, J.Y. A Review of the Immunomodulatory Activities of Polysaccharides Isolated from Panax Species. J. Ginseng Res. 2022, 46, 23–32. [Google Scholar] [CrossRef]
- Ren, L.; Zhang, J.; Zhang, T. Immunomodulatory Activities of Polysaccharides from Ganoderma on Immune Effector Cells. Food Chem. 2021, 340, 127933. [Google Scholar] [CrossRef]
- Zhao, Y.; Yan, B.; Wang, Z.; Li, M.; Zhao, W. Natural Polysaccharides with Immunomodulatory Activities. Mini-Rev. Med. Chem. 2019, 20, 96–106. [Google Scholar] [CrossRef]
- Zhong, W.; Liu, S.; Yang, H.; Li, E. Effect of Selected Yeast on Physicochemical and Oenological Properties of Blueberry Wine Fermented with Citrate-Degrading Pichia Fermentans. LWT 2021, 145, 111261. [Google Scholar] [CrossRef]
- Scutarașu, E.C.; Teliban, I.V.; Zamfir, C.I.; Luchian, C.E.; Colibaba, L.C.; Niculaua, M.; Cotea, V.V. Effect of Different Winemaking Conditions on Organic Acids Compounds of White Wines. Foods 2021, 10, 2569. [Google Scholar] [CrossRef]
- Aung, T.; Park, S.-S.; Kim, M.-J. Influence of Lactobacillus (LAB) Fermentation on the Enhancement of Branched Chain Amino Acids and Antioxidant Properties in Bran among Wheat By-Products. Fermentation 2022, 8, 732. [Google Scholar] [CrossRef]
- Nie, J.; Fu, X.; Wang, L.; Xu, J.; Gao, X. Impact of Monascus Purpureus Fermentation on Antioxidant Activity, Free Amino Acid Profiles and Flavor Properties of Kelp (Saccharina japonica). Food Chem. 2023, 400, 133990. [Google Scholar] [CrossRef]
- Chu, R.; Uaila, E.; Ismail, T.; Lazarte, C.E. Effect of Short-Term Lactic Fermentation on Polyphenol Profile and Antioxidant Capacity in White and Red Quinoa Varieties. Foods 2024, 13, 2413. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, K.; Ray, M.; Adak, A.; Halder, S.K.; Das, A.; Jana, A.; Parua (Mondal), S.; Vágvölgyi, C.; Das Mohapatra, P.K.; Pati, B.R.; et al. Role of Probiotic Lactobacillus Fermentum KKL1 in the Preparation of a Rice Based Fermented Beverage. Bioresour. Technol. 2015, 188, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Baryłko-Pikielna, N.; Kostyra, E. Sensory Interaction of Umami Substances with Model Food Matrices and Its Hedonic Effect. Food Qual. Prefer. 2007, 18, 751–758. [Google Scholar] [CrossRef]
- Cai, W. Diversity of Microbiota, Microbial Functions, and Flavor in Different Types of Low-Temperature Daqu. Food Res. Int. 2021, 150, 110734. [Google Scholar] [CrossRef]
- Liang, Z.; Fang, Z.; Pai, A.; Luo, J.; Gan, R.; Gao, Y.; Lu, J.; Zhang, P. Glycosidically Bound Aroma Precursors in Fruits: A Comprehensive Review. Crit. Rev. Food Sci. Nutr. 2022, 62, 215–243. [Google Scholar] [CrossRef]
- Chen, C. Influence of 4 Lactic Acid Bacteria on the Flavor Profile of Fermented Apple Juice. Food Biosci. 2019, 27, 30–36. [Google Scholar] [CrossRef]
- Luo, Y.-Y.; Guo, Y.; Hu, X.-Y.; Liu, W.-H.; Liu, B.-Q.; Yang, J.; Tu, Z.-C.; Huang, Y.-H. Flavor Improvement of Fermented Soybean Foods by Co-Fermentation with Bacillus Velezensis and Lactiplantibacillus Plantarum. LWT 2023, 186, 115257. [Google Scholar] [CrossRef]
- Clark, A.J.; Soni, B.K.; Sharkey, B.; Acree, T.; Lavin, E.; Bailey, H.M.; Stein, H.H.; Han, A.; Elie, M.; Nadal, M. Shiitake Mycelium Fermentation Improves Digestibility, Nutritional Value, Flavor and Functionality of Plant Proteins. LWT 2022, 156, 113065. [Google Scholar] [CrossRef]
- Procopio, S.; Qian, F.; Becker, T. Function and Regulation of Yeast Genes Involved in Higher Alcohol and Ester Metabolism during Beverage Fermentation. Eur. Food Res. Technol. 2011, 233, 721–729. [Google Scholar] [CrossRef]
- Liu, X.; Xu, S.; Wang, M.; Wang, L.; Liu, J. Effect of Mixed Fermentation with Pichia Fermentans, Hanseniaspora Uvarum, and Wickeramomyces Anomala on the Quality of Fig (Ficus carica L.) Wines. J. Food Process. Preserv. 2021, 45, e15169. [Google Scholar] [CrossRef]
- Yan, Y. Mixed Fermentation of Blueberry Pomace with L. Rhamnosus GG and L. Plantarum-1_Enhance the Active Ingredient, Antioxidant Activity and Health-Promoting Benefits. Food Chem. Toxicol. 2019, 131, 110541. [Google Scholar] [CrossRef]
- Goldberg, E.M.; Wang, K.; Goldberg, J.; Aliani, M. Factors Affecting the Ortho- and Retronasal Perception of Flavors: A Review. Crit. Rev. Food Sci. Nutr. 2018, 58, 913–923. [Google Scholar] [CrossRef] [PubMed]
- Günther-Jordanland, K.; Dawid, C.; Hofmann, T. Quantitation and Taste Contribution of Sensory Active Molecules in Oat (Avena sativa L.). J. Agric. Food Chem. 2020, 68, 10097–10108. [Google Scholar] [CrossRef] [PubMed]
- Milardi, D.; Gazit, E.; Radford, S.E.; Xu, Y.; Gallardo, R.U.; Caflisch, A.; Westermark, G.T.; Westermark, P.; Rosa, C.L.; Ramamoorthy, A. Proteostasis of Islet Amyloid Polypeptide: A Molecular Perspective of Risk Factors and Protective Strategies for Type II Diabetes. Chem. Rev. 2021, 121, 1845–1893. [Google Scholar] [CrossRef] [PubMed]
- Nam, H.; Jung, H.; Karuppasamy, S.; Park, Y.S.; Cho, Y.S.; Lee, J.Y.; Seong, S.-I.; Suh, J.G. Anti-Diabetic Effect of the Soybean Extract Fermented by Bacillus Subtilis MORI in Db/Db Mice. Food Sci. Biotechnol. 2012, 21, 1669–1676. [Google Scholar] [CrossRef]
- Yang, H.J.; Kwon, D.Y.; Kim, M.J.; Kang, S.; Park, S. Meju, Unsalted Soybeans Fermented with Bacillus Subtilis and Aspergilus Oryzae, Potentiates Insulinotropic Actions and Improves Hepatic Insulin Sensitivity in Diabetic Rats. Nutr. Metab. 2012, 9, 37. [Google Scholar] [CrossRef]
- Kwon, D.Y.; Jang, J.S.; Hong, S.M.; Lee, J.E.; Sung, S.R.; Park, H.R.; Park, S. Long-Term Consumption of Fermented Soybean-Derived Chungkookjang Enhances Insulinotropic Action Unlike Soybeans in 90% Pancreatectomized Diabetic Rats. Eur. J. Nutr. 2007, 46, 44–52. [Google Scholar] [CrossRef]
- Isas, A.S.; Escobar, F.; Álvarez-Villamil, E.; Molina, V.; Mateos, R.; Lizarraga, E.; Mozzi, F.; Van Nieuwenhove, C. Fermentation of Pomegranate Juice by Lactic Acid Bacteria and Its Biological Effect on Mice Fed a High-Fat Diet. Food Biosci. 2023, 53, 102516. [Google Scholar] [CrossRef]
- Yeung, A.W.K.; Tzvetkov, N.T.; Durazzo, A.; Lucarini, M.; Souto, E.B.; Santini, A.; Gan, R.-Y.; Jozwik, A.; Grzybek, W.; Horbańczuk, J.O.; et al. Natural Products in Diabetes Research: Quantitative Literature Analysis. Nat. Prod. Res. 2021, 35, 5813–5827. [Google Scholar] [CrossRef]
- Díaz-López, A.; Bulló, M.; Martínez-González, M.A.; Corella, D.; Estruch, R.; Fitó, M.; Gómez-Gracia, E.; Fiol, M.; García de la Corte, F.J.; Ros, E.; et al. Dairy Product Consumption and Risk of Type 2 Diabetes in an Elderly Spanish Mediterranean Population at High Cardiovascular Risk. Eur. J. Nutr. 2016, 55, 349–360. [Google Scholar] [CrossRef]
- Alihosseini, N.; Moahboob, S.A.; Farrin, N.; Mobasseri, M.; Taghizadeh, A.; Ostadrahimi, A.R. Effect of probiotic fermented milk (kefir) on serum level of insulin and homocysteine in type 2 diabetes patients. Acta Endocrinol. 2017, 13, 431–436. [Google Scholar] [CrossRef] [PubMed]
- El-Bashiti, T.A.; Zabut, B.M.; Safia, F.F.A. Effect of Probiotic Fermented Milk (Kefir) on Some Blood Biochemical Parameters Among Newly Diagnosed Type 2 Diabetic Adult Males in Gaza Governorate. Curr. Res. Nutr. Food Sci. J. 2019, 7, 568–575. [Google Scholar] [CrossRef]
- Hove, K.D.; Brøns, C.; Færch, K.; Lund, S.S.; Rossing, P.; Vaag, A. Effects of 12 Weeks of Treatment with Fermented Milk on Blood Pressure, Glucose Metabolism and Markers of Cardiovascular Risk in Patients with Type 2 Diabetes: A Randomised Double-Blind Placebo-Controlled Study. Eur. J. Endocrinol. 2015, 172, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Ostadrahimi, A.; Taghizadeh, A.; Mobasseri, M.; Farrin, N.; Payahoo, L.; Beyramalipoor Gheshlaghi, Z.; Vahedjabbari, M. Effect of Probiotic Fermented Milk (Kefir) on Glycemic Control and Lipid Profile in Type 2 Diabetic Patients: A Randomized Double-Blind Placebo-Controlled Clinical Trial. Iran. J. Public Health 2015, 44, 228–237. [Google Scholar]
- Kannel, W.B.; Dawber, T.R.; Kagan, A.; Revotskie, N.; Stokes, J. Factors of Risk in the Development of Coronary Heart Disease—Six-Year Follow-up Experience. Ann. Intern. Med. 1961, 55, 33–50. [Google Scholar] [CrossRef]
- Jeon, Y.B.; Lee, J.-J.; Chang, H.C. Characterization of Juice Fermented with Lactobacillus Plantarum EM and Its Cholesterol-lowering Effects on Rats Fed a High-fat and High-cholesterol Diet. Food Sci. Nutr. 2019, 7, 3622–3634. [Google Scholar] [CrossRef]
- Lim, J.-H.; Jung, E.-S.; Choi, E.-K.; Jeong, D.-Y.; Jo, S.-W.; Jin, J.-H.; Lee, J.-M.; Park, B.-H.; Chae, S.-W. Supplementation with Aspergillus Oryzae-Fermented Kochujang Lowers Serum Cholesterol in Subjects with Hyperlipidemia. Clin. Nutr. 2015, 34, 383–387. [Google Scholar] [CrossRef]
- Štšepetova, J.; Rätsep, M.; Gerulis, O.; Jõesaar, A.; Mikelsaar, M.; Songisepp, E. Impact of Lactiplantibacillus plantarum Inducia on Metabolic and Antioxidative Response in Cholesterol and BMI Variable Indices: Randomised, Double-Blind, Placebo-Controlled Trials. Benef. Microbes 2022, 14, 1–16. [Google Scholar] [CrossRef]
- Bendich, A.; Olson, J.A. Biological Actions of Carotenoids. FASEB J. 1989, 3, 1927–1932. [Google Scholar] [CrossRef]
- D’Odorico, A.; Martines, D.; Kiechl, S.; Egger, G.; Oberhollenzer, F.; Bonvicini, P.; Sturniolo, G.C.; Naccarato, R.; Willeit, J. High Plasma Levels of α- and β-Carotene Are Associated with a Lower Risk of Atherosclerosis: Results from the Bruneck Study. Atherosclerosis 2000, 153, 231–239. [Google Scholar] [CrossRef]
- Piermarocchi, S.; Saviano, S.; Parisi, V.; Tedeschi, M.; Panozzo, G.; Scarpa, G.; Boschi, G.; Lo Giudice, G. Carotenoids in Age-Related Maculopathy Italian Study (CARMIS): Two-Year Results of a Randomized Study. Eur. J. Ophthalmol. 2011, 22, 216–225. [Google Scholar]
- Hozawa, A.; Jacobs, D.R.; Steffes, M.W.; Gross, M.D.; Steffen, L.M.; Lee, D.-H. Circulating Carotenoid Concentrations and Incident Hypertension: The Coronary Artery Risk Development in Young Adults (CARDIA) Study. J. Hypertens. 2009, 27, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Frias, J.; Miranda, M.L.; Doblado, R.; Vidal-Valverde, C. Effect of Germination and Fermentation on the Antioxidant Vitamin Content and Antioxidant Capacity of Lupinus Albus L. Var. Multolupa. Food Chem. 2005, 92, 211–220. [Google Scholar] [CrossRef]
- Hunaefi, D.; Akumo, D.N.; Smetanska, I. Effect of Fermentation on Antioxidant Properties of Red Cabbages. Food Biotechnol. 2013, 27, 66–85. [Google Scholar] [CrossRef]
- Vento, M.; Della Croce, C.M.; Bellani, L.; Tassi, E.L.; Echeverria, M.C.; Giorgetti, L. Effect of Sprouting, Fermentation and Cooking on Antioxidant Content and Total Antioxidant Activity in Quinoa and Amaranth. Int. J. Mol. Sci. 2024, 25, 10972. [Google Scholar] [CrossRef]
- Verni, M.; Verardo, V.; Rizzello, C.G. How Fermentation Affects the Antioxidant Properties of Cereals and Legumes. Foods 2019, 8, 362. [Google Scholar] [CrossRef]
- Carciochi, R.A.; Galván-D’Alessandro, L.; Vandendriessche, P.; Chollet, S. Effect of Germination and Fermentation Process on the Antioxidant Compounds of Quinoa Seeds. Plant Foods Hum. Nutr. 2016, 71, 361–367. [Google Scholar] [CrossRef]
- Morifuji, M.; Ichikawa, S.; Kitade, M.; Fukasawa, T.; Asami, Y.; Manabe, Y.; Sugawara, T. Exopolysaccharides from Milk Fermented by Lactic Acid Bacteria Enhance Dietary Carotenoid Bioavailability in Humans in a Randomized Crossover Trial and in Rats. Am. J. Clin. Nutr. 2020, 111, 903–914. [Google Scholar] [CrossRef]
- Li, S.; Ma, C.; Gong, G.; Liu, Z.; Chang, C.; Xu, Z. The Impact of Onion Juice on Milk Fermentation by Lactobacillus Acidophilus. LWT 2016, 65, 543–548. [Google Scholar] [CrossRef]
- Xiao, Y.; Xing, G.; Rui, X.; Li, W.; Chen, X.; Jiang, M.; Dong, M. Enhancement of the Antioxidant Capacity of Chickpeas by Solid State Fermentation with Cordyceps Militaris SN-18. J. Funct. Foods 2014, 10, 210–222. [Google Scholar] [CrossRef]
- Ooi, T.S.; Ting, A.S.Y.; Siow, L.F. Influence of Selected Native Yeast Starter Cultures on the Antioxidant Activities, Fermentation Index and Total Soluble Solids of Malaysia Cocoa Beans: A Simulation Study. LWT 2020, 122, 108977. [Google Scholar] [CrossRef]
- Reyes, A.; Haynes, M.; Hanson, N.; Angly, F.E.; Heath, A.C.; Rohwer, F.; Gordon, J.I. Viruses in the Faecal Microbiota of Monozygotic Twins and Their Mothers. Nature 2010, 466, 334–338. [Google Scholar] [CrossRef] [PubMed]
- Lozupone, C.A.; Stombaugh, J.I.; Gordon, J.I.; Jansson, J.K.; Knight, R. Diversity, Stability and Resilience of the Human Gut Microbiota. Nature 2012, 489, 220–230. [Google Scholar] [CrossRef] [PubMed]
- Gou, H.-Z.; Zhang, Y.-L.; Ren, L.-F.; Li, Z.-J.; Zhang, L. How Do Intestinal Probiotics Restore the Intestinal Barrier? Front. Microbiol. 2022, 13, 929346. [Google Scholar] [CrossRef]
- Ohland, C.L.; MacNaughton, W.K. Probiotic Bacteria and Intestinal Epithelial Barrier Function. Am. J. Physiol.-Gastrointest. Liver Physiol. 2010, 298, G807–G819. [Google Scholar] [CrossRef]
- Corr, S.C.; Hill, C.; Gahan, C.G.M. Chapter 1 Understanding the Mechanisms by Which Probiotics Inhibit Gastrointestinal Pathogens. Adv. Food Nutr. Res. 2009, 56, 1–15. [Google Scholar]
- Hynönen, U.; Palva, A. Lactobacillus Surface Layer Proteins: Structure, Function and Applications. Appl. Microbiol. Biotechnol. 2013, 97, 5225–5243. [Google Scholar] [CrossRef]
- Fukushima, Y.; Miyaguchi, S.; Yamano, T.; Kaburagi, T.; Iino, H.; Ushida, K.; Sato, K. Improvement of Nutritional Status and Incidence of Infection in Hospitalised, Enterally Fed Elderly by Feeding of Fermented Milk Containing Probiotic Lactobacillus Johnsonii La1 (NCC533). Br. J. Nutr. 2007, 98, 969–977. [Google Scholar] [CrossRef]
- Makino, S.; Ikegami, S.; Kume, A.; Horiuchi, H.; Sasaki, H.; Orii, N. Reducing the Risk of Infection in the Elderly by Dietary Intake of Yoghurt Fermented with Lactobacillus Delbrueckii Ssp. Bulgaricus OLL1073R-1. Br. J. Nutr. 2010, 104, 998–1006. [Google Scholar] [CrossRef]
- Taylor, B.C.; Lejzerowicz, F.; Poirel, M.; Shaffer, J.P.; Jiang, L.; Aksenov, A.; Litwin, N.; Humphrey, G.; Martino, C.; Miller-Montgomery, S.; et al. Consumption of Fermented Foods Is Associated with Systematic Differences in the Gut Microbiome and Metabolome. mSystems 2020, 5, e00901–e00919. [Google Scholar] [CrossRef]
- Wastyk, H.C.; Fragiadakis, G.K.; Perelman, D.; Dahan, D.; Merrill, B.D.; Yu, F.B.; Topf, M.; Gonzalez, C.G.; Van Treuren, W.; Han, S.; et al. Gut-Microbiota-Targeted Diets Modulate Human Immune Status. Cell 2021, 184, 4137–4153. [Google Scholar] [CrossRef] [PubMed]
- Hervert-Hernández, D.; and Goñi, I. Dietary Polyphenols and Human Gut Microbiota: A Review. Food Rev. Int. 2011, 27, 154–169. [Google Scholar] [CrossRef]
- Lee, H.C.; Jenner, A.M.; Low, C.S.; Lee, Y.K. Effect of Tea Phenolics and Their Aromatic Fecal Bacterial Metabolites on Intestinal Microbiota. Res. Microbiol. 2006, 157, 876–884. [Google Scholar] [CrossRef]
- Duda-Chodak, A.; Tarko, T.; Satora, P.; Sroka, P. Interaction of Dietary Compounds, Especially Polyphenols, with the Intestinal Microbiota: A Review. Eur. J. Nutr. 2015, 54, 325–341. [Google Scholar] [CrossRef]
- Manach, C.; Williamson, G.; Morand, C.; Scalbert, A.; Rémésy, C. Bioavailability and Bioefficacy of Polyphenols in Humans. I. Review of 97 Bioavailability Studies2. Am. J. Clin. Nutr. 2005, 81, 230S–242S. [Google Scholar] [CrossRef]
- Hur, S.J.; Lee, S.Y.; Kim, Y.-C.; Choi, I.; Kim, G.-B. Effect of Fermentation on the Antioxidant Activity in Plant-Based Foods. Food Chem. 2014, 160, 346–356. [Google Scholar] [CrossRef]
- Rodríguez, H.; Curiel, J.A.; Landete, J.M.; de las Rivas, B.; de Felipe, F.L.; Gómez-Cordovés, C.; Mancheño, J.M.; Muñoz, R. Food Phenolics and Lactic Acid Bacteria. Int. J. Food Microbiol. 2009, 132, 79–90. [Google Scholar] [CrossRef]
- Gan, R.-Y.; Shah, N.P.; Wang, M.-F.; Lui, W.-Y.; Corke, H. Fermentation Alters Antioxidant Capacity and Polyphenol Distribution in Selected Edible Legumes. Int. J. Food Sci. Tech. 2016, 51, 875–884. [Google Scholar] [CrossRef]
- Marques, F.Z.; Nelson, E.; Chu, P.-Y.; Horlock, D.; Fiedler, A.; Ziemann, M.; Tan, J.K.; Kuruppu, S.; Rajapakse, N.W.; El-Osta, A.; et al. High-Fiber Diet and Acetate Supplementation Change the Gut Microbiota and Prevent the Development of Hypertension and Heart Failure in Hypertensive Mice. Circulation 2017, 135, 964–977. [Google Scholar] [CrossRef]
- Korcz, E.; Kerényi, Z.; Varga, L. Dietary Fibers, Prebiotics, and Exopolysaccharides Produced by Lactic Acid Bacteria: Potential Health Benefits with Special Regard to Cholesterol-Lowering Effects. Food Funct. 2018, 9, 3057–3068. [Google Scholar] [CrossRef]
- Kim, M.H.; Kang, S.G.; Park, J.H.; Yanagisawa, M.; Kim, C.H. Short-Chain Fatty Acids Activate GPR41 and GPR43 on Intestinal Epithelial Cells to Promote Inflammatory Responses in Mice. Gastroenterology 2013, 145, 396–406. [Google Scholar] [CrossRef] [PubMed]
- Kabat, A.M.; Srinivasan, N.; Maloy, K.J. Modulation of Immune Development and Function by Intestinal Microbiota. Trends Immunol. 2014, 35, 507–517. [Google Scholar] [CrossRef] [PubMed]
- Woo, J.K.; Choi, S.; Kang, J.-H.; Kim, D.E.; Hurh, B.-S.; Jeon, J.-E.; Kim, S.Y.; Oh, S.H. Fermented Barley and Soybean (BS) Mixture Enhances Intestinal Barrier Function in Dextran Sulfate Sodium (DSS)-Induced Colitis Mouse Model. BMC Complement. Altern. Med. 2016, 16, 498. [Google Scholar] [CrossRef] [PubMed]
- Cuervo, M.P.; Castillo, A.; Santiago-Connolly, L.M. Overview of Biological Hazards and Foodborne Diseases. In Encyclopedia of Food Safety, 2nd ed.; Smithers, G.W., Ed.; Academic Press: Oxford, UK, 2024; pp. 1–18. [Google Scholar]
- Barbosa, J.; Albano, H.; Silva, C.P.; Teixeira, P. Microbiological Contamination of Reusable Plastic Bags for Food Transportation. Food Control 2019, 99, 158–163. [Google Scholar] [CrossRef]
- Cornes, M.P. Exogenous Sample Contamination. Sources and Interference. Clin. Biochem. 2016, 49, 1340–1345. [Google Scholar] [CrossRef]
- Yao, Y.; Zhong, X.; Zhou, Y.; Zhang, H.; Zhao, D.; Zhang, W.; Liu, Y.; Xu, J.; Xie, C.; Yu, C.; et al. Exploring the Characteristics of Burkholderia Gladioli Pathovar Cocovenenans: Growth, Bongkrekic Acid Production, and Potential Risks of Food Contamination in Wet Rice Noodles and Vermicelli. Food Microbiol. 2024, 120, 104449. [Google Scholar] [CrossRef]
- Adebiyi, J.A.; Kayitesi, E.; Adebo, O.A.; Changwa, R.; Njobeh, P.B. Food Fermentation and Mycotoxin Detoxification: An African Perspective. Food Control 2019, 106, 106731. [Google Scholar] [CrossRef]
- Liu, L.; Xie, M.; Wei, D. Biological Detoxification of Mycotoxins: Current Status and Future Advances. Int. J. Mol. Sci. 2022, 23, 1064. [Google Scholar] [CrossRef]
- Oguntoyinbo, F.A. Development of Hazard Analysis Critical Control Points (HACCP) and Enhancement of Microbial Safety Quality during Production of Fermented Legume Based Condiments in Nigeria. Niger. Food J. 2012, 30, 59–66. [Google Scholar] [CrossRef]
- Radu, E.; Dima, A.; Dobrota, E.M.; Badea, A.-M.; Madsen, D.Ø.; Dobrin, C.; Stanciu, S. Global Trends and Research Hotspots on HACCP and Modern Quality Management Systems in the Food Industry. Heliyon 2023, 9, e18232. [Google Scholar] [CrossRef]
- Suzzi, G.; Gardini, F. Biogenic Amines in Dry Fermented Sausages: A Review. Int. J. Food Microbiol. 2003, 88, 41–54. [Google Scholar] [CrossRef] [PubMed]
- Gardini, F.; Özogul, Y.; Suzzi, G.; Tabanelli, G.; Özogul, F. Technological Factors Affecting Biogenic Amine Content in Foods: A Review. Front. Microbiol. 2016, 7, 1218. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Capillas, C.; Jiménez-Colmenero, F. Biogenic Amines in Meat and Meat Products. Crit. Rev. Food Sci. Nutr. 2004, 44, 489–599. [Google Scholar] [CrossRef]
- Feddern, V.; Mazzuco, H.; Fonseca, F.N.; de Lima, G.J.M.M. A Review on Biogenic Amines in Food and Feed: Toxicological Aspects, Impact on Health and Control Measures. Anim. Prod. Sci. 2019, 59, 608–618. [Google Scholar] [CrossRef]
- Li, Y.; Xiong, D.; Yuan, L.; Fan, P.; Xiao, Y.; Chen, J.; Feng, W. Transcriptome and Protein Networks to Elucidate the Mechanism Underlying Nitrite Degradation by Lactiplantibacillus Plantarum. Food Res. Int. 2022, 156, 111319. [Google Scholar] [CrossRef]
- Wang, X.; Ren, H.; Wang, W.; Xie, Z.J. Effects of a Starter Culture on Histamine Reduction, Nitrite Depletion and Oxidative Stability of Fermented Sausages. J. Food Saf. 2016, 36, 195–202. [Google Scholar] [CrossRef]
- Kononiuk, A.D.; Karwowska, M. Comparison of Selected Parameters Related to Food Safety of Fallow Deer and Beef Uncured Fermented Sausages with Freeze-Dried Acid Whey Addition. Meat Sci. 2020, 161, 108015. [Google Scholar] [CrossRef]
- Bondonno, C.P.; Dalgaard, F.; Blekkenhorst, L.C.; Murray, K.; Lewis, J.R.; Croft, K.D.; Kyrø, C.; Torp-Pedersen, C.; Gislason, G.; Tjønneland, A.; et al. Vegetable Nitrate Intake, Blood Pressure and Incident Cardiovascular Disease: Danish Diet, Cancer, and Health Study. Eur. J. Epidemiol. 2021, 36, 813–825. [Google Scholar] [CrossRef]
- EFSA Panel on Biological Hazards (BIOHAZ). Scientific Opinion on Risk Based Control of Biogenic Amine Formation in Fermented Foods. EFSA J. 2011, 9, 2393. [Google Scholar] [CrossRef]
- Abuhlega, T.A.; Ali, M.R. Biogenic Amines in Fish: Prevention and Reduction. J. Food Process. Preserv. 2022, 46, e16883. [Google Scholar] [CrossRef]
- Kim, S.-H.; Kang, K.H.; Kim, S.H.; Lee, S.; Lee, S.-H.; Ha, E.-S.; Sung, N.-J.; Kim, J.G.; Chung, M.J. Lactic Acid Bacteria Directly Degrade N-Nitrosodimethylamine and Increase the Nitrite-Scavenging Ability in Kimchi. Food Control 2017, 71, 101–109. [Google Scholar] [CrossRef]
- Venegas-Ortega, M.G.; Flores-Gallegos, A.C.; Martínez-Hernández, J.L.; Aguilar, C.N.; Nevárez-Moorillón, G.V. Production of Bioactive Peptides from Lactic Acid Bacteria: A Sustainable Approach for Healthier Foods. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1039–1051. [Google Scholar] [CrossRef] [PubMed]
- Doeun, D.; Davaatseren, M.; Chung, M.-S. Biogenic Amines in Foods. Food Sci. Biotechnol. 2017, 26, 1463–1474. [Google Scholar] [CrossRef] [PubMed]
- Thakur, S.; Kumar, P.; Gupta, N. Exploring Regional Influences on Bioactive Components in Tea Leaves and Their Effect on Sensory Quality. J. Food Compos. Anal. 2025, 144, 107683. [Google Scholar] [CrossRef]
- Ye, J.-H.; Ye, Y.; Yin, J.-F.; Jin, J.; Liang, Y.-R.; Liu, R.-Y.; Tang, P.; Xu, Y.-Q. Bitterness and Astringency of Tea Leaves and Products: Formation Mechanism and Reducing Strategies. Trends Food Sci. Technol. 2022, 123, 130–143. [Google Scholar] [CrossRef]
| Fermented Soy Products | Birthplace | Nutritional Value | References |
|---|---|---|---|
| Soy sauce | China | Contains isoflavones and gamma-aminobutyric acid, which enhance antioxidant activity | [37] |
| Soy milk | China | As a source of protein and flavoring ingredients, it has antioxidant and antihypertensive properties | [38] |
| Miso | Japan | Estrogen-like activity, antioxidant and angiotensin converting enzyme inhibitory activity of isoflavones | [39] |
| Tempeh | Indonesia | It has a variety of biological activities such as antioxidant, antidiabetic, and antihypertensive | [40] |
| Natto | Japan | Contains many peptides and at least 17 amino acids, making nutrients more accessible | [41] |
| Raw Materials | Fermentation Strains | Anti-Oxidative Effects | References |
| Papaya | Lactobacillus acidophilus | The free radical scavenging activity of DPPH and ABTS decreased, indicating that the growth of Lactobacillus acidophilus requires the scavenging of free radicals [154]. | [103] |
| Lactiplantibacillus plantarum | The free radical scavenging activity of DPPH and ABTS was improved, and the antioxidant capacity was enhanced. | ||
| Wheat bran | Lactiplantibacillus plantarum KCTC 3104 | The free radical scavenging activity was higher than that of raw wheat bran, and the equivalent antioxidant capacity of Trolox was enhanced. | [114] |
| Enterococcus faecalis KCTC 3206 | |||
| Saccharin japonica | Monascus purpureus | The free radical scavenging activity of ABTS increased by 1.8 times. | [115] |
| Chickpea | Cordyceps militaris SN-18 | The antioxidant activity was enhanced with the increase of total phenol content. | [155] |
| Cocoa Beans | Pichia kudriavzevii | The content of total phenol and total flavonoids was increased, and it has strong antioxidant potential. | [156] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, B.; Nie, P.; Xu, H. Plant-Based Fermented Foods: Classification, Biochemical Transformations, and Health Benefits. Fermentation 2025, 11, 364. https://doi.org/10.3390/fermentation11070364
Peng B, Nie P, Xu H. Plant-Based Fermented Foods: Classification, Biochemical Transformations, and Health Benefits. Fermentation. 2025; 11(7):364. https://doi.org/10.3390/fermentation11070364
Chicago/Turabian StylePeng, Beini, Penghui Nie, and Hengyi Xu. 2025. "Plant-Based Fermented Foods: Classification, Biochemical Transformations, and Health Benefits" Fermentation 11, no. 7: 364. https://doi.org/10.3390/fermentation11070364
APA StylePeng, B., Nie, P., & Xu, H. (2025). Plant-Based Fermented Foods: Classification, Biochemical Transformations, and Health Benefits. Fermentation, 11(7), 364. https://doi.org/10.3390/fermentation11070364








