Abstract
In recent years, plant-based fermented foods (PBFs) have become popular all over the world due to their high nutritional value. Compared with traditional foods, PBFs can effectively address dietary issues of high fat content, excessive calories, and elevated cholesterol levels in food formulations, while providing higher nutritional value and enhanced sensory properties (taste and flavor) than conventional plant-based products. These characteristics make PBFs more suitable for people’s yearning for a healthy diet. This review discussed the close relationship between PBFs and human health, elaborating on the definition of PBFs, common types, and the beneficial effects that occur during the fermentation process for human health. Furthermore, we also explored the nutritional value of PBFs. Herein, PBFs are not only rich in probiotics, organic acids, and various bioactive substances that promote gut health and boost immunity but also play a positive role in preventing certain chronic diseases. Finally, this article looks forward to the future development trends of PBFs, predicting their significant potential in healthy eating and sustainability.
1. Introduction
People have found that the significant increase in most chronic non-communicable diseases is related to the global dietary pattern trending towards high fat, high salt, and high sugar [1]. Data from the World Health Organization (WHO) show that the annual incidence of chronic non-communicable diseases causes approximately 43 million deaths globally, which is equivalent to 75% of the global non-pandemic-related deaths [2]. According to the prediction in a research report, by 2050, the number of overweight and obese adults globally may reach 3.8 billion [3], and 15.6% of the global population aged 5–14 suffers from obesity [4], for which the excessive intake of high-calorie processed foods is to blame [5].
Thanks to the view of being “healthier” [6], plant-based foods have come into the public’s view. The concept of plant-based foods is derived from the American Plant Food Association’s “Fruits, vegetables, nuts and whole grains, associated with improved nutrition, but highly processed foods [7].” In a substitution analysis, nuts, whole grains, legumes, and fish appear to be healthier protein sources than eggs, processed meats, unprocessed red meats, and poultry and can also reduce the risk of cardiovascular disease and premature death [8].
Nonetheless, common plant-based foods are deficient in some aspects in terms of appearance, taste, flavor, texture, and nutrition [9]. For example, common plant proteins such as soybeans and peas generally have the problem of lacking essential amino acids (such as methionine) [10,11]. Although plant-based dairy alternatives (PBDA) avoid the problem of lactose intolerance, their protein biological value (BV) is 20–40% lower than that of milk, and it is difficult to match their flavor and texture with those of traditional dairy products [12,13]. These deficiencies directly restrict the application value of plant-based diets in the prevention and control of chronic diseases.
Against this backdrop, fermentation technology, regarded as a “biological enhancer,” demonstrates unique advantages [14]. Actually, there have been numerous studies in recent years proving that fermented foods contain bioactive compounds and have beneficial properties [15,16]. During the fermentation process of food, microorganisms will trigger various biochemical reactions, which are helpful for the release of vitamins, amino acids, short-chain fatty acids (SCFAs), phenolic compounds, extracellular polysaccharides (EPSs), organic acids, and bioactive peptides [17,18,19,20]. These substances are of great benefit to human health: Vitamins, as precursors of many coenzymes, play a key role in the metabolism of nucleic acid precursors, post-synthetic modification of proteins, or control of calcium binding in bones [21]; EPSs have hypocholesterolemia, antioxidant, and immunomodulator properties [22]; SCFAs, such as 4-carbobutyric acid, have an alleviating effect on inflammation [23]; and complex polyphenols and flavonoids are enzymatically hydrolyzed into bioactive and more bioavailable components with strong antioxidant activity [24]. Long-term consumption of PBFs contributes to maintaining the equilibrium of the gut microbiota, boosting immunity, and decreasing the risk of cardiovascular diseases [25,26].
Since PBFs can bring a lot of benefits to human health, they are widely acknowledged as a highly valuable category of health food. Therefore, PBFs are highly popular among those who advocate for a healthy lifestyle and have become an essential element in the modern dietary framework. Given the significance people place on PBFs, this manuscript has described the synergistic effect of their two components on human health from three aspects: the classification of PBFs, the efficacy of fermented foods (Figure 1), and the effect of plant-based foods and the changes of plant-based foods before and after fermentation.
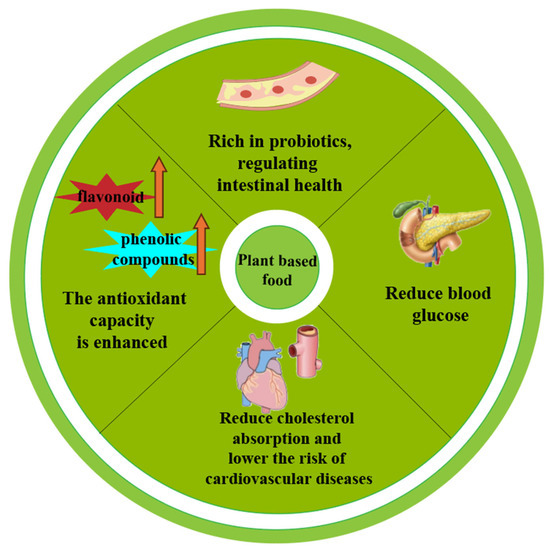
Figure 1.
PBFs’ benefits for the human body.(PS: The arrows in the picture indicate the increase in the amount of substances.).
2. Reaction Mechanisms and Common Types of PBFs
Fermented foods are “foods produced through the growth of desired microorganisms and the enzymatic transformation of food ingredients” [21]. There has been extensive research on fermented foods and human health, and researchers now generally believe that fermented foods are good for human health. Fermented foods are also called “functional foods” because the microorganisms contained in them can produce a variety of nutrients and improve human health [27].
2.1. Common Classification of PBFs
Based on the differences in raw food materials, this section introduces the common categories of PBFs on the market and explains the benefits of fermentation (Figure 2).
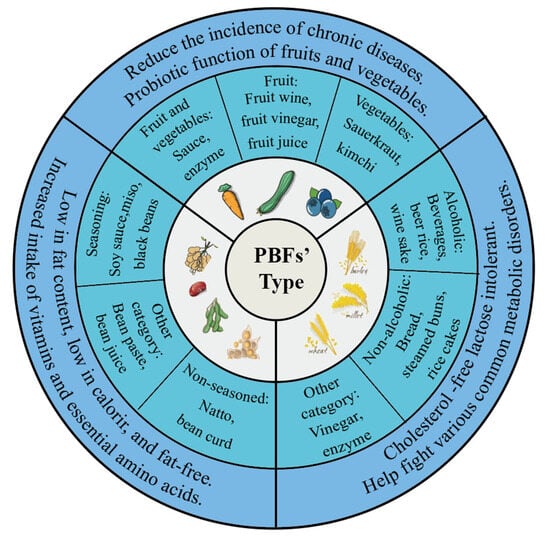
Figure 2.
The common types of PBFs.
2.1.1. Fermented Fruits and Vegetables
Fermented fruit and vegetable foods are a category of functional fruit and vegetable products made from fresh fruits and vegetables as raw materials, which undergo a series of preprocessing and processing steps and are inoculated with fermenting microorganisms. Fermented fruit and vegetable drinks contain a variety of nutrients and chemicals that have anti-inflammatory, antibacterial, antioxidant, and anti-cancer benefits [28,29]. Many studies have also shown that fruit and vegetable intake is associated with a lower incidence of chronic diseases such as coronary heart disease, cancer, diabetes, Alzheimer’s disease, and metabolic syndrome, as well as atherosclerosis caused by unhealthy lifestyle habits [30]. Fruits and vegetables have extremely high nutritional value, but compared with other foods, fruits and vegetables are easily affected by various environmental pressures during production and post-harvest processing, handling, and storage, resulting in deterioration of fruits and vegetables [31]. This susceptibility will not only cause huge economic losses but also cause the production of toxins, causing harm to the human body [32]. The use of probiotic fermentation technology to develop fermented fruits and vegetables with special nutritional and health care functions can prolong their shelf life [31,33].
2.1.2. Fermented Soy
Since ancient times, soy has been an indispensable part of a healthy diet in many Asian countries. Various suitable climates and geographical regions have created a large-scale crop, making soybeans a staple food in Asia. Due to insufficient meat consumption, fermented soy products have played a crucial role as a source of protein in Asian diets [34,35].
Fermented soy products are a category of functional fermented plant proteins made from high-protein plants such as soybeans, walnuts, almonds, etc. by inoculating fermenting bacteria and undergoing food processing treatments.
Compared with ordinary soy products, fermented soy products undergo a series of chemical reactions under the action of enzymes secreted by microorganisms, which leads to an increase in the content of vitamins, essential amino acids, and fatty acids, which greatly enhances the flavor and nutritional value of the product (Table 1). Soy protein has the advantages of being low fat, low calorie, and cholesterol-free and is lactose free, making it the best choice for lactose-intolerant patients and vegetarians [36].

Table 1.
The origins and nutritional values of various fermented bean products.
2.1.3. Fermented Grains
Fermented cereal foods are a category of functional fermented cereal products made from grains through the process of fermentation. The fermentation process can improve the sensory characteristics and nutritional properties of cereal foods, and it is beneficial to human health [42,43]. Epidemiological studies have shown that grains may help combat various common metabolic disorders, such as diabetes, various cancers, cardiovascular diseases, and obesity [44,45]. In addition, the taste and flavor of fermented grain foods are also better than those of ordinary grain products [46,47]. Cereal fermented beverages are non-dairy products and are considered possible carriers of probiotic strains. Soy-based fermented beverages and fermented cereal foods are the alternative for vegetarians and lactose-intolerant consumers [48].
2.2. Reaction Mechanisms of PBFs
The fermentation mechanism of PBFs is mainly driven by the metabolic activities of microorganisms, involving the transformation of carbohydrates, proteins, and polyphenols, ultimately resulting in unique flavor, texture, and functional characteristics. The manuscript will discuss this from three aspects: the role of microorganisms, metabolic pathways, and substrate transformation.
2.2.1. Synergistic Effects of Microbial Communities
Fermenting microorganisms play a central role in the fermentation of plant-based foods. Through fermentation, microorganisms can transform the nutrients in plant raw materials, making them more easily digestible and absorbed, while also producing new flavors and nutrients. During the fermentation process, lactic acid bacteria can convert the carbohydrates in plant raw materials into lactic acid through the glycolysis pathway, reducing the pH value of the food. This not only plays a certain role in preservation but also endows the food with a unique sour taste and flavor [49]. For example, lactic acid bacteria can produce various enzymes during the fermentation process to hydrolyze proteins into peptides and amino acids and produce flavor precursor substances [50,51,52]. Yeast can produce carbon dioxide gas during the fermentation process, giving food a fluffy texture. It can also produce some volatile compounds to enhance the aroma of the food [53,54]. Molds secrete hydrolytic enzymes such as amylase and protease to decompose plant cell walls and macromolecular substrates, releasing fermentable sugars, amino acids, and other flavor compounds, thereby enhancing the umami taste of fermented products [55,56]. During the production process of some food products, these microorganisms can also interact with each other to produce flavor compounds that enhance the aroma of the products [57] or overcome the deficiencies in single-strain fermentation and improve the quality of the products [58,59].
2.2.2. Generation and Function of Metabolites
During the fermentation process of PBFs, microbial metabolism plays a crucial role and can produce a variety of bioactive substances with important functions:
In terms of antioxidant activity, lactic acid bacteria and yeast exhibit remarkable capabilities. They generate antioxidant substances such as γ-aminobutyric acid (GABA) and glutathione through redox reactions [60,61,62]. GABA, a naturally occurring non-protein amino acid, is mainly formed through an irreversible α-decarboxylation reaction of L-glutamic acid or its salts under the action of glutamic acid decarboxylase [63] (this enzyme is mainly found in bacteria [64,65,66]). It has various physiological functions, among which antioxidant activity is one of its important characteristics [67]. Glutathione is a water-soluble tripeptide composed of glutamic acid, cysteine, and glycine [68]. It can be released from yeast cells through autolysis [69]. It is an important molecule containing sulfhydryl residues, which are the basis for the antioxidant properties of glutathione [70].
The fermentation process also produces prebiotics and probiotics. Prebiotics are selectively fermented ingredients that are beneficial to digestive system health and can stimulate the growth of probiotics [71]. Currently, common carbohydrates have all been used to perform the functions of prebiotics (such as lactulose, fructooligosaccharides, β-glucan, trans-galactooligosaccharides, etc.) [72]. As an exopolysaccharide, β-glucan can effectively stimulate the host’s immune response [73]. It has been proven to exhibit potential anti-cancer activity and is frequently utilized as an adjuvant in clinical applications to enhance drug efficacy [74,75,76].
2.2.3. Dynamic Regulation of Substrate Conversion
During the substrate conversion process of PBFs, the dynamic regulation mechanism is achieved through the specific enzyme system secreted by microorganisms, which significantly improves the bioavailability of anti-nutritional factors (phytic acid, tannins, enzyme inhibitors, etc.) in the raw materials. Anti-nutritional factors are usually associated with reduced protein digestibility and mineral absorption rate. Therefore, foods with high contents of such substances are always considered to be low-nutritional-value foods [77]. However, fermentation seems to reduce the content of anti-nutritional factors in foods and increase the in vitro protein digestibility [78,79].
Taking soybeans, grains, and vegetables as examples, the naturally occurring phytic acid (inositol hexaphosphate) in them forms stable chelates with divalent minerals (such as iron, zinc, and calcium) through phosphate groups [80,81]. These chelates are difficult to be digested and absorbed by humans, which will reduce the nutritional value of foods [82,83]. Some common LABs can catalyze and hydrolyze phytic acid into inositol and free phosphate groups by synthesizing microbial phytase, reducing the concentration of phytic acid and thus improving the bioavailability of mineral ions [84,85]. For example, in grains fermented by Lactobacillus plantarum subsp. plantarum, Lactobacillus fermentum, and Lactobacillus casei subspecies, the percentages of phytate reduction at the end of fermentation were 52.58%, 46.39%, and 37.11% respectively [86]. Research has proven that phytic acid is the main inhibitor of iron absorption. When the phytic acid consumed by the testers decreased from its natural amount of 4.9–8.4 to less than 0.01 mg/g isolated, the absorption of iron increased by four to five times [87]. This has important clinical significance for the prevention of nutrition-related chronic diseases such as iron deficiency anemia.
Tannins are a group of phenolic compounds present in the secondary metabolism of plants. They can form stable complexes with proteins, other polymers (such as cellulose, hemicellulose, pectin), and minerals, reducing the catalytic effect of digestive enzymes on proteins [88,89,90]. In an experiment investigating the nutritional characteristics of sorghum flour, the phytic acid, tannins, and oxalates in sorghum flour fermented by Lactobacillus plantarum decreased by 77%, 96.7%, and 67.85%, respectively [91]. Lactobacillus plantarum hydrolyzes tannins into gallic acid and glucose by secreting tannase. The formed gallic acid is decarboxylated to pyrogallol by gallic acid decarboxylase, which improves protein absorption [92].
3. Benefits of PBFs
3.1. Beneficial Substance Produced by Metabolic Processes
PBFs are inherently rich in a variety of nutrients, such as protein, dietary fiber, vitamins, minerals, and beneficial microbial metabolizers [32]. After the fermentation process, the content of these nutrients is retained, and their bioavailability may increase due to chemical changes during the fermentation process [15,93]. For example, some of the active substances, such as bacteriocins in milk during fermentation, are increased [94], which have been shown to prevent infection by some pathogenic microorganisms [95].
3.1.1. Organic Acids
Organic acids refer to organic compounds that are acidic and contain one or more carboxyl groups, which play an important role in maintaining the nutritional value and sensory quality of food [96]. At the same time, organic acids can also improve human health: pyruvic acid provides energy for the human body through the tricarboxylic acid cycle; citric acid is an important intermediate product in various metabolic pathways in animals and plants, and sodium citrate is a common anticoagulant in clinical practice; and malic acid can quickly provide energy for the human body, achieving the goals of resisting fatigue and protecting the liver, kidneys, and heart [97].
Citric acid, malic acid, and tartaric acid are used as acidity regulators, which can maintain or change the pH value of food [98]. However, for some fruits like kiwifruit and citrus fruits, their juices are not suitable for direct consumption due to the high content of citric acid. Fermentation can effectively reduce the content of citric acid and other substances in food [99,100], thereby enhancing the taste of the juice. Take the fermentation of papaya as an example. Lactic acid bacteria decompose metabolic sugars (free sucrose, fructose, and glucose) through fermentation to form organic acids such as lactic acid and acetic acid [101,102]. The total organic acid content of fermented papaya increases significantly [103] (Table 2). Pyruvic acid can be used as a preservative to prevent food from spoiling and effectively extend the shelf life. Therefore, the fermented papaya is less prone to spoilage and can be preserved for a long time.
3.1.2. Active Substances
Bioactive compounds are found in small amounts in plant-based foods as secondary metabolites and are additional nutrients with positive health effects [104]. These bioactive substances beneficial to the human body show relatively obvious changes before and after fermentation. (Table 3). For example, the lowest total flavonoid content in raw soybeans was 2.36 mg GAE/g, while the total flavonoid content in fermented soybean products varied from 3.67 mg GAE/g in spicy fermented bean curd to 12.37 mg GAE/g in black bean and pig knuckle. Probably affected by soybean raw materials, the total phenol content varied. Generally speaking, significant changes in the total phenol content values were found among various fermented soybean products and raw soybeans (p < 0.05) [105].
There is growing evidence that the consumption of bioactive compounds can improve health and reduce the risk of chronic disease [106]. Flavan-3-ols have a positive impact on cardiometabolic outcomes, including reducing the risk of diabetes and cardiovascular-related outcomes [107,108]. Some polysaccharides can stimulate the proliferation and activation of immune cells and enhance the defensive ability of the immune system [109,110,111].

Table 2.
Changes in the organic acid content of different PBF products before and after fermentation.
Table 2.
Changes in the organic acid content of different PBF products before and after fermentation.
| Raw Material | Fermentation Strain | Organic Acid Content g/L | References | |
|---|---|---|---|---|
| Before Fermentation | After Fermentation | |||
| Papaya | Lactobacillus | 0.55 ± 0.05 (pyruvate) | 1.13 ± 0.15 (pyruvate) | [103] |
| 53.2 ± 2.8 (malic acid) | 77.6 ± 3.5 (malic acid) | |||
| Lactiplantibacillus plantarum | 0.55 ± 0.05 (pyruvate) | 1.20 ± 0.11 (pyruvate) | ||
| 53.2 ± 2.8 (malic acid) | 74.2 ± 2.0 (malic acid) | |||
| Blueberry | LacidophilusSaccharomyces cerevisiae RV002, Pichia fermentans JT-1-3, Saccharomyces cerevisiae NP-7-5 | 1.73±0.08 (citric acid | 0.98 ± 0.02 (citric acid) | [112] |
| 1.53 ± 0.10 (malic acid) | 0.99 ± 0.03 (malic acid) | |||
| 0.9 ± 0.04 (tartaric acid) | 0.52 ± 0.06 (tartaric acid) | |||
| Grape | Saccharomyces cerevisiae | 5.07 ± 0.02 (tartaric acid) | 3.97 ± 0.04 (tartaric acid) | [113] |
| 1.87 ± 0.02 (malic acid) | 1.43 ± 0.00 (malic acid) | |||

Table 3.
Changes in the total phenol content and total flavonoid content of different PBF products before and after fermentation.
Table 3.
Changes in the total phenol content and total flavonoid content of different PBF products before and after fermentation.
| Raw Material | Fermentation Strain | Biologically Active Ingredient mg/g | References | |||
|---|---|---|---|---|---|---|
| Before Fermentation | After Fermentation | |||||
| Total Phenol Content | Total Flavonoid Content | Total Phenol Content | Total Flavonoid Content | |||
| Papaya | Lactobacillus acidophilus | 0.032 ± 0.006 | 0.50 ± 0.06 | 0.027 ± 0.009 | 1.11 ± 0.17 | [103] |
| Lactiplantibacillus plantarum | 0.032 ± 0.008 | 0.029 ± 0.006 | 1.45 ± 0.13 | |||
| Wheat bran | Lactobacillus acidophilus KCTC 3164 | 1.61 ± 0.62 | 0.88 ± 0.11 | 2.80 ± 0.27 | 1.01 ± 0.08 | [114] |
| Lactobacillus helveticus KCTC 3545 | 1.98 ± 0.21 | 0.71 ± 0.07 | ||||
| Enterococcus faecalis KCTC 3206 | 2.33 ± 0.09 | 0.71 ± 0.08 | ||||
| Saccharin japonica | Monascus purpureus | 1.658 | 0.227 | 8.443 | 0.463 | [115] |
| White quinoa | Lactiplantibacillus plantarum 299v | 4.68 ± 0.05 | - | 7.78 ± 0.07 | - | [116] |
| Rice | Lactobacillus fermentum KKL1 | 11.8 | 0.04 | 63.42 | 45.36 | [117] |
3.2. Improve Food Sensory Properties
With the improvement in living standards, people’s pursuit of flavor is also becoming more and more intense. The sense of taste affects the selection, intake, absorption, and digestion of food [118]. The final sensory quality of fermented food and the overall product acceptance largely depend on the content and composition of flavor substances [119]. The smell of food is also closely related to volatile aroma compounds. There are many aroma precursors in fruits, such as sugars, glycosidic bonds, and amino acids [120], which usually lack unique aroma characteristics. However, after a series of biochemical reactions such as fermentation, these precursors can be converted into complex aroma compounds, which contribute to the development of rich and diverse flavors [121].
In the experiment, the soybean co-cultured by the two strains of Bacillus velezensis SDL1 and Lactiplantibacillus plantarum Ly8 enhanced the synthesis of branched-chain amino acids and threonine by reducing the acid content and increased the content of some esters, acetyl urea and pyrazine, achieving its superior flavor characteristics [122]. In another experiment, no strong beany odor associated with the presence in the unfermented protein mixture was detected in the fermented pea and rice protein mixture. In addition, the unpleasant odor compounds egg thronal, methyl mercaptan, and bergamot-like compounds in the unfermented protein blend were significantly reduced by 40%, 78%, and 99%, respectively, in the fermented protein blend, demonstrating that the sensory properties of the fermented pea and rice protein concentration blend were improved compared to the unfermented protein mixture [123].
For reducing the unpleasant odors in the raw materials, there is also a method by which yeast can produce pleasant odor-active compounds during the fermentation process [124]. It has a very wide range of applications in the food field. Research has found that for foods fermented with aroma-producing yeasts, the contents of esters and other substances are increased. For example, three types of aroma-producing yeasts can biosynthesize ethyl acetate and isoamyl acetate through esterification and alcoholysis pathways, greatly increasing the concentrations of esters and alcohol flavor compounds in high-salt liquid fermentation soy sauce during the fermentation process [54]. Moreover, fig wines fermented with non-Saccharomyces contain more odorant active substances than fig wines fermented with Saccharomyces cerevisiae, achieving the purpose of enhancing the aroma [125]. While organic acids play an important role in the sensory properties and stability of alcoholic beverages [126], volatile and non-volatile organic acids are particularly noteworthy factors. These organic acids play a crucial role in shaping the sour aroma through the positive and postnasal sense of smell in the nasal passage [127]. Non-volatile organic acids can also produce drinks with sour taste or mouthfeel [128].
4. PBFs’s Potential Benefits to Human Health
There is increasing focus on the potential mechanisms of PBFs in the prevention of chronic non-communicable diseases (diseases that exist for a long time and usually progress slowly, including cardiovascular diseases, cancers, chronic respiratory diseases, and diabetes). Studies have shown that after microbial fermentation, the physicochemical properties of plant-based foods undergo significant changes. Bioactive components are significantly enriched through microbial transformation pathways, while antinutritional factors and potential harmful metabolites are degraded or transformed; in addition, the fermentation process can enhance the bioavailability of functional components and limit the intake of some pathogenic factors by regulating intestinal absorption efficiency. In this section, we reviewed using PBFs as a new intervention strategy for the prevention of chronic diseases (Figure 3).
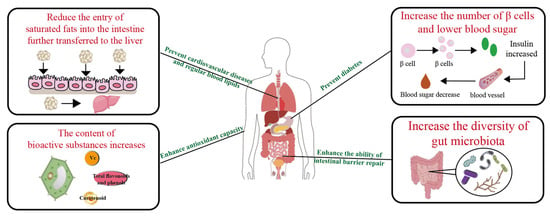
Figure 3.
The pathways by which fermentation exerts an effect on human health.
4.1. Regulate Blood Sugar and Improve Insulin Resistance
Diabetes mellitus (DM) is a non-communicable chronic metabolic disease characterized by hyperglycemia, deficient insulin secretion, and insulin action. It has become a major public health problem worldwide, with significant economic and social repercussions [129].
Insulin and glucagon are synthesized by beta cells and alpha cells, respectively, and are key factors in regulating blood sugar stability. Many studies have found that fermented soy products can potentially improve pancreatic damage and promote insulin secretion, thereby minimizing hyperglycemia conditions [130,131]. Kwon et al.’s study also found that giving fermented soy products to 90% of rats with pancreatic excision diabetes had a significant increase in the number of beta cells, that is, insulin secretion was improved, while unfermented soy did not have this effect [132]. In animal determination experiments, the blood glucose levels of mice fed fermented pomegranate juice were significantly reduced compared with mice in the HFD group. Another study found that CD-1 mice with drug-induced diabetes had a significant decrease in blood glucose levels after taking pomegranate juice for four months [133]. The research results of fermented soybean products in improving pancreatic injury and promoting insulin secretion and of pomegranate juice in lowering blood glucose levels provide new research directions and intervention ideas for the prevention and treatment of diabetes.
Diabetes is a major global public health issue. PBFs show potential in alleviating T2DM [134]. Among the 3,833 participants in the study by Díaz-López et al., individuals with higher intakes of total dairy products, fermented dairy products, low-fat dairy products, and yogurt had a 32% to 41% reduced risk of developing T2DM [135]. Similar conclusions can be found in several other human experiments: The fasting blood glucose and glycated hemoglobin levels of individuals who consumed fermented dairy products were significantly reduced at the end of the experiment, indicating that fermented dairy products may prevent T2DM and related complications [136,137,138,139].
Regrettably, most of these studies are still at the stage of animal experiments. In the future, more high-quality clinical studies are urgently needed to further verify the efficacy and safety of PBFs in the human body and clarify their mechanism of action and effective dose, thereby providing more scientific and reliable strategies for the prevention and treatment of diabetes and helping to solve the economic and social burden caused by diabetes.
4.2. Reduce Cholesterol and Triglyceride Absorption
Cholesterol levels have long been considered the most important risk factor for the development of cardiovascular disease. As early as 1961, studies showed that saturated fatty acids increase cholesterol levels, which in turn increases the risk of developing cardiovascular disease [140]. Many animal and clinical experiments have shown that some probiotics can lower blood lipids. Probiotics can reduce total cholesterol levels by lowering density lipoprotein (LDL) cholesterol [26,45].
In another experiment, researchers found that the serum TG, TC, and LDL-C of hypercholesterolemic mice were significantly elevated, while the serum HDL-C level decreased. Notably, mice fed with fermented fruit juice containing Lactiplantibacillus plantarum EM and a high-fat/high-cholesterol diet showed a significant reduction in serum TG, TC, and LDL-C levels, which suggested that taking EM juice can improve hypercholesterolemia caused by a high-fat/high-cholesterol diet. Additionally, the fecal excretion of TL, TG, and TC after consuming EM juice was more than 1.33 × 109 L. plantarum EM cells/mL, which was significantly higher than that of other groups. This proves that the juice fermented by Lp. plantarum EM is effective in preventing hypercholesterolemia [141].
The same conclusion was also drawn in human experiments. The researchers recruited 30 subjects who had no other diseases except hyperlipidemia. They were asked to consume a fixed amount of fermented red chili sauce for each meal. After 12 weeks, the total cholesterol level decreased from 215.5 ± 16.1 mg/dL to 194.5 ± 25.4 mg/dL [142]. In another two-parallel, double-blind, placebo-controlled intervention, the total cholesterol, low-density lipoprotein cholesterol (LDL-c), and non-high-density cholesterol of participants who received the tested Lactobacillus-fermented yogurt daily also decreased significantly [143].
These research findings have provided rich theoretical basis and research directions for in-depth exploration of the application of fermented foods in the prevention of cardiovascular diseases and regulation of blood lipids. However, more large-scale and multi-center human clinical trials are still needed to further clarify the mechanism of action, the optimal dosage, and the applicable population of probiotic fermented foods in the human body, to promote their practical application in cardiovascular health maintenance.
4.3. Enhances Antioxidant Capacity
Proteins, flavonoids, phenolic compounds, and carotenoids in plants are generally considered natural antioxidants and have attracted attention because of their special nutritional value. Multiple studies have proven that carotenoids have potential health-protective effects against many degenerative diseases, including cardiovascular diseases, age-related macular degeneration, and certain types of cancer [144,145,146,147]. (Flavonoids and phenolic compounds are mentioned in Section 3.1.2).
However, these substances in food may be destroyed by processing, reducing the already low bioavailability. Fermentation can convert conjugated phenols into free phenols by using microbial enzymes and can also change the composition by changing the protein composition, so as to achieve the purpose of improving the antioxidant capacity of food [148].(Table 4).Plant-based foods are an important source of natural antioxidants in the human diet. During the fermentation process, microorganisms can enhance the antioxidant properties of plant-based foods by releasing active components bound to the cell wall [149]. Similarly, in the fermentation experiment of amaranth, it was also concluded that the fermentation process has a positive impact on antioxidant capacity by increasing phenolic and/or flavonoid compounds (induced by microbial hydrolysis reactions) and through their microbial synthesis [150,151,152]. Similarly, in a clinical study, researchers were pleasantly surprised to find that the simultaneous intake of β-carotene and milk fermented by lactic acid bacteria significantly increased the absorption rate of dietary β-carotene in humans and rats [153].

Table 4.
Changes in anti-oxidative effects of different PBFs products before and after fermentation.
These research achievements have wide applications: In the food sector, they provide a theoretical basis for developing functional foods with high antioxidant activity. Future research can further optimize the combination of strains and fermentation processes to precisely control the functional properties of plant-based foods.
4.4. Regulation of Intestinal Microbiota
The human gut microbiome is a large and diverse community that contains many types of life: bacteria, archaea, eukaryotic viruses, viruses, and parasites [157,158]. These microbial florae provide a venue for PBFs to participate in the physiological activities of their hosts. Consuming fermented foods can improve intestinal barrier integrity and intestinal immunity through different mechanisms and maintain intestinal homeostasis.
PBFs can influence gut health by virtue of the probiotics they contain. PBFs are rich in probiotics such as lactic acid bacteria and Bifidobacterium. After these probiotics enter the gut, they affect the gut barrier function by regulating the expression of genes and proteins involved in tight junction signal transduction in intestinal epithelial cells [159,160]. Meanwhile, beneficial bacteria can secrete some antibacterial substances or compete with pathogens for nutrients and space, preventing the attachment and colonization of harmful bacteria. In this way, the proportion of beneficial bacteria in the gut microbiota can be increased, and the balance of the gut microecosystem can be maintained [161,162,163,164]. In an experiment evaluating the effects of PBF intake on the gut microbiota of consumers and non-consumers, researchers examined the presence of various beneficial bacteria in the intestines of 115 subjects. They found that several microorganisms associated with fermented food consumers originated from the microorganisms in fermented foods and confirmed that the abundance of gut microbiota in consumers was significantly higher than that in non-consumers [165]. In another 17-week experiment on the effects of a fermented diet on the human microbiome and immune system of adults, researchers also found that a diet high in fermented foods increased the diversity of the human microbiota and reduced inflammatory markers [166].
Metabolites produced during food fermentation, such as polyphenols and short-chain fatty acids, can regulate the intestinal environment and inhibit inflammation through chemical signals. Many studies have shown that polyphenols can stimulate the growth of symbiotic bacteria and beneficial microbial communities while inhibiting the growth of pathogenic bacteria by altering the gut microbiota [167,168,169]. However, polyphenols are easily recognized by the body as exogenous substances, so their bioavailability is relatively low. Researchers measured the plasma concentration and urinary excretion of adults after a single-dose intake of polyphenols and found that a small portion of dietary polyphenols (5–10% of the total intake) can be absorbed and utilized by the small intestine after deglycosylation. Still, the bioavailability of most polyphenols remains low [170]. Fermentation can decompose complex fibers (such as cellulose and pectin) into fermented foods into soluble dietary fibers through various hydrolases (such as amylase and β-glucosidase) secreted by microorganisms and release bioactive compounds (such as polyphenols and flavonoids) originally wrapped by the fibers, thereby improving the bioavailability of polyphenols [171,172]. Taking the fermentation of beans as an example, enzymatic hydrolysis can significantly release the bound polyphenols (such as anthocyanins and protocatechuic acid) originally wrapped by fibers, converting them into free polyphenols and increasing their bioavailability by approximately 80% [173]. Short-chain fatty acids (SCFAs) can also balance the gut microbiota by inducing a more favorable environment for the growth of beneficial gut microorganisms or influencing mucus concentration [174,175]. In addition, SCFAs activate the mitogen kinase signaling pathway through G protein-coupled receptors on intestinal epithelial cells, promote the production of immune factors, improve the intestinal immune function of mice, and enhance the intestinal barrier function [176,177]. In a mouse model of inflammatory bowel disease fed with fermented barley and soybeans, the intestinal morphology was restored, and the levels of tight junction proteins were increased, which was beneficial to intestinal integrity [178].
In summary, PBFs can regulate the gut microbiota, enhance the gut barrier function, and inhibit the inflammatory response. Their characteristic of improving gut health through multiple pathways provides a scientific basis for targeted nutritional interventions in gut-related diseases such as inflammatory bowel disease and metabolic syndrome. Currently, there are still very few clinical studies on diseases. In the future, it is necessary to further explore their molecular mechanisms and clinical translation pathways.
5. Industrialization Challenges of PBFs
The first four chapters of this paper systematically expound the remarkable advantages of PBFs in improving the sensory characteristics of food and enhancing nutritional functions. These advantages are strongly promoting PBFs to gradually enter the market, showing certain market potential. However, to achieve large-scale industrialization, it is still necessary to break through multi-dimensional bottlenecks:
5.1. Potential Safety Risks
Microbial contamination is considered an important factor leading to frequent food safety accidents and foodborne diseases [179]. To ensure the nutritional value and safety of food during fermentation, storage, transportation, and marketing, we must take effective measures to prevent possible contamination at each stage.
5.1.1. Exogenous Pollution
The open fermentation environment makes it easy for microorganisms (such as molds and bacteria) in water, air, the human body, or mechanical equipment to enter the fermentation system [180,181,182]. These microorganisms will compete with the target fermenting microorganisms for nutrients during the fermentation process, affecting the fermentation effect and even producing toxins [183]. They may also change the metabolic pathway of fermentation, producing undesirable metabolic products, which in turn affect the quality and safety of fermented foods. Researchers attempted to design LAB to express specific enzymes capable of degrading mycotoxins or producing antibacterial peptides with enhanced efficacy, but the efficiency was limited [184].
We hope that by adopting the laminar flow clean system, steam sterilization device, and air filtration technology, we can prevent foreign microorganisms from competing for nutrients and interfering with the target fermentation metabolism and avoid the formation of toxins (such as aflatoxin) and abnormal metabolic products. By optimizing fermentation parameters (pH, temperature, dissolved oxygen) and real-time monitoring of the fermentation process, we can ensure the reproducibility of beneficial metabolic pathways (such as phenolic conversion and SCFAs production) and prevent the formation of undesirable metabolic products. Simultaneously implementing the whole-process HACCP system and setting up microbial and toxin detection controls at critical control points (raw material acceptance, pre-treatment, main fermentation, sterilization, and packaging) is of crucial practical significance in reducing potential hazards and improving food quality [185,186].
5.1.2. Endogenous Contamination
Under normal circumstances, microorganisms in food are generally harmless. However, under certain specific conditions, they may secrete some risk substances harmful to the human body (such as biogenic amines and nitrosamines). During the fermentation process, some microorganisms will decompose proteins and amino acids in food to produce biogenic amines (such as histamine and tyramine) [187,188]. Research shows that excessive intake of biogenic amines can cause headaches, palpitations, or allergic reactions, posing a safety risk to special populations (such as those taking monoamine oxidase inhibitors) [189,190]. Meanwhile, due to the non-standard use of chemical nitrogen fertilizers, there is often a phenomenon of excessively high nitrite residues in plant raw materials [191]. Under low pH conditions, nitrites react with amines or amides naturally present in plants to form carcinogenic nitrosamines [192,193,194].
To reduce the occurrence of the above situations, we believe that strict strain screening and functional regulation should be carried out. According to the characteristics of raw materials (such as protein/phenol content) and different metabolic goals, non-pathogenic strains with specific functions (such as lactic acid bacteria and yeasts) should be selected. For example, strains carrying amino acid decarboxylase genes (hdc, tdc) should be excluded through whole-genome sequencing to inhibit the synthesis of biogenic amines [35,195,196]. Alternatively, strains with nitrate reductase activity (such as Lactobacillus plantarum) can be screened to degrade nitrite [197,198]. Online biosensors can be used to regulate temperature, dissolved oxygen content, and pH value in real time (for example, the activity of decarboxylase is the lowest when the pH is between 4.2 and 4.8) and limit the conditions that are favorable for microorganisms to undergo chemical reactions and produce undesirable metabolic products. At the same time, techniques such as gas chromatography can be used to monitor their content and block pollution occurrence at any time [199].
Through effective control of risk substances such as biogenic amines, nitrites, and nitrosamines, the safety of PBFs can be maximally ensured, allowing consumers to consume them with greater peace of mind.
5.2. Inconsistency of Product Efficacy
5.2.1. Fluctuation of Bioactive Components in Raw Materials
The bioactive components of plant raw materials show significant fluctuations due to differences in origin, variety, and harvest season, which directly affect the stability of the content and composition of functional components in the final product.
For example, catechins are the compounds responsible for the bitterness and astringency in tea. The total catechin content in the fresh buds and young leaves of green tea picked during the summer and monsoon crop seasons is significantly high [200]. In the subsequent tea processing, more methods need to be adopted to reduce the bitterness and astringency of tea and tea products [201].
To address the issue of fluctuations in the bioactive components of raw materials and enhance the stability and quality of products, it is necessary to establish a global raw material database. This database should integrate information on plant raw materials from different origins, varieties, and harvest seasons. Meanwhile, combined with geographical indication certification, it can accurately identify the specific origins and production characteristics of high-quality raw materials. With quality control standards for active ingredients, quantitative analysis can be conducted along with maintaining strict control of the key active ingredients in raw materials. Through data mining and intelligent algorithms, raw material combinations with stronger mutual compatibility can be screened out from the massive information in the database to ensure the stability of the fermentation process and the consistency of product efficacy, providing a solid raw material foundation for the industrial production of high-quality PBFs.
5.2.2. Process Sensitivity and Standardization Challenges
All microorganisms have their optimal growth environments, and different target products also require changes in the fermentation environment. Therefore, PBFs have very strict requirements for fermentation conditions. A slight deviation can lead to a slowdown in the growth and metabolic rate of microorganisms or even their death, and the production rates of key metabolites (such as phenols and short-chain fatty acids) will also fluctuate. It is urgent to develop an intelligent fermentation system. This system will be centered around an AI-driven sensor network, which can analyze metabolic dynamics in real time and achieve automatic feedback adjustment of process parameters such as strain ratio and fermentation duration.
Once fluctuations in the production rate of key metabolites are detected, the system will immediately adjust the corresponding parameters to ensure that the fermentation process is always in the optimal state, thereby stabilizing the production rate of key metabolites and guaranteeing the stability and reliability of the PBFs product quality.
6. Summary and Prospect
In conclusion, the raw materials of PBFs are mainly divided into three categories: fruits and vegetables, soy products, and grains. PBFs significantly enhance nutritional value and health functions through microbial transformation. All of them can balance organic acids through fermentation, achieving natural preservation and palatability synergy. Fermentation also promotes the transformation of antioxidant substances such as phenols and carotenoids, driving the innovation of functional foods. On the sensory level, aroma-producing yeasts and organic acids work together to build a multi-level flavor system. In terms of health benefits, the metabolic products of PBFs can regulate the balance of intestinal flora and enhance the immune barrier, and their bioactive peptides and antioxidant substances also have protective effects such as lowering blood pressure and blood lipids.
Although certain achievements have been made in the research of PBFs, the development of PBFs still faces multiple challenges. Currently, the raw materials and forms of PBFs on the market are relatively monotonous. The raw materials mainly consist of soybeans and grains, while the potential of nuts, seeds, and materials of both medicinal and edible origin (such as wolfberry and polygonatum) has not been fully exploited. The product forms are mostly concentrated in beverages or foods, but innovative forms such as fermented seasonings and medicines (such as fermented Chinese herbal preparations) are worthy of exploration. For instance, in response to the low content of active ingredients or toxicity issues of Chinese herbal medicines, microbial fermentation technology can effectively enhance their efficacy, reduce toxicity, and improve flavor, providing a new path for the modernization of Chinese medicine. The existing achievements are mostly based on in vitro experiments or animal models; however, the potential mechanism between PBFs and immune regulation, as well as metabolic regulation, in the complex physiological environment of the human body are still unclear.
In the future, large-scale multi-center randomized controlled trials combined with multi-omics technologies are needed to precisely analyze the actual effects of PBFs in the prevention and treatment of chronic diseases. Technological innovation is the key bottleneck for the industry to make breakthroughs. The biological utilization rate and product texture of plant proteins still need to be improved through strain selection and process optimization. Meanwhile, controlling production costs and standardizing production processes are essential for large-scale application. Only by promoting the diversification of raw materials, in-depth understanding of mechanisms, and intensive technology in a coordinated manner can we expect to achieve the industrial transformation of PBFs from “experience-driven” to “data-driven,” making them truly a new generation of healthy foods with controllable safety and clear efficacy.
Author Contributions
Conceptualization, B.P.; methodology, B.P. and P.N.; software, B.P.; validation, B.P. and P.N.; formal analysis, B.P.; investigation, B.P.; writing—original draft preparation, B.P.; writing—review and editing, H.X. and P.N.; visualization, B.P.; supervision, H.X.; project administration, H.X.; funding acquisition, H.X. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Research Foundation from Academic and Technical Leaders of Major Disciplines in Jiangxi Province, China (20194BCJ22004).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Cordain, L.; Eaton, S.B.; Sebastian, A.; Mann, N.; Lindeberg, S.; Watkins, B.A.; O’Keefe, J.H.; Brand-Miller, J. Origins and Evolution of the Western Diet: Health Implications for the 21st Century1,2. Am. J. Clin. Nutr. 2005, 81, 341–354. [Google Scholar] [CrossRef] [PubMed]
- World Healthy Organization Noncommunicable Diseases. Available online: https://www.who.int/news-room/fact-sheets/detail/noncommunicable-diseases (accessed on 27 May 2025).
- Ng, M.; Gakidou, E.; Lo, J.; Abate, Y.H.; Abbafati, C.; Abbas, N.; Abbasian, M.; Abd ElHafeez, S.; Abdel-Rahman, W.M.; Abd-Elsalam, S.; et al. Global, Regional, and National Prevalence of Adult Overweight and Obesity, 1990–2021, with Forecasts to 2050: A Forecasting Study for the Global Burden of Disease Study 2021. Lancet 2025, 405, 813–838. [Google Scholar] [CrossRef] [PubMed]
- Kerr, J.A.; Patton, G.C.; Cini, K.I.; Abate, Y.H.; Abbas, N.; Abd Al Magied, A.H.A.; Abd ElHafeez, S.; Abd-Elsalam, S.; Abdollahi, A.; Abdoun, M.; et al. Global, Regional, and National Prevalence of Child and Adolescent Overweight and Obesity, 1990–2021, with Forecasts to 2050: A Forecasting Study for the Global Burden of Disease Study 2021. Lancet 2025, 405, 785–812. [Google Scholar] [CrossRef] [PubMed]
- Swinburn, B.; Sacks, G.; Ravussin, E. Increased Food Energy Supply Is More than Sufficient to Explain the US Epidemic of Obesity1. Am. J. Clin. Nutr. 2009, 90, 1453–1456. [Google Scholar] [CrossRef]
- Cena, H.; Calder, P.C. Defining a Healthy Diet: Evidence for the Role of Contemporary Dietary Patterns in Health and Disease. Nutrients 2020, 12, 334. [Google Scholar] [CrossRef]
- McClements, D.J.; Grossmann, L. The Science of Plant-Based Foods: Constructing next-Generation Meat, Fish, Milk, and Egg Analogs. Compr. Rev. Food Sci. Food Saf. 2021, 20, 4049–4100. [Google Scholar] [CrossRef]
- Zhong, V.W.; Allen, N.B.; Greenland, P.; Carnethon, M.R.; Ning, H.; Wilkins, J.T.; Lloyd-Jones, D.M.; Van Horn, L. Protein Foods from Animal Sources, Incident Cardiovascular Disease and All-Cause Mortality: A Substitution Analysis. Int. J. Epidemiol. 2021, 50, 223–233. [Google Scholar] [CrossRef]
- Reyes-Jurado, F.; Soto-Reyes, N.; Dávila-Rodríguez, M.; Lorenzo-Leal, A.C.; Jiménez-Munguía, M.T.; Mani-López, E.; López-Malo, A. Plant-Based Milk Alternatives: Types, Processes, Benefits, and Characteristics. Food Rev. Int. 2023, 39, 2320–2351. [Google Scholar] [CrossRef]
- Friedman, M.; Brandon, D.L. Nutritional and Health Benefits of Soy Proteins. J. Agric. Food Chem. 2001, 49, 1069–1086. [Google Scholar] [CrossRef]
- Krishnan, H.B. Engineering Soybean for Enhanced Sulfur Amino Acid Content. Crop Sci. 2005, 45, 454–461. [Google Scholar] [CrossRef]
- Sethi, S.; Tyagi, S.K.; Anurag, R.K. Plant-Based Milk Alternatives an Emerging Segment of Functional Beverages: A Review. J. Food Sci. Technol. 2016, 53, 3408–3423. [Google Scholar] [CrossRef] [PubMed]
- Tangyu, M.; Muller, J.; Bolten, C.J.; Wittmann, C. Fermentation of Plant-Based Milk Alternatives for Improved Flavour and Nutritional Value. Appl. Microbiol. Biotechnol. 2019, 103, 9263–9275. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Sedó Molina, G.E.; Tovar, M.; Minh Quoc, H.; Hansen, E.B.; Bang-Berthelsen, C.H. Isolation and Characterization of Plant-Based Lactic Acid Bacteria from Spontaneously Fermented Foods Using a New Modified Medium. LWT 2024, 192, 115695. [Google Scholar] [CrossRef]
- Şanlier, N.; Gökcen, B.B.; Sezgin, A.C. Health Benefits of Fermented Foods. Crit. Rev. Food Sci. Nutr. 2019, 59, 506–527. [Google Scholar] [CrossRef]
- Rul, F.; Béra-Maillet, C.; Champomier-Vergès, M.C.; El-Mecherfi, K.E.; Foligné, B.; Michalski, M.C.; Milenkovic, D.; Savary-Auzeloux, I. Underlying Evidence for the Health Benefits of Fermented Foods in Humans. Food Funct. 2022, 13, 4804–4824. [Google Scholar] [CrossRef]
- Beltrán-Barrientos, L.; Hernández-Mendoza, A.; González-Córdova, A.; Astiazarán-García, H.; Esparza-Romero, J.; Vallejo-Córdoba, B. Mechanistic Pathways Underlying the Antihypertensive Effect of Fermented Milk with Lactococcus Lactis NRRL B-50571 in Spontaneously Hypertensive Rats. Nutrients 2018, 10, 262. [Google Scholar] [CrossRef]
- Rocchetti, G.; Miragoli, F.; Zacconi, C.; Lucini, L.; Rebecchi, A. Impact of Cooking and Fermentation by Lactic Acid Bacteria on Phenolic Profile of Quinoa and Buckwheat Seeds. Food Res. Int. 2019, 119, 886–894. [Google Scholar] [CrossRef]
- Revuelta, J.L.; Serrano-Amatriain, C.; Ledesma-Amaro, R.; Jiménez, A. Formation of Folates by Microorganisms: Towards the Biotechnological Production of This Vitamin. Appl. Microbiol. Biotechnol. 2018, 102, 8613–8620. [Google Scholar] [CrossRef]
- Zhang, D.; Jian, Y.-P.; Zhang, Y.-N.; Li, Y.; Gu, L.-T.; Sun, H.-H.; Liu, M.-D.; Zhou, H.-L.; Wang, Y.-S.; Xu, Z.-X. Short-Chain Fatty Acids in Diseases. Cell Commun. Signal. 2023, 21, 212. [Google Scholar] [CrossRef]
- Marco, M.L.; Sanders, M.E.; Gänzle, M.; Arrieta, M.C.; Cotter, P.D.; De Vuyst, L.; Hill, C.; Holzapfel, W.; Lebeer, S.; Merenstein, D.; et al. The International Scientific Association for Probiotics and Prebiotics (ISAPP) Consensus Statement on Fermented Foods. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 196–208. [Google Scholar] [CrossRef]
- Zhou, Y.; Cui, Y.; Qu, X. Exopolysaccharides of Lactic Acid Bacteria: Structure, Bioactivity and Associations: A Review. Carbohydr. Polym. 2019, 207, 317–332. [Google Scholar] [CrossRef] [PubMed]
- Sam, Q.H.; Ling, H.; Yew, W.S.; Tan, Z.; Ravikumar, S.; Chang, M.W.; Chai, L.Y.A. The Divergent Immunomodulatory Effects of Short Chain Fatty Acids and Medium Chain Fatty Acids. Int. J. Mol. Sci. 2021, 22, 6453. [Google Scholar] [CrossRef] [PubMed]
- Gaggìa, F.; Baffoni, L.; Galiano, M.; Nielsen, D.S.; Jakobsen, R.R.; Castro-Mejía, J.L.; Bosi, S.; Truzzi, F.; Musumeci, F.; Dinelli, G.; et al. Kombucha Beverage from Green, Black and Rooibos Teas: A Comparative Study Looking at Microbiology, Chemistry and Antioxidant Activity. Nutrients 2018, 11, 1. [Google Scholar] [CrossRef] [PubMed]
- Bell, V.; Ferrão, J.; Pimentel, L.; Pintado, M.; Fernandes, T. One Health, Fermented Foods, and Gut Microbiota. Foods 2018, 7, 195. [Google Scholar] [CrossRef]
- Mikelsaar, M.; Sepp, E.; Štšepetova, J.; Hütt, P.; Zilmer, K.; Kullisaar, T.; Zilmer, M. Regulation of Plasma Lipid Profile by Lactobacillus fermentum (Probiotic Strain ME-3 DSM14241) in a Randomised Controlled Trial of Clinically Healthy Adults. BMC Nutr. 2015, 1, 27. [Google Scholar] [CrossRef]
- Orisakwe, O.E.; Amadi, C.N.; Frazzoli, C.; Dokubo, A. Nigerian Foods of Probiotics Relevance and Chronic Metal Exposure: A Systematic Review. Environ. Sci. Pollut. Res. 2020, 27, 19285–19297. [Google Scholar] [CrossRef]
- Chiou, S.-Y.; Sung, J.-M.; Huang, P.-W.; Lin, S.-D. Antioxidant, Antidiabetic, and Antihypertensive Properties of Echinacea Purpurea Flower Extract and Caffeic Acid Derivatives Using In Vitro Models. J. Med. Food 2017, 20, 171–179. [Google Scholar] [CrossRef]
- Yahia, E.M.; García-Solís, P.; Celis, M.E.M. Chapter 2—Contribution of Fruits and Vegetables to Human Nutrition and Health. In Postharvest Physiology and Biochemistry of Fruits and Vegetables; Yahia, E.M., Ed.; Woodhead Publishing: Sawston, UK, 2019; Volume 2, pp. 19–45. [Google Scholar]
- del Río-Celestino, M.; Font, R. The Health Benefits of Fruits and Vegetables. Foods 2020, 9, 369. [Google Scholar] [CrossRef]
- Elik, A.; Yanik, D.K.; Istanbullu, Y.; Guzelsoy, N.A.; Yavuz, A.; Gogus, F. Strategies to Reduce Post-Harvest Losses for Fruits and Vegetables. Int. J. Sci. Technol. Res. 2019, 5, 29–39. [Google Scholar]
- Yuan, X.; Wang, T.; Sun, L.; Qiao, Z.; Pan, H.; Zhong, Y.; Zhuang, Y. Recent Advances of Fermented Fruits: A Review on Strains, Fermentation Strategies, and Functional Activities. Food Chem. X 2024, 22, 101482. [Google Scholar] [CrossRef]
- Paramithiotis, S.; Das, G.; Shin, H.-S.; Patra, J.K. Fate of Bioactive Compounds during Lactic Acid Fermentation of Fruits and Vegetables. Foods 2022, 11, 733. [Google Scholar] [CrossRef] [PubMed]
- Rai, A.; Kumaraswamy, J. Health Benefits of Functional Proteins in Fermented Foods. In Health Benefits Fermented Foods Beverages; CRC Press: Boca Raton, FL, USA, 2015; Chapter 14; pp. 457–476. [Google Scholar]
- Park, Y.K.; Lee, J.H.; Mah, J.-H. Occurrence and Reduction of Biogenic Amines in Traditional Asian Fermented Soybean Foods: A Review. Food Chem. 2019, 278, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Cerdá-Bernad, D.; Valero-Cases, E.; Pastor, J.-J.; Frutos, M.J.; Pérez-Llamas, F. Probiotic Red Quinoa Drinks for Celiacs and Lactose Intolerant People: Study of Functional, Physicochemical and Probiotic Properties during Fermentation and Gastrointestinal Digestion. Int. J. Food Sci. Nutr. 2022, 73, 49–59. [Google Scholar] [CrossRef]
- Shi, H.; Li, J.; Zhang, Y.; Ding, K.; Zhao, G.; Duan, X.; Hadiatullah, H. Effect of Wheat Germination on Nutritional Properties and the Flavor of Soy Sauce. Food Biosci. 2022, 48, 101738. [Google Scholar] [CrossRef]
- Kumari, M.; Kokkiligadda, A.; Dasriya, V.; Naithani, H. Functional Relevance and Health Benefits of Soymilk Fermented by Lactic Acid Bacteria. J. Appl. Microbiol. 2022, 133, 104–119. [Google Scholar] [CrossRef]
- Sasaki, H.; Pham Thi Ngoc, D.; Nishikawa, M.; Kanauchi, M. Lipopolysaccharide Neutralizing Protein in Miso, Japanese Fermented Soybean Paste. J. Food Sci. 2020, 85, 2498–2505. [Google Scholar] [CrossRef]
- He, X.; Rong, P.; Liu, H.; Gan, B.; Wu, D.; Li, H.; Gan, R. Co-Fermentation of Edible Mushroom By-Products with Soybeans Enhances Nutritional Values, Isoflavone Aglycones, and Antioxidant Capacity of Douchi Koji. Foods 2022, 11, 2943. [Google Scholar] [CrossRef]
- Escamilla, D.M.; Rosso, M.L.; Holshouser, D.L.; Chen, P.; Zhang, B. Improvement of Soybean Cultivars for Natto Production through the Selection of Seed Morphological and Physiological Characteristics and Seed Compositions: A Review. Plant Breed. 2019, 138, 131–139. [Google Scholar] [CrossRef]
- Ferri, M.; Serrazanetti, D.I.; Tassoni, A.; Baldissarri, M.; Gianotti, A. Improving the Functional and Sensorial Profile of Cereal-Based Fermented Foods by Selecting Lactobacillus Plantarum Strains via a Metabolomics Approach. Food Res. Int. 2016, 89, 1095–1105. [Google Scholar] [CrossRef]
- Sakandar, H.A.; Hussain, R.; Kubow, S.; Sadiq, F.A.; Huang, W.; Imran, M. Sourdough Bread: A Contemporary Cereal Fermented Product. J. Food Process. Preserv. 2019, 43, e13883. [Google Scholar] [CrossRef]
- Rosa-Sibakov, N.; Poutanen, K.; Micard, V. How Does Wheat Grain, Bran and Aleurone Structure Impact Their Nutritional and Technological Properties? Trends Food Sci. Technol. 2015, 41, 118–134. [Google Scholar] [CrossRef]
- Giacco, R.; Della Pepa, G.; Luongo, D.; Riccardi, G. Whole Grain Intake in Relation to Body Weight: From Epidemiological Evidence to Clinical Trials. Nutr. Metab. Cardiovasc. Dis. 2011, 21, 901–908. [Google Scholar] [CrossRef] [PubMed]
- Nionelli, L.; Pontonio, E.; Gobbetti, M.; Rizzello, C.G. Use of Hop Extract as Antifungal Ingredient for Bread Making and Selection of Autochthonous Resistant Starters for Sourdough Fermentation. Int. J. Food Microbiol. 2018, 266, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Palla, M.; Agnolucci, M.; Calzone, A.; Giovannetti, M.; Di Cagno, R.; Gobbetti, M.; Rizzello, C.G.; Pontonio, E. Exploitation of Autochthonous Tuscan Sourdough Yeasts as Potential Starters. Int. J. Food Microbiol. 2019, 302, 59–68. [Google Scholar] [CrossRef]
- Menezes, A.G.T.; Ramos, C.L.; Dias, D.R.; Schwan, R.F. Combination of Probiotic Yeast and Lactic Acid Bacteria as Starter Culture to Produce Maize-Based Beverages. Food Res. Int. 2018, 111, 187–197. [Google Scholar] [CrossRef]
- Tamang, J.P.; Cotter, P.D.; Endo, A.; Han, N.S.; Kort, R.; Liu, S.Q.; Mayo, B.; Westerik, N.; Hutkins, R. Fermented Foods in a Global Age: East Meets West. Compr. Rev. Food Sci. Food Saf. 2020, 19, 184–217. [Google Scholar] [CrossRef]
- Liu, R.; Gao, G.; Bai, Y.; Hou, L. Fermentation of High-Salt Liquid–State Soy Sauce without Any Additives by Inoculation of Lactic Acid Bacteria and Yeast. Food Sci. Technol. Int. 2020, 26, 642–654. [Google Scholar] [CrossRef]
- Liang, Z.; Yi, M.; Sun, J.; Zhang, T.; Wen, R.; Li, C.; Reshetnik, E.I.; Gribanova, S.L.; Liu, L.; Zhang, G. Physicochemical Properties and Volatile Profile of Mung Bean Flour Fermented by Lacticaseibacillus Casei and Lactococcus Lactis. LWT 2022, 163, 113565. [Google Scholar] [CrossRef]
- Niamah, A.K.; Al-fekaiki, D.F.; Thyab Gddoa Al-Sahlany, S.; Verma, D.K.; Patel, A.R.; Singh, S. Investigating the Effect of Addition of Probiotic Microorganisms (Bacteria or Yeast) to Yoghurt on the Viability and Volatile Aromatic Profiles. J. Food Meas. Charact. 2023, 17, 5463–5473. [Google Scholar] [CrossRef]
- Díaz-Montaño, D.M.; Délia, M.-L.; Estarrón-Espinosa, M.; Strehaiano, P. Fermentative Capability and Aroma Compound Production by Yeast Strains Isolated from Agave Tequilana Weber Juice. Enzym. Microb. Technol. 2008, 42, 608–616. [Google Scholar] [CrossRef]
- Jiang, X.; Peng, D.; Zhang, W.; Duan, M.; Ruan, Z.; Huang, S.; Zhou, S.; Fang, Q. Effect of Aroma-Producing Yeasts in High-Salt Liquid-State Fermentation Soy Sauce and the Biosynthesis Pathways of the Dominant Esters. Food Chem. 2021, 344, 128681. [Google Scholar] [CrossRef] [PubMed]
- Sanjukta, S.; Rai, A.K. Production of Bioactive Peptides during Soybean Fermentation and Their Potential Health Benefits. Trends Food Sci. Technol. 2016, 50, 1–10. [Google Scholar] [CrossRef]
- Xu, D.; Pan, L.; Zhao, H.; Zhao, M.; Sun, J.; Liu, D. Breeding and Identification of Novel Koji Molds with High Activity of Acid Protease by Genome Recombination between Aspergillus Oryzae and Aspergillus Niger. J. Ind. Microbiol. Biotechnol. 2011, 38, 1255–1265. [Google Scholar] [CrossRef] [PubMed]
- Harada, R.; Yuzuki, M.; Ito, K.; Shiga, K.; Bamba, T.; Fukusaki, E. Influence of Yeast and Lactic Acid Bacterium on the Constituent Profile of Soy Sauce during Fermentation. J. Biosci. Bioeng. 2017, 123, 203–208. [Google Scholar] [CrossRef]
- Esfahani, B.N.; Kadivar, M.; Shahedi, M.; Soleimanian-Zad, S. Reduction of Acrylamide in Whole-Wheat Bread by Combining Lactobacilli and Yeast Fermentation. Food Addit. Contam. Part A 2017, 34, 1904–1914. [Google Scholar] [CrossRef]
- Rinaldi, M.; Paciulli, M.; Caligiani, A.; Scazzina, F.; Chiavaro, E. Sourdough Fermentation and Chestnut Flour in Gluten-Free Bread: A Shelf-Life Evaluation. Food Chem. 2016, 224, 144–152. [Google Scholar] [CrossRef]
- Maras, B.; Sweeney, G.; Barra, D.; Bossa, F.; John, R.A. The Amino Acid Sequence of Glutamate Decarboxylase from Escherichia Coli. Eur. J. Biochem. 1992, 204, 93–98. [Google Scholar] [CrossRef]
- Hao, R.; Schmit, J.C. Cloning of the Gene for Glutamate Decarboxylase and Its Expression during Conidiation in Neurospora Crassa. Biochem. J. 1993, 293, 735–738. [Google Scholar] [CrossRef]
- Santos, L.O.; Silva, P.G.P.; Lemos Junior, W.J.F.; de Oliveira, V.S.; Anschau, A. Glutathione Production by Saccharomyces Cerevisiae: Current State and Perspectives. Appl. Microbiol. Biotechnol. 2022, 106, 1879–1894. [Google Scholar] [CrossRef]
- Narayan, V.S.; Nair, P.M. Metabolism, Enzymology and Possible Roles of 4-Aminobutyrate in Higher Plants. Phytochemistry 1990, 29, 367–375. [Google Scholar] [CrossRef]
- Bertoldi, M.; Carbone, V.; Borri Voltattorni, C. Ornithine and Glutamate Decarboxylases Catalyse an Oxidative Deamination of Their Alpha-Methyl Substrates. Biochem. J. 1999, 342, 509–512. [Google Scholar] [CrossRef] [PubMed]
- Kato, Y.; Kato, Y.; Furukawa, K.; Hara, S. Cloning and Nucleotide Sequence of the Glutamate Decarboxylase-Encoding Gene gadA from Aspergillus Oryzae. Biosci. Biotechnol. Biochem. 2002, 66, 2600–2605. [Google Scholar] [CrossRef] [PubMed]
- Rice, E.W.; Johnson, C.H.; Dunnigan, M.E.; Reasoner, D.J. Rapid Glutamate Decarboxylase Assay for Detection of Escherichia Coli. Appl. Environ. Microbiol. 1993, 59, 4347–4349. [Google Scholar] [CrossRef]
- Diana, M.; Quílez, J.; Rafecas, M. Gamma-Aminobutyric Acid as a Bioactive Compound in Foods: A Review. J. Funct. Foods 2014, 10, 407–420. [Google Scholar] [CrossRef]
- Junior, W.J.F.L.; Treu, L.; Nadai, C.; Duarte, V.d.S.; Campanaro, S.; Fabrega-Prats, M.; Giacomini, A.; Corcih, V. Genomic Insights into the Glutathione Metabolism of the Non-Conventional Wine Yeast Starmerella bacillaris. OENO One 2021, 55, 105–117. [Google Scholar]
- Lavigne, V.; Pons, A.; Dubourdieu, D. Assay of Glutathione in Must and Wines Using Capillary Electrophoresis and Laser-Induced Fluorescence Detection: Changes in Concentration in Dry White Wines during Alcoholic Fermentation and Aging. J. Chromatogr. A 2007, 1139, 130–135. [Google Scholar] [CrossRef]
- Kiriyama, K.; Hara, K.Y.; Kondo, A. Oxidized Glutathione Fermentation Using Saccharomyces Cerevisiae Engineered for Glutathione Metabolism. Appl. Microbiol. Biotechnol. 2013, 97, 7399–7404. [Google Scholar] [CrossRef]
- Joshi, D.; Roy, S.; Banerjee, S. Chapter 19—Prebiotics: A Functional Food in Health and Disease. In Natural Products and Drug Discovery; Mandal, S.C., Mandal, V., Konishi, T., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 507–523. [Google Scholar]
- Muñoz-González, C.; Brule, M.; Martin, C.; Feron, G.; Canon, F. Influence of Prebiotic Fructans on Retronasal Aroma from Elderly Individuals. Molecules 2021, 26, 2906. [Google Scholar] [CrossRef]
- Brown, G.D.; Gordon, S. Fungal β-Glucans and Mammalian Immunity. Immunity 2003, 19, 311–315. [Google Scholar] [CrossRef]
- Kogan, G.; Šandula, J.; Korolenko, T.A.; Falameeva, O.V.; Poteryaeva, O.N.; Zhanaeva, S.Y.; Levina, O.A.; Filatova, T.G.; Kaledin, V.I. Increased Efficiency of Lewis Lung Carcinoma Chemotherapy with a Macrophage Stimulator—Yeast Carboxymethyl Glucan. Int. Immunopharmacol. 2002, 2, 775–781. [Google Scholar] [CrossRef]
- Ohno, N.; Furukawa, M.; Miura, N.N.; Adachi, Y.; Motoi, M.; Yadomae, T. Antitumor Beta Glucan from the Cultured Fruit Body of Agaricus Blazei. Biol. Pharm. Bull. 2001, 24, 820. [Google Scholar] [CrossRef] [PubMed]
- Cao, Q.-Z.; Lin, Z.-B. Antitumor and Anti-Angiogenic Activity of Ganoderma Lucidum Polysaccharides Peptide. Acta Pharmacol. Sin. 2004, 25, 833–838. [Google Scholar] [PubMed]
- Scalbert, A.; Mila, I.; Expert, D.; Marmolle, F.; Albrecht, A.M.; Hurrell, R.; Huneau, J.F.; Tomé, D. Polyphenols, Metal Ion Complexation and Biological Consequences. Basic Life Sci. 2000, 66, 545–554. [Google Scholar]
- Raihanatu, M.B.; Modu, S.; Falmata, A.S.; Shettima, Y.A.; Heman, M. Effect of Processing (Sprouting and Fermentation) of Five Local Varieties of Sorghum on Some Biochemical Parameters. Biokemistri 2011, 23, 91–96. [Google Scholar]
- Correia, I.; Nunes, A.; Duarte, I.F.; Barros, A.; Delgadillo, I. Sorghum Fermentation Followed by Spectroscopic Techniques. Food Chem. 2005, 90, 853–859. [Google Scholar] [CrossRef]
- Persson, H.; Türk, M.; Nyman, M.; Sandberg, A.-S. Binding of Cu2+, Zn2+, and Cd2+ to Inositol Tri-, Tetra-, Penta-, and Hexaphosphates. J. Agric. Food Chem. 1998, 46, 3194–3200. [Google Scholar] [CrossRef]
- Holm, P.B.; Kristiansen, K.N.; Pedersen, H.B. Transgenic Approaches in Commonly Consumed Cereals to Improve Iron and Zinc Content and Bioavailability. J. Nutr. 2002, 132, 514S–516S. [Google Scholar] [CrossRef]
- Dong, Q.; Saneoka, H. Physiological Characteristics, Phytase Activity, and Mineral Bioavailability of a Low-Phytate Soybean Line during Germination. Plant Foods Hum. Nutr. 2020, 75, 383–389. [Google Scholar] [CrossRef]
- Vats, P.; Bhattacharyya, M.S.; Banerjee, U.C. Use of Phytases (Myo-Inositolhexakisphosphate Phosphohydrolases) for Combatting Environmental Pollution: A Biological Approach. Crit. Rev. Environ. Sci. Technol. 2005, 35, 469–486. [Google Scholar] [CrossRef]
- Ali, N.; Paul, S.; Gayen, D.; Sarkar, S.N.; Datta, K.; Datta, S.K. Development of Low Phytate Rice by RNAi Mediated Seed-Specific Silencing of Inositol 1,3,4,5,6-Pentakisphosphate 2-Kinase Gene (IPK1). PLoS ONE 2013, 8, e68161. [Google Scholar] [CrossRef]
- Schlemmer, U.; Frølich, W.; Prieto, R.M.; Grases, F. Phytate in Foods and Significance for Humans: Food Sources, Intake, Processing, Bioavailability, Protective Role and Analysis. Mol. Nutr. Food Res. 2009, 53, S330–S375. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, S.M.B.; Gholamhosseinpour, A.; Mousavi Khaneghah, A. Fermentation of Acorn Dough by Lactobacilli Strains: Phytic Acid Degradation and Antioxidant Activity. LWT 2019, 100, 144–149. [Google Scholar] [CrossRef]
- Hurrell, R.; Juillerat, M.; Reddy, M.; Lynch, S.; Dassenko, S.; Cook, J. Soy Protein, Phytate, and Iron Absorption in Humans. Am. J. Clin. Nutr. 1992, 56, 573–578. [Google Scholar] [CrossRef]
- Martínez, T.F.; Moyano, F.J. Effect of Tannic Acid on in Vitro Enzymatic Hydrolysis of Some Protein Sources. J. Sci. Food Agric. 2003, 83, 456–464. [Google Scholar] [CrossRef]
- Makkar, H.P.; Francis, G.; Becker, K. Protein Concentrate from Jatropha Curcas Screw-Pressed Seed Cake and Toxic and Antinutritional Factors in Protein Concentrate. J. Sci. Food Agric. 2008, 88, 1542–1548. [Google Scholar] [CrossRef]
- Lajtha, L. Chemistry of Vegetable Tannins. Nature 1966, 212, 883. [Google Scholar]
- Ojha, P.; Adhikari, R.; Karki, R.; Mishra, A.; Subedi, U.; Karki, T.B. Malting and Fermentation Effects on Antinutritional Components and Functional Characteristics of Sorghum Flour. Food Sci. Nutr. 2018, 6, 47–53. [Google Scholar] [CrossRef]
- Kumar, R.A.; Gunasekaran, P.; Lakshmanan, M. Biodegradation of Tannic Acid by Citrobacter Freundii Isolated from a Tannery Effluent. J. Basic Microbiol. 1999, 39, 161–168. [Google Scholar] [CrossRef]
- Liu, L.; Chen, X.; Hao, L.; Zhang, G.; Jin, Z.; Li, C.; Yang, Y.; Rao, J.; Chen, B. Traditional Fermented Soybean Products: Processing, Flavor Formation, Nutritional and Biological Activities. Crit. Rev. Food Sci. Nutr. 2022, 62, 1971–1989. [Google Scholar] [CrossRef]
- Nielsen, S.D.; Jakobsen, L.M.A.; Geiker, N.R.W.; Bertram, H.C. Chemically Acidified, Live and Heat-Inactivated Fermented Dairy Yoghurt Show Distinct Bioactive Peptides, Free Amino Acids and Small Compounds Profiles. Food Chem. 2022, 376, 131919. [Google Scholar] [CrossRef]
- Hutchinson, A.N.; Tingö, L.; Brummer, R.J. The Potential Effects of Probiotics and ω-3 Fatty Acids on Chronic Low-Grade Inflammation. Nutrients 2020, 12, 2402. [Google Scholar] [CrossRef] [PubMed]
- Strazzullo, P.; D’Elia, L.; Kandala, N.-B.; Cappuccio, F.P. Salt Intake, Stroke, and Cardiovascular Disease: Meta-Analysis of Prospective Studies. BMJ 2009, 339, b4567. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Yu, F.; Kang, J.; Li, Q.; Warusawitharana, H.K.; Li, B. Quality Chemistry, Physiological Functions, and Health Benefits of Organic Acids from Tea (Camellia sinensis). Molecules 2023, 28, 2339. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Pu, D.; Zhou, X.; Zhang, Y. Recent Progress in the Study of Taste Characteristics and the Nutrition and Health Properties of Organic Acids in Foods. Foods 2022, 11, 3408. [Google Scholar] [CrossRef]
- Li, M.; Qin, J.; Zhong, B.; Hao, F.; Wu, Z. Improving Acidity and Flavors of Citrus Juice as Well as Its Antioxidant Activity by Cofermentation with Deacidification Bacteria Combination. Food Biosci. 2023, 53, 102592. [Google Scholar] [CrossRef]
- Zhong, W.; Chen, T.; Yang, H.; Li, E. Isolation and Selection of Non-Saccharomyces Yeasts Being Capable of Degrading Citric Acid and Evaluation Its Effect on Kiwifruit Wine Fermentation. Fermentation 2020, 6, 25. [Google Scholar] [CrossRef]
- Piard, J.C.; Desmazeaud, M. Inhibiting Factors Produced by Lactic Acid Bacteria. 1. Oxygen Metabolites and Catabolism End-Products. Lait 1991, 71, 525–541. [Google Scholar] [CrossRef]
- Camu, N.; De Winter, T.; Verbrugghe, K.; Cleenwerck, I.; Vandamme, P.; Takrama, J.S.; Vancanneyt, M.; De Vuyst, L. Dynamics and Biodiversity of Populations of Lactic Acid Bacteria and Acetic Acid Bacteria Involved in Spontaneous Heap Fermentation of Cocoa Beans in Ghana. Appl. Environ. Microbiol. 2007, 73, 1809–1824. [Google Scholar] [CrossRef]
- Chen, R.; Chen, W.; Chen, H.; Zhang, G.; Chen, W. Comparative Evaluation of the Antioxidant Capacities, Organic Acids, and Volatiles of Papaya Juices Fermented by Lactobacillus Acidophilus and Lactobacillus Plantarum. J. Food Qual. 2018, 2018, 9490435. [Google Scholar] [CrossRef]
- del Río, C.; Segura-Carretero, A. Neuroprotection with Bioactive Compounds. Nutrients 2023, 15, 4612. [Google Scholar] [CrossRef]
- Xu, L.; Du, B.; Xu, B. A Systematic, Comparative Study on the Beneficial Health Components and Antioxidant Activities of Commercially Fermented Soy Products Marketed in China. Food Chem. 2015, 174, 202–213. [Google Scholar] [CrossRef] [PubMed]
- Bursać Kovačević, D.; Brdar, D.; Fabečić, P.; Barba, F.J.; Lorenzo, J.M.; Putnik, P. Chapter 2—Strategies to Achieve a Healthy and Balanced Diet: Fruits and Vegetables as a Natural Source of Bioactive Compounds. In Agri-Food Industry Strategies for Healthy Diets and Sustainability; Barba, F.J., Putnik, P., Kovačević, D.B., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 51–88. [Google Scholar]
- Larsson, S.C.; Åkesson, A.; Gigante, B.; Wolk, A. Chocolate Consumption and Risk of Myocardial Infarction: A Prospective Study and Meta-Analysis. Heart 2016, 102, 1017–1022. [Google Scholar] [CrossRef] [PubMed]
- Fraga, C.G.; Actis-Goretta, L.; Ottaviani, J.I.; Carrasquedo, F.; Lotito, S.B.; Lazarus, S.; Schmitz, H.H.; Keen, C.L. Regular Consumption of a Flavanol-Rich Chocolate Can Improve Oxidant Stress in Young Soccer Players. J. Immunol. Res. 2005, 12, 606407. [Google Scholar] [CrossRef]
- Hu, Y.; He, Y.; Niu, Z.; Shen, T.; Zhang, J.; Wang, X.; Hu, W.; Cho, J.Y. A Review of the Immunomodulatory Activities of Polysaccharides Isolated from Panax Species. J. Ginseng Res. 2022, 46, 23–32. [Google Scholar] [CrossRef]
- Ren, L.; Zhang, J.; Zhang, T. Immunomodulatory Activities of Polysaccharides from Ganoderma on Immune Effector Cells. Food Chem. 2021, 340, 127933. [Google Scholar] [CrossRef]
- Zhao, Y.; Yan, B.; Wang, Z.; Li, M.; Zhao, W. Natural Polysaccharides with Immunomodulatory Activities. Mini-Rev. Med. Chem. 2019, 20, 96–106. [Google Scholar] [CrossRef]
- Zhong, W.; Liu, S.; Yang, H.; Li, E. Effect of Selected Yeast on Physicochemical and Oenological Properties of Blueberry Wine Fermented with Citrate-Degrading Pichia Fermentans. LWT 2021, 145, 111261. [Google Scholar] [CrossRef]
- Scutarașu, E.C.; Teliban, I.V.; Zamfir, C.I.; Luchian, C.E.; Colibaba, L.C.; Niculaua, M.; Cotea, V.V. Effect of Different Winemaking Conditions on Organic Acids Compounds of White Wines. Foods 2021, 10, 2569. [Google Scholar] [CrossRef]
- Aung, T.; Park, S.-S.; Kim, M.-J. Influence of Lactobacillus (LAB) Fermentation on the Enhancement of Branched Chain Amino Acids and Antioxidant Properties in Bran among Wheat By-Products. Fermentation 2022, 8, 732. [Google Scholar] [CrossRef]
- Nie, J.; Fu, X.; Wang, L.; Xu, J.; Gao, X. Impact of Monascus Purpureus Fermentation on Antioxidant Activity, Free Amino Acid Profiles and Flavor Properties of Kelp (Saccharina japonica). Food Chem. 2023, 400, 133990. [Google Scholar] [CrossRef]
- Chu, R.; Uaila, E.; Ismail, T.; Lazarte, C.E. Effect of Short-Term Lactic Fermentation on Polyphenol Profile and Antioxidant Capacity in White and Red Quinoa Varieties. Foods 2024, 13, 2413. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, K.; Ray, M.; Adak, A.; Halder, S.K.; Das, A.; Jana, A.; Parua (Mondal), S.; Vágvölgyi, C.; Das Mohapatra, P.K.; Pati, B.R.; et al. Role of Probiotic Lactobacillus Fermentum KKL1 in the Preparation of a Rice Based Fermented Beverage. Bioresour. Technol. 2015, 188, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Baryłko-Pikielna, N.; Kostyra, E. Sensory Interaction of Umami Substances with Model Food Matrices and Its Hedonic Effect. Food Qual. Prefer. 2007, 18, 751–758. [Google Scholar] [CrossRef]
- Cai, W. Diversity of Microbiota, Microbial Functions, and Flavor in Different Types of Low-Temperature Daqu. Food Res. Int. 2021, 150, 110734. [Google Scholar] [CrossRef]
- Liang, Z.; Fang, Z.; Pai, A.; Luo, J.; Gan, R.; Gao, Y.; Lu, J.; Zhang, P. Glycosidically Bound Aroma Precursors in Fruits: A Comprehensive Review. Crit. Rev. Food Sci. Nutr. 2022, 62, 215–243. [Google Scholar] [CrossRef]
- Chen, C. Influence of 4 Lactic Acid Bacteria on the Flavor Profile of Fermented Apple Juice. Food Biosci. 2019, 27, 30–36. [Google Scholar] [CrossRef]
- Luo, Y.-Y.; Guo, Y.; Hu, X.-Y.; Liu, W.-H.; Liu, B.-Q.; Yang, J.; Tu, Z.-C.; Huang, Y.-H. Flavor Improvement of Fermented Soybean Foods by Co-Fermentation with Bacillus Velezensis and Lactiplantibacillus Plantarum. LWT 2023, 186, 115257. [Google Scholar] [CrossRef]
- Clark, A.J.; Soni, B.K.; Sharkey, B.; Acree, T.; Lavin, E.; Bailey, H.M.; Stein, H.H.; Han, A.; Elie, M.; Nadal, M. Shiitake Mycelium Fermentation Improves Digestibility, Nutritional Value, Flavor and Functionality of Plant Proteins. LWT 2022, 156, 113065. [Google Scholar] [CrossRef]
- Procopio, S.; Qian, F.; Becker, T. Function and Regulation of Yeast Genes Involved in Higher Alcohol and Ester Metabolism during Beverage Fermentation. Eur. Food Res. Technol. 2011, 233, 721–729. [Google Scholar] [CrossRef]
- Liu, X.; Xu, S.; Wang, M.; Wang, L.; Liu, J. Effect of Mixed Fermentation with Pichia Fermentans, Hanseniaspora Uvarum, and Wickeramomyces Anomala on the Quality of Fig (Ficus carica L.) Wines. J. Food Process. Preserv. 2021, 45, e15169. [Google Scholar] [CrossRef]
- Yan, Y. Mixed Fermentation of Blueberry Pomace with L. Rhamnosus GG and L. Plantarum-1_Enhance the Active Ingredient, Antioxidant Activity and Health-Promoting Benefits. Food Chem. Toxicol. 2019, 131, 110541. [Google Scholar] [CrossRef]
- Goldberg, E.M.; Wang, K.; Goldberg, J.; Aliani, M. Factors Affecting the Ortho- and Retronasal Perception of Flavors: A Review. Crit. Rev. Food Sci. Nutr. 2018, 58, 913–923. [Google Scholar] [CrossRef] [PubMed]
- Günther-Jordanland, K.; Dawid, C.; Hofmann, T. Quantitation and Taste Contribution of Sensory Active Molecules in Oat (Avena sativa L.). J. Agric. Food Chem. 2020, 68, 10097–10108. [Google Scholar] [CrossRef] [PubMed]
- Milardi, D.; Gazit, E.; Radford, S.E.; Xu, Y.; Gallardo, R.U.; Caflisch, A.; Westermark, G.T.; Westermark, P.; Rosa, C.L.; Ramamoorthy, A. Proteostasis of Islet Amyloid Polypeptide: A Molecular Perspective of Risk Factors and Protective Strategies for Type II Diabetes. Chem. Rev. 2021, 121, 1845–1893. [Google Scholar] [CrossRef] [PubMed]
- Nam, H.; Jung, H.; Karuppasamy, S.; Park, Y.S.; Cho, Y.S.; Lee, J.Y.; Seong, S.-I.; Suh, J.G. Anti-Diabetic Effect of the Soybean Extract Fermented by Bacillus Subtilis MORI in Db/Db Mice. Food Sci. Biotechnol. 2012, 21, 1669–1676. [Google Scholar] [CrossRef]
- Yang, H.J.; Kwon, D.Y.; Kim, M.J.; Kang, S.; Park, S. Meju, Unsalted Soybeans Fermented with Bacillus Subtilis and Aspergilus Oryzae, Potentiates Insulinotropic Actions and Improves Hepatic Insulin Sensitivity in Diabetic Rats. Nutr. Metab. 2012, 9, 37. [Google Scholar] [CrossRef]
- Kwon, D.Y.; Jang, J.S.; Hong, S.M.; Lee, J.E.; Sung, S.R.; Park, H.R.; Park, S. Long-Term Consumption of Fermented Soybean-Derived Chungkookjang Enhances Insulinotropic Action Unlike Soybeans in 90% Pancreatectomized Diabetic Rats. Eur. J. Nutr. 2007, 46, 44–52. [Google Scholar] [CrossRef]
- Isas, A.S.; Escobar, F.; Álvarez-Villamil, E.; Molina, V.; Mateos, R.; Lizarraga, E.; Mozzi, F.; Van Nieuwenhove, C. Fermentation of Pomegranate Juice by Lactic Acid Bacteria and Its Biological Effect on Mice Fed a High-Fat Diet. Food Biosci. 2023, 53, 102516. [Google Scholar] [CrossRef]
- Yeung, A.W.K.; Tzvetkov, N.T.; Durazzo, A.; Lucarini, M.; Souto, E.B.; Santini, A.; Gan, R.-Y.; Jozwik, A.; Grzybek, W.; Horbańczuk, J.O.; et al. Natural Products in Diabetes Research: Quantitative Literature Analysis. Nat. Prod. Res. 2021, 35, 5813–5827. [Google Scholar] [CrossRef]
- Díaz-López, A.; Bulló, M.; Martínez-González, M.A.; Corella, D.; Estruch, R.; Fitó, M.; Gómez-Gracia, E.; Fiol, M.; García de la Corte, F.J.; Ros, E.; et al. Dairy Product Consumption and Risk of Type 2 Diabetes in an Elderly Spanish Mediterranean Population at High Cardiovascular Risk. Eur. J. Nutr. 2016, 55, 349–360. [Google Scholar] [CrossRef]
- Alihosseini, N.; Moahboob, S.A.; Farrin, N.; Mobasseri, M.; Taghizadeh, A.; Ostadrahimi, A.R. Effect of probiotic fermented milk (kefir) on serum level of insulin and homocysteine in type 2 diabetes patients. Acta Endocrinol. 2017, 13, 431–436. [Google Scholar] [CrossRef] [PubMed]
- El-Bashiti, T.A.; Zabut, B.M.; Safia, F.F.A. Effect of Probiotic Fermented Milk (Kefir) on Some Blood Biochemical Parameters Among Newly Diagnosed Type 2 Diabetic Adult Males in Gaza Governorate. Curr. Res. Nutr. Food Sci. J. 2019, 7, 568–575. [Google Scholar] [CrossRef]
- Hove, K.D.; Brøns, C.; Færch, K.; Lund, S.S.; Rossing, P.; Vaag, A. Effects of 12 Weeks of Treatment with Fermented Milk on Blood Pressure, Glucose Metabolism and Markers of Cardiovascular Risk in Patients with Type 2 Diabetes: A Randomised Double-Blind Placebo-Controlled Study. Eur. J. Endocrinol. 2015, 172, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Ostadrahimi, A.; Taghizadeh, A.; Mobasseri, M.; Farrin, N.; Payahoo, L.; Beyramalipoor Gheshlaghi, Z.; Vahedjabbari, M. Effect of Probiotic Fermented Milk (Kefir) on Glycemic Control and Lipid Profile in Type 2 Diabetic Patients: A Randomized Double-Blind Placebo-Controlled Clinical Trial. Iran. J. Public Health 2015, 44, 228–237. [Google Scholar]
- Kannel, W.B.; Dawber, T.R.; Kagan, A.; Revotskie, N.; Stokes, J. Factors of Risk in the Development of Coronary Heart Disease—Six-Year Follow-up Experience. Ann. Intern. Med. 1961, 55, 33–50. [Google Scholar] [CrossRef]
- Jeon, Y.B.; Lee, J.-J.; Chang, H.C. Characterization of Juice Fermented with Lactobacillus Plantarum EM and Its Cholesterol-lowering Effects on Rats Fed a High-fat and High-cholesterol Diet. Food Sci. Nutr. 2019, 7, 3622–3634. [Google Scholar] [CrossRef]
- Lim, J.-H.; Jung, E.-S.; Choi, E.-K.; Jeong, D.-Y.; Jo, S.-W.; Jin, J.-H.; Lee, J.-M.; Park, B.-H.; Chae, S.-W. Supplementation with Aspergillus Oryzae-Fermented Kochujang Lowers Serum Cholesterol in Subjects with Hyperlipidemia. Clin. Nutr. 2015, 34, 383–387. [Google Scholar] [CrossRef]
- Štšepetova, J.; Rätsep, M.; Gerulis, O.; Jõesaar, A.; Mikelsaar, M.; Songisepp, E. Impact of Lactiplantibacillus plantarum Inducia on Metabolic and Antioxidative Response in Cholesterol and BMI Variable Indices: Randomised, Double-Blind, Placebo-Controlled Trials. Benef. Microbes 2022, 14, 1–16. [Google Scholar] [CrossRef]
- Bendich, A.; Olson, J.A. Biological Actions of Carotenoids. FASEB J. 1989, 3, 1927–1932. [Google Scholar] [CrossRef]
- D’Odorico, A.; Martines, D.; Kiechl, S.; Egger, G.; Oberhollenzer, F.; Bonvicini, P.; Sturniolo, G.C.; Naccarato, R.; Willeit, J. High Plasma Levels of α- and β-Carotene Are Associated with a Lower Risk of Atherosclerosis: Results from the Bruneck Study. Atherosclerosis 2000, 153, 231–239. [Google Scholar] [CrossRef]
- Piermarocchi, S.; Saviano, S.; Parisi, V.; Tedeschi, M.; Panozzo, G.; Scarpa, G.; Boschi, G.; Lo Giudice, G. Carotenoids in Age-Related Maculopathy Italian Study (CARMIS): Two-Year Results of a Randomized Study. Eur. J. Ophthalmol. 2011, 22, 216–225. [Google Scholar]
- Hozawa, A.; Jacobs, D.R.; Steffes, M.W.; Gross, M.D.; Steffen, L.M.; Lee, D.-H. Circulating Carotenoid Concentrations and Incident Hypertension: The Coronary Artery Risk Development in Young Adults (CARDIA) Study. J. Hypertens. 2009, 27, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Frias, J.; Miranda, M.L.; Doblado, R.; Vidal-Valverde, C. Effect of Germination and Fermentation on the Antioxidant Vitamin Content and Antioxidant Capacity of Lupinus Albus L. Var. Multolupa. Food Chem. 2005, 92, 211–220. [Google Scholar] [CrossRef]
- Hunaefi, D.; Akumo, D.N.; Smetanska, I. Effect of Fermentation on Antioxidant Properties of Red Cabbages. Food Biotechnol. 2013, 27, 66–85. [Google Scholar] [CrossRef]
- Vento, M.; Della Croce, C.M.; Bellani, L.; Tassi, E.L.; Echeverria, M.C.; Giorgetti, L. Effect of Sprouting, Fermentation and Cooking on Antioxidant Content and Total Antioxidant Activity in Quinoa and Amaranth. Int. J. Mol. Sci. 2024, 25, 10972. [Google Scholar] [CrossRef]
- Verni, M.; Verardo, V.; Rizzello, C.G. How Fermentation Affects the Antioxidant Properties of Cereals and Legumes. Foods 2019, 8, 362. [Google Scholar] [CrossRef]
- Carciochi, R.A.; Galván-D’Alessandro, L.; Vandendriessche, P.; Chollet, S. Effect of Germination and Fermentation Process on the Antioxidant Compounds of Quinoa Seeds. Plant Foods Hum. Nutr. 2016, 71, 361–367. [Google Scholar] [CrossRef]
- Morifuji, M.; Ichikawa, S.; Kitade, M.; Fukasawa, T.; Asami, Y.; Manabe, Y.; Sugawara, T. Exopolysaccharides from Milk Fermented by Lactic Acid Bacteria Enhance Dietary Carotenoid Bioavailability in Humans in a Randomized Crossover Trial and in Rats. Am. J. Clin. Nutr. 2020, 111, 903–914. [Google Scholar] [CrossRef]
- Li, S.; Ma, C.; Gong, G.; Liu, Z.; Chang, C.; Xu, Z. The Impact of Onion Juice on Milk Fermentation by Lactobacillus Acidophilus. LWT 2016, 65, 543–548. [Google Scholar] [CrossRef]
- Xiao, Y.; Xing, G.; Rui, X.; Li, W.; Chen, X.; Jiang, M.; Dong, M. Enhancement of the Antioxidant Capacity of Chickpeas by Solid State Fermentation with Cordyceps Militaris SN-18. J. Funct. Foods 2014, 10, 210–222. [Google Scholar] [CrossRef]
- Ooi, T.S.; Ting, A.S.Y.; Siow, L.F. Influence of Selected Native Yeast Starter Cultures on the Antioxidant Activities, Fermentation Index and Total Soluble Solids of Malaysia Cocoa Beans: A Simulation Study. LWT 2020, 122, 108977. [Google Scholar] [CrossRef]
- Reyes, A.; Haynes, M.; Hanson, N.; Angly, F.E.; Heath, A.C.; Rohwer, F.; Gordon, J.I. Viruses in the Faecal Microbiota of Monozygotic Twins and Their Mothers. Nature 2010, 466, 334–338. [Google Scholar] [CrossRef] [PubMed]
- Lozupone, C.A.; Stombaugh, J.I.; Gordon, J.I.; Jansson, J.K.; Knight, R. Diversity, Stability and Resilience of the Human Gut Microbiota. Nature 2012, 489, 220–230. [Google Scholar] [CrossRef] [PubMed]
- Gou, H.-Z.; Zhang, Y.-L.; Ren, L.-F.; Li, Z.-J.; Zhang, L. How Do Intestinal Probiotics Restore the Intestinal Barrier? Front. Microbiol. 2022, 13, 929346. [Google Scholar] [CrossRef]
- Ohland, C.L.; MacNaughton, W.K. Probiotic Bacteria and Intestinal Epithelial Barrier Function. Am. J. Physiol.-Gastrointest. Liver Physiol. 2010, 298, G807–G819. [Google Scholar] [CrossRef]
- Corr, S.C.; Hill, C.; Gahan, C.G.M. Chapter 1 Understanding the Mechanisms by Which Probiotics Inhibit Gastrointestinal Pathogens. Adv. Food Nutr. Res. 2009, 56, 1–15. [Google Scholar]
- Hynönen, U.; Palva, A. Lactobacillus Surface Layer Proteins: Structure, Function and Applications. Appl. Microbiol. Biotechnol. 2013, 97, 5225–5243. [Google Scholar] [CrossRef]
- Fukushima, Y.; Miyaguchi, S.; Yamano, T.; Kaburagi, T.; Iino, H.; Ushida, K.; Sato, K. Improvement of Nutritional Status and Incidence of Infection in Hospitalised, Enterally Fed Elderly by Feeding of Fermented Milk Containing Probiotic Lactobacillus Johnsonii La1 (NCC533). Br. J. Nutr. 2007, 98, 969–977. [Google Scholar] [CrossRef]
- Makino, S.; Ikegami, S.; Kume, A.; Horiuchi, H.; Sasaki, H.; Orii, N. Reducing the Risk of Infection in the Elderly by Dietary Intake of Yoghurt Fermented with Lactobacillus Delbrueckii Ssp. Bulgaricus OLL1073R-1. Br. J. Nutr. 2010, 104, 998–1006. [Google Scholar] [CrossRef]
- Taylor, B.C.; Lejzerowicz, F.; Poirel, M.; Shaffer, J.P.; Jiang, L.; Aksenov, A.; Litwin, N.; Humphrey, G.; Martino, C.; Miller-Montgomery, S.; et al. Consumption of Fermented Foods Is Associated with Systematic Differences in the Gut Microbiome and Metabolome. mSystems 2020, 5, e00901–e00919. [Google Scholar] [CrossRef]
- Wastyk, H.C.; Fragiadakis, G.K.; Perelman, D.; Dahan, D.; Merrill, B.D.; Yu, F.B.; Topf, M.; Gonzalez, C.G.; Van Treuren, W.; Han, S.; et al. Gut-Microbiota-Targeted Diets Modulate Human Immune Status. Cell 2021, 184, 4137–4153. [Google Scholar] [CrossRef] [PubMed]
- Hervert-Hernández, D.; and Goñi, I. Dietary Polyphenols and Human Gut Microbiota: A Review. Food Rev. Int. 2011, 27, 154–169. [Google Scholar] [CrossRef]
- Lee, H.C.; Jenner, A.M.; Low, C.S.; Lee, Y.K. Effect of Tea Phenolics and Their Aromatic Fecal Bacterial Metabolites on Intestinal Microbiota. Res. Microbiol. 2006, 157, 876–884. [Google Scholar] [CrossRef]
- Duda-Chodak, A.; Tarko, T.; Satora, P.; Sroka, P. Interaction of Dietary Compounds, Especially Polyphenols, with the Intestinal Microbiota: A Review. Eur. J. Nutr. 2015, 54, 325–341. [Google Scholar] [CrossRef]
- Manach, C.; Williamson, G.; Morand, C.; Scalbert, A.; Rémésy, C. Bioavailability and Bioefficacy of Polyphenols in Humans. I. Review of 97 Bioavailability Studies2. Am. J. Clin. Nutr. 2005, 81, 230S–242S. [Google Scholar] [CrossRef]
- Hur, S.J.; Lee, S.Y.; Kim, Y.-C.; Choi, I.; Kim, G.-B. Effect of Fermentation on the Antioxidant Activity in Plant-Based Foods. Food Chem. 2014, 160, 346–356. [Google Scholar] [CrossRef]
- Rodríguez, H.; Curiel, J.A.; Landete, J.M.; de las Rivas, B.; de Felipe, F.L.; Gómez-Cordovés, C.; Mancheño, J.M.; Muñoz, R. Food Phenolics and Lactic Acid Bacteria. Int. J. Food Microbiol. 2009, 132, 79–90. [Google Scholar] [CrossRef]
- Gan, R.-Y.; Shah, N.P.; Wang, M.-F.; Lui, W.-Y.; Corke, H. Fermentation Alters Antioxidant Capacity and Polyphenol Distribution in Selected Edible Legumes. Int. J. Food Sci. Tech. 2016, 51, 875–884. [Google Scholar] [CrossRef]
- Marques, F.Z.; Nelson, E.; Chu, P.-Y.; Horlock, D.; Fiedler, A.; Ziemann, M.; Tan, J.K.; Kuruppu, S.; Rajapakse, N.W.; El-Osta, A.; et al. High-Fiber Diet and Acetate Supplementation Change the Gut Microbiota and Prevent the Development of Hypertension and Heart Failure in Hypertensive Mice. Circulation 2017, 135, 964–977. [Google Scholar] [CrossRef]
- Korcz, E.; Kerényi, Z.; Varga, L. Dietary Fibers, Prebiotics, and Exopolysaccharides Produced by Lactic Acid Bacteria: Potential Health Benefits with Special Regard to Cholesterol-Lowering Effects. Food Funct. 2018, 9, 3057–3068. [Google Scholar] [CrossRef]
- Kim, M.H.; Kang, S.G.; Park, J.H.; Yanagisawa, M.; Kim, C.H. Short-Chain Fatty Acids Activate GPR41 and GPR43 on Intestinal Epithelial Cells to Promote Inflammatory Responses in Mice. Gastroenterology 2013, 145, 396–406. [Google Scholar] [CrossRef] [PubMed]
- Kabat, A.M.; Srinivasan, N.; Maloy, K.J. Modulation of Immune Development and Function by Intestinal Microbiota. Trends Immunol. 2014, 35, 507–517. [Google Scholar] [CrossRef] [PubMed]
- Woo, J.K.; Choi, S.; Kang, J.-H.; Kim, D.E.; Hurh, B.-S.; Jeon, J.-E.; Kim, S.Y.; Oh, S.H. Fermented Barley and Soybean (BS) Mixture Enhances Intestinal Barrier Function in Dextran Sulfate Sodium (DSS)-Induced Colitis Mouse Model. BMC Complement. Altern. Med. 2016, 16, 498. [Google Scholar] [CrossRef] [PubMed]
- Cuervo, M.P.; Castillo, A.; Santiago-Connolly, L.M. Overview of Biological Hazards and Foodborne Diseases. In Encyclopedia of Food Safety, 2nd ed.; Smithers, G.W., Ed.; Academic Press: Oxford, UK, 2024; pp. 1–18. [Google Scholar]
- Barbosa, J.; Albano, H.; Silva, C.P.; Teixeira, P. Microbiological Contamination of Reusable Plastic Bags for Food Transportation. Food Control 2019, 99, 158–163. [Google Scholar] [CrossRef]
- Cornes, M.P. Exogenous Sample Contamination. Sources and Interference. Clin. Biochem. 2016, 49, 1340–1345. [Google Scholar] [CrossRef]
- Yao, Y.; Zhong, X.; Zhou, Y.; Zhang, H.; Zhao, D.; Zhang, W.; Liu, Y.; Xu, J.; Xie, C.; Yu, C.; et al. Exploring the Characteristics of Burkholderia Gladioli Pathovar Cocovenenans: Growth, Bongkrekic Acid Production, and Potential Risks of Food Contamination in Wet Rice Noodles and Vermicelli. Food Microbiol. 2024, 120, 104449. [Google Scholar] [CrossRef]
- Adebiyi, J.A.; Kayitesi, E.; Adebo, O.A.; Changwa, R.; Njobeh, P.B. Food Fermentation and Mycotoxin Detoxification: An African Perspective. Food Control 2019, 106, 106731. [Google Scholar] [CrossRef]
- Liu, L.; Xie, M.; Wei, D. Biological Detoxification of Mycotoxins: Current Status and Future Advances. Int. J. Mol. Sci. 2022, 23, 1064. [Google Scholar] [CrossRef]
- Oguntoyinbo, F.A. Development of Hazard Analysis Critical Control Points (HACCP) and Enhancement of Microbial Safety Quality during Production of Fermented Legume Based Condiments in Nigeria. Niger. Food J. 2012, 30, 59–66. [Google Scholar] [CrossRef]
- Radu, E.; Dima, A.; Dobrota, E.M.; Badea, A.-M.; Madsen, D.Ø.; Dobrin, C.; Stanciu, S. Global Trends and Research Hotspots on HACCP and Modern Quality Management Systems in the Food Industry. Heliyon 2023, 9, e18232. [Google Scholar] [CrossRef]
- Suzzi, G.; Gardini, F. Biogenic Amines in Dry Fermented Sausages: A Review. Int. J. Food Microbiol. 2003, 88, 41–54. [Google Scholar] [CrossRef] [PubMed]
- Gardini, F.; Özogul, Y.; Suzzi, G.; Tabanelli, G.; Özogul, F. Technological Factors Affecting Biogenic Amine Content in Foods: A Review. Front. Microbiol. 2016, 7, 1218. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Capillas, C.; Jiménez-Colmenero, F. Biogenic Amines in Meat and Meat Products. Crit. Rev. Food Sci. Nutr. 2004, 44, 489–599. [Google Scholar] [CrossRef]
- Feddern, V.; Mazzuco, H.; Fonseca, F.N.; de Lima, G.J.M.M. A Review on Biogenic Amines in Food and Feed: Toxicological Aspects, Impact on Health and Control Measures. Anim. Prod. Sci. 2019, 59, 608–618. [Google Scholar] [CrossRef]
- Li, Y.; Xiong, D.; Yuan, L.; Fan, P.; Xiao, Y.; Chen, J.; Feng, W. Transcriptome and Protein Networks to Elucidate the Mechanism Underlying Nitrite Degradation by Lactiplantibacillus Plantarum. Food Res. Int. 2022, 156, 111319. [Google Scholar] [CrossRef]
- Wang, X.; Ren, H.; Wang, W.; Xie, Z.J. Effects of a Starter Culture on Histamine Reduction, Nitrite Depletion and Oxidative Stability of Fermented Sausages. J. Food Saf. 2016, 36, 195–202. [Google Scholar] [CrossRef]
- Kononiuk, A.D.; Karwowska, M. Comparison of Selected Parameters Related to Food Safety of Fallow Deer and Beef Uncured Fermented Sausages with Freeze-Dried Acid Whey Addition. Meat Sci. 2020, 161, 108015. [Google Scholar] [CrossRef]
- Bondonno, C.P.; Dalgaard, F.; Blekkenhorst, L.C.; Murray, K.; Lewis, J.R.; Croft, K.D.; Kyrø, C.; Torp-Pedersen, C.; Gislason, G.; Tjønneland, A.; et al. Vegetable Nitrate Intake, Blood Pressure and Incident Cardiovascular Disease: Danish Diet, Cancer, and Health Study. Eur. J. Epidemiol. 2021, 36, 813–825. [Google Scholar] [CrossRef]
- EFSA Panel on Biological Hazards (BIOHAZ). Scientific Opinion on Risk Based Control of Biogenic Amine Formation in Fermented Foods. EFSA J. 2011, 9, 2393. [Google Scholar] [CrossRef]
- Abuhlega, T.A.; Ali, M.R. Biogenic Amines in Fish: Prevention and Reduction. J. Food Process. Preserv. 2022, 46, e16883. [Google Scholar] [CrossRef]
- Kim, S.-H.; Kang, K.H.; Kim, S.H.; Lee, S.; Lee, S.-H.; Ha, E.-S.; Sung, N.-J.; Kim, J.G.; Chung, M.J. Lactic Acid Bacteria Directly Degrade N-Nitrosodimethylamine and Increase the Nitrite-Scavenging Ability in Kimchi. Food Control 2017, 71, 101–109. [Google Scholar] [CrossRef]
- Venegas-Ortega, M.G.; Flores-Gallegos, A.C.; Martínez-Hernández, J.L.; Aguilar, C.N.; Nevárez-Moorillón, G.V. Production of Bioactive Peptides from Lactic Acid Bacteria: A Sustainable Approach for Healthier Foods. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1039–1051. [Google Scholar] [CrossRef] [PubMed]
- Doeun, D.; Davaatseren, M.; Chung, M.-S. Biogenic Amines in Foods. Food Sci. Biotechnol. 2017, 26, 1463–1474. [Google Scholar] [CrossRef] [PubMed]
- Thakur, S.; Kumar, P.; Gupta, N. Exploring Regional Influences on Bioactive Components in Tea Leaves and Their Effect on Sensory Quality. J. Food Compos. Anal. 2025, 144, 107683. [Google Scholar] [CrossRef]
- Ye, J.-H.; Ye, Y.; Yin, J.-F.; Jin, J.; Liang, Y.-R.; Liu, R.-Y.; Tang, P.; Xu, Y.-Q. Bitterness and Astringency of Tea Leaves and Products: Formation Mechanism and Reducing Strategies. Trends Food Sci. Technol. 2022, 123, 130–143. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).