Research Progress on Nutritional Properties of Noni (Morinda citrifolia L.) Fruit and Its Fermented Foods
Abstract
1. Introduction
2. Noni Fruit Nutritional Composition
2.1. Polysaccharides
2.2. Polyphenols
2.3. Iridoids
2.4. Anthraquinones
2.5. Alkaloids
2.6. Other Nutrients
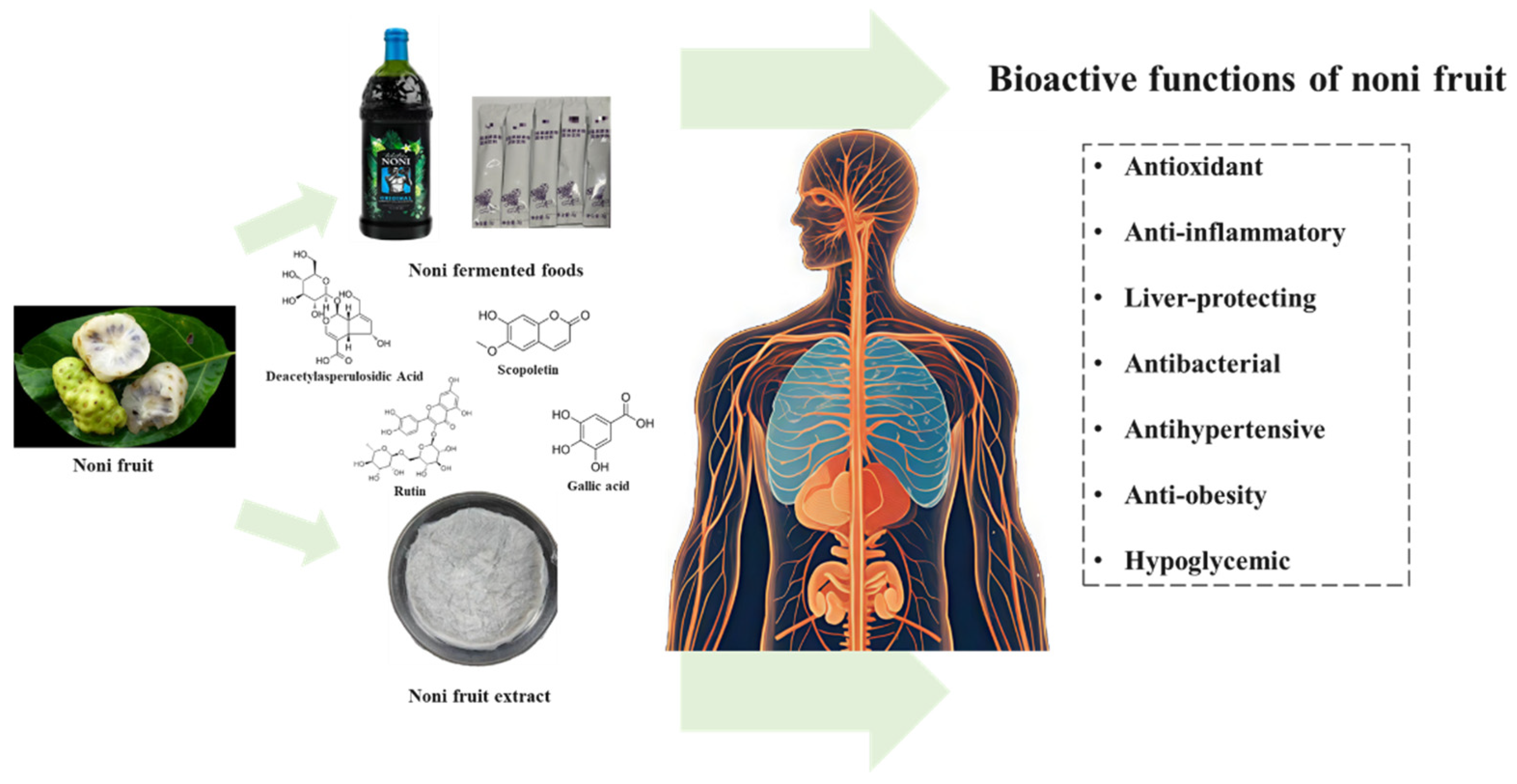
3. Health Benefits of Noni Fruit
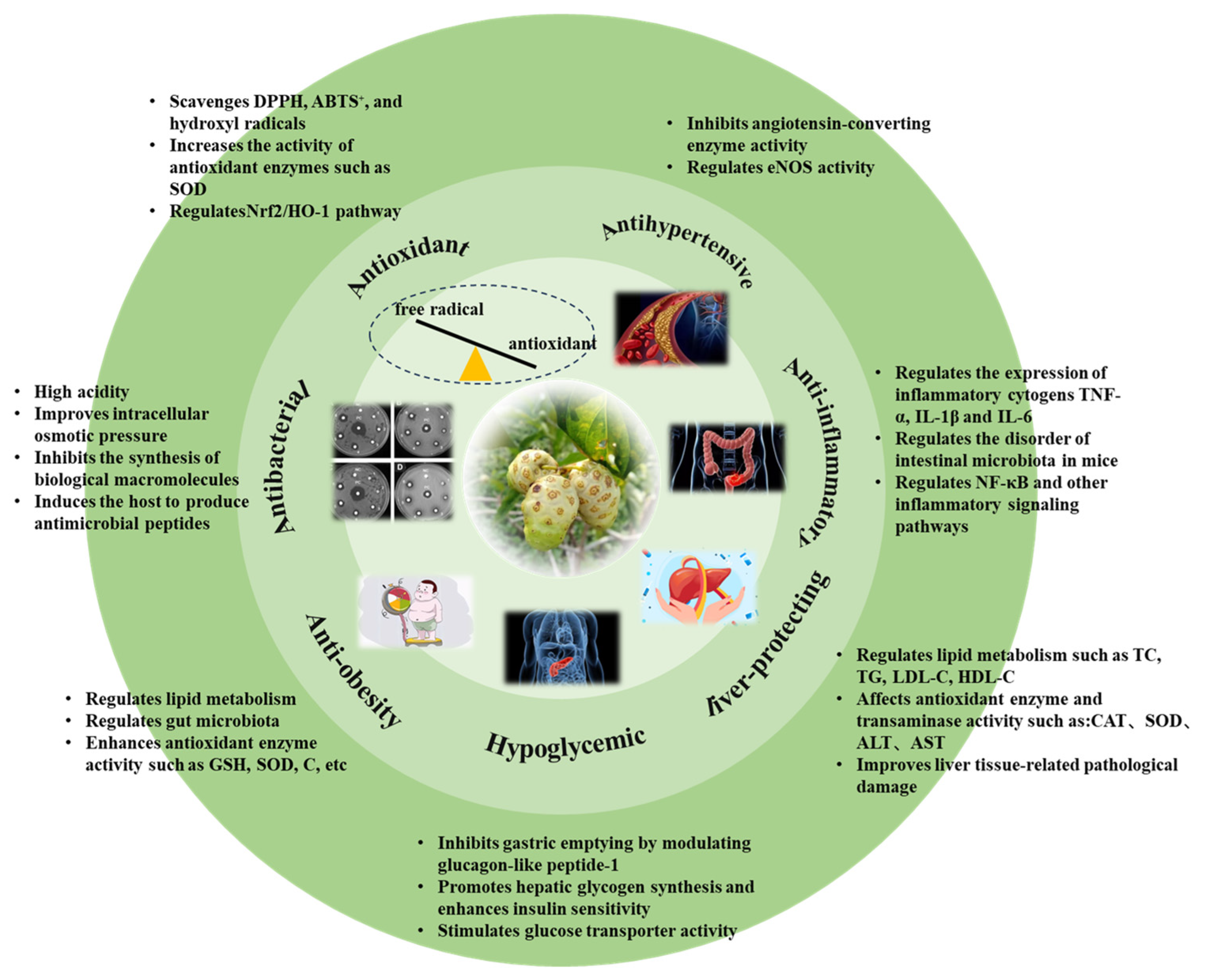
3.1. Antioxidants
3.2. Anti-Inflammatory Effects
3.3. Liver-Protecting Effects
3.4. Antibacterial Effects
3.5. Antihypertensive Effects
3.6. Anti-Obesity Effects
3.7. Hypoglycemic Effects
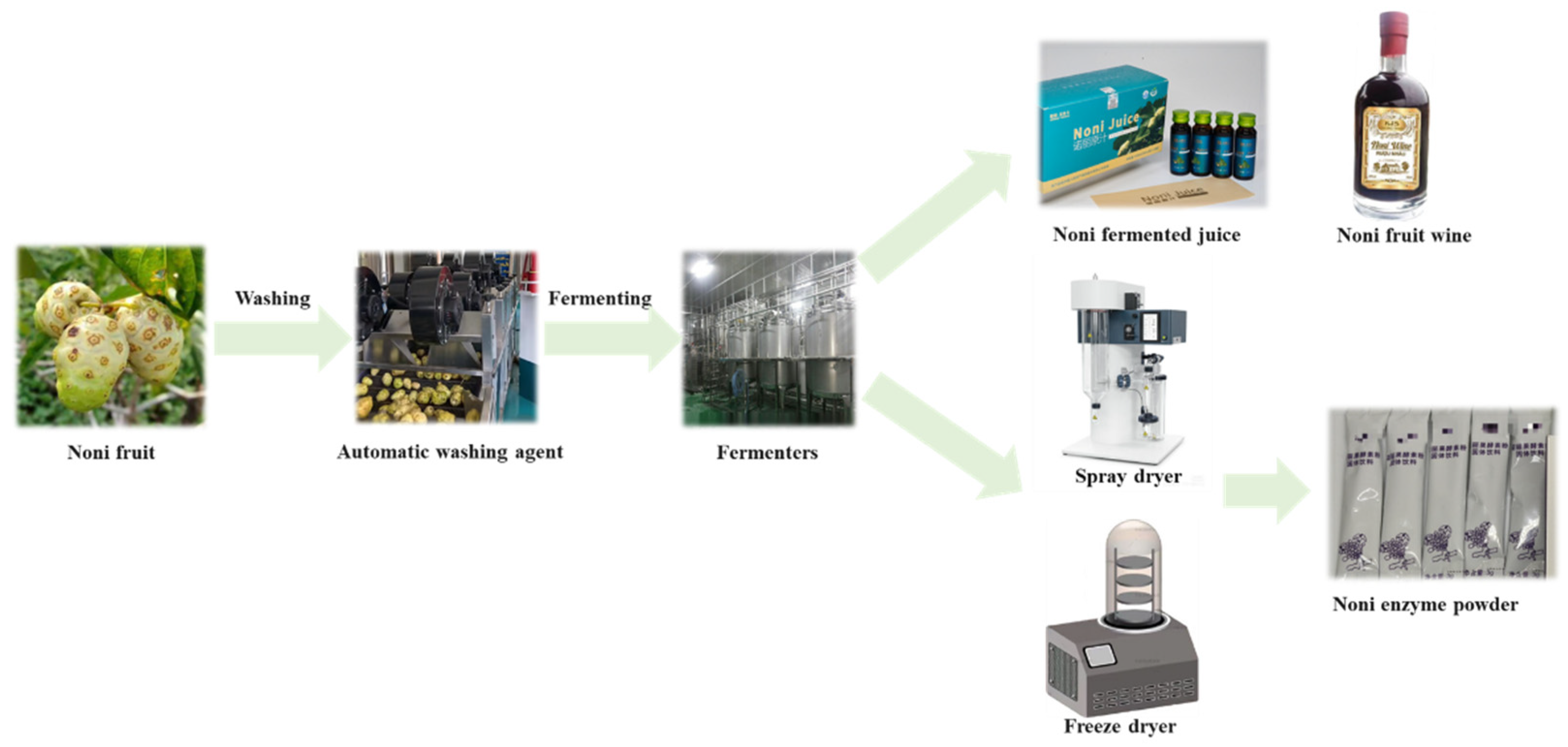
4. Fermented Noni Fruit Foods
4.1. Types and Production Processes of Fermented Noni Fruit Foods
4.2. The Effects of Fermenting Strains on the Quality and Metabolites of Fermented Noni Juice
4.3. The Effects of Fermenting Strains on the Main Flavors of Fermented Noni Juice
5. Summary and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, X.; Lin, X.; Hu, X.; Li, C.; Wang, L.; Fei, T. Noni (Morinda citrifolia) fruit and by-products: A comprehensive review of its chemical compositions, health-promoting effects, safety assessment and industrial applications. Trends Food Sci. Technol. 2024, 153, 104690. [Google Scholar] [CrossRef]
- Zhang, R.; Hong, Q.; Li, D.; Zhou, L.; Wang, X.; Wang, R.; Liu, S.; Zhang, L.; Li, C. Postharvest ripening improves the texture and active ingredients of noni fruit (Morinda citrifolia L.) for processing. Postharvest Biol. Technol. 2024, 217, 113089. [Google Scholar] [CrossRef]
- Guo, M.; Mao, B.; Ahmed Sadiq, F.; Hao, Y.; Cui, S.; Yi, M.; Hong, Q.; Lee, Y.; Zhao, J. Effects of noni fruit and fermented noni juice against acute alcohol induced liver injury in mice. J. Funct. Foods 2020, 70, 103995. [Google Scholar] [CrossRef]
- Sousa, S.G.; Oliveira, L.A.; de Aguiar Magalhães, D.; de Brito, T.; Batista, J.; Pereira, C.; de Souza Costa, M.; Mazulo, J.; de Carvalho Filgueiras, M.; Vasconselos, D.; et al. Chemical structure and anti-inflammatory effect of polysaccharide extracted from Morinda citrifolia Linn (Noni). Carbohydr. Polym. 2018, 197, 515–523. [Google Scholar] [CrossRef]
- Kim, H.; Rahmawati, L.; Hong, Y.H.; Choi, S.; Cho, J. NK cell-mediated immunostimulatory effects of ethanol extract of Morinda citrifolia (noni) fruit. BMC Complement. Med. 2022, 22, 222. [Google Scholar] [CrossRef]
- Mo, W.; Zou, J.; Wu, M.; Peng, Z.; He, W.; Li, W.; Wu, X. Noni (Morinda citrifolia L.) fruit polysaccharide ameliorated high-fat diet-induced obesity by modulating gut microbiota and improving bile acid metabolism. J. Funct. Foods 2023, 101, 105408. [Google Scholar] [CrossRef]
- Zhang, B.; Yu, Z.; Zhou, X.; Nong, X.; Li, X.; Wang, H.; Wang, H.; Chen, G. New alkaloids from the noni juice with potential α-glucosidase inhibitory activity. Fitoterapia 2021, 153, 104946. [Google Scholar] [CrossRef]
- Meng, Y.; Zhang, B.; Zhang, D.; Yu, Z.; Long, C.; Li, S.; Zhang, X.; Li, X.; Chen, G. Chemical constituents from noni (Morinda citrifolia L.) fruit juice and their chemotaxonomic significance. Biochem. Syst. Ecol. 2024, 117, 104910. [Google Scholar] [CrossRef]
- Jin, M.; Wang, Y.; Yang, X.; Yin, H.; Nie, S.; Wu, X. Structure characterization of a polysaccharide extracted from noni (Morinda citrifolia L.) and its protective effect against DSS-induced bowel disease in mice. Food Hydrocoll. 2019, 90, 189–197. [Google Scholar] [CrossRef]
- Yang, X.; Mo, W.; Zheng, C.; Li, W.; Tang, J.; Wu, X. Alleviating effects of noni fruit polysaccharide on hepatic oxidative stress and inflammation in rats under a high-fat diet and its possible mechanisms. Food Funct. 2020, 11, 2953–2968. [Google Scholar] [CrossRef]
- Chang, Z.; Gu, C.; Wang, M.; Chen, J.; Zhou, J.; Yue, M.; Zhang, C.; Liu, F.; Feng, Z. Structural characterization of noni (Morinda citrifolia L.) pectin and its inhibitory activity on pancreatic lipase. Int. J. Biol. Macromol. 2024, 283, 137521. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Liang, Z.; Li, J.; Manzoor, M.; Liu, H.; Han, Z.; Zeng, X. Variation in physicochemical properties and bioactivities of Morinda citrifolia L. (Noni) polysaccharides at different stages of maturity. Front Nutr. 2023, 9, 1094906. [Google Scholar] [CrossRef]
- Chen, X.; Gu, C.; Zhu, K.; Xu, F.; Feng, Z.; Zhang, Y. Insight into the effects of different ripeness levels on the quality and flavor chemistry of Noni fruit (Morinda citrifolia L.). Food Chem. 2024, 434, 137408. [Google Scholar] [CrossRef]
- Zuo, L.; Lu, Y.; Jiang, S.; Yan, Y.; Tong, Y.; Chen, S.; Wang, P. Study on monosaccharide compositions analysis and antioxidant activity in vitro of polysaccharides from Noni. Sci. Technol. Food Ind. 2017, 38, 56–60. (In Chinese) [Google Scholar] [CrossRef]
- Muñoz-Almagro, N.; Wilde, P.J.; Montilla, A.; Villamiel, M. Development of low-calorie gels from sunflower pectin extracted by the assistance of ultrasound. LWT Food Sci. Technol. 2025, 222, 117609. [Google Scholar] [CrossRef]
- El Khayat Driaa, Y.; Maarir, H.; Mennani, M.; Grimi, N.; Moubarik, A.; Boussetta, N. Ultrasound, pulsed electric fields, and high-voltage electrical discharges assisted extraction of cellulose and lignin from walnut shells. Int. J. Biol. Macromol. 2025, 292, 139319. [Google Scholar] [CrossRef] [PubMed]
- Lal, A.M.N.; Prince, M.V.; Kothakota, A.; Pandiselvam, R.; Thirumdas, R.; Mahanti, N.; Sreeja, R. Pulsed electric field combined with microwave-assisted extraction of pectin polysaccharide from jackfruit waste. Innov. Food Sci. Emerg. Technol. 2021, 74, 102844. [Google Scholar] [CrossRef]
- Li, J.; Niu, D.; Zhang, Y.; Zeng, X. Physicochemical properties, antioxidant and antiproliferative activities of polysaccharides from Morinda citrifolia L. (Noni) based on different extraction methods. Int. J. Biol. Macromol. 2020, 150, 114–121. [Google Scholar] [CrossRef]
- Cui, Y.; Wang, R.; Cao, S.; Ismael, M.; Wang, X.; Lü, X. A galacturonic acid-rich polysaccharide from Diospyros kaki peel: Isolation, characterization, rheological properties and antioxidant activities in vitro. Food Chem. 2023, 416, 135781. [Google Scholar] [CrossRef]
- Teng, H.; Chen, L. Polyphenols and bioavailability: An update. Crit. Rev. Food Sci. 2019, 59, 2040–2051. [Google Scholar] [CrossRef]
- Liu, H.; Guo, X.; Jiang, K.; Shi, B.; Liu, L.; Hou, R.; Chen, G.; Farag, M.; Yan, N.; Liu, L. Dietary polyphenols regulate appetite mechanism via gut-brain axis and gut homeostasis. Food Chem. 2024, 446, 138739. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Wang, L.; Zhang, L.; Wan, S.; Li, C.; Liu, S. Solvents effect on phenolics, iridoids, antioxidant activity, antibacterial activity, and pancreatic lipase inhibition activity of noni (Morinda citrifolia L.) fruit extract. Food Chem. 2022, 377, 131989. [Google Scholar] [CrossRef]
- Ozturk, B.; Parkinson, C.; Gonzalez-Miquel, M. Extraction of polyphenolic antioxidants from orange peel waste using deep eutectic solvents. Sep. Purif. Technol. 2018, 206, 1–13. [Google Scholar] [CrossRef]
- Wang, S.; Liu, Z.; Zhao, S.; Zhang, L.; Li, C.; Liu, S. Effect of combined ultrasonic and enzymatic extraction technique on the quality of noni (Morinda citrifolia L.) juice. Ultrason. Sonochem. 2023, 92, 106231. [Google Scholar] [CrossRef] [PubMed]
- Dzah, C.S.; Kpodo, F.M.K.; Asante-Donyinah, D.; Boateng, N. The influence of Morinda citrifolia fruit maturity level, parts and storage length on total phenols, ascorbic acid, antioxidant activity and ethylene gas emission. Food Chem. Adv. 2024, 4, 100599. [Google Scholar] [CrossRef]
- Chen, J.; Chen, X.; Zhang, Y.; Feng, Z.; Zhu, K.; Xu, F.; Gu, C. Bioactivity and influence on colonic microbiota of polyphenols from noni (Morinda citrifolia L.) fruit under simulated gastrointestinal digestion. Food Chem. X 2024, 21, 101076. [Google Scholar] [CrossRef]
- Liu, H.; Jiang, W.; Cao, J.; Li, Y. Changes in Extractable and Non-extractable Polyphenols and Their Antioxidant Properties during Fruit On-tree Ripening in Five Peach Cultivars. Hortic. Plant J. 2019, 5, 137–144. [Google Scholar] [CrossRef]
- Su, C.; Yang, M.; Chen, S.; Fu, C.; Zhang, L.; Liu, S.; Kang, J.; Li, C. Multiple metabolite profiles uncover remarkable bioactive compounds and metabolic characteristics of noni fruit (Morinda citrifolia L.) at various stages of ripeness. Food Chem. 2024, 450, 139357. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Chen, C.; Fang, Y. Evaluation of main nutritional components and antioxidant activities of Morindacitrifolia L. from 2 kinds of different regions of cultivation. J. Food Saf. Qual. 2022, 13, 5902–5909. (In Chinese) [Google Scholar] [CrossRef]
- Masuda, M.; Murata, K.; Fukuhama, A.; Naruto, S.; Fujita, T.; Uwaya, A.; Isami, F.; Matsuda, H. Inhibitory effects of constituents of Morinda citrifolia seeds on elastase and tyrosinase. J. Nat. Med. 2009, 63, 267–273. [Google Scholar] [CrossRef]
- Wei, Q.Z.; Yu, Z.X.; Xu, M.; Xu, W.; Cen, F.; Zhang, Y.; Li, X.; Chen, G. Studies on the Chemical Constituents from the Seeds of Noni and Their Anti-inflammatory Bioactivity. Chin. J. Org. Chem. 2024, 44, 1357–1362. [Google Scholar] [CrossRef]
- Deng, S.; West, B.J.; Jensen, C.J. A quantitative comparison of phytochemical components in global noni fruits and their commercial products. Food Chem. 2010, 122, 267–270. [Google Scholar] [CrossRef]
- Wang, L.; Meng, X.; Zhou, H.; Liu, Y.; Zhang, Y.; Liang, H.; Hou, G.; Kang, W.; Liu, Z. Iridoids and active ones in patrinia: A review. Heliyon 2023, 9, e16518. [Google Scholar] [CrossRef] [PubMed]
- Hao, Z.; Wang, X.; Yang, M.; Cao, B.; Zeng, M.; Zhou, S.; Li, M.; Cao, Y.; Xie, S.; Zheng, X.; et al. Minor iridoid glycosides from the fruits of Cornus officinalis Sieb. et Zucc. and their anti-diabetic bioactivities. Phytochemistry 2023, 205, 113505. [Google Scholar] [CrossRef] [PubMed]
- Ge, J.; Liu, Z.; Zhong, Z.; Wang, L.; Zhuo, X.; Li, J.; Jiang, X.; Ye, X.; Xie, T.; Bai, R. Natural terpenoids with anti-inflammatory activities: Potential leads for anti-inflammatory drug discovery. Bioorganic. Chem. 2022, 124, 105817. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Gu, C.; Wang, M.; Chen, J.; Chang, H.; Chang, Z.; Zhou, J.; Yue, M.; Zhang, W.; Zhang, Q.; et al. Effect of temperature regulation on microbial community, volatile flavours, amino acid profiles, and iridoid glycosides during noni (Morinda citrifolia L.) fruit fermentation. Food Chem. 2025, 462, 140966. [Google Scholar] [CrossRef]
- Deng, S.; West, B.J.; Palu, A.K.; Jensen, C.J. Determination and comparative analysis of major iridoids in different parts and cultivation sources of Morinda citrifolia. Phytochem. Anal. 2011, 22, 26–30. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, L.; Li, X.; Li, J.; Cheng, C. Determination of asperulosidic acid in Noni fresh fruit and juice by HPLC. Food Ferment. Ind. 2014, 40, 205–208. (In Chinese) [Google Scholar] [CrossRef]
- Zhang, C.; Chen, X.; Guo, X.; Guo, R.; Zhu, L.; Qiu, X.; Yu, X.; Chai, J.; Gu, C.; Feng, Z. A novel strategy for improving the antioxidant, iridoid, and flavor properties of Noni (Morinda citrifolia L.) fruit juice by lactic acid bacteria fermentation. LWT 2023, 184, 115075. [Google Scholar] [CrossRef]
- Zhang, C.; Gu, C.; Su, F.; Wang, M.; Chen, J.; Chang, Z.; Zhou, J.; Yue, M.; Liu, F.; Feng, Z. Iridoid glycosides from noni (Morinda citrifolia L.) fruit pomace: A novel booster strategy for its extraction and will its α-glucosidase inhibitory be increased by acetylation? LWT Food Sci. Technol. 2024, 207, 116626. [Google Scholar] [CrossRef]
- Saar-Reismaa, P.; Koel, M.; Tarto, R.; Vaher, M. Extraction of bioactive compounds from Dipsacus fullonum leaves using deep eutectic solvents. J. Chromatogr. A 2022, 1677, 463330. [Google Scholar] [CrossRef] [PubMed]
- Rente, D.; Paiva, A.; Duarte, A.R. The Role of Hydrogen Bond Donor on the Extraction of Phenolic Compounds from Natural Matrices Using Deep Eutectic Systems. Molecules 2021, 26, 2336. [Google Scholar] [CrossRef]
- Lu, S.; Zhang, Z.; Liang, Y.; Zhong, Q.; Li, G.; Hu, Z. Iridoid Glycosides from Phlomis Medicinalis Diels: Optimized Extraction and Hemostasis Evaluation. Chem. Biodivers. 2022, 19, e202100936. [Google Scholar] [CrossRef]
- Yu, L.; Cao, L.; Chang, Y.; Duan, C.; Liu, C.; Zhao, X.; Yue, G.; Wang, X.; Fu, Y. Enhanced extraction performance of iridoids, phenolic acids from Eucommia ulmoides leaves by tailor-made ternary deep eutectic solvent. Microchem. J. 2021, 161, 105788. [Google Scholar] [CrossRef]
- Su, C.; Kang, J.; Liu, S.; Li, C. Exploring the influence of fruit ripeness on the microbiome, bioactive components, and flavor profiles of naturally fermented noni (Morinda citrifolia L.) juice. Food Chem. 2025, 482, 144192. [Google Scholar] [CrossRef]
- Akihisa, T.; Matsumoto, K.; Tokuda, H.; Yasukawa, K.; Seino, K.; Nakamoto, K.; Kuninaga, H.; Suzuki, T.; Kimura, Y. Anti-inflammatory and Potential Cancer Chemopreventive Constituents of the Fruits of Morinda citrifolia (Noni). J. Nat. Prod. 2007, 70, 754–757. [Google Scholar] [CrossRef] [PubMed]
- Su, B.; Pawlus, A.D.; Jung, H.; Keller, W.; McLaughlin, J.; Kinghorn, A.D. Chemical Constituents of the Fruits of Morindac itrifolia (Noni) and Their Antioxidant Activity. J. Nat. Prod. 2005, 68, 592–595. [Google Scholar] [CrossRef]
- Tang, J.; Liu, H.; Zhou, Z.; Liu, J. Iridoids from Morinda citrifolia. Chin. J. Med. Chem. 2009, 19, 379–381. (In Chinese) [Google Scholar]
- Su, G.Y.; Chen, M.L.; Wang, K.W. Natural New Bioactive Anthraquinones from Rubiaceae. Mini-Rev. Org. Chem. 2020, 17, 872–883. [Google Scholar] [CrossRef]
- Okoń, E.; Kukula-Koch, W.; Jarząb, A.; Gaweł-Bęben, K.; Bator, E.; Michalak-Tomczyk, M.; Jachuła, J.; Antosiewicz-Klimczak, B.; Odrzywolski, A.; Koch, W.; et al. The Activity of 1,8-Dihydroanthraquinone Derivatives in Nervous System Cancers. Molecules 2024, 29, 5989. [Google Scholar] [CrossRef]
- Bussmann, R.W.; Hennig, L.; Giannis, A.; Ortwein, J.; Kutchan, T.; Feng, X. Anthraquinone Content in Noni (Morinda citrifolia L.). Evid. -Based Complement. Altern. Med. 2013, 2013, 208378. [Google Scholar] [CrossRef] [PubMed]
- Deng, S.; West, B.J.; Jensen, C.J.; Basar, S.; Westendorf, J. Development and validation of an RP-HPLC method for the analysis of anthraquinones in noni fruits and leaves. Food Chem. 2009, 116, 505–508. [Google Scholar] [CrossRef]
- Nualsanit, T.; Rojanapanthu, P.; Gritsanapan, W.; Lee, S.; Lawson, D.; Baek, S. Damnacanthal, a noni component, exhibits antitumorigenic activity in human colorectal cancer cells. J. Nutr. Biochem. 2012, 23, 915–923. [Google Scholar] [CrossRef] [PubMed]
- Kanokmedhakul, K.; Kanokmedhakul, S.; Phatchana, R. Biological activity of Anthraquinones and Triterpenoids from Prismatomeris fragrans. J. Ethnopharmacol. 2005, 100, 284–288. [Google Scholar] [CrossRef] [PubMed]
- García-Vilas, J.A.; Quesada, A.R.; Medina, M.A. Damnacanthal, a noni anthraquinone, inhibits c-Met and is a potent antitumor compound against Hep G2 human hepatocellular carcinoma cells. Sci. Rep. 2015, 5, 8021. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.F.; Ni, C.L.; Huang, Y.L.; Sheu, S.J.; Chen, C.C. Lignans and anthraquinones from the fruits of Morinda citrifolia. Nat. Prod. Res. 2007, 21, 1199–1204. [Google Scholar] [CrossRef]
- Pawlus, A.D.; Su, B.; Keller, W.J.; Kinghorn, A.D. An Anthraquinone with Potent Quinone Reductase-Inducing Activity and Other Constituents of the Fruits of Morinda citrifolia (Noni). J. Nat. Prod. 2005, 68, 1720–1722. [Google Scholar] [CrossRef]
- Sina, H.; Dramane, G.; Tchekounou, P.; Assogba, M.F.; Chabi-Sika, K.; Boya, B.; Socohou, A.; Adjanohoun, A.; Baba-Moussa, L. Phytochemical composition and in vitro biological activities of Morinda citrifolia fruit juice. Saudi J. Biol. Sci. 2021, 28, 1331–1335. [Google Scholar] [CrossRef]
- Shen, Z.; Guan, R.; Du, M.; Bian, Y.; Wang, Y.; Suo, X.; Xiong, S.; Liu, Y.; Fu, Y. Chemical constituents from fruits of Morinda citrifolia and their inhibitory effects on proliferation of synoviocytes in vitro. China J. Chin. Mater. Medica 2023, 48, 105–113. (In Chinese) [Google Scholar] [CrossRef]
- Almeida, É.S.; de Oliveira, D.; Hotza, D. Properties and Applications of Morinda citrifolia (Noni): A Review. Compr. Rev. Food Sci. Food Saf. 2019, 18, 883–909. [Google Scholar] [CrossRef]
- West, B.J.; Deng, S.; Jensen, C.J. Nutrient and phytochemical analyses of processed noni puree. Food Res. Int. 2011, 44, 2295–2301. [Google Scholar] [CrossRef]
- Vasconcelos, I.P.D.; Silva, R.E.V.; Costa, P.M.C.; Rodrigues, L.J. Nutrition and bioactive potential of the noni fruit cultivated from the Mato Grosso State. Ciência Rural 2021, 51. [Google Scholar] [CrossRef]
- Hernández Ramírez, J.A.; Ulloa, J.A.; Ulloa Rangel, B.E.; Rosas Ulloa, P. Valorization of the Noni (Morinda citrifolia) Seeds as Source of a Protein Concentrate and Its Physicochemical, Functional, and Structural Characterization. Waste Biomass Valorization 2024, 15, 2033–2043. [Google Scholar] [CrossRef]
- Du, S.; Wang, H.; Zhang, B.; Popkin, B.M. Dietary Potassium Intake Remains Low and Sodium Intake Remains High, and Most Sodium is Derived from Home Food Preparation for Chinese Adults, 1991–2015 Trends. J. Nutr. 2020, 150, 1230–1239. [Google Scholar] [CrossRef]
- Peng, Y.; Zhao, D.; Li, M.; Wen, X.; Ni, Y. Production and functional characteristics of low-sodium high-potassium soy protein for the development of healthy soy-based foods. Int. J. Biol. Macromol. 2023, 226, 1332–1340. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.; Chen, M.; Su, C.X.; West, B.J. In Vivo Antioxidant Activity of Deacetylasperulosidic Acid in Noni. J. Anal. Methods Chem. 2013, 2013, 804504. [Google Scholar] [CrossRef]
- Genc, S.; Cicek, B.; Yeni, Y.; Kuzucu, M.; Hacimuftuoglu, A.; Bolat, I.; Yildirim, S.; Zaker, H.; Zachariou, A.; Sofikitis, N.; et al. Morinda citrifolia protective effects on paclitaxel-induced testis parenchyma toxicity: An experimental study. Reprod. Toxicol. 2024, 127, 108611. [Google Scholar] [CrossRef]
- Kwon, S.; Kothari, D.; Jung, H.; Lim, J.; Kim, W.; Kwon, H.; Han, S.; Seo, S.; Choi, Y.; Kim, S. Noni juice-fortified yogurt mitigates dextran sodium sulfate-induced colitis in mice through the modulation of inflammatory cytokines. J. Funct. Foods 2021, 86, 104652. [Google Scholar] [CrossRef]
- Dussossoy, E.; Bichon, F.; Bony, E.; Portet, K.; Brat, P.; Vaillant, F.; Michel, A.; Poucheret, P. Pulmonary anti-inflammatory effects and spasmolytic properties of Costa Rican noni juice (Morinda citrifolia L.). J. Ethnopharmacol. 2016, 192, 264–272. [Google Scholar] [CrossRef]
- Qu, T.; Zhang, J.; Wang, R.; Liu, S.; Ren, F.; Li, C. Fermented Noni Juice Alleviates DSS Colitis in Mice by Mediating Intestinal Microbiota. J. Chin. Inst. Food Sci. Technol. 2022, 22, 118–126. (In Chinese) [Google Scholar] [CrossRef]
- Liu, Y.; Bi, Y.; Yin, Y.; Chen, C.; Meng, X.; Wang, M.; Wang, M.; Chen, X.; Li, Z.; Liu, X.; et al. Protective Effects and Molecular Mechanism of Fermented noni (Morinda citrifolia) Fruit Juice on Hyperuricemia Cell Model. Sci. Technol. Food Ind. 2023, 44, 370–376. [Google Scholar] [CrossRef]
- Wang, R.; Wang, L.; Wu, H.; Zhang, L.; Hu, X.; Li, C.; Liu, S. Noni (Morinda citrifolia L.) fruit phenolic extract supplementation ameliorates NAFLD by modulating insulin resistance, oxidative stress, inflammation, liver metabolism and gut microbiota. Food Res. Int. 2022, 160, 111732. [Google Scholar] [CrossRef]
- Lin, Y.; Lin, H.; Chen, Y.; Yang, D.; Li, C.; Chang, Y. Hepatoprotective effects of naturally fermented noni juice against thioacetamide-induced liver fibrosis in rats. J. Chin. Med. Assoc. 2017, 80, 212–221. [Google Scholar] [CrossRef]
- Lin, Y.; Chang, Y.; Yang, D.; Tzang, B.; Chen, Y. Beneficial effects of noni (Morinda citrifolia L.) juice on livers of high-fat dietary hamsters. Food Chem. 2013, 140, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Tailulu, A.; Li, M.; Ye, B.; Al-qudaimi, R.; Cao, F.; Liu, W.; Shi, P. Antimicrobial and anticancer activities of Hainan dry noni fruit alcoholic extracts and their novel compounds identification using UPLC-Q-Exactive Obitrap-MS/MS. J. Pharm. Biomed. 2022, 220, 114989. [Google Scholar] [CrossRef]
- Li, J.; Chen, J.; Li, X.; Cheng, C.; Xiao, D. Studies on the Antimicrobial Properties and Substances in Xisha Noni Juice. J. Chin. Inst. Food Sci. Technol. 2015, 15, 143–149. (In Chinese) [Google Scholar] [CrossRef]
- Kang, J.; Song, K.B. Antibacterial activity of the noni fruit extract against Listeria monocytogenes and its applicability as a natural sanitizer for the washing of fresh-cut produce. Food Microbiol. 2019, 84, 103260. [Google Scholar] [CrossRef]
- Wigati, D.; Anwar, K.; Sudarsono; Nugroho, A. E. Hypotensive Activity of Ethanolic Extracts of Morinda citrifolia L. Leaves and Fruit in Dexamethasone-Induced Hypertensive Rat. J. Evid. Based Complement. Altern. Med. 2017, 22, 107–113. [Google Scholar] [CrossRef]
- Wang, R.; Wang, L.; Wang, S.; Wang, J.; Su, C.; Zhang, L.; Li, C.; Liu, S. Phenolics from noni (Morinda citrifolia L.) fruit alleviate obesity in high fat diet-fed mice via modulating the gut microbiota and mitigating intestinal damage. Food Chem. 2023, 402, 134232. [Google Scholar] [CrossRef]
- Shoeb, A.; Alwar, M.C.; Shenoy, P.J.; Gokul, P. Effect of Morinda citrifolia (Noni) Fruit Juice on High Fat Diet Induced Dyslipidemia in Rats. J. Clin. Diagn. Res. 2016, 10, FF06. [Google Scholar] [CrossRef]
- Mota, J.C.; Almeida, P.P.; Freitas, M.Q.; Stockler-Pinto, M.B.; Guimarães, J.T. Far from being a simple question: The complexity between in vitro and in vivo responses from nutrients and bioactive compounds with antioxidant potential. Food Chem. 2023, 402, 134351. [Google Scholar] [CrossRef] [PubMed]
- Su, C.; Kang, J.; Zhang, L.; Liu, S.; Li, C. Insight into the changes in active metabolite profiles of noni (Morinda citrifolia L.) fruit subjected to different drying treatments. Food Res. Int. 2024, 193, 114858. [Google Scholar] [CrossRef]
- Zhou, S.; Tan, J.; Chen, G. Purification, structural characterization and antioxidant mechanism of Noni polysaccharide based on Nrf2/HO-1/NQO1 signaling pathway. J. Food Meas. Charact. 2025, 19, 128–150. [Google Scholar] [CrossRef]
- Han, H.; Song, K.B. Noni (Morinda citrifolia) fruit polysaccharide films containing blueberry (Vaccinium corymbosum) leaf extract as an antioxidant packaging material. Food Hydrocoll. 2021, 112, 106372. [Google Scholar] [CrossRef]
- Lin, X.; Chen, S.; Wang, R.; Li, C.; Wang, L. Fabrication, characterization and biological properties of pectin and/or chitosan-based films incorporated with noni (Morinda citrifolia) fruit extract. Food Hydrocoll. 2023, 134, 108025. [Google Scholar] [CrossRef]
- Dong, Y.; Hao, M.; Xu, Y.; Feng, L.; Li, D.; Lian, Y.; Wu, D.; Dai, Z. A novel lutein-stevioside complex inhibits DSS-induced colitis. Food Prod. Process. Nutr. 2025, 7, 34. [Google Scholar] [CrossRef]
- Bourgonje, A.R.; Feelisch, M.; Faber, K.N.; Pasch, A.; Dijkstra, G.; van Goor, H. Oxidative Stress and Redox-Modulating Therapeutics in Inflammatory Bowel Disease. Trends Mol. Med. 2020, 26, 1034–1046. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, Y.; Zhou, M.; Wu, Y.; Guan, X. Study on the interaction between grain polyphenols and intestinal microorganisms: A review. Food Biosci. 2023, 53, 102536. [Google Scholar] [CrossRef]
- Chen, G.; Xie, M.; Wan, P.; Chen, D.; Dai, Z.; Ye, H.; Hu, B.; Zeng, X.; Liu, Z. Fuzhuan Brick Tea Polysaccharides Attenuate Metabolic Syndrome in High-Fat Diet Induced Mice in Association with Modulation in the Gut Microbiota. J. Agric. Food Chem. 2018, 66, 2783–2795. [Google Scholar] [CrossRef]
- Morales-Lozoya, V.; Espinoza-Gómez, H.; Flores-López, L.Z.; Sotelo-Barrera, E.L.; Núñez-Rivera, A.; Cadena-Nava, R.D.; Alonso-Nuñez, G.; Rivero, I.A. Study of the effect of the different parts of Morinda citrifolia L. (noni) on the green synthesis of silver nanoparticles and their antibacterial activity. Appl. Surf. Sci. 2021, 537, 147855. [Google Scholar] [CrossRef]
- West, B.J.; Palmer, S.K.; Deng, S.; Palu, A.K. Antimicrobial Activity of an Iridoid Rich Extract from “Morinda citrifolia” Fruit. Curr. Res. J. Biol. Sci. 2012, 4, 52–54. [Google Scholar]
- Purwaningroom, D.L.; Maghfirah, S.; Rifai, M.; Widodo, N. Exploring the mechanism of the anti-hypertension properties of Morinda citrifolia through a bioinformatics approach. Kuwait J. Sci. 2021, 48, 1–10. [Google Scholar] [CrossRef]
- Yoshitomi, H.; Zhou, J.; Nishigaki, T.; Li, W.; Liu, T.; Wu, L.; Gao, M. Morinda citrifolia (Noni) fruit juice promotes vascular endothelium function in hypertension via glucagon-like peptide-1receptor-CaMKKβ-AMPK-eNOS pathway. Phytother. Res. 2020, 34, 2341–2350. [Google Scholar] [CrossRef] [PubMed]
- Gilani, A.H.; Mandukhail, S.; Iqbal, J.; Yasinzai, M.; Aziz, N.; Khan, A.; Najeeb-ur-Rehman. Antispasmodic and vasodilator activities of Morinda citrifolia root extract are mediated through blockade of voltage dependent calcium channels. BMC Complement. Altern. Med. 2010, 10, 2. [Google Scholar] [CrossRef] [PubMed]
- Nayak, B.S.; Marshall, J.R.; Isitor, G.; Adogwa, A. Hypoglycemic and Hepatoprotective Activity of Fermented Fruit Juice of Morinda citrifolia (Noni) in Diabetic Rats. Evid. -Based Complement. Altern. Med. 2011, 2011, 875293. [Google Scholar] [CrossRef] [PubMed]
- Inada, A.C.; Silva, G.T.; Silva, L.P.; Alves, F.M.; Filiú, W.F.; Asato, M.A.; Junior, W.H.; Corsino, J.; Figueiredo, P.D.; Garcez, F.D.; et al. Therapeutic Effects of Morinda citrifolia Linn. (Noni) Aqueous Fruit Extract on the Glucose and Lipid Metabolism in High-Fat/High-Fructose-Fed Swiss Mice. Nutrients 2020, 12, 3439. [Google Scholar] [CrossRef]
- Matsuda, H.; Li, Y.; Yamahara, J.; Yoshikawa, M. Inhibition of Gastric Emptying by Triterpene Saponin, Momordin Ic, in Mice: Roles of Blood Glucose, Capsaicin-Sensitive Sensory Nerves, and Central Nervous System. J. Pharmacol. Exp. Ther. 1999, 289, 729–734. [Google Scholar] [CrossRef]
- Wang, R.; Zhang, L.; Zhang, Q.; Zhang, J.; Liu, S.; Li, C.; Wang, L. Glycolipid Metabolism and Metagenomic Analysis of the Therapeutic Effect of a Phenolics-Rich Extract from Noni Fruit on Type 2 Diabetic Mice. J. Agric. Food Chem. 2022, 70, 2876–2888. [Google Scholar] [CrossRef]
- Arkorful, D.A.; Appiah, F.; Owusu, J.; Nartey, E.; Darban, I. Effect of Prefermentation Treatment on the Bioactive Compounds of Noni (Morinda citrifolia L.) Juice. J. Food Process. Preserv. 2024, 2024, 7605531. [Google Scholar] [CrossRef]
- Pruthviraj; Naik, M.K.; Naik, R.G.; Nandish, M.S.; Ekabote, S.D.; Sreenivasa, M.Y. Antibacterial and plant growth promoting attributes of Limosilactobacillus sp. MYSN3 isolated from noni fruit. S. Afr. J. Bot. 2023, 162, 559–567. [Google Scholar] [CrossRef]
- Wang, M.; Gu, C.; Chang, Z.; Chen, J.; Zhou, J.; Yue, M.; Liu, F.; Feng, Z. Nutrient Consumption Patterns of Saccharomyces cerevisiae and Their Application in Fruit Wine Fermentation. Fermentation 2024, 10, 539. [Google Scholar] [CrossRef]
- Piñón-Balderrama, C.I.; Leyva-Porras, C.; Terán-Figueroa, Y.; Espinosa-Solís, V.; Álvarez-Salas, C.; Saavedra-Leos, M. Encapsulation of Active Ingredients in Food Industry by Spray-Drying and Nano Spray-Drying Technologies. Processes 2020, 8, 889. [Google Scholar] [CrossRef]
- Zhang, C.; Ada Khoo, S.L.; Chen, X.D.; Quek, S.Y. Microencapsulation of fermented noni juice via micro-fluidic-jet spray drying: Evaluation of powder properties and functionalities. Powder Technol. 2020, 361, 995–1005. [Google Scholar] [CrossRef]
- Kim, Y.; Pyeon, J.; Lee, J.; Kim, E.; La, I.; Lee, O.; Kim, K.; Sung, J.; Kim, Y. Chemical fingerprint analysis of fermented Morinda citrifolia L. (Noni) juice by UHPLC Q-TOF/MS combined with chemometric analysis. Appl. Biol. Chem. 2024, 67, 59. [Google Scholar] [CrossRef]
- Wang, Z.; Dou, R.; Yang, R.; Cai, K.; Li, C.; Li, W. Changes in Phenols, Polysaccharides and Volatile Profiles of Noni (Morinda citrifolia L.) Juice during Fermentation. Molecules 2021, 26, 2604. [Google Scholar] [CrossRef]
- Zhang, L.; Hong, Q.; Yu, C.; Wang, R.; Li, C.; Liu, S. Acetobacter sp. improves the undesirable odors of fermented noni (Morinda citrifolia L.) juice. Food Chem. 2023, 401, 134126. [Google Scholar] [CrossRef]
- Cheng, Y.; Li, P.; Hu, B.; Xu, L.; Liu, S.; Yu, H.; Guo, Y.; Xie, Y.; Yao, W.; Qian, H. Correlation analysis reveals the intensified fermentation via Lactobacillus plantarum improved the flavor of fermented noni juice. Food Biosci. 2021, 43, 101234. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, S.; Chen, Y.; Guo, J.; Li, C.; Zhang, J. Metatranscriptomic approach reveals the functional and enzyme dynamics of core microbes during noni fruit fermentation. Food Res. Int. 2021, 141, 109999. [Google Scholar] [CrossRef]
- de Ruijter, J.C.; Aisala, H.; Jokinen, I.; Krogerus, K.; Rischer, H.; Toivari, M. Production and sensory analysis of grape flavoured beer by co-fermentation of an industrial and a genetically modified laboratory yeast strain. Eur. Food Res. Technol. 2023, 249, 1991–2000. [Google Scholar] [CrossRef]
- Bennis, N.X.; Bieseman, J.; Daran, J.G. Unlocking lager’s flavour palette by metabolic engineering of Saccharomyces pastorianus for enhanced ethyl ester production. Metab. Eng. 2024, 85, 180–193. [Google Scholar] [CrossRef]
- van Wyk, N.; Kroukamp, H.; Espinosa, M.I.; von Wallbrunn, C.; Wendland, J.; Pretorius, I.S. Blending wine yeast phenotypes with the aid of CRISPR DNA editing technologies. Int. J. Food Microbiol. 2020, 324, 108615. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Xiong, J.; Yu, H.; Chen, C.; Lou, X. Flavor optimization in dairy fermentation: From strain screening and metabolic diversity to aroma regulation. Trends Food Sci. Technol. 2023, 141, 104194. [Google Scholar] [CrossRef]

| Carbohydrate | China’s Hainan Noni Fruit | Brazilian Noni Fruit | Vietnam Noni Fruit |
|---|---|---|---|
| Galacturonic acid | 58.4 | 29.1 | 53.6 |
| Galactose | 4.4 | 30.9 | 17.9 |
| Rhamnose | 4.8 | 5.4 | 9.5 |
| Arabinose | 2.2 | 31.0 | 13.6 |
| Mannose | Not analyzed | 3.6 | 0.7 |
| Xylose | Not analyzed | Not identified | 1.2 |
| Glucose | 21.1 | Not identified | 2.2 |
| Glucuronic acid | Not analyzed | Not identified | 1.1 |
| Fucose | Not analyzed | Not analyzed | 0.3 |
| Polyphenol Compound | Monomers | Place of Origin, Variety | Fruit Organizations | Maturity Level | Concentration | Reference |
|---|---|---|---|---|---|---|
| Phenolic acid | Gallic acid | Hainan, China and Fiji | Fresh fruit (dry powder) | Not analyzed | 1181.29–1497.42 μg/g | [29] |
| Protocatechuic acid | 155.34–330.01 μg/g | |||||
| Caffeic acid | 25.12–251.52 μg/g | |||||
| Chlorogenic acid | 97.31–128.36 μg/g | |||||
| 4-Hydroxybenzoic acid | 16.54–126.19 μg/g | |||||
| Tannins | 383.90–653.16 μg/g | |||||
| Gentianic acid | Hainan, China | 336.96–390.63 μg/g | [22] | |||
| Salicylic acid | 169.08–3109.23 μg/g | |||||
| Ferulic acid | 0–29.69 μg/g | |||||
| Ursolic acid | French Polynesia | Seeds (dry powder) | not analyzed | [30] | ||
| Flavonoids | Rhinocerosin | Hainan, China and Fiji | Fresh fruit (dry powder) | 0–6.67 μg/g | [29] | |
| Naringin | 77.12–487.25 μg/g | |||||
| Eriocitrin | 41.37–134.24 μg/g | |||||
| Floridzin | 0–55.38 μg/g | |||||
| Nobiletin | 0–25.31 μg/g | |||||
| Epicatechin | 0–176.64 μg/g | |||||
| Catechin | 0–388.01 μg/g | |||||
| Kaempferol | 0–28.50 μg/g | |||||
| Quercetin-3-rhamnoside | 431.07–860.21 μg/g | |||||
| Rutin | 298.55–514.72 μg/g | |||||
| Naringin dihydrochalcone | 0–48.37 μg/g | |||||
| Naringenin | 0–15.30 μg/g | |||||
| Hesperidin | 0–8.21 μg/g | |||||
| Neohesperidin | 82.46–140.29 μg/g | |||||
| Isorhamnetin 3-O-galactoside | Hainan, China | 131.52–246.51 μg/g | [22] | |||
| Kaempferol 3-O-rutinoside | 39.45–84.86 μg/g | |||||
| Kaempferol 3-O-glucoside | 699.47–949.50 μg/g | |||||
| Quercetin | 232.88–528.30 μg/g | |||||
| Brazilin | Unripe | 0.28 ± 0.02 μg/kg | [26] | |||
| Partially mature | 0.34 ± 0.02 μg/kg | |||||
| Mature | 0.32 ± 0.01 μg/kg | |||||
| Overripe | 0.34 ± 0.01 μg/kg | |||||
| Robinetin | Unripe | 1.85 ± 0.07 μg/kg | ||||
| Partially mature | 1.34 ± 0.08 μg/kg | |||||
| Mature | 1.70 ± 0.28 μg/kg | |||||
| Overripe | 1.15 ± 0.09 μg/kg | |||||
| Spinosin | Unripe | 6.46 ± 0.35 μg/kg | ||||
| Partially mature | 5.48 ± 0.13 μg/kg | |||||
| Mature | 3.68 ± 0.23 μg/kg | |||||
| Overripe | 3.89 ± 0.08 μg/kg | |||||
| Isorhamnetin 3-O-neohesperidin | Unripe | 0.36 ± 0.01 μg/kg | ||||
| Partially mature | 0.21 ± 0.02 μg/kg | |||||
| Mature | 0.24 ± 0.02 μg/kg | |||||
| Overripe | 0.12 ± 0.01 μg/kg | |||||
| Tricin | Unripe | 0.01 ± 0.00 μg/kg | ||||
| Partially mature | 0.07 ± 0.05 μg/kg | |||||
| Mature | not detected | |||||
| Overripe | 0.02 ± 0.01 μg/kg | |||||
| Lignins | Vanillin | Not analyzed | 6.77–11.20 μg/g | [22] | ||
| Moricitan A | Seeds (dry powder) | 0.3 mg/kg | [31] | |||
| 3,3′-Bisdemethylpinoresinol | French Polynesia | Not analyzed | [30] | |||
| Coumarins | Scopolamine | Tahiti and Jasmine Island in French Polynesia, Tonga, Dominican Republic, Okinawa, Thailand, and Hawaii | Fresh fruit (fruit pulp) | 0.064–6.87 mg/g | [32] |
| Chemical Composition | Chemical Formula | Place of Origin, Variety | Extraction Methods | Concentration | Reference |
|---|---|---|---|---|---|
| Asperulosidic acid | C18H24O12 | Okinawa, Japan | Methanol extraction 3 times and n-butanol extraction | 2.57 mg/kg md | [46] |
| 6α-Hydroxypolyglucoside | C17H26O11 | Nature’s Sunshine Products, Inc., Lehi, UT, USA | 7.50 mg/kg md | [47] | |
| 6beta,7beta-epoxy-8-epi-splendoside | C17H24O12 | 1.80 mg/kg md | |||
| Borreriagenin | C10H14O5 | unspecified | |||
| 4-epi-dunnisinin | C11H14O5 | Taiwan Hsieh Co, Taiwan, China | A total of 95% ethanol extraction 4 times and ethyl acetate extraction | 4.40 mg/kg md | [48] |
| Asperuloside | C18H22O11 | 50.00 mg/kg md | |||
| Deacetylasperulosidic acid | C16H20O10 | 4.00 mg/kg md | |||
| deacetylasperulosidic acid | C16H22O11 | Hainan, China | A total of 75% ethanol extraction | 22.65 mg/g md | [22] |
| Chemical Composition | Chemical Formula | Place of Origin, Variety | Fruit Organization | Concentration | Reference |
|---|---|---|---|---|---|
| 3-Hydroxy-1,2-dimethoxy-anthraquinone | C16H12O5 | Haikou, Hainan, China | Seeds (dry powder) | 0.40 mg/kg | [31] |
| 2-Hydroxy-1,5-dimethoxy-6-(methoxymethyl)-9,10-anthracenedione | C18H16O6 | 0.30 mg/kg | |||
| 2,4-Dihydroxy-3,8-dimethoxy-7-(methoxymethyl)-9,10-anthracenedione | C18H16O7 | 0.80 mg/kg | |||
| 1,6-Dihydroxy-5-methoxy-2-(methoxymethyl)-9,10-anthracenedione | C17H14O6 | Haikou, Hainan, China | 0.50 mg/kg | [31] | |
| Fiji noni fruit | Fresh fruit (dry powder) | 0.80 mg/kg | [56] | ||
| 1,5,7-Trihydroxy-6-methoxy-2-methoxymethylanthraquinone | C16H12O6 | Fiji noni fruit | 0.15 mg/kg | ||
| 2-Methoxy-1,3,6-trihydroxyanthraquinone | C15H10O6 | Nature’s Sunshine Products, Inc. | 0.18 mg/kg | [57] |
| Health Benefits | Research Target | Mechanisms Explored | Reference |
|---|---|---|---|
| Antioxidant effects | Noni fruit polysaccharides | Noni fruit polysaccharides increased the content of Superoxide Dismutase (SOD) in serum; decreased the content of Malondialdehyde (MDA) in liver tissue; downregulated Nuclear Factor Erythroid 2-Related Factor 2 (Nrf2), Heme Oxygenase-1 (HO-1), NAD (P) H:Quinone Oxidoreductase 1 (NQO1) proteins, and genes related to the oxidative stress signaling pathway in RAW264.7 cells; and inhibited the abnormal decrease in Catalase (CAT), SOD, and Glutathione Peroxidase (GSH-Px) levels and the abnormal increase in MDA, respectively. | [62] |
| Noni fruit deacetylasperulosidic acid | Noni fruit deacetylasperulosidic acid decreased MDA content and increased SOD activity in rat serum. | [66] | |
| Noni fruit extract | Noni fruit extract decreased MDA levels and increased SOD activity in rat testis. | [67] | |
| Noni fruit polysaccharide | Noni fruit polysaccharides reduced hepatic MDA levels and increased hepatic trolox equivalent antioxidant capacity levels and SOD and GSH-Px activities. | [10] | |
| Anti-inflammatory effects | Noni juice-fortified yogurt | The addition of noni juice to yogurt alleviates dextrose sodium sulfate (DSS)-induced colitis in mice through downregulation of Interleukin-6 (IL-6) and Interferon-γ (IFN-γ) mRNA expression and up-regulation of Interleukin-10 (IL-10) mRNA expression. | [68] |
| Noni juice | Noni juice treatment of rats with pneumonia reduced lung macrophages by 20–26%, lymphocytes by 34–58%, eosinophils by 30–53%, and neutrophils by 28–70%, and Noni juice showed dose-dependent Nitric Oxide (NO) scavenging effects. | [69] | |
| Noni juice | Treatment of DSS-induced colitis in mice with noni juice reduced serum Tumor Necrosis Factor-α (TNF-α) and IL-6 production and regulated intestinal flora communities to normal levels. | [70] | |
| Fermented noni juice | Fermented noni juice can reduce TNF-α and Interleukin-1β (IL-1β) protein expression levels and inhibit the Nuclear Factor kappa-light-chain-enhancer of activated B cells (NF-κB) signaling pathway in the renal cortical proximal tubular epithelial cells of hyperuricemia. | [71] | |
| Noni fruit polysaccharide | Noni fruit polysaccharides were able to reverse the trend of body weight loss and promote the expression of intestinal tight junction proteins (ZO-1 and occludin proteins) and mucus secretion in mice with colon injury, thereby reducing the damage to the colonic mucosal barrier caused by DSS. | [9] | |
| Noni fruit polysaccharides | Noni fruit polysaccharides reduced scores of paw tissue damage, decreased myeloperoxidase (MPO) activity, and inhibited leukocyte migration to sites of inflammation in dysfunctional mice. | [4] | |
| Liver-protecting effects | Noni fruit polysaccharides | Noni fruit polysaccharides reduce the triglyceride (TG) and total cholesterol (TC) content in the liver of rats on a high-fat diet, reduce serum alanine aminotransferase (ALT) and AST (aspartate aminotransferase) activity, regulate intestinal flora, reduce the production of short-chain fatty acids, decrease the permeability of the colonic barrier, and alleviate metabolic endotoxemia. | [10] |
| Noni fruit polyphenols | Noni fruit polyphenols reduced TC and TG levels and inhibited the increase in ALT and AST activities in the liver of mice with high-fat-diet-induced non-alcoholic steatohepatitis. Noni fruit phenolic administration ameliorates hepatic inflammation in high-fat-diet mice by inhibiting the NF-κB pathway. | [72] | |
| Noni juice and fermented noni juice | Noni juice and fermented noni juice reduced AST, ALT, alkaline phosphatase (ALP), and glutamyltransferase (GGT) activities in the serum of mice with acute alcoholic liver injury; maintained the integrity of hepatocytes; and increased the activities of GSH, GSH-PX, SOD, and CAT in the liver while inhibiting the production of MDA. | [3] | |
| Fermented noni juice | Fermented noni juice dose-dependently decreased AST and ALT activities in the serum of rats induced by thioacetamide. Juice treatment increased the activities of GSH, SOD, CAT, and GSH-PX in liver and increased the antioxidant capacity of liver. | [73] | |
| Noni fruit polyphenols | Supplementation with fermented noni juice reduced TC levels in the liver and serum of hamsters on a high-fat diet, alleviated microvesicular steatosis around the central vein of liver tissue, and reduced ALT activity in liver. | [74] | |
| Antibacterial effects | Noni fruit extract | The effective minimum inhibitory concentration (MIC) of noni fruit ethanolic extract was 35.43 mg/mL against Escherichia coli and 47.80 mg/mL against Candida albicans. However, the MIC of noni fruit methanolic extract was 117.40 mg/mL against Escherichia coli and 108.01 mg/mL against Candida albicans, and noni fruit methanolic extract had a weaker antibacterial effect. | [75] |
| Noni fruit polyphenols | The MIC values of 70% ethanol prepared polyphenolic extract of noni fruit against Listeria monocytogenes, Staphylococcus aureus, Bacillus subtilis, Escherichia coli, Salmonella spp., and Pseudomonas aeruginosa were 0.098, 0.049, 0.024, 0.024, 3.125, and 0.781 mg/mL, respectively. | [22] | |
| Noni juice | The diameter of the circle of inhibition of a mixture of acids consisting of malic, 3-methylvaleric, acetic, and capric acids was 16.49 mm, which was not significantly different from that of noni juice, which was 16.55 mm. | [76] | |
| Noni fruit extract | Increasing the concentration of noni fruit extract in the discs enhanced the inhibition of Listeria monocytogenes, and the diameter of the inhibition circle of the discs impregnated with 80 mg of noni fruit extract was 22.43 mm. | [77] | |
| Antihypertensive effects | Noni fruit extract | Treatment of dexamethasone-induced hypertensive rats with ethanolic extract of noni fruit resulted in a decrease in systolic and diastolic blood pressure by 11.39% and 10.57%, respectively, compared to a model group. | [78] |
| Anti-obesity effects | Noni fruit polysaccharides | Noni fruit polysaccharides reduced the body weight and serum levels of TC, TG, Low-Density Lipoprotein Cholesterol (LDL-C), and total bile acids and increased the serum levels of High-Density Lipoprotein Cholesterol (HDL-C) in high-fat mice. | [6] |
| Noni fruit polyphenols | Noni fruit polyphenols reduced body weight gain and improved steatosis as well as lipid accumulation in mice on a high-fat diet. Noni fruit polyphenols reduced the serum levels of TC, TG, and LDL-C and increased HDL-C. | [79] | |
| Noni juice | Noni juice reduced TC, TG, and LDL-C content in serum and increased HDL-C content in serum of mice on a high-fat diet. | [80] | |
| Fermented noni juice | Fermented noni juice was able to reduce the liver coefficient and visceral fat coefficient in mice on a high-fat diet in a dose-dependent manner. It reduced the content of TG and TC in the serum and liver of mice and improved hepatic steatosis. | [74] |
| Place of Origin, Variety | Fermentation Strains | Aroma Category | Related Characteristic Aroma Substances | Description of the Main Aromas | Active Ingredients and Functions | Reference |
|---|---|---|---|---|---|---|
| Wanning City, Hainan Province, China | Natural fermentation | Esters, alcohols, acids, ketones, etc. | Methyl caproate, methyl hexadecanoate, methyl salicylate, ethanol, 2-heptanol, caprylic acid, hexanoic acid, and 2-heptanone | Floral, fruity, cheesy, and sweaty | The total phenol content decreased first, then increased, and subsequently decreased again during the fermentation process. The highest total phenol content reached 199.77 mg GAE/100 mL and 185.60 mg GAE/100 mL in laboratory-fermented juice and factory-fermented juice, respectively, on the 28th day of fermentation. The total flavonoid content showed an increasing trend in both fermentation modes, reaching a maximum value of 36.73 mg RE/100 mL on the 63rd day of laboratory fermentation. | [105] |
| Haikou City, Hainan Province, China | Lactobacillus plantarum | Esters, alcohols, acids, ketones, and aldehydes | Caprylic acid, capric acid, ethyl caproate, methyl caproate, linalool, 2-heptanol, etc. | Cheesy scent, sweaty scent, citrus and floral scent, and mushroom scent | Not analyzed | [107] |
| Wanning City, Hainan Province, China | Lactococcus lactis | Acids, esters, aldehydes, alcohols, and ketones | Caprylic acid, nonanal, 1-nonanol, and 2-heptanone, etc. | Cheesy flavor, pungent acidity, and fruity flavor | Compared with a control group, the total phenol content in Lactococcus lactis juice was higher and showed an increase followed by a decrease. At the end of fermentation, the total phenol content was 67.00 mg GAE/100 mL, and the total flavonoid content was 30.11 mg RE/100 mL. Additionally, the content of deacetylasperulosidic acid increased significantly to 32.66 mg/100 mL. | [39] |
| Wanning City, Hainan Province, China | Acetobacter | Acids, esters, alcohols, ketones, aldehydes, and terpenes | Caprylic acid, capric acid, ethyl acetate, ethyl propionate, 1-octen-3-one, 2-pentanone, and α-watercressene | Floral, fruity, grassy, and mushroomy | With a prolongation of the fermentation time, the content of asperulosidic acid in fermented noni juice decreased continuously. The deacetylasperulosidic acid showed the opposite trend, with a concentration of more than 1200 μg/mL after 20 d of fermentation. The total phenol content showed a decreasing trend, but the total flavonoid content did not change significantly. | [106] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ni, Q.; Zhang, Z.; Niu, L.; Yang, R.; Xiong, L.; Li, D.; Dai, Z. Research Progress on Nutritional Properties of Noni (Morinda citrifolia L.) Fruit and Its Fermented Foods. Fermentation 2025, 11, 358. https://doi.org/10.3390/fermentation11070358
Ni Q, Zhang Z, Niu L, Yang R, Xiong L, Li D, Dai Z. Research Progress on Nutritional Properties of Noni (Morinda citrifolia L.) Fruit and Its Fermented Foods. Fermentation. 2025; 11(7):358. https://doi.org/10.3390/fermentation11070358
Chicago/Turabian StyleNi, Qianjin, Zhi Zhang, Liying Niu, Runqiang Yang, Lingming Xiong, Dajing Li, and Zhuqing Dai. 2025. "Research Progress on Nutritional Properties of Noni (Morinda citrifolia L.) Fruit and Its Fermented Foods" Fermentation 11, no. 7: 358. https://doi.org/10.3390/fermentation11070358
APA StyleNi, Q., Zhang, Z., Niu, L., Yang, R., Xiong, L., Li, D., & Dai, Z. (2025). Research Progress on Nutritional Properties of Noni (Morinda citrifolia L.) Fruit and Its Fermented Foods. Fermentation, 11(7), 358. https://doi.org/10.3390/fermentation11070358







