Enhancing Bioconversion of Crude Glycerol into Butanol and 1,3-Propanediol After Pretreatment by Coupling Fermentation and In Situ Recovery: Effect of Initial pH Control
Abstract
1. Introduction
2. Materials and Methods
2.1. Sources of Crude Glycerol and Pretreatment
2.2. Strain Maintenance
2.3. Formulation of the Fermentation Medium
2.4. Reactor Fermentation
2.5. Analytical Methods
3. Results and Discussion
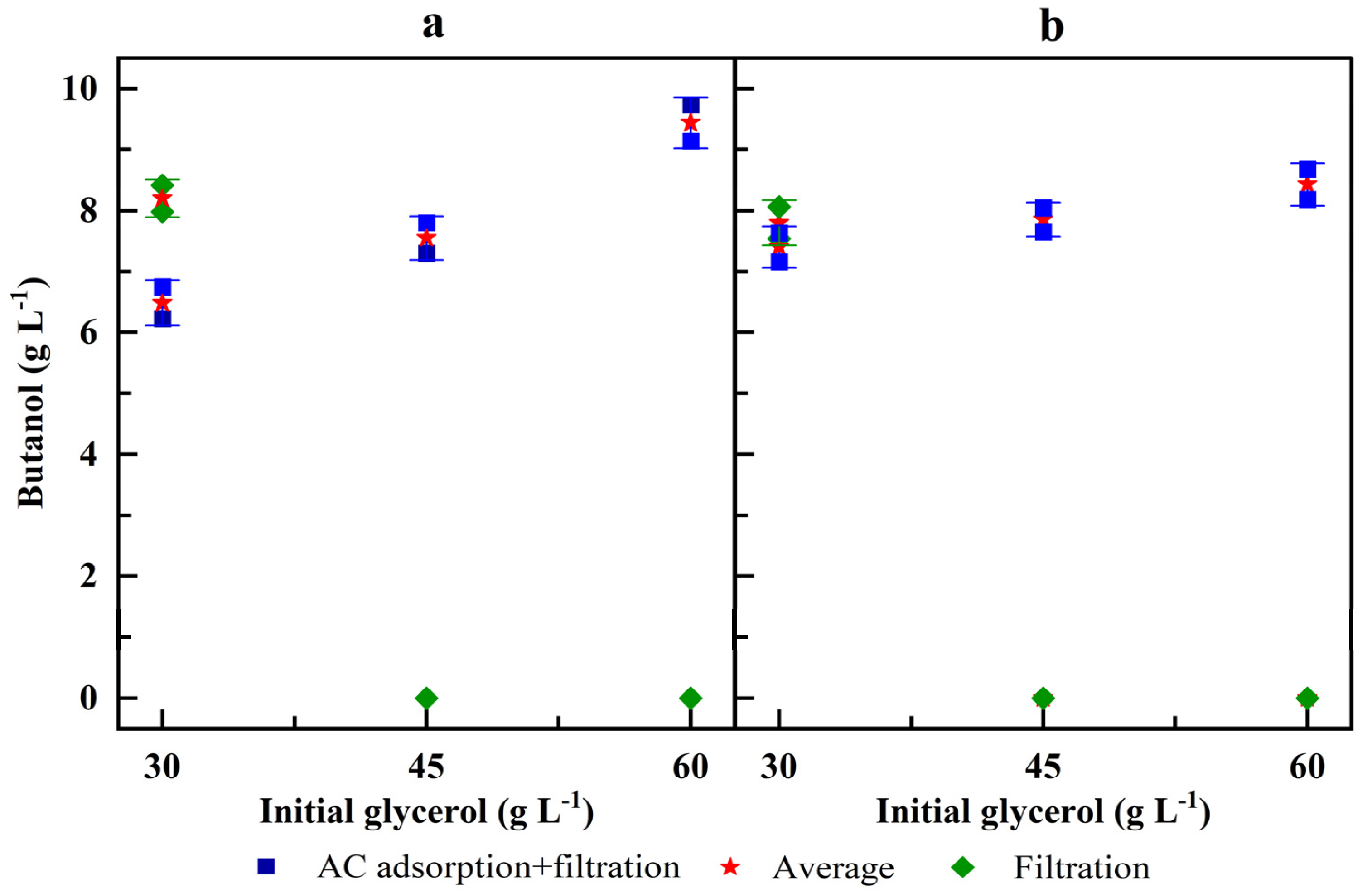
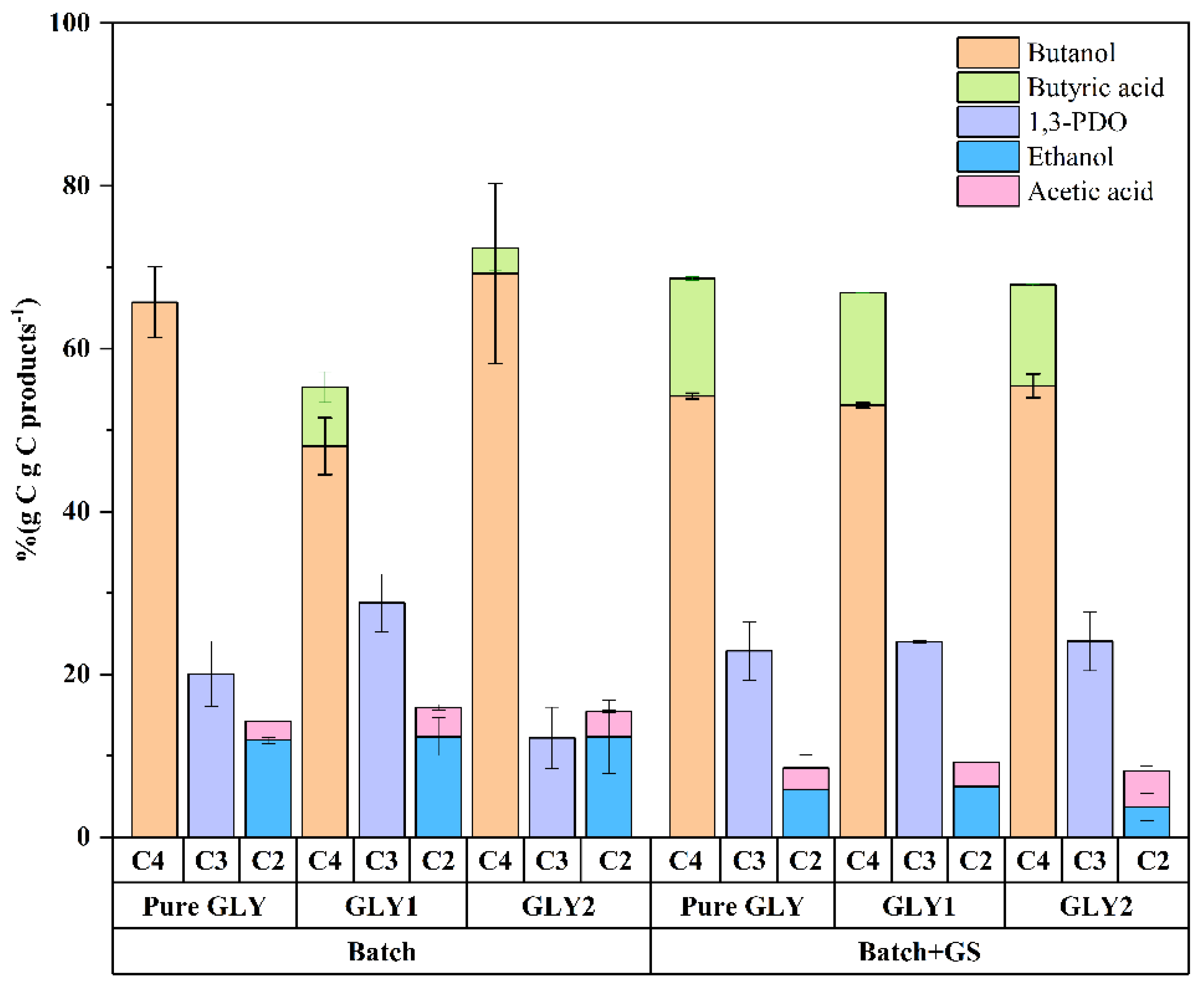
3.1. Effect of the Pretreatment of Crude Glycerol on Butanol Production
3.2. Effect of the Medium Formulation on Butanol Production
3.3. Effect of the Medium pH on Butanol Production
3.4. Fermentation of Crude Glycerol Under pH Control
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- European Parliament and Council Directive (EU). 2023/2413 of the European Parliament and of the Council of 18 October 2023 Amending Directive (EU) 2018/2001, Regulation (EU) 2018/1999 and Directive 98/70/EC as Regards the Promotion of Energy from Renewable Sources, and Repealing Council Directive (EU) 2015/652. 2023. Available online: https://eur-lex.europa.eu/eli/dir/2023/2413/oj/eng (accessed on 30 April 2025).
- Energy Institute. Statistical Review of World Energy, 72nd ed.; Energy Institute: London, UK, 2023. [Google Scholar]
- Kongjao, S.; Damronglerd, S.; Hunsom, M. Purification of Crude Glycerol Derived from Waste Used-Oil Methyl Ester Plant. Korean J. Chem. Eng. 2010, 27, 944–949. [Google Scholar] [CrossRef]
- Attarbachi, T.; Kingsley, M.D.; Spallina, V. New Trends on Crude Glycerol Purification: A Review. Fuel 2023, 340, 127485. [Google Scholar] [CrossRef]
- Ripoll, M.; Betancor, L. Opportunities for the Valorization of Industrial Glycerol via Biotransformations. Curr. Opin. Green Sustain. Chem. 2021, 28, 100430. [Google Scholar] [CrossRef]
- Sarchami, T.; Johnson, E.; Rehmann, L. Optimization of Fermentation Condition Favoring Butanol Production from Glycerol by Clostridium Pasteurianum DSM 525. Bioresour. Technol. 2016, 208, 73–80. [Google Scholar] [CrossRef]
- Rigaki, A.; Webb, C.; Theodoropoulos, C. Double Substrate Limitation Model for the Bio-Based Production of Succinic Acid from Glycerol. Biochem. Eng. J. 2020, 153, 107391. [Google Scholar] [CrossRef]
- Melo, N.T.M.; Pontes, G.C.; Procópio, D.P.; Cunha, G.C.d.G.; Eliodório, K.P.; Paes, H.C.; Basso, T.O.; Parachin, N.S. Evaluation of Product Distribution in Chemostat and Batch Fermentation in Lactic Acid-Producing Komagataella Phaffii Strains Utilizing Glycerol as Substrate. Microorganisms 2020, 8, 781. [Google Scholar] [CrossRef] [PubMed]
- Oh, H.J.; Kim, K.Y.; Lee, K.M.; Lee, S.M.; Gong, G.; Oh, M.K.; Um, Y. Butyric Acid Production with High Selectivity Coupled with Acetic Acid Consumption in Sugar-Glycerol Mixture Fermentation by Clostridium Tyrobutyricum ATCC25755. J. Ind. Eng. Chem. 2019, 75, 44–51. [Google Scholar] [CrossRef]
- Sun, Y.Q.; Shen, J.T.; Yan, L.; Zhou, J.J.; Jiang, L.L.; Chen, Y.; Yuan, J.L.; Feng, E.M.; Xiu, Z.L. Advances in Bioconversion of Glycerol to 1,3-Propanediol: Prospects and Challenges. Process Biochem. 2018, 71, 134–146. [Google Scholar] [CrossRef]
- Parate, R.; Mane, R.; Dharne, M.; Rode, C. Mixed Bacterial Culture Mediated Direct Conversion of Bio-Glycerol to Diols. Bioresour. Technol. 2018, 250, 86–93. [Google Scholar] [CrossRef]
- Andersen, V.F.; Anderson, J.E.; Wallington, T.J.; Mueller, S.A.; Nielsen, O.J. Vapor Pressures of Alcohol-Gasoline Blends. Energy Fuels 2010, 24, 3647–3654. [Google Scholar] [CrossRef]
- Munch, G.; Mittler, J.; Rehmann, L. Increased Selectivity for Butanol in Clostridium Pasteurianum Fermentations via Butyric Acid Addition or Dual Feedstock Strategy. Fermentation 2020, 6, 67. [Google Scholar] [CrossRef]
- Biebl, H. Fermentation of Glycerol by Clostridium Pasteurianum—Batch and Continuous Culture Studies. J. Ind. Microbiol. Biotechnol. 2001, 27, 18–26. [Google Scholar] [CrossRef]
- Gallardo, R.; Alves, M.; Rodrigues, L.R. Influence of Nutritional and Operational Parameters on the Production of Butanol or 1,3-Propanediol from Glycerol by a Mutant Clostridium Pasteurianum. New Biotechnol. 2017, 34, 59–67. [Google Scholar] [CrossRef]
- Dabrock, B.; Bahl, H.; Gottschalk, G. Parameters Affecting Solvent Production by Clostridium Pasteurianum. Appl. Environ. Microbiol. 1992, 58, 1233–1239. [Google Scholar] [CrossRef]
- Branduardi, P.; de Ferra, F.; Longo, V.; Porro, D. Microbial N-Butanol Production from Clostridia to Non-Clostridial Hosts. Eng. Life. Sci. 2014, 14, 16–26. [Google Scholar] [CrossRef]
- Malaviya, A.; Jang, Y.S.; Lee, S.Y. Continuous Butanol Production with Reduced Byproducts Formation from Glycerol by a Hyper Producing Mutant of Clostridium Pasteurianum. Appl. Microbiol. Biotechnol. 2012, 93, 1485–1494. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, K.M.; Grosse-Honebrink, A.; Derecka, K.; Rotta, C.; Zhang, Y.; Minton, N.P. Towards Improved Butanol Production through Targeted Genetic Modification of Clostridium Pasteurianum. Metab. Eng. 2017, 40, 124–137. [Google Scholar] [CrossRef] [PubMed]
- Sedlar, K.; Kolek, J.; Provaznik, I.; Patakova, P. Reclassification of Non-Type Strain Clostridium Pasteurianum NRRL B-598 as Clostridium Beijerinckii NRRL B-598. J. Biotechnol. 2017, 244, 1–3. [Google Scholar] [CrossRef]
- Kolek, J.; Patáková, P.; Melzoch, K.; Sigler, K.; Řezanka, T. Changes in Membrane Plasmalogens of Clostridium Pasteurianum during Butanol Fermentation as Determined by Lipidomic Analysis. PLoS ONE 2015, 10, e0122058. [Google Scholar] [CrossRef][Green Version]
- Jensen, T.Ø.; Kvist, T.; Mikkelsen, M.J.; Christensen, P.V.; Westermann, P. Fermentation of Crude Glycerol from Biodiesel Production by Clostridium Pasteurianum. J. Ind. Microbiol. Biotechnol. 2012, 39, 709–717. [Google Scholar] [CrossRef]
- Zhu, H.; Liu, G.; Yuan, J.; Chen, T.; Xin, F.; Jiang, M.; Fan, Y.; Jin, W. In-Situ Recovery of Bio-Butanol from Glycerol Fermentation Using PDMS/Ceramic Composite Membrane. Sep. Purif. Technol. 2019, 229, 115811. [Google Scholar] [CrossRef]
- Manosak, R.; Limpattayanate, S.; Hunsom, M. Sequential-Refining of Crude Glycerol Derived from Waste Used-Oil Methyl Ester Plant via a Combined Process of Chemical and Adsorption. Fuel Process. Technol. 2011, 92, 92–99. [Google Scholar] [CrossRef]
- Venkataramanan, K.P.; Barquero, J.J.; Kurniawan, Y.; Taconi, K.A.; Bothun, G.D.; Scholz, C. Impact of Impurities in Biodiesel-Derived Crude Glycerol on the Fermentation by Clostridium Pasteurianum ATCC 6013. Appl. Microbiol. Biotechnol. 2012, 93, 1325–1335. [Google Scholar] [CrossRef]
- Taconi, K.A.; Venkataramanan, K.P.; Johnson, D.T. Growth and Solvent Production by Clostridium Pasteurianum ATCCVR 6013TM Utilizing Biodiesel-Derived Crude Glycerol as the Sole Carbon Source. Environ. Prog. Sustain. Energy 2009, 28, 100–110. [Google Scholar] [CrossRef]
- Gallardo, R.; Alves, M.; Rodrigues, L.R. Modulation of Crude Glycerol Fermentation by Clostridium Pasteurianum DSM 525 towards the Production of Butanol. Biomass Bioenergy 2014, 71, 134–143. [Google Scholar] [CrossRef]
- Kumar, L.R.; Yellapu, S.K.; Tyagi, R.D.; Drogui, P. Purified Crude Glycerol by Acid Treatment Allows to Improve Lipid Productivity by Yarrowia Lipolytica SKY7. Process Biochem. 2020, 96, 165–173. [Google Scholar] [CrossRef]
- Biebl, H.; Pfennig, N. Isolation of Members of the Family Rhodospirillaceae. In The Prokaryotes; Springer: Berlin/Heidelberg, Germany, 1991; pp. 267–273. [Google Scholar]
- ISO 12937; Petroleum Products—Determination of Water—Coulometric Karl Fischer Titration Method. ISO: Geneva, Switzerland, 2000.
- Gheshlaghi, R.; Scharer, J.M.; Moo-Young, M.; Chou, C.P. Metabolic Pathways of Clostridia for Producing Butanol. Biotechnol. Adv. 2009, 27, 764–781. [Google Scholar] [CrossRef]
- Kubiak, P.; Leja, K.; Myszka, K.; Celińska, E.; Spychała, M.; Szymanowska-Powałowska, D.; Czaczyk, K.; Grajek, W. Physiological Predisposition of Various Clostridium Species to Synthetize 1,3-Propanediol from Glycerol. Process Biochem. 2012, 47, 1308–1319. [Google Scholar] [CrossRef]
- Groeger, C.; Wang, W.; Sabra, W.; Utesch, T.; Zeng, A.P. Metabolic and Proteomic Analyses of Product Selectivity and Redox Regulation in Clostridium Pasteurianum Grown on Glycerol under Varied Iron Availability. Microb. Cell Factories 2017, 16, 64. [Google Scholar] [CrossRef]
- Regestein, L.; Doerr, E.W.; Staaden, A.; Rehmann, L. Impact of Butyric Acid on Butanol Formation by Clostridium Pasteurianum. Bioresour. Technol. 2015, 196, 153–159. [Google Scholar] [CrossRef]
- Moon, C.; Hwan Lee, C.; Sang, B.I.; Um, Y. Optimization of Medium Compositions Favoring Butanol and 1,3-Propanediol Production from Glycerol by Clostridium Pasteurianum. Bioresour. Technol. 2011, 102, 10561–10568. [Google Scholar] [CrossRef] [PubMed]
- Monot, F.; Martin, J.R.; Petitdemange, H.; Gay, R. Acetone and Butanol Production by Clostridium Acetobutylicum in a Synthetic Medium. Appl. Environ. Microbiol. 1982, 44, 1318–1324. [Google Scholar] [CrossRef] [PubMed]
- Capilla, M.; San-Valero, P.; Izquierdo, M.; Penya-roja, J.M.; Gabaldón, C. The Combined Effect on Initial Glucose Concentration and pH Control Strategies for Acetone-Butanol-Ethanol (ABE) Fermentation by Clostridium Acetobutylicum DSM 792. Biochem. Eng. J. 2021, 167, 107910. [Google Scholar] [CrossRef]
- Venkataramanan, K.P.; Kurniawan, Y.; Boatman, J.J.; Haynes, C.H.; Taconi, K.A.; Martin, L.; Bothun, G.D.; Scholz, C. Homeoviscous Response of Clostridium Pasteurianum to Butanol Toxicity during Glycerol Fermentation. J. Biotechnol. 2014, 179, 8–14. [Google Scholar] [CrossRef]
- Kalafatakis, S.; Skiadas, I.V.; Gavala, H.N. Determining Butanol Inhibition Kinetics on the Growth of Clostridium Pasteurianum Based on Continuous Operation and Pulse Substrate Additions. J. Chem. Technol. Biotechnol. 2019, 94, 1559–1566. [Google Scholar] [CrossRef]
- Groeger, C.; Sabra, W.; Zeng, A.P. Simultaneous Production of 1,3-Propanediol and n-Butanol by Clostridium Pasteurianum: In Situ Gas Stripping and Cellular Metabolism. Eng. Life. Sci. 2016, 16, 664–674. [Google Scholar] [CrossRef]




| Fat Source | GLY1 | GLY2 |
|---|---|---|
| Glycerol (g L−1) | 508.3 | 546.9 |
| Water (g L−1) | 602.7 | 629.2 |
| Solids (g L−1) | 9.27 | 9.54 |
| Ash (g L−1) | 0.69 | 1.45 |
| MONG (g L−1) | 66.8 | 42.6 |
| Methanol (g L−1) | n.d. | 6.66 |
| GLY1 | GLY2 | ||||||
|---|---|---|---|---|---|---|---|
| Initial | After Filtration | After GAC Adsorption + Filtration | Initial | After Filtration | After GAC Adsorption + Filtration | ||
| Glycerol (g L−1) | 102.5 | 100.6 | 97.7 | 100.9 | 98.9 | 97.7 | |
| Solids (g L−1) | 1.19 | 0.93 | 0.34 | 1.36 | 0.70 | 0.24 | |
| Ash (g L−1) | 0.05 | 0.05 | n.d. | n.d. | n.d. | n.d. | |
| MONG (g L−1) | 11.2 | 10.2 | 4.3 | 9.3 | 8.5 | 3.6 | |
| Methanol (g L−1) | n.d. | n.d. | n.d. | 1.21 | 1.16 | n.d. | |
| FFD | Glycerol Consumption (g L−1) | Biomass Growth (g-dw L−1) | Product Formation (g L−1) | |||||
|---|---|---|---|---|---|---|---|---|
| Run | X1 | X2 | X3 | Butanol | Ethanol | 1,3-PDO | ||
| 1 | 5.00 | 1.00 | 0.00 | 26.49 | 2.78 | 8.77 | 3.21 | 3.92 |
| 2 | 5.00 | 1.00 | 6.00 | 26.60 | 2.93 | 10.00 | 2.10 | 2.60 |
| 3 | 0.01 | 3.00 | 0.00 | 12.85 | 1.34 | 3.85 | 0.90 | 4.10 |
| 5 | 5.00 | 3.00 | 0.00 | 30.05 | 2.63 | 9.29 | 2.37 | 3.81 |
| 6 | 5.00 | 3.00 | 6.00 | 27.86 | 2.62 | 9.05 | 2.70 | 3.69 |
| 8 | 0.01 | 3.00 | 6.00 | 16.31 | 1.45 | 4.77 | 1.10 | 4.42 |
| 9 | 0.01 | 1.00 | 6.00 | 6.41 | 0.96 | 2.56 | 1.05 | 3.31 |
| 10 | 0.01 | 1.00 | 0.00 | 10.25 | 0.95 | 2.50 | 1.25 | 3.12 |
| 4, 7, 11 | 2.50 | 2.00 | 3.00 | 29.73 ± 1.66 | 2.85 ± 0.07 | 8.68 ± 0.31 | 2.74 ± 0.04 | 3.52 ± 0.07 |
| Initial Glycerol (g L−1) | Supplemented Iron | Equivalent Fe2+ (g L−1) | Butanol Production (g L−1) | 1,3-PDO Production (g L−1) | Butanol Yield (g g−1) a | 1,3-PDO Yield (g g−1) b | Reference |
|---|---|---|---|---|---|---|---|
| 25 | 0.1 g L−1 FeSO4·7H2O | 2.0 10−2 | 5.5 c | 7.6 c | 0.24 d | 0.33 d | [26] |
| 20 | 0.02 g L−1 FeSO4·7H2O | 4.0 10−3 | ~6.0 e | ~1.8 e | n.a. | n.a | [35] |
| 80 | 0.01 g L−1 FeSO4·7H2O | 2.0 10−3 | 10.0 | 6.6 | 0.25 | 0.16 | [18] |
| 30 | 0.05 g L−1 FeSO4 | 1.8 10−2 | 8.7 | ~1.0 e | 0.29 | -- | [34] |
| 50 | 0.01 g L−1 FeSO4·7H2O | 2.0 10−3 | 6.5 | ~3.3 e | 0.16 | ~0.08 c | [6] |
| 0.1 g L−1 FeSO4·7H2O | 2.0 10−2 | 8.6 | ~4.5 | 0.21 | ~0.11 c | ||
| 45 | 0.943 mg L−1 FeCl2 | 4.2 10−4 | 7.1 | 6.8 | 0.20 | 0.19 | [15] |
| 20 | 0.05 g L−1 FeSO4 | 1.8 10−2 | ~5.7 e | n.a. | ~0.28 c | n.a. | [13] |
| 60 | 5 mg L−1 FeSO4 | 1.8 10−3 | 8.8 | 3.9 | 0.33 | 0.15 | This study (run 1-Table 3) |
| pH Control | Glycerol Consumption (g L−1) | Product Formation (g L−1) | Product Yield (g g−1glycerol consumed) | Maximum Productivity (g L−1 h−1) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Butanol | Ethanol | 1,3-PDO | Butanol | Ethanol | 1,3-PDO | Butanol | Ethanol | 1,3-PDO | ||
| ≥4.5 | 18.84 | 5.78 | n.d. | 1.16 | 0.31 | -- | 0.06 | 0.55 | -- | 0.32 |
| ≥5.5 | 29.32 | 7.51 | 2.05 | 2.17 | 0.26 | 0.07 | 0.07 | 0.76 | 0.39 | 0.31 |
| ≥6.5 | 38.18 | 9.09 | 2.11 | 3.80 | 0.25 | 0.06 | 0.10 | 1.20 | 0.32 | 0.31 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ortega, A.; Valles, A.; Capilla, M.; Gabaldón, C.; Álvarez-Hornos, F.J.; Marzal, P. Enhancing Bioconversion of Crude Glycerol into Butanol and 1,3-Propanediol After Pretreatment by Coupling Fermentation and In Situ Recovery: Effect of Initial pH Control. Fermentation 2025, 11, 339. https://doi.org/10.3390/fermentation11060339
Ortega A, Valles A, Capilla M, Gabaldón C, Álvarez-Hornos FJ, Marzal P. Enhancing Bioconversion of Crude Glycerol into Butanol and 1,3-Propanediol After Pretreatment by Coupling Fermentation and In Situ Recovery: Effect of Initial pH Control. Fermentation. 2025; 11(6):339. https://doi.org/10.3390/fermentation11060339
Chicago/Turabian StyleOrtega, Alejandro, Alejo Valles, Miguel Capilla, Carmen Gabaldón, Francisco Javier Álvarez-Hornos, and Paula Marzal. 2025. "Enhancing Bioconversion of Crude Glycerol into Butanol and 1,3-Propanediol After Pretreatment by Coupling Fermentation and In Situ Recovery: Effect of Initial pH Control" Fermentation 11, no. 6: 339. https://doi.org/10.3390/fermentation11060339
APA StyleOrtega, A., Valles, A., Capilla, M., Gabaldón, C., Álvarez-Hornos, F. J., & Marzal, P. (2025). Enhancing Bioconversion of Crude Glycerol into Butanol and 1,3-Propanediol After Pretreatment by Coupling Fermentation and In Situ Recovery: Effect of Initial pH Control. Fermentation, 11(6), 339. https://doi.org/10.3390/fermentation11060339







