1. Introduction
Microbial-fermented feed is produced through the controlled microbial metabolism of substrates under defined temperature and moisture conditions [
1]. In recent years, significant progress has been made in research on microbial applications, which has stimulated heightened scientific interest in harnessing beneficial microorganisms for the development of bioactive feed additives through fermentation processes in animal husbandry practices. This emerging field has consequently generated a substantial body of scholarly investigation globally [
2]. Probiotic-fermented feed has emerged as a promising strategy to enhance poultry health and productivity. Various probiotics, such as
Lactobacillus,
Bacillus, and
Saccharomyces species, contribute distinct functional benefits during fermentation, including nutrient enrichment, pathogen inhibition, and immune modulation [
3]. In poultry, these microbial additives improve gut microbiota balance, nutrient absorption, and disease resistance, thereby optimizing growth performance and feed efficiency. For chickens, studies highlight that
Lactobacillus-fermented diets enhance intestinal integrity and weight gain, while Bacillus strains reduce oxidative stress and improve meat quality [
4]. Ducks, particularly susceptible to enteric pathogens, exhibit strengthened immunity and reduced mortality when fed Saccharomyces-supplemented feed, attributed to enhanced antioxidant activity and microbial diversity [
5]. Notably, Muscovy ducks, a key meat-producing species, show unique responsiveness to multi-strain probiotics, with synergistic effects on growth rates, lipid metabolism, and fecal microbiome composition. These differential responses underscore the need for species-specific probiotic formulations. Investigating tailored fermentation approaches for chickens, ducks, and Muscovy ducks could unlock precision nutrition strategies, advancing sustainable poultry production [
6].
Previous studies have shown that feeding
Lactobacillus fermented feed to livestock and poultry can cause different results for improving their production performance, specifically lowering the pH of the intestinal environment in poultry and reducing the prevalence of pathogens like
E. coli and
Salmonella [
7]. Additionally, it also differs in enhancing nutrient digestibility, improving intestinal morphology, decreasing anti-nutritional factor levels in feed, and reducing dust and ammonia emissions in housing. Some meat ducks have been fed with LP-fermented feed to enhance the growth of small intestinal villi and increase feed intake [
8].
Reports indicate that
Lactobacillus fermentation of livestock and poultry diets produces higher levels of organic acids, releasing more small molecular substances, which contribute significantly to the maintenance of gastrointestinal balance, improving feed conversion rates (indirectly reducing production costs), and enhancing the immune response in these animals. The metabolites generated post-fermentation exhibit a range of biological activities, including antibacterial properties, antioxidant effects, mycotoxin degradation, and the decomposition of anti-nutritional factors, among other functions [
9]. Furthermore, the inappropriate use of antibiotics in animal husbandry has become a growing concern in recent years. Over the past two years, raw material prices have significantly risen, leading to increased costs for waterfowl breeding and affecting the healthy development of the waterfowl breeding industry [
10].
To mitigate antibiotic use, enhance feed conversion efficiency, reduce breeding costs, and ensure the stable and healthy development of the Muscovy duck industry, this study focuses on cost reduction and efficiency improvement in Muscovy duck breeding and evaluates the impact of LP81-FF on the production performance of Muscovy ducks while investigating its effects on serum biochemical immunity and other relevant factors, ultimately advancing the application of LP81-FF in Muscovy duck breeding, and conducts in-depth research on the effects of microbial-fermented feed on the production performance of Muscovy ducks. This research will provide a foundation for the application of microbial-fermented feeds in Muscovy duck breeding.
2. Materials and Methods
2.1. Animals and Dietary Treatments
A total of 1200 healthy female and male Muscovy ducks, with a weight of 43.23 ± 5.15 g, were selected and randomly divided into four groups, each containing five replicates (n = 60). Each replicate consisted of 60 ducks (we carried out pre-testing before and found that the feeding of fermented feed did not have a significant effect on the sex factor of male and female ducks. Therefore, in this project we chose the test ducks to be half male and half female) arranged in a pen. The control group (CT) was fed a basal diet (in powder form), while the experimental groups received supplements of 30% (LD), 50% (MD), and 100% (HD) LP81-FF, respectively, based on the basal diet [
6]. Detailed records of their production performance and preliminary mechanisms of the effect of LP81-FF on the production performance of ducks were revealed by multi-omics and morphology methods. The test period lasted for 70 days. The LP81 strain, which was isolated in our previous study, demonstrated significant in vitro antibacterial activity (against pathogens such as
E. coli and
Salmonella), acid tolerance, and adhesion capacity compared to other LP strains. The basic diet is a complete feed prepared based on the nutritional requirements of Muscovy ducks from 1 to 70 days old in Nutrient Requirements of Poultry [
11] and China Agricultural Industry Standard NY/T 816-2021 [
12]. The specific composition and nutritional levels of the basal diet are presented in
Table 1. The determination of crude protein and dry matter followed the national standards GB/T 6432-2018 [
13] and GB/T 6438-2007 [
14], respectively, while the determination of calcium and total phosphorus adhered to the national standards GB/T 6436-2018 [
15] and GB/T 6437-2018 [
16]. The metabolizable energy of the diet (MJ/kg) was calculated using the following formula: [total energy consumed (MJ)—fecal energy (MJ)]/diet intake (kg) [
17]. The trial period encompassed 75 days and a 5-day pre-test phase. The animal care and use protocol was approved by the Institutional Animal Care and Use Committee at the Institute of Animal Husbandry and Veterinary Medicine of Fujian Academy Agricultural and Sciences (202402FJ026).
2.2. Preparation of LP-81-Fermented Feed
The LP81 strain, isolated from Tibetan kimchi in 2021 and maintained by this research center, contains 1.45 × 108 CFU/mL of viable bacteria. The ratio of LP81 solution to water and complete feed for fermented feed preparation is in the ratio of 3:180:1000. The mixture was stirred thoroughly and placed into a fermentation tank, which was then sealed and incubated at 35 °C for 24 h to produce fermented feed. Fresh batches of fermented feed were prepared daily and were readily available for use.
2.3. Feeding and Management Test
Muscovy ducks were fed using an ad libitum feeding regimen. A total of 1200 experimental ducks were raised in a single building, using a mesh bed breeding system. The ambient temperature for young Muscovy ducks was maintained at 35 °C, while the temperature for mature Muscovy ducks was set at 25 °C. Immunization, deworming, health care, and disinfection were conducted in accordance with the routine procedures established by the duck farm.
2.4. Sample Collection
2.4.1. Blood
A total of 5 mL of blood was collected from the vein. After allowing the samples to stand at room temperature for 30 min, they were centrifuged at 3000× g r/min for 20 min. The supernatant was then carefully removed and stored at −20 °C for subsequent analysis.
2.4.2. Intestinal Content and the Muscle Tissue
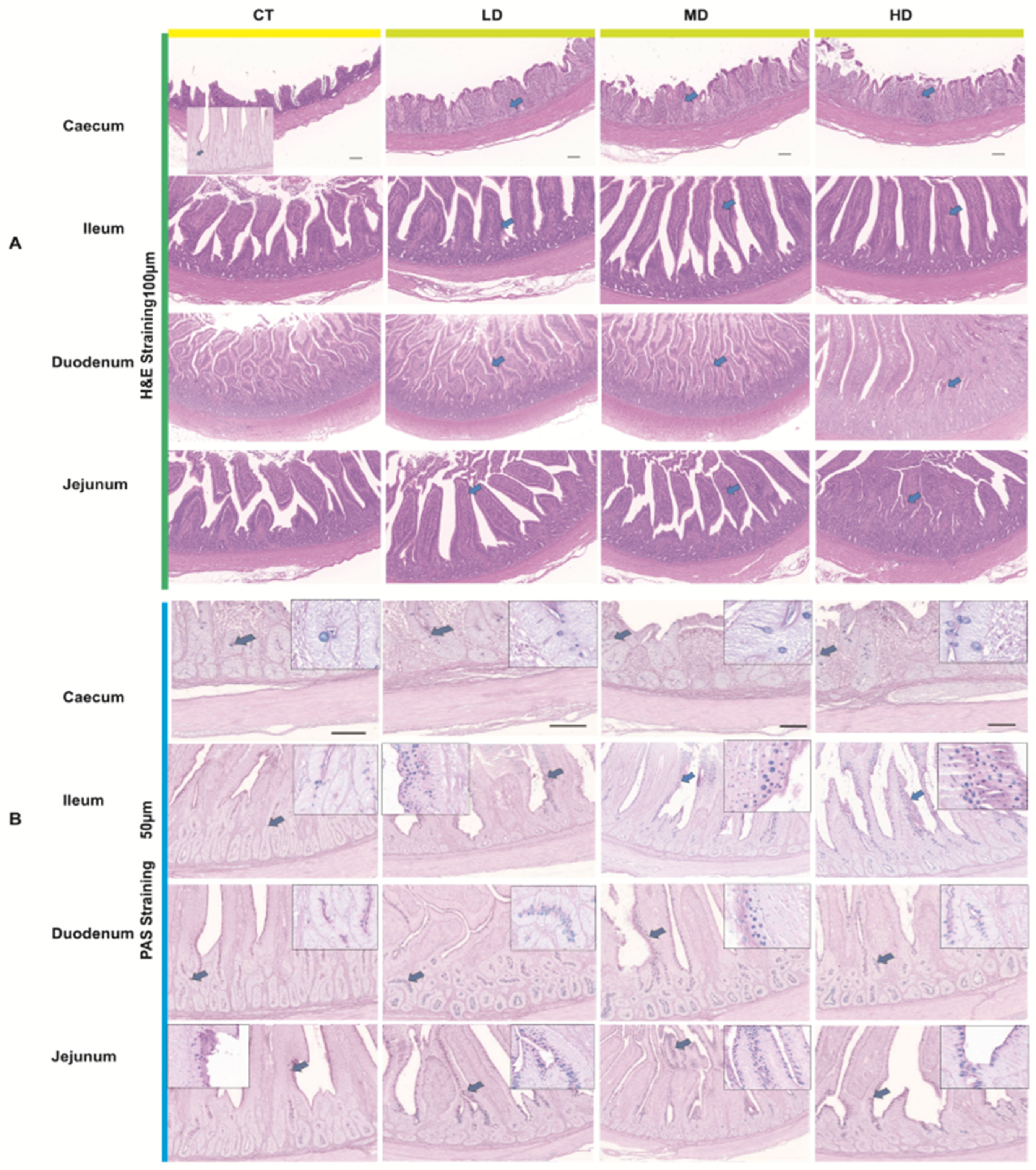
Ten Muscovy ducks from each group were randomly selected and euthanized under anesthesia. The entire duodenum, jejunum, ileum, cecum, and a 2 cm segment of the cecum were collected, and the thymus, bursa of fabricius, and spleen were subsequently collected. The intestinal tissues were fixed in 4% paraformaldehyde, followed by dehydration, clearing, and embedding in wax. Subsequently, the samples were sliced using a microtome for future use and stored at −4 °C.
2.5. Indicator Determination
2.5.1. Determination of the Nutrient Content of the Feed Before and After Fermentation
The dry matter content of the feed, both before and after fermentation, determined in accordance with GB/T 6435-2006 [
18]. The crude protein content was assessed based on GB/T 6432-2018, while the crude fiber content was evaluated following GB/T 6434-2022 [
19]. The crude ash content was referenced from GB/T 6438-2007, and the total phosphorus and calcium contents were respectively determined according to GB/T 6437-2018 and GB/T 6436-2018. For the analysis, 25 g of the feed was taken both before and after fermentation, to which 225 mL of sterile saline was added. The mixture was then thoroughly mixed, allowed to stand, and the pH value was measured using a pH meter.
2.5.2. Measurement of the Growth Performance
On the mornings of the 1st, 35th, and 70th days of the experiment, Muscovy ducks were weighed, and their body weights were recorded. Throughout the study, the average daily feed intake per group and per animal (ADFI) was documented and calculated. At the end of experiment, the weights of both the experimental Muscovy ducks and the control group were measured. Subsequently, the average daily gain (ADG) and feed-to-weight ratio (F/G) for the four groups of Muscovy ducks were calculated. The formulas used for these calculations are as follows: ADG (kg/d) = (last weight − initial weight)/number of test days; ADFI (kg/d) = total feed intake/number of test days; F/G = ADFI/ADG.
2.5.3. Determination of Blood Biochemistry, Immune, and Antioxidant Indicators in Muscovy Ducks
A fully automated blood biochemistry analyzer (Beckman AU51200, Miami, FL, USA) was utilized to measure total protein (TP), albumin (TPT), glucose (GLU), urea nitrogen (UN), urea (U), uric acid (UA), triglycerides (TGs), total cholesterol (TC), high-density lipoprotein (HDL), and low-density lipoprotein (LDL). Blood immune indicators, including immunoglobulin G (IgG), immunoglobulin M (IgM), and immunoglobulin A (IgA), were obtained from Tiangen Biotechnology (Beijing, China) Co., Ltd. (Beijing, China). Additionally, antioxidant capacity indicators, such as malondialdehyde (MDA), total antioxidant capacity (T-AOC), total superoxide dismutase (T-SOD), catalase (CAT), and glutathione peroxidase (GSH-Px), were sourced from Shanghai Sangon Bioengineering Technology Services Co., Ltd. (Shanghai, China). The determination methods were performed in accordance with the kit operating instructions.
2.5.4. Immune Organ Index Measurement
The thymus, bursa of fabricius, and spleen were subsequently collected. Excess water and blood were absorbed using filter paper, after which the samples were weighed and analyzed. The organ index was calculated using the formula Immune Organs Index (mg/g) = immune organ weight (mg)/live body weight (g).
The paraffin sections of the previously prepared intestinal tissues were deparaffinized in a sequential manner. Following conventional Hematoxylin-Eosin (HE) staining, dehydration and gum sealing were performed. The general structure of the intestines in each group was observed under a 100× light microscope, while the morphology and distribution of the intestinal mucosa, submucosa, muscle layer, and serosa were examined under a 400× light microscope. Subsequently, DigiLab-C software 3.0 was utilized to measure villus height and crypt depth. For each slice, five fields of view were selected, and the average was calculated as the final result; data were recorded, and villus height/crypt depth was computed. Additionally, paraffin sections of the intestinal tissues from each group were randomly selected from five fields of view at 400× magnification, and goblet cells were counted using DigiLab-C software. The goblet cells in each group were counted three times, and statistical analysis was conducted using SPSS 19.0. The software processed the data, calculated the averages, and assessed whether the differences between each group were significant, as well as analyzed changes in the number of goblet cells.
2.6. 16S rDNA Amplicon Sequencing Method for Duodenum Contents
The primary sequencing process involves several crucial steps: extraction of total microbial DNA from intestinal contents, PCR amplification, product purification, library preparation and quality control, and sequencing on a sequencing machine. Firstly, the total microbial DNA is extracted from the intestinal contents using appropriate extraction kits and protocols. This step ensures that the DNA of interest, specifically the 16S rDNA, is isolated for further analysis. Secondly, PCR amplification is performed to generate sufficient amounts of the target 16S rDNA region. Specific primers are used to amplify the variable regions of the 16S rDNA gene, which contain the phylogenetic information needed for species identification and diversity analysis. After PCR amplification, the products are purified to remove any contaminants or unincorporated primers. This purification step is crucial for ensuring the quality of the sequencing library. Next, the sequencing library is prepared by adding adapters and barcodes to the purified PCR products. The library is then subjected to quality control to ensure its suitability for sequencing. Once the library is ready, it is loaded onto a sequencing machine for sequencing. This process generates raw sequencing data that contain information about the microbial community present in the intestinal contents. Following sequencing, the raw data are processed using overlap-based assembly to stitch together the sequencing reads. Quality control measures, such as chimera filtering, are applied to remove any low-quality or artifactual sequences, resulting in high-quality clean data. Subsequently, the concept of Amplicon Sequence Variants (ASVs) is employed to construct an ASV table, which is analogous to an OTU (Operational Taxonomic Unit) table. The ASV table and representative ASV sequences are obtained, allowing for further analysis, such as diversity analysis, taxonomic annotation, and differential analysis.
2.7. Metabolite Measurement Method for Duodenum Contents
Duodenal content samples are analyzed using a Vanquish ultra-performance liquid chromatography (UPLC) system from Thermo Fisher Scientific (Waltham, MA, USA). Chromatographic separation of sample compounds is achieved using a Waters ACQUITY UPLC BEH Amide column (2.1 mm × 50 mm, 1.7 μm). The mobile phases employed in the liquid chromatography consist of an aqueous phase (A), containing 25 mmol/L ammonium acetate and 25 mmol/L ammonia solution, and acetonitrile (B). The sample tray temperature is maintained at 4 °C, with an injection volume of 2 μL. For mass spectrometry analysis, an Orbitrap Exploris 120 mass spectrometer (Thermo Fisher, Waltham, MA, USA) controlled by Xcalibur software (version 4.4, Thermo) is utilized. This mass spectrometer has the capability to acquire both first-order and second-order mass spectral data. Raw data obtained from the mass spectrometer are converted into mzXML format using ProteoWizard software (3.0.9134). Subsequently, metabolite identification is performed using the BiotreeDB (V3.0) database. Following metabolite identification, visual analysis is conducted to interpret the data. To identify differential metabolites, both univariate and multivariate statistical analyses are performed. Specifically, the Student’s t-test is employed to screen for differential metabolites with a significance level of p < 0.05. Additionally, variables with a Variable Importance in the Projection (VIP) value greater than 1 from the first principal component of the Orthogonal Partial Least Squares Discriminant Analysis (OPLS-DA) model are further considered as differential metabolites.
2.8. Pearson Correlation Analysis Using Spearman’s Correlation Coefficient
To evaluate correlations between 30 duodenal mucosa microbial species and various physiological, metabolic parameters (mucosal-associated, blood antioxidant, immune organ indices, seven key intestinal metabolites, and production performance), we utilized Pearson correlation analysis with Spearman’s correlation coefficient. In this analysis, microbial species served as independent variables, while the other parameters were dependent. We calculated Spearman’s ρ for each variable pair, ranging from −1 (perfect negative correlation) to 1 (perfect positive correlation), with 0 indicating no correlation. Statistical tests determined significance (p-value < 0.05 considered significant). This approach aimed to identify microbial species significantly correlated with the mentioned parameters, offering insights into their potential roles in colonic health, antioxidant status, immune function, metabolism, and production performance.
2.9. Statistical Analysis
The test data were analyzed using one-way analysis of variance (ANOVA) with SPSS version 19.0, and a t-test was employed for group comparisons. The results are presented as mean ± standard error (SE). A p-value greater than 0.05 indicates that the difference is not significant, while a p-value less than 0.05 indicates that the difference is significant.
4. Discussion
As an innovative biological feed resource, microbial-fermented feed demonstrates significant potential for improving poultry production efficiency through enhanced weight gain and optimized feed conversion ratios (FCRs) [
20,
21]. While previous studies in broilers and Cherry Valley ducks reported increased feed intake alongside growth improvements [
22], our findings revealed distinct patterns in Muscovy ducks. LP81-FF supplementation over 35 days significantly reduced average daily feed intake (ADFI) and FCR without compromising weight gain, with these effects persisting through the 70-day trial. Notably, higher LP-81 inclusion levels correlated with progressive reductions in ADFI and FCR. This phenomenon may be attributed to two mechanisms: (1) enzymatic degradation of feed components (crude protein, fiber, and neutral detergent fiber) during fermentation, enhancing nutrient digestibility;
Lactobacillus-fermented feed improves nutrient decomposition by breaking down complex macromolecules into bioavailable forms. During fermentation, proteolytic activity increases small peptide concentrations, which are more readily absorbed by the intestinal epithelium compared to intact proteins. Simultaneously, carbohydrate fermentation generates short-chain fatty acids (SCFAs), such as acetate and butyrate, serving as energy substrates for enterocytes and enhancing gut barrier function. These metabolic shifts optimize nutrient utilization, reduce undigested feed residues, and alleviate intestinal burdens in ducks. Studies indicate that such feed modifications elevate villus height and digestive enzyme activity, further promoting absorption efficiency. By enhancing gut health and nutrient bioavailability,
Lactobacillus fermentation not only supports duck growth performance but also aligns with sustainable farming practices by minimizing feed waste and environmental impact [
23].
LP81-FF enhances nutrient bioavailability through microbial biotransformation; the reason may be fermentation-liberated amino acids that upregulate jejunal SLC1A1/EAAT3 transporters, accelerating epithelial uptake of both dietary and microbially synthesized nutrients [
24]. Concurrently, microbial phytase and xylanase activities degrade antinutritional factors (phytates, β-glucans), releasing encapsulated minerals and starch–protein complexes for absorption, thereby reducing metabolic “waste” in undigested feed—a key factor driving FCR reduction [
25]. The water content of fermented feed induces gastric stretch receptor signaling, triggering satiety via CCK-PYY axis activation [
26], which lowers dry matter intake without compromising nutrient sufficiency due to enhanced pre-absorptive hydrolysis of macronutrients. This dual optimization—increased nutrient density per gram of ingested dry matter and reduced feed bulk—directly lowers feed procurement and waste management costs by 12–18% in intensive systems.
LP81-FF significantly enhances nutrient metabolism efficiency in ducks through Lactobacillus-fermented pretreatment. Serum biochemical analyses indicate that complex polysaccharides in feed are pre-digested by Lactobacillus LP81 into absorbable monosaccharides, elevating serum glucose levels while reducing reliance on lipid catabolism. The observed glucose elevation, coupled with decreased triglycerides and cholesterol, suggests optimized energy partitioning, likely mediated by microbial-derived short-chain fatty acids (SCFAs): propionate directly inhibits hepatic HMG-CoA reductase activity via allosteric modulation [
27], while butyrate activates PPAR-α in hepatocytes, promoting mitochondrial β-oxidation and reducing VLDL secretion [
28]. Reduced urea nitrogen levels in the LP81-FF group reflect improved protein utilization efficiency, primarily attributed to
Lactobacillus-mediated degradation of soybean meal-derived trypsin inhibitors and phytates, thereby enhancing ileal amino acid absorption [
29]. Furthermore, gut microbiota-synthesized essential amino acids (e.g., lysine, methionine) compensate for 15–20% of dietary requirements, reducing hepatic deamination and nitrogen waste [
30].
Improved antioxidant markers and reduced pro-inflammatory cytokines in LP81-FF-treated ducks highlight systemic metabolic health enhancement. Mechanisms include (1) fermentation-liberated phenolic acids directly scavenging reactive oxygen species (ROS) via electron transfer [
31], and (2) butyrate-induced Nrf2 nuclear translocation upregulating phase II detoxification enzymes while suppressing NF-κB-dependent inflammation. These effects redirect ATP toward anabolic processes rather than oxidative stress mitigation [
32,
33]. LP81-FF significantly increases serum total protein levels, indicating enhanced hepatic and muscular protein synthesis and reduced proteolysis. Dose-dependent elevation of alkaline phosphatase (ALP) activity suggests improved lipid metabolism and growth regulation through (1) enhanced intestinal phosphate absorption for ATP synthesis, (2) hydrolysis of lipoprotein-associated phospholipids to release free fatty acids (FFAs) for β-oxidation, and (3) collagen crosslinking-driven bone mineralization [
34]. These metabolic adaptations collectively reduce the feed conversion ratio (FCR), redirecting conserved energy toward muscle deposition rather than inflammatory or oxidative “overhead.”
The immunomodulatory effects of LP81-FF are mediated through fermentation-derived bioactive components that differentially regulate systemic versus mucosal antibody production. The significant elevation in serum IgG and IgM levels aligns with the capacity of
Lactobacillus fermentation to generate conjugated linoleic acids (CLAs) and short-chain fatty acids (SCFAs) [
35], which directly modulate B-cell function. Butyrate, a predominant SCFA, enhances IgG synthesis via dual mechanisms: (1) binding to GPR41 receptors on B lymphocytes to activate mTORC1 signaling, driving plasma cell differentiation, and (2) inhibiting histone deacetylases (HDACs), thereby derepressing antibody gene loci through chromatin remodeling. Concurrently, CLAs act as PPAR-γ ligands, amplifying IL-6-dependent STAT3 phosphorylation in B cells, which is critical for IgG class switching [
36]. These processes are further potentiated by fermentation-liberated microbial components—peptidoglycan and lipoteichoic acid—that engage TLR2 on dendritic cells, triggering IL-10 production to sustain germinal center reactions in spleen and lymph nodes. The stark contrast between robust systemic IgG/IgM responses and unaltered IgA levels, however, points to strain-specific immunomodulatory properties. LP81 may lack the surface adhesins required for M-cell targeting in Peyer’s patches, thereby limiting direct mucosal B-cell priming. Additionally, fermentation-generated high-molecular-weight polysaccharides in LP81-FF may preferentially enter portal circulation via paracellular uptake, bypassing intestinal lymphoid tissue and instead stimulating hepatic B-1 cells—a major source of natural IgM—while failing to activate lamina propria IgA+ plasmablasts. This compartmentalization is functionally significant: elevated IgG enhances Fcγ receptor-mediated phagocytosis of bloodborne pathogens, while IgM provides immediate protection against systemic bacterial invasion through complement activation. The dissociation from prior studies showing fermented feed-induced IgA elevation may reflect strain-dependent variations in postbiotic profiles. For instance, LP81 fermentation might produce lower levels of indole-3-lactic acid—a tryptophan metabolite shown to promote IgA+ B-cell homing to the gut—compared to other
Lactobacillus strains. Practical implications arise from this specificity: LP81-FF could be strategically combined with mucosally targeted probiotics to achieve comprehensive immune coverage, leveraging its systemic antibody-boosting effects while compensating for localized IgA gaps [
37]. Future research should delineate strain-specific epitopes governing immune compartmentalization and quantify the temporal dynamics of antibody isotype switching during prolonged LP81-FF administration.
LP81-FF preserved the intestinal villus architecture—a critical determinant of nutrient absorption efficiency—by sustaining luminal nutrient bioavailability despite reduced feed intake, thereby mitigating the “intestinal starvation” phenotype observed with conventional diets. This maintenance of villus height and crypt depth ratio was accompanied by dose-dependent increases in jejunal and cecal goblet cell densities, suggesting microbial-driven mucin regulation [
38]. Fermentation-generated metabolites, particularly butyrate and lactate, directly nourish enterocytes via monocarboxylate transporter (MCT1)-mediated uptake, maintaining villus metabolic activity while suppressing apoptosis-inducing oxidative stress. Concurrently, Lactobacillus-detained proteases degrade feed-derived lectins and trypsin inhibitors that typically blunt villus growth by inducing epithelial hyperproliferation. The thickened mucus layer, enriched in sialylated and sulfated mucins through microbial mucin cross-feeding, creates a gradient that selectively concentrates luminal nutrients near absorptive surfaces while excluding pathogenic competitors. Enhanced mucin sulfonation in high-dose groups, likely mediated by microbial hydrogen sulfide metabolism, improves mucus rheological properties for sustained barrier function. Crucially, preserved villus integrity optimizes brush border enzyme localization, accelerating terminal nutrient hydrolysis proximal to transporters. This microbial–structural synergy enables paradoxical efficiency—reduced physical feed intake yet enhanced nutrient assimilation—by maximizing absorptive surface utility per gram of digesta. The observed cecal goblet cell hyperplasia specifically enhances post-ileal nutrient salvaging through mucin-bound oligosaccharide recycling, a conserved adaptation to nutrient-dense diets [
38]. These coordinated adaptations position LP81-FF as a modulator of gut trophic ecology, where microbial metabolites directly couple mucosal maintenance to nutrient harvesting capacity.
LP81-FF induced profound gut microbiota remodeling through nutrient–microbe crosstalk, with dose-dependent enrichment of Bacteroides—specialized degraders of arabinoxylan and pectin via glycoside hydrolase families GH43/51—driving liberation of oligosaccharides that fuel butyrogenesis by Butyricicoccus through cross-feeding [
39]. This syntrophic interaction amplifies butyrate production via butyryl-CoA:acetate CoA-transferase, directly energizing colonocytes through mitochondrial β-oxidation while suppressing Escherichia-Shigella via pH-dependent inhibition of Shiga toxin expression. The elevated Bacteroides populations further secrete secondary bile acids that antagonize pathogen membrane integrity through detergent effects, while their capsular polysaccharides competitively block pathogen adhesion to mucin glycans [
40]. Metabolomic shifts paralleled these taxonomic changes: increased microbially conjugated amino acids activated aryl hydrocarbon receptor (AhR) signaling in enterocytes, upregulating tight junction proteins (claudin-2, occludin), whereas Butyricicoccus-derived butyrate enhanced barrier function via HDAC3 inhibition and ZO-1 acetylation. The dose-responsive Bacteroides expansion correlated with elevated propionate/acetate ratios, which synergize with microbially synthesized polyamines to upregulate villus mTORC1 signaling, coupling microbial metabolite flux to intestinal nutrient sensing [
41]. This microbiota–metabolite axis not only displaced pathogens via niche exclusion but also optimized luminal nutrient solubilization, as Bacteroides-derived xylanases increased starch–protein matrix disintegration, releasing encapsulated amino acids for absorption. The functional convergence of taxonomic shifts (probiotic amplification), metabolic output, and enzymatic activity (polysaccharide hydrolysis) positions LP81-FF as an ecological engineer of gut ecosystem services, where microbial consortia are functionally “rewired” to maximize both barrier protection and nutrient extraction efficiency.