Predicting Organic Acid Variation in White Wine Malolactic Fermentation Using a Logistic Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Grapes’ Harvest and Vinification
2.2. Wine Characterization
2.3. Extraction of Volatile Compounds and Gas Chromatography-Mass Spectrometry (GC-MS) Analysis
2.4. Statistical Analysis
3. Results and Discussion
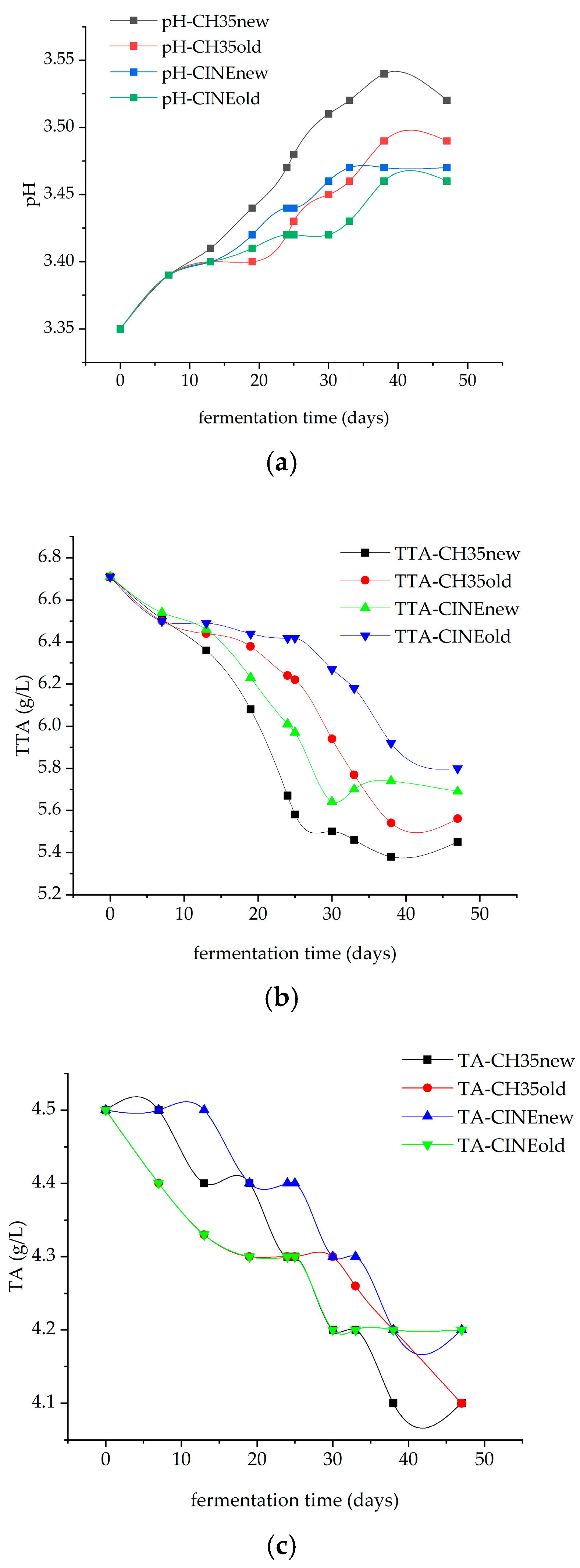
3.1. Variation in pH and Acidity During Fermentation
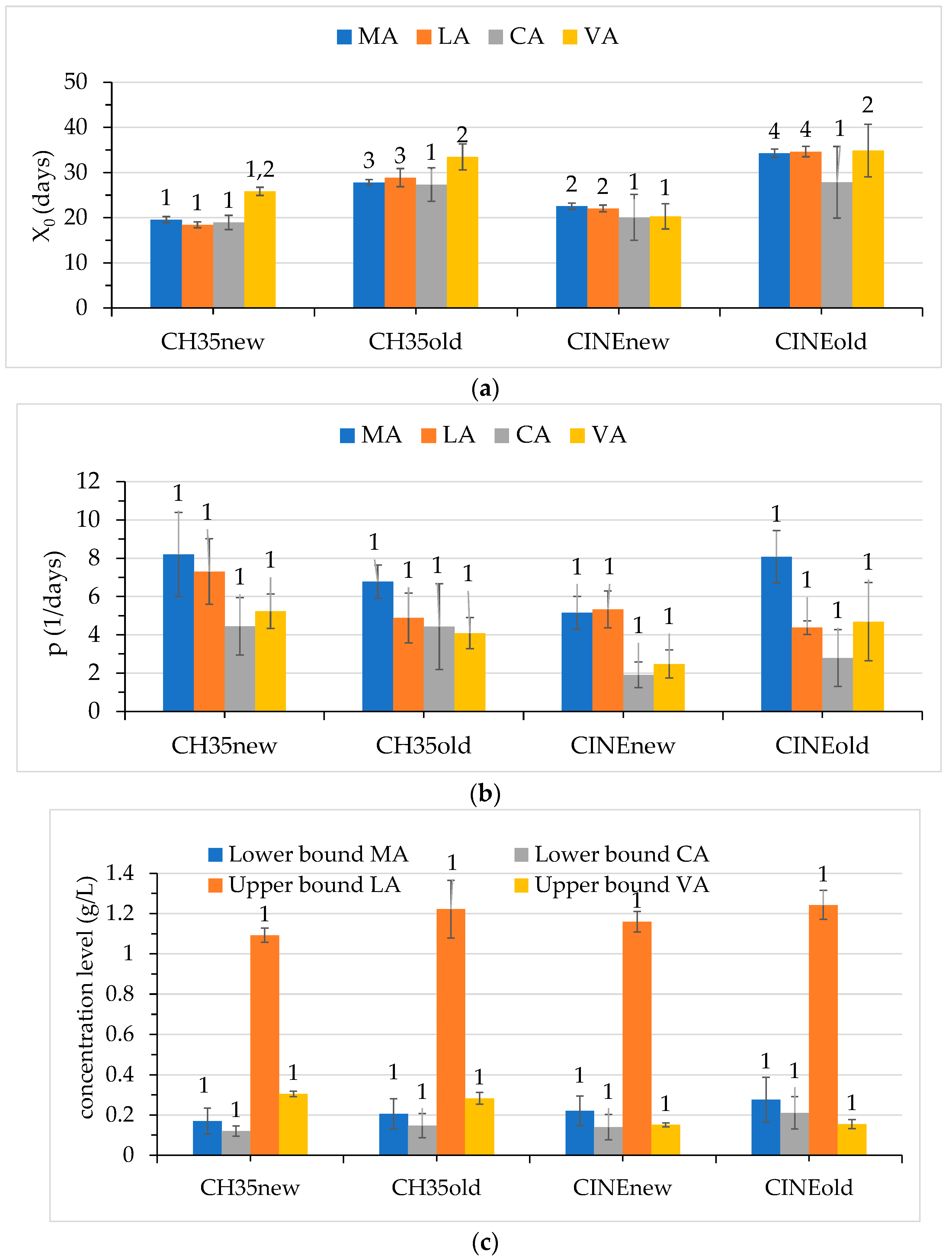
3.2. Variation in the Organic Acids During Malolactic Fermentation
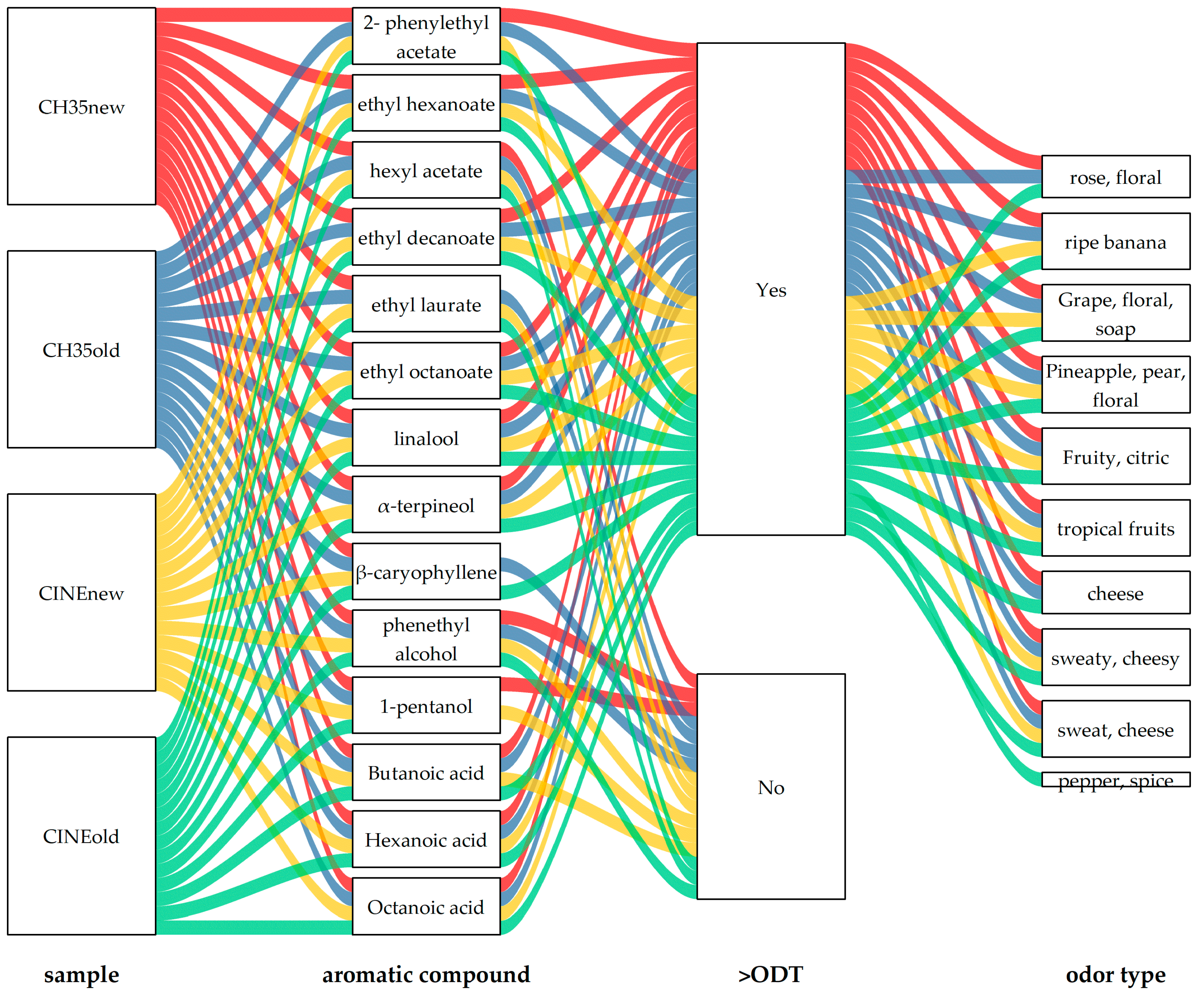
3.3. Volatile Profile of Wines
| Chemical Group | Aromatic Compound | ODT (mg/L) | Ch35new | CH35old | CINEnew | CINEold | Aromatic Description | Literature Reference |
|---|---|---|---|---|---|---|---|---|
| Esters | 2- phenylethyl acetate | 0.25 | 0.537 ± 0.038 b | 0.479 ± 0.031 b | 0.103 ± 0.012 a | 0.486 ± 0.022 b | rose, floral | [53] |
| ethyl hexanoate | 0.005 | 1.305 ± 0.064 b | 1.100 ± 0.148 b | 0.235 ± 0.032 a | 1.347 ± 0.073 b | ripe banana | [53] | |
| hexyl acetate | 1.50 | 0.156 ± 0.009 b | 0.214 ± 0.003 c | 0.037 ± 0.005 a | 0.251 ± 0.016 d | sweet, perfume | [54] | |
| ethyl decanoate | 0.20 | 2.904 ± 0.048 d | 1.480 ± 0.014 b | 0.464 ± 0.019 a | 1.623 ± 0.006 c | grape, floral, soap | [54] | |
| ethyl laurate | 1.50 | ND * | 0.165 ± 0.021 b | 0.049 ± 0.002 a | 0.237 ± 0.013 c | flowers, fruity | [55] | |
| ethyl octanoate | 0.002 | 2.986 ± 0.033 d | 2.222 ± 0.065 b | 0.532 ± 0.117 a | 2.485 ± 0.073 c | pineapple, pear, floral | [56] | |
| Terpenes | linalool | 0.025 | 0.170 ± 0.034 b | 0.158 ± 0.015 b | 0.038 ± 0.006 a | 0.200 ± 0.020 b | fruity, citric | [55] |
| α-terpineol | 0.01 | 0.051 ± 0.001 b | 0.048 ± 0.004 b | 0.010 ± 0.001 a | 0.060 ± 0.004 c | tropical fruits | [57] | |
| β-caryophyllene | 0.064 | ND | 0.056 ± 0.007 a | ND | 0.306 ± 0.051 b | pepper, spice | [58] | |
| Higher alcohols | phenethyl alcohol | 10 | 9.609 ± 0.042 d | 7.934 ± 0.272 b | 1.996 ± 0.177 a | 8.820 ± 0.015 c | flowers, rose | [59] |
| 1-pentanol | 64 | 6.135 ± 0.335 a | ND | 7.220 ± 0.389 b | ND | sweet, balsamic | [54] |
| Aromatic Compound | ODT (mg/L) | CH35new | CH35old | CINEnew | CINEold | Aromatic Description | Literature |
|---|---|---|---|---|---|---|---|
| Butanoic acid | 0.17 | 0.611 ± 0.022 b | 0.574 ± 0.013 b | 0.115 ± 0.007 a | 0.582 ± 0.043 b | cheese | [60] |
| Hexanoic acid | 0.40 | 5.217 ± 0.027 c | 4.196 ± 0.331 b | 0.962 ± 0.092 a | 4.151 ± 0.235 b | sweaty, cheesy | [54] |
| Octanoic acid | 0.50 | 2.220 ± 0.151 b | 7.779 ± 0.352 c | 1.495 ± 0.112 a | 7.483 ± 0.203 c | sweat, cheese | [59] |
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MLF | Malolactic Fermentation |
| O. oeni | Oenococcus oeni |
| LAB | Lactic Acid Bacteria |
| TA | Total Titratable Acidity |
| VA | Volatile Acidity |
| MA | Malic Acid |
| LA | Lactic Acid |
| CA | Citric Acid |
| AA | Acetic Acid |
| ODT | Odor Perception Threshold |
References
- Maicas, S. Advances in Wine Fermentation. Fermentation 2021, 7, 187. [Google Scholar] [CrossRef]
- Paramithiotis, S.; Stasinou, V.; Tzamourani, A.; Kotseridis, Y.; Dimopoulou, M. Malolactic Fermentation—Theoretical Advances and Practical Considerations. Fermentation 2022, 8, 521. [Google Scholar] [CrossRef]
- Mendes Ferreira, A.; Mendes-Faia, A. The Role of Yeasts and Lactic Acid Bacteria on the Metabolism of Organic Acids during Winemaking. Foods 2020, 9, 1231. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.Q. Malolactic fermentation in wine—Beyond deacidification. J. Appl. Microbiol. 2002, 92, 589–601. [Google Scholar] [CrossRef]
- Capozzi, V.; Tufariello, M.; De Simone, N.; Fragasso, M.; Grieco, F. Biodiversity of Oenological Lactic Acid Bacteria: Species- and Strain-Dependent Plus/Minus Effects on Wine Quality and Safety. Fermentation 2021, 7, 24. [Google Scholar] [CrossRef]
- Soufleros, E.H.; Bouloumpasi, E.; Zotou, A.; Loukou, Z. Determination of biogenic amines in Greek wines by HPLC and ultraviolet detection after dansylation and examination of factors affecting their presence and concentration. Food Chem. 2007, 101, 704–716. [Google Scholar] [CrossRef]
- Moreira, L.; Milheiro, J.; Filipe-Ribeiro, L.; Cosme, F.; Nunes, F.M. Exploring factors influencing the levels of biogenic amines in wine and microbiological strategies for controlling their occurrence in winemaking. Food Res. Int. 2024, 190, 114558. [Google Scholar] [CrossRef]
- Wüthrich, B. Allergic and intolerance reactions to wine. Allergol. Sel. 2018, 2, 80–88. [Google Scholar] [CrossRef]
- Paraskevopoulos, Y. Optimization of the management conditions of malolactic fermentation in red wines of the Nemea region. Electron. J. Sci. Technol. 2008, 3. [Google Scholar]
- Lonvaud-Funel, A. Biogenic amines in wines: Role of lactic acid bacteria. FEMS Microbiol. Lett. 2001, 199, 9–13. [Google Scholar] [CrossRef]
- Davis, C.R.; Wibowo, D.; Eschenbruch, R.; Lee, T.H.; Fleet, G.H. Practical Implications of Malolactic Fermentation: A Review. Am. J. Enol. Vitic. 1985, 36, 290. [Google Scholar] [CrossRef]
- EFSA. Scientific Opinion on risk based control of biogenic amine formation in fermented foods-European Food Safety Authority Panel on Biological Hazards (BIOHAZ). EFSA J. 2011, 9, 2393–2486. [Google Scholar] [CrossRef]
- Guerrini, S.; Mangani, S.; Granchi, L.; Vincenzini, M. Biogenic amine production by Oenococcus oeni. Curr. Microbiol. 2002, 44, 374–378. [Google Scholar] [CrossRef]
- Vargas-Luna, C.; Godoy, L.; Benavides, S.; Ceppi de Lecco, C.; Urtubia, A.; Franco, W. Screening and Selection of Native Lactic Acid Bacteria Isolated from Chilean Grapes. Foods 2025, 14, 143. [Google Scholar] [CrossRef]
- Sablayrolles, J.M. Control of alcoholic fermentation in winemaking: Current situation and prospect. Food Res. Int. 2009, 42, 418–424. [Google Scholar] [CrossRef]
- Cano-López, M.; Pardo-Minguez, F.; López-Roca, J.M.; Gómez-Plaza, E. Chromatic characteristics and anthocyanin profile of a micro-oxygenated red wine after oak or bottle maturation. Eur. Food Res. Technol. 2007, 225, 127–132. [Google Scholar] [CrossRef]
- Anli, R.E.; Cavuldak, Ö.A. A review of microoxygenation application in wine. J. Inst. Brew. 2012, 118, 368–385. [Google Scholar] [CrossRef]
- Hernández-Orte, P.; Lapeña, A.C.; Escudero, A.; Astrain, J.; Baron, C.; Pardo, I.; Polo, L.; Ferrer, S.; Cacho, J.; Ferreira, V. Effect of micro-oxygenation on the evolution of aromatic compounds in wines: Malolactic fermentation and ageing in wood. LWT Food Sci. Technol. 2009, 42, 391–401. [Google Scholar] [CrossRef]
- Zhang, T.; Liao, Z.; Li, Z.; Liu, Y.; Liu, Y.; Song, Y.; Qin, Y. Dynamic changes in dissolved oxygen concentration, microbial communities, and volatile compounds during industrial oak-barrel fermentation of Sauvignon Blanc wine. Food Res. Int. 2024, 197, 115250. [Google Scholar] [CrossRef]
- Bartowsky, E.J.; Costello, P.J.; Chambers, P.J. Emerging trends in the application of malolactic fermentation. Aust. J. Grape Wine Res. 2015, 21, 663–669. [Google Scholar] [CrossRef]
- Lerm, E.; Engelbrecht, L.; Du Toit, M. Malolactic fermentation: The ABC’s of MLF. S. Afr. J. Enol. Vitic. 2010, 31, 186–212. [Google Scholar] [CrossRef]
- Lombardi, S.J.; Pannella, G.; Iorizzo, M.; Testa, B.; Succi, M.; Tremonte, P.; Sorrentino, E.; Di Renzo, M.; Strollo, D.; Coppola, R. Inoculum Strategies and Performances of Malolactic Starter Lactobacillus plantarum M10: Impact on Chemical and Sensorial Characteristics of Fiano Wine. Microorganisms 2020, 8, 516. [Google Scholar] [CrossRef]
- Ripper, M. Die schweflige Säure im Weine und deren Bestimmung. (Mitteilungen aus Schmitt’s Laboratorium, [Untersuchungs- Amt, chemische Versuchsstation und hygienisches Institut] zu Wiesbaden.). J. Für Prakt. Chem. 1892, 46, 428–473. [Google Scholar] [CrossRef]
- ISO 12099:2017; Animal Feeding Stuffs, Cereals and Milled Cereal Products. Guidelines for the Application of Near-Infrared Spectrometry. International Organization for Standardization: London, UK, 2017.
- Lenti, L.; Nartea, A.; Orhotohwo, O.L.; Pacetti, D.; Fiorini, D. Development and Validation of a New GC-FID Method for the Determination of Short and Medium Chain Free Fatty Acids in Wine. Molecules 2022, 27, 8195. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Li, L.; Xiao, S.; Chen, Z.; Sarsaiya, S.; Zhang, S.; ShangGuan, Y.; Liu, H.; Xu, D. Callus growth kinetics and accumulation of secondary metabolites of Bletilla striata Rchb.f. using a callus suspension culture. PLoS ONE 2020, 15, e0220084. [Google Scholar] [CrossRef]
- Fleet, G.H.; Heard, G.M. Yeast-Growth during Fermentation. In Wine, Microbiology and Biotechnology; Fleet, G.H., Ed.; Harwood Academic: Lausanne, Switerland, 1993; pp. 27–54. [Google Scholar]
- Guzzo, J.; Jobin, M.-P.; Diviès, C. Increase of sulfite tolerance in Oenococcus oeni by means of acidic adaptation. FEMS Microbiol. Lett. 1998, 160, 43–47. [Google Scholar] [CrossRef]
- Henick-Kling, T. Malolactic fermentation. In Wine, Microbiology and Biotechnology; Fleet, G.H., Ed.; CRC Press: Boca Raton, FL, USA, 1993; pp. 289–326. [Google Scholar]
- Volschenk, H.; Vuuren, H.; Viljoen-Bloom, M. Malic Acid in Wine: Origin, Function and Metabolism during Vinification. S. Afr. J. Enol. Vitic. 2006, 27, 123–136. [Google Scholar] [CrossRef]
- Lucio, O.; Pardo, I.; Krieger-Weber, S.; Heras, J.M.; Ferrer, S. Selection of Lactobacillus strains to induce biological acidification in low acidity wines. LWT 2016, 73, 334–341. [Google Scholar] [CrossRef]
- Bartowsky, E.J. Oenococcus oeni and malolactic fermentation—Moving into the molecular arena. Aust. J. Grape Wine Res. 2005, 11, 174–187. [Google Scholar] [CrossRef]
- Eicher, C.; Coulon, J.; Favier, M.; Alexandre, H.; Reguant, C.; Grandvalet, C. Citrate metabolism in lactic acid bacteria: Is there a beneficial effect for Oenococcus oeni in wine? Front. Microbiol. 2023, 14, 1283220. [Google Scholar] [CrossRef]
- Lorentzen, M.P.G.; Lucas, P.M. Distribution of Oenococcus oeni populations in natural habitats. Appl. Microbiol. Biotechnol. 2019, 103, 2937–2945. [Google Scholar] [CrossRef]
- Popescu-Mitroi, I.; Radu, D.; Stoica, F. The study of glycerol metabolism in the malolactic fermentation of red wines. Rom. Biotechnol. Lett. 2014, 19, 9019–9027. [Google Scholar]
- McDaniel, M.; Henderson, L.A.; Watson, B.T.; Heatherbell, D. Sensory Panel Training and Screening for Descriptive Analysis of the Aroma of Pinot Noir Wine Fermented by Several Strains of Malolactic Bacteria. In Descriptive Sensory Analysis in Practice; Wiley: Hoboken, NJ, USA, 2004; pp. 351–369. [Google Scholar]
- Davis, C.R.; Wibowo, D.J.; Lee, T.H.; Fleet, G.H. Growth and Metabolism of Lactic Acid Bacteria during and after Malolactic Fermentation of Wines at Different pH. Appl. Environ. Microbiol. 1986, 51, 539–545. [Google Scholar] [CrossRef]
- Izquierdo-Cañas, P.M.; Mena-Morales, A.; García-Romero, E. Malolactic fermentation before or during wine aging in barrels. LWT—Food Sci. Technol. 2016, 66, 468–474. [Google Scholar] [CrossRef]
- Malherbe, S.; Bauer, F.; Du Toit, M. Understanding Problem Fermentations—A Review. S. Afr. J. Enol. Vitic. 2007, 28, 169–186. [Google Scholar] [CrossRef]
- Hornsey, I.S. The Yeast and Fermentation. In The Chemistry and Biology of Winemaking; Hornsey, I.S., Ed.; The Royal Society of Chemistry: London, UK, 2007; pp. 114–238. [Google Scholar]
- Ferrari, G. Influence of must nitrogen composition on wine and spirit quality and relation with aromatic composition and defects—A review. J. Int. Des Sci. De La Vigne Vin 2002, 36, 1–10. [Google Scholar] [CrossRef]
- Knoll, C.; Fritsch, S.; Schnell, S.; Grossmann, M.; Krieger-Weber, S.; du Toit, M.; Rauhut, D. Impact of different malolactic fermentation inoculation scenarios on Riesling wine aroma. World J. Microbiol. Biotechnol. 2012, 28, 1143–1153. [Google Scholar] [CrossRef]
- Siebert, T.E.; Barker, A.; Pearson, W.; Barter, S.R.; de Barros Lopes, M.A.; Darriet, P.; Herderich, M.J.; Francis, I.L. Volatile Compounds Related to ‘Stone Fruit’ Aroma Attributes in Viognier and Chardonnay Wines. J. Agric. Food Chem. 2018, 66, 2838–2850. [Google Scholar] [CrossRef]
- Qu, J.; Chen, X.; Wang, X.; He, S.; Tao, Y.; Jin, G. Esters and higher alcohols regulation to enhance wine fruity aroma based on oxidation-reduction potential. LWT 2024, 200, 116165. [Google Scholar] [CrossRef]
- Celik, Z.D.; Cabaroglu, T.; Krieger-Weber, S. Impact of malolactic fermentation on the volatile composition of Turkish Kalecik karası red wines. J. Inst. Brew. 2019, 125, 92–99. [Google Scholar] [CrossRef]
- Billet, K.; Thibon, C.; Badet, M.L.; Wirgot, N.; Noret, L.; Nikolantonaki, M.; Gougeon, R.D. White wines aged in barrels with controlled tannin potential exhibit correlated long-term oxidative stability in bottle. Food Chem. X 2024, 24, 101907. [Google Scholar] [CrossRef]
- Ashrafi, B.; Rashidipour, M.; Gholami, E.; Sattari, E.; Marzban, A.; Kheirandish, F.; Khaksarian, M.; Taherikalani, M.; Soroush, S. Investigation of the phytochemicals and bioactivity potential of essential oil from Nepeta curvidens Boiss. & Balansa. S. Afr. J. Bot. 2020, 135, 109–116. [Google Scholar] [CrossRef]
- Shi, X.; Liu, Y.; Ma, Q.; Wang, J.; Luo, J.; Suo, R.; Sun, J. Effects of low temperature on the dynamics of volatile compounds and their correlation with the microbial succession during the fermentation of Longyan wine. LWT 2022, 154, 112661. [Google Scholar] [CrossRef]
- Knoll, C.; Fritsch, S.; Schnell, S.; Grossmann, M.; Rauhut, D.; du Toit, M. Influence of pH and ethanol on malolactic fermentation and volatile aroma compound composition in white wines. LWT Food Sci. Technol. 2011, 44, 2077–2086. [Google Scholar] [CrossRef]
- Navarro, M.; Kontoudakis, N.; Gómez-Alonso, S.; García-Romero, E.; Canals, J.M.; Hermosín-Gutíerrez, I.; Zamora, F. Influence of the volatile substances released by oak barrels into a Cabernet Sauvignon red wine and a discolored Macabeo white wine on sensory appreciation by a trained panel. Eur. Food Res. Technol. 2018, 244, 245–258. [Google Scholar] [CrossRef]
- Rapp, A.; Mandery, H. Wine aroma. Experientia 1986, 42, 873–884. [Google Scholar] [CrossRef]
- Costello, P. The chemistry of malolactic fermentation. In Malolactic Fermentation in Wine; Morenzoni, R., Specht, K.S., Eds.; Lallemand Inc.: Montreal, QC, Canada, 2005; pp. 4:1–4:11. [Google Scholar]
- Izquierdo Cañas, P.M.; García Romero, E.; Gómez Alonso, S.; Palop Herreros, M.L.L. Changes in the aromatic composition of Tempranillo wines during spontaneous malolactic fermentation. J. Food Compos. Anal. 2008, 21, 724–730. [Google Scholar] [CrossRef]
- Kelebek, H.; Selli, S. Determination of volatile, phenolic, organic acid and sugar components in a Turkish cv. Dortyol (Citrus sinensis L. Osbeck) orange juice. J. Sci. Food Agric. 2011, 91, 1855–1862. [Google Scholar] [CrossRef]
- Jiang, B.; Zhang, Z. Volatile compounds of young wines from cabernet sauvignon, cabernet gernischet and chardonnay varieties grown in the loess plateau region of china. Molecules 2010, 15, 9184–9196. [Google Scholar] [CrossRef]
- Bayram, M.; Kayalar, M. White wines from Narince grapes: Impact of two different grape provenances on phenolic and volatile composition. OENO One 2018, 52, 81–92. [Google Scholar] [CrossRef]
- Chigo-Hernandez, M.M.; Tomasino, E. Aroma Perception of Limonene, Linalool and α-Terpineol Combinations in Pinot Gris Wine. Foods 2023, 12, 2389. [Google Scholar] [CrossRef] [PubMed]
- Sommano, S.R.; Chittasupho, C.; Ruksiriwanich, W.; Jantrawut, P. The Cannabis Terpenes. Molecules 2020, 25, 5792. [Google Scholar] [CrossRef] [PubMed]
- Selli, S.; Cabaroglu, T.; Canbas, A.; Erten, H.; Nurgel, C.; Lepoutre, J.P.; Gunata, Z. Volatile composition of red wine from cv. Kalecik Karasι grown in central Anatolia. Food Chem. 2004, 85, 207–213. [Google Scholar] [CrossRef]
- de Revel, G.; Martin, N.; Pripis-Nicolau, L.; Lonvaud-Funel, A.; Bertrand, A. Contribution to the Knowledge of Malolactic Fermentation Influence on Wine Aroma. J. Agric. Food Chem. 1999, 47, 4003–4008. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karampatea, A.; Skendi, A.; Manoledaki, M.; Bouloumpasi, E. Predicting Organic Acid Variation in White Wine Malolactic Fermentation Using a Logistic Model. Fermentation 2025, 11, 288. https://doi.org/10.3390/fermentation11050288
Karampatea A, Skendi A, Manoledaki M, Bouloumpasi E. Predicting Organic Acid Variation in White Wine Malolactic Fermentation Using a Logistic Model. Fermentation. 2025; 11(5):288. https://doi.org/10.3390/fermentation11050288
Chicago/Turabian StyleKarampatea, Aikaterini, Adriana Skendi, Maria Manoledaki, and Elisavet Bouloumpasi. 2025. "Predicting Organic Acid Variation in White Wine Malolactic Fermentation Using a Logistic Model" Fermentation 11, no. 5: 288. https://doi.org/10.3390/fermentation11050288
APA StyleKarampatea, A., Skendi, A., Manoledaki, M., & Bouloumpasi, E. (2025). Predicting Organic Acid Variation in White Wine Malolactic Fermentation Using a Logistic Model. Fermentation, 11(5), 288. https://doi.org/10.3390/fermentation11050288





