Consortium of Candida utilis, Kluyveromyces marxianus and Saccharomyces cerevisiae Yeasts for Vinasse Fermentation of Agave americana L. Liquor for Biomass Production and Reduction in Chemical Oxygen Demand
Abstract
1. Introduction
2. Materials and Methods
3. Results
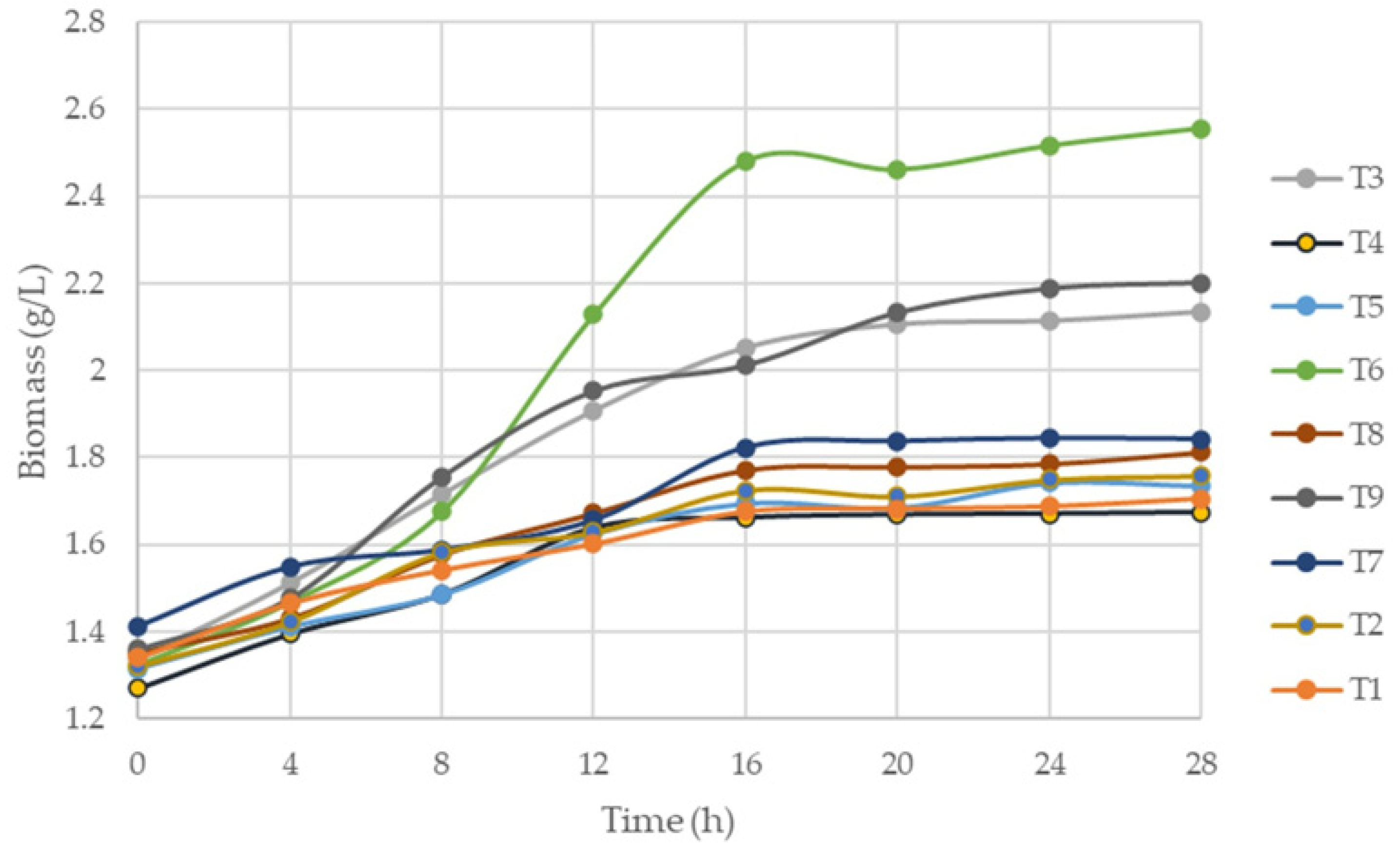
3.1. Biomass Production
Analysis of Biomass Yield Results
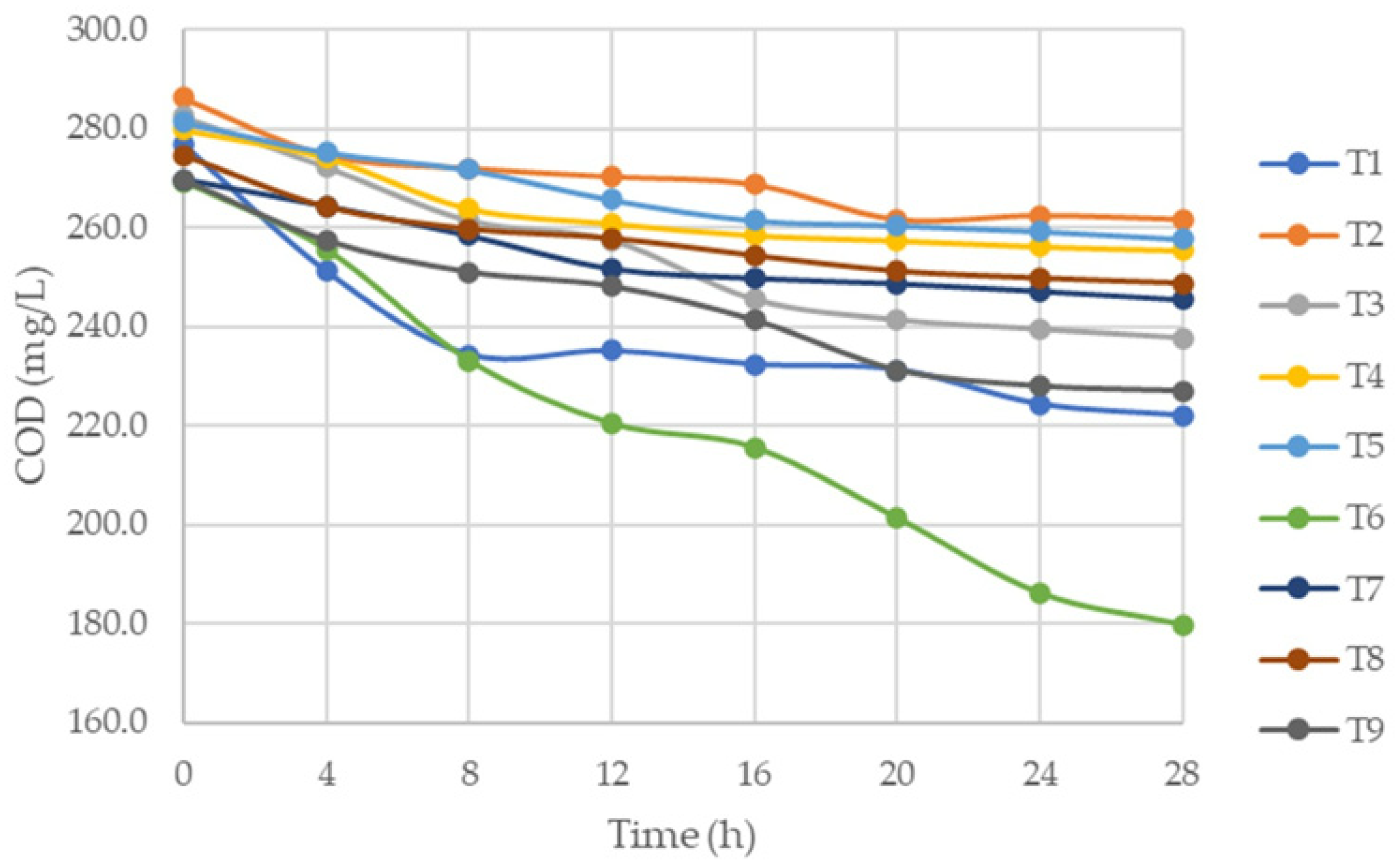
3.2. Reduction in Chemical Oxygen Demand (COD)
Analysis of the Results of COD Reduction in the Aerobic Fermentation Process of Blue Cabuya Vinasse
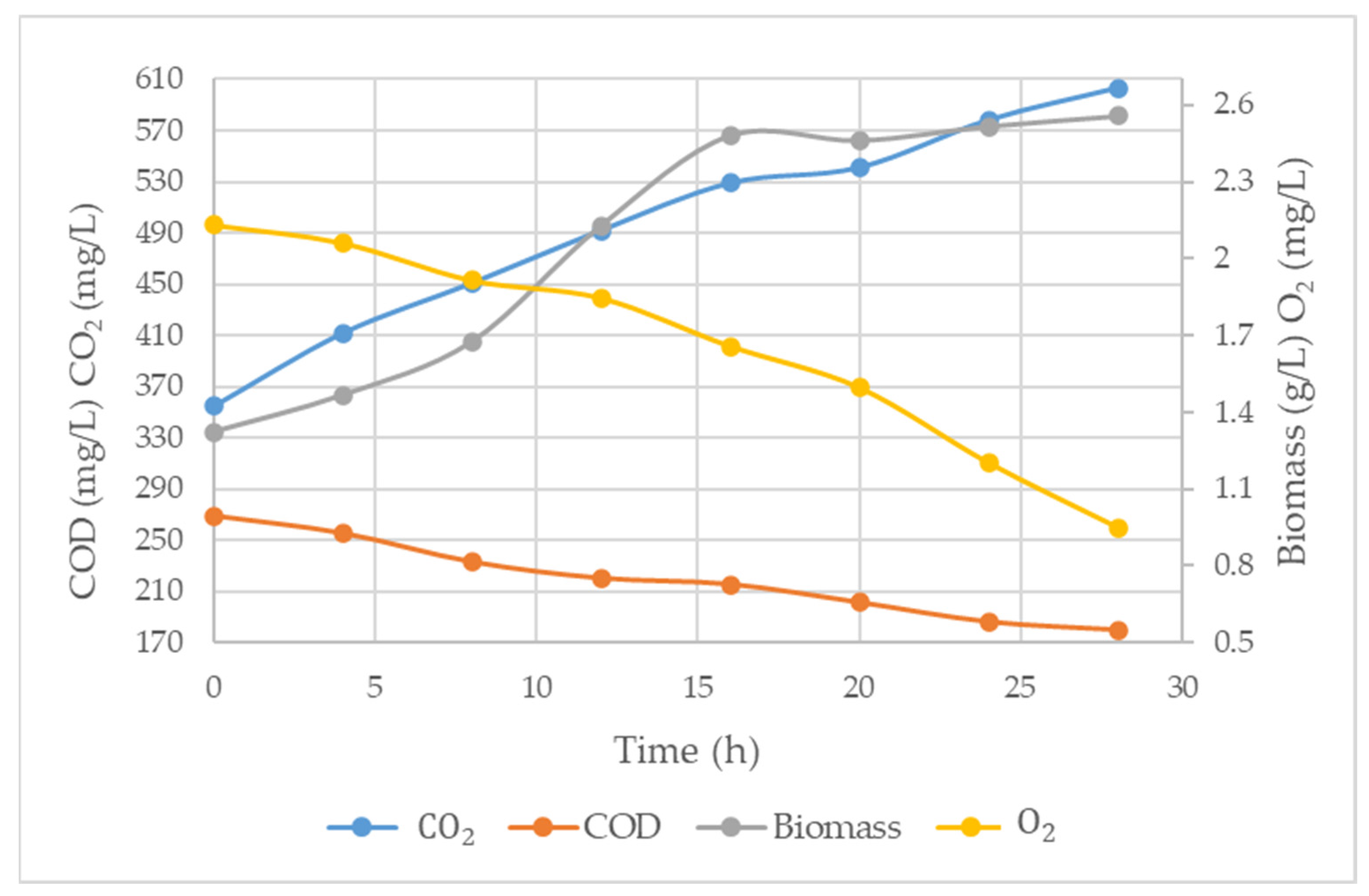
3.3. Biomass and CO2 Production, COD Reduction and O2 Consumption Trial T6
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gentry, H.S. Agaves of Continental North America; The University of Arizona Press: Tucson, AZ, USA, 1982; pp. 1–668. [Google Scholar]
- Valenzuela, A. El Agave Tequilero, su Cultivo e Industria; Lı’tteris Editores: Mexico city, Mexico, 1997; pp. 1–204. [Google Scholar]
- Robles Calderón, R. Condiciones óptimas del proceso de fermentación alcohólica del jugo de la Cabuya Azul (Agave americana L.), empleando cepas de levadura Saccharomyces cerevisiae. Available online: https://cybertesis.unmsm.edu.pe/item/9414face-4e32-4038-ac5d-b2e6d311f755 (accessed on 12 March 2025).
- López-López, A.; Davila-Vazquez, G.; León-Becerril, E.; Villegas-García, E.; Gallardo-Valdez, J. Tequila vinasses: Generation and full scale treatment processes. Rev. Environ. Sci. Biotechnol. 2010, 9, 109–116. [Google Scholar] [CrossRef]
- Consejo Regulador del Tequila. Manual Técnico Tequilero. D.R. ©; Consejo Regulador del Tequila ISBN: Guadalajara, Jalisco, México, 2019; pp. 128–171. [Google Scholar]
- Santiago-Santiago, A.; Arana-Coronado, O.; Matus, J.; Paz, J.; Toledo-cervantes, A.; Méndez-Acosta, H. Use of real options to evaluate the profitability of biogas production from stillage in the tequila industry. Agrociencia 2023, 1–11. [Google Scholar] [CrossRef]
- Otieno, B.; Apollo, S. Energy recovery from biomethanation of vinasse and its potential application in ozonation post-treatment for removal of biorecalcitrant organic compounds. J. Water Process Eng. 2021, 39, 101723. [Google Scholar] [CrossRef]
- Robles-González, V.; Galíndez-Mayer, J.; Rinderknecht-Seijas, N.; Poggi-Varaldo, H.M. Treatment of mezcal vinasses: A review. J. Biotechnol. 2012, 157, 524–546. [Google Scholar] [CrossRef]
- Azevedo-Santos, V.M.; Fernandes, J.A.; de Souza Andrade, G.; de Moraes, P.M.; Magurran, A.E.; Pelicice, F.M.; Giarrizzo, T. An overview of vinasse pollution in aquatic ecosystems in Brazil. Environ. Manag. 2024, 74, 1037–1044. [Google Scholar] [CrossRef] [PubMed]
- Rulli, M.M.; Del Gobbo, L.M.; Colin, V.L. Harmful effects of sugarcane vinasse on water bodies: Conventional remediation technologies. In Green Sustainable Process for Chemical and Environmental Engineering and Science: Natural Materials-Based Green Composites 2: Biomass; Elsevier: Amsterdam, The Netherlands, 2023; pp. 375–394. [Google Scholar] [CrossRef]
- Tamashiro, J.R.; Lima, I.S.; Paiva, F.F.G.D.; Silva, L.H.P.; Oliveira, D.V.M.D.; Baffa, O.; Kinoshita, A. Treatment of Sugarcane Vinasse Using Heterogeneous Photocatalysis with Zinc Oxide Nanoparticles. Sustainability 2022, 14, 16052. [Google Scholar] [CrossRef]
- Portocarrero, R.D.L.Á.; Chalco Vera, J.; Vallejo, J.I.; De Gerónimo, E.; Costa, J.L.; Aparicio, V.C. Long-term vinasse application enhanced the initial dissipation of atrazine and ametryn in a sugarcane field in Tucumán, Argentina. Integr. Environ. Assess. Manag. 2024, 20, 1075–1086. [Google Scholar] [CrossRef] [PubMed]
- Carrilho, E.N.V.M.; Labuto, G.; Kamogawa, M.Y. Destination of Vinasse, a Residue From Alcohol Industry: Resource Recovery and Prevention of Pollution. In Environmental Materials and Waste: Resource Recovery and Pollution Prevention; Elsevier: Amsterdam, The Netherlands, 2016; pp. 21–43. [Google Scholar] [CrossRef]
- Fuess, L.T.; Rodrigues, I.J.; Garcia, M.L. Fertirrigation with sugarcane vinasse: Foreseeing potential impacts on soil and water resources through vinasse characterization. J. Environ. Sci. Health—Part A Toxic/Hazard. Subst. Environ. Eng. 2017, 52, 1063–1072. [Google Scholar] [CrossRef]
- Soobadar, A.; Ng Kee Kwong, K.F.R. Impact of High Rates of Vinasse on Some Pertinent Soil Characteristics and on Sugarcane Yield in Mauritius. J. Sustain. Agric. 2012, 36, 36–53. [Google Scholar] [CrossRef]
- Cajo, L.; Nizama, L.; Carreño, C. Efecto de la concentración del inóculo y la melaza como suplemento de la vinaza de destilería para la producción de biomasa de Candida utilis nativa. Sci. Agropecu. 2011, 2, 65–72. [Google Scholar] [CrossRef][Green Version]
- Díaz-Vázquez, D.; Garibay, M.V.; Fernández del Castillo, A.; Orozco-Nunnelly, D.A.; Senés-Guerrero, C.; Gradilla-Hernández, V. Yeast community composition impacts on tequila industry waste treatment for pollution control and waste-to-product synthesis. Front. Chem. Eng. 2022, 4, 1013873. [Google Scholar] [CrossRef]
- Santos, F.; Eichler, P.; Machado, G.; De Mattia, M.S.; De Souza, G. Chapter 2—By-products of the sugarcane industry. In Sugarcane Biorefinery, Technology and Perspectives; Santos, F., Rabelo, S.C., De Matos, M., Eichler, P., Eds.; Academic Press: New York, NY, USA, 2020; pp. 21–48. [Google Scholar] [CrossRef]
- Lane, M.M.; Morrissey, J.P. Kluyveromyces marxianus: A yeast emerging from its sister’s shadow. Fungal Biol. Rev. 2010, 24, 17–26. [Google Scholar] [CrossRef]
- Karim, A.; Gerliani, L.N.; Aïder, M. Kluyveromyces marxianus: An emerging yeast cell factory for applications in food and biotechnology. Int. J. Food Microbiol. 2020, 333, 108818. [Google Scholar] [CrossRef]
- Kieliszek, M.; Kot, A.; Bzducha-Wróbel, A.; Błażejak, S.; Gientka, I.; Kurcz, A. Biotechnological use of Candida yeasts in the food industry: A review. Fungal Biol. Rev. 2017, 31, 185–198. [Google Scholar] [CrossRef]
- de Melo Pereira, G.V.; Leal, B.; De Carvallo, D.; Karp, S.G.; De Dea, J.; Orado, J.; Oliveira, B.; Soccol, C.R. What Is Candida Doing in My Food? A Review and Safety Alert on Its Use as Starter Cultures in Fermented Foods. Microorganisms 2022, 10, 1855. [Google Scholar] [CrossRef]
- de Rodríguez-Romero, J.J.; Aceves-Lara, C.A.; Silva, C.F.; Gschaedler, A.; Amaya-Delgado, L.; Arrizon, J. 2-Phenylethanol and 2-phenylethylacetate production by nonconventional yeasts using tequila vinasses as a substrate. Biotechnol. Rep. 2020, 25, e00420. [Google Scholar] [CrossRef]
- dos Reis, K.C.; Coimbra, J.M.; Duarte, W.F.; Schwan, R.F.; Silva, C.F. Biological treatment of vinasse with yeast and simultaneous production of single-cell protein for feed supplementation. Int. J. Environ. Sci. Technol. 2019, 16, 763–774. [Google Scholar] [CrossRef]
- Wioleta, M.; Zielińska, M. Distillery Stillage: Characteristics, Treatment, and Valorization. Appl. Biochem. Biotechnol. 2020, 192, 770–793. [Google Scholar] [CrossRef]
- Mohana, S.; Acharya, B.K.; Madamwar, D. Distillery spent wash: Treatment technologies and potential applications. J. Hazard. Mater. 2009, 163, 12–25. [Google Scholar] [CrossRef]
- Pandey, R.A.; Malhotra, A.; Tankhiwale, S.; Pande, S.; Pathe, P.P.; Kaul, S.N. Treatment of biologically treated distillery effluent—A case study. Int. J. Environ. Stud. 2003, 60, 263–275. [Google Scholar] [CrossRef]
- Utami, I.; Kautsar, D.; Akbar, R.; Muljani, S. Vinasse Treatment With Aerobic Microbial Method Using Activated Sludge. J. Phys. Conf. Ser. 2020, 1569, 042059. [Google Scholar] [CrossRef]
- Florencia, R.; Abumalé, C.; Edna, R.; Francisco, P.; Selene, L.; Rocío, M.; Víctor, M.; Roel, S.; Joel, M. Anaerobic Treatment of Vinasse from Sugarcane Ethanol Production in Expanded Granular Sludge Bed Bioreactor. J. Chem. Eng. Process Technol. 2018, 91, 3. [Google Scholar]
- Rodrigues Reis, C.; Hu, B. Vinasse from sugarcane ethanol production: Better treatment or better utilization? Front. Energy Res. 2017, 5, 7. [Google Scholar] [CrossRef]
- Burton, F.; George, T.; Stensel, H. Waste Water Engineering Treatment and Reuse; MC Graw-Hill Companies: New York, NY, USA, 2003. [Google Scholar]
- Liu, X.; Jia, B.; Sun, X.; Ai, J.; Wang, L.; Wang, C.; Zhao, F.; Zhan, J.; Huang, W. Effect of initial ph on growth characteristics and fermentation properties of Saccharomyces cerevisiae. J. Food Sci. 2015, 80, M800–M808. [Google Scholar] [CrossRef]
- Michel-Cuello, C.; Fonseca, G.G.; Cervantes, E.M.; Rivera, N.A. Effect of temperature and ph environment on the hydrolysis of maguey fructans to obtain fructose syrup. Rev. Mex. Ing. Química 2015, 14, 615–622. [Google Scholar]
- Lesaffre. Fermentis: Fermented Beverages, Beer, Wine and Spirits. Fermentis by Lesaffre. Available online: https://www.lesaffre.com/activities/food-taste-pleasure/fermented-beverages/ (accessed on 12 March 2025).
- Alemán-Nava, G.S.; Gatti, I.A.; Parra-Saldivar, V.; Dallemand, J.F.; Rittmann, B.E.; Iqbal, H.M.N. Biotechnological revalorization of Tequila waste and by-product streams for cleaner production—A review from bio-refinery perspective. J. Clean. Prod. 2018, 172, 3713–3720. [Google Scholar] [CrossRef]
- Alfaro, J.M.; Reyes, S.I.; Cristobal, H.A.; Martearena, M.R.; Baigorí, M.D.; Pera, L.M. Remediation of sugarcane vinasse using Rhodotorula glutinis or Rhodotorula mucilaginosa: Biomass morphology and its potential technological applications. Biocatal. Agric. Biotechnol. 2024, 58, 103193. [Google Scholar] [CrossRef]
- Pires, J.F.; Ferreira, G.M.R.; Reis, K.C.; Schwan, R.F.; Silva, C.F. Mixed yeasts inocula for simultaneous production of SCP and treatment of vinasse to reduce soil and fresh water pollution. J. Environ. Manag. 2016, 182, 455–463. [Google Scholar] [CrossRef]
- Nurcholis, M.; Lertwattanasakul, N.; Rodrussamee, N.; Kosaka, T.; Murata, M.; Yamada, M. Integration of comprehensive data and biotechnological tools for industrial applications of Kluyveromyces marxianus. Appl. Microbiol. Biotechnol. 2020, 104, 475–488. [Google Scholar] [CrossRef]
- Silva, C.F.; Arcuri, S.L.; Campos, C.R.; Vilela, D.M.; Alves, J.G.L.F.; Schwan, R.F. Using the residue of spirit production and bio-ethanol for protein production by yeasts. Waste Manag. 2011, 31, 108–114. [Google Scholar] [CrossRef]
- Rogeri, R.C.; Fuess, L.T.; Eng, F.; Do Vale Borges, A.; Neves de Araujo, M.; Rissato, M.; Da Silva, A. Strategies to control pH in the dark fermentation of sugarcane vinasse: Impacts on sulfate reduction, biohydrogen production and metabolite distribution. J. Environ. Manag. 2023, 325, 116495. [Google Scholar] [CrossRef] [PubMed]
- Mendoza, L.M.; De Nadra, M.C.M.; Bru, E.; Farías, M.E. Influence of wine-related physicochemical factors on the growth and metabolism of non-Saccharomyces and Saccharomyces yeasts in mixed culture. J. Ind. Microbiol. Biotechnol. 2009, 36, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Amillastre, E.; Aceves-Lara, C.A.; Uribelarrea, J.L.; Alfenore, S.; Guillouet, S.E. Dynamic model of temperature impact on cell viability and major product formation during fed-batch and continuous ethanolic fermentation in Saccharomyces cerevisiae. Bioresour. Technol. 2012, 117, 242–250. [Google Scholar] [CrossRef] [PubMed]





| Vinasse | Temperature | pH | Trial |
|---|---|---|---|
| % | °C | ||
| 100 | 28 | 3.0 | T1 |
| 4.0 | T2 | ||
| 5.0 | T3 | ||
| 30 | 3.0 | T4 | |
| 4.0 | T5 | ||
| 5.0 | T6 | ||
| 32 | 3.0 | T7 | |
| 4.0 | T8 | ||
| 5.0 | T9 |
| Time (h) | Biomass (g/L) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| T1 | T2 | T3 | T4 | T5 | T6 | T7 | T8 | T9 | |
| 0 | 1.34 | 1.32 | 1.34 | 1.269 | 1.315 | 1.323 | 1.412 | 1.352 | 1.36 |
| 4 | 1.465 | 1.422 | 1.512 | 1.394 | 1.411 | 1.467 | 1.548 | 1.431 | 1.477 |
| 8 | 1.541 | 1.582 | 1.746 | 1.485 | 1.485 | 1.675 | 1.587 | 1.577 | 1.756 |
| 12 | 1.601 | 1.628 | 1.908 | 1.638 | 1.625 | 2.128 | 1.655 | 1.672 | 1.952 |
| 16 | 1.675 | 1.725 | 2052 | 1.661 | 1.695 | 2.48 | 1.822 | 1.771 | 2.011 |
| 20 | 1.682 | 1.711 | 2.105 | 1.668 | 1.685 | 2.461 | 1.836 | 1.778 | 2.132 |
| 24 | 1.687 | 1.769 | 2.113 | 1.67 | 1.742 | 2.516 | 1.843 | 1.799 | 2.187 |
| 28 | 1.705 | 1.758 | 2.134 | 1.673 | 1.735 | 2.556 | 1.841 | 1.812 | 2.200 |
| Tests of Inter-Subject Effects | |||||
|---|---|---|---|---|---|
| Dependent Variable: Biomass | |||||
| Origen | Type III | gl | Square | F | Sig. |
| Sum of Squares | Root Mean | ||||
| Corrected model | 5.378 a | 15 | 0.359 | 21.115 | 0.000 |
| Intersection | 213.642 | 1 | 213.642 | 12,820.645 | 0.000 |
| Time | 3.356 | 7 | 0.479 | 28.767 | 0.000 |
| Trial | 2.022 | 8 | 0.253 | 15.170 | 0.000 |
| Mistake | 0.933 | 56 | 0.017 | ||
| Total | 219.953 | 72 | |||
| Total corrected | 6.311 | 71 | |||
| Time (h) | COD (mg/L) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| T1 | T2 | T3 | T4 | T5 | T6 | T7 | T8 | T9 | |
| 0 | 276.9 | 286.2 | 282.36 | 279.7 | 281.3 | 269.3 | 269.7 | 274.7 | 269.7 |
| 4 | 251.2 | 274.6 | 272.3 | 274.1 | 275.2 | 255.6 | 264.3 | 264.2 | 257.4 |
| 8 | 234.3 | 271.9 | 261.4 | 263.9 | 271.7 | 233.1 | 258.4 | 259.7 | 251.0 |
| 12 | 235.1 | 270.2 | 257.4 | 260.8 | 265.6 | 220.4 | 251.7 | 257.8 | 248.1 |
| 16 | 232.3 | 268.6 | 245.4 | 258.5 | 261.4 | 215.5 | 249.8 | 254.3 | 241.3 |
| 20 | 231.3 | 261.5 | 241.3 | 257.4 | 260.4 | 201.3 | 248.7 | 251.2 | 231.3 |
| 24 | 224.3 | 262.3 | 239.4 | 256.2 | 259.2 | 186.1 | 247.2 | 249.7 | 228.0 |
| 28 | 222.0 | 261.5 | 237.58 | 255.4 | 257.6 | 179.7 | 245.5 | 248.7 | 227.0 |
| Tests of Inter-Subject Effects | |||||
|---|---|---|---|---|---|
| Dependent Variable: COD | |||||
| Origen | Type III Sum of Squares | gl | Square Root Media | F | Sig. |
| Corrected model | 27,280.506 a | 15 | 1818.700 | 27.039 | 0.000 |
| Intersection | 4,575,413.334 | 1 | 4,575,413.334 | 68,023.247 | 0.000 |
| Time | 11,662.813 | 7 | 1666.116 | 24.770 | 0.000 |
| Trial | 15,617.694 | 8 | 1952.212 | 29.024 | 0.000 |
| Mistake | 3766.700 | 56 | 67.262 | ||
| Total | 4,606,460.540 | 72 | |||
| Total corrected | 31,047.206 | 71 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calderón, R.R.; Boza, F.A.; Benmites-Alfaro, E.; Gómez, O.T.; Flores, J.C. Consortium of Candida utilis, Kluyveromyces marxianus and Saccharomyces cerevisiae Yeasts for Vinasse Fermentation of Agave americana L. Liquor for Biomass Production and Reduction in Chemical Oxygen Demand. Fermentation 2025, 11, 281. https://doi.org/10.3390/fermentation11050281
Calderón RR, Boza FA, Benmites-Alfaro E, Gómez OT, Flores JC. Consortium of Candida utilis, Kluyveromyces marxianus and Saccharomyces cerevisiae Yeasts for Vinasse Fermentation of Agave americana L. Liquor for Biomass Production and Reduction in Chemical Oxygen Demand. Fermentation. 2025; 11(5):281. https://doi.org/10.3390/fermentation11050281
Chicago/Turabian StyleCalderón, Roberto Robles, Francisco Alcántara Boza, Elmer Benmites-Alfaro, Oscar Tinoco Gómez, and Jaqueline Chirre Flores. 2025. "Consortium of Candida utilis, Kluyveromyces marxianus and Saccharomyces cerevisiae Yeasts for Vinasse Fermentation of Agave americana L. Liquor for Biomass Production and Reduction in Chemical Oxygen Demand" Fermentation 11, no. 5: 281. https://doi.org/10.3390/fermentation11050281
APA StyleCalderón, R. R., Boza, F. A., Benmites-Alfaro, E., Gómez, O. T., & Flores, J. C. (2025). Consortium of Candida utilis, Kluyveromyces marxianus and Saccharomyces cerevisiae Yeasts for Vinasse Fermentation of Agave americana L. Liquor for Biomass Production and Reduction in Chemical Oxygen Demand. Fermentation, 11(5), 281. https://doi.org/10.3390/fermentation11050281






