Non-Targeted Metabolomics Analysis Unravels Changes in Non-Volatile Metabolites in Folium nelumbinis (Lotus Leaf) Induced by Aspergillus cristatus-Mediated Fermentation
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Fermentation Processing
2.3. Sensory Evaluation
2.4. Determination of Total Flavonoids and Alkaloids
2.5. Metabolite Extraction
2.6. UHPLC-MS/MS) Analysis
2.7. Data Analysis
3. Results and Discussion
3.1. Effect of A. cristatus Fermentation on the Sensory Quality of Lotus Leaf
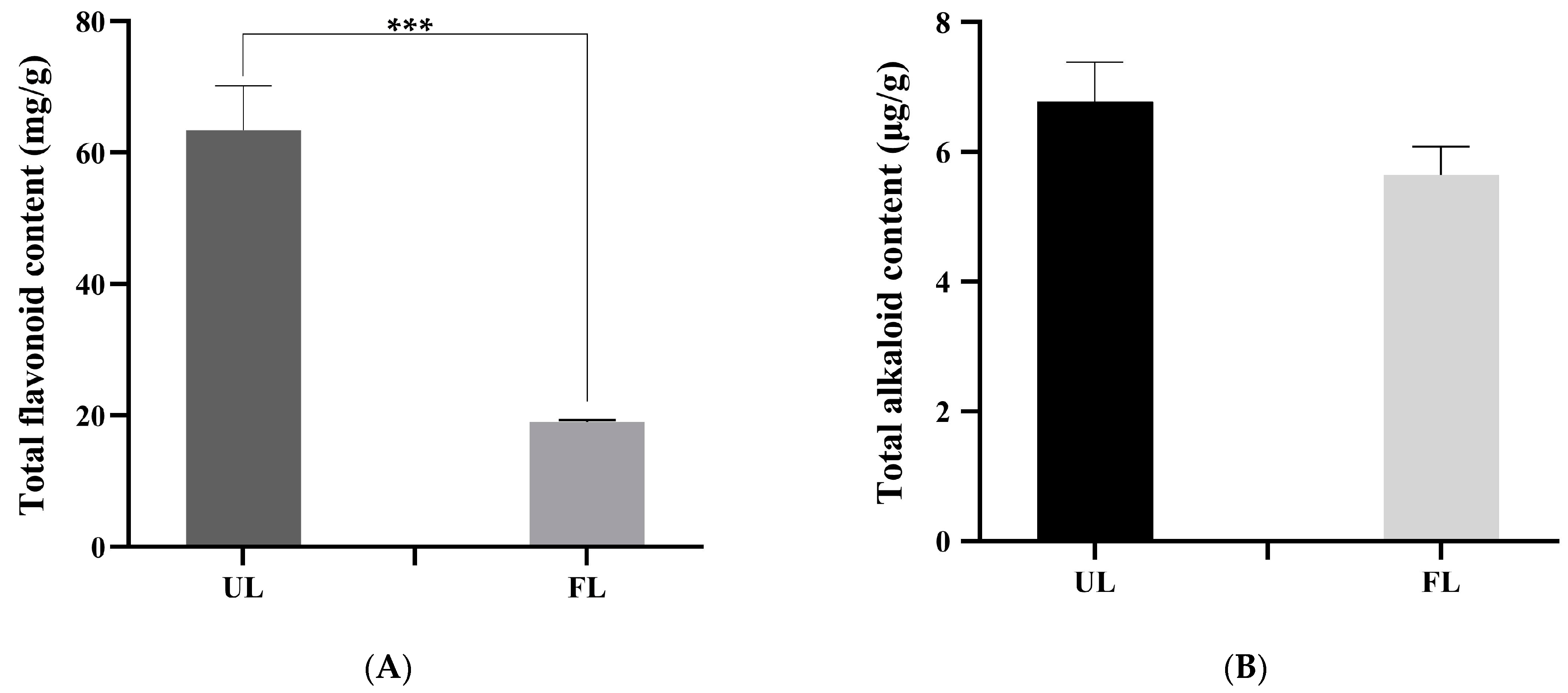
3.2. Effect of A. cristatus Fermentation on the Main Active Components of Lotus Leaf
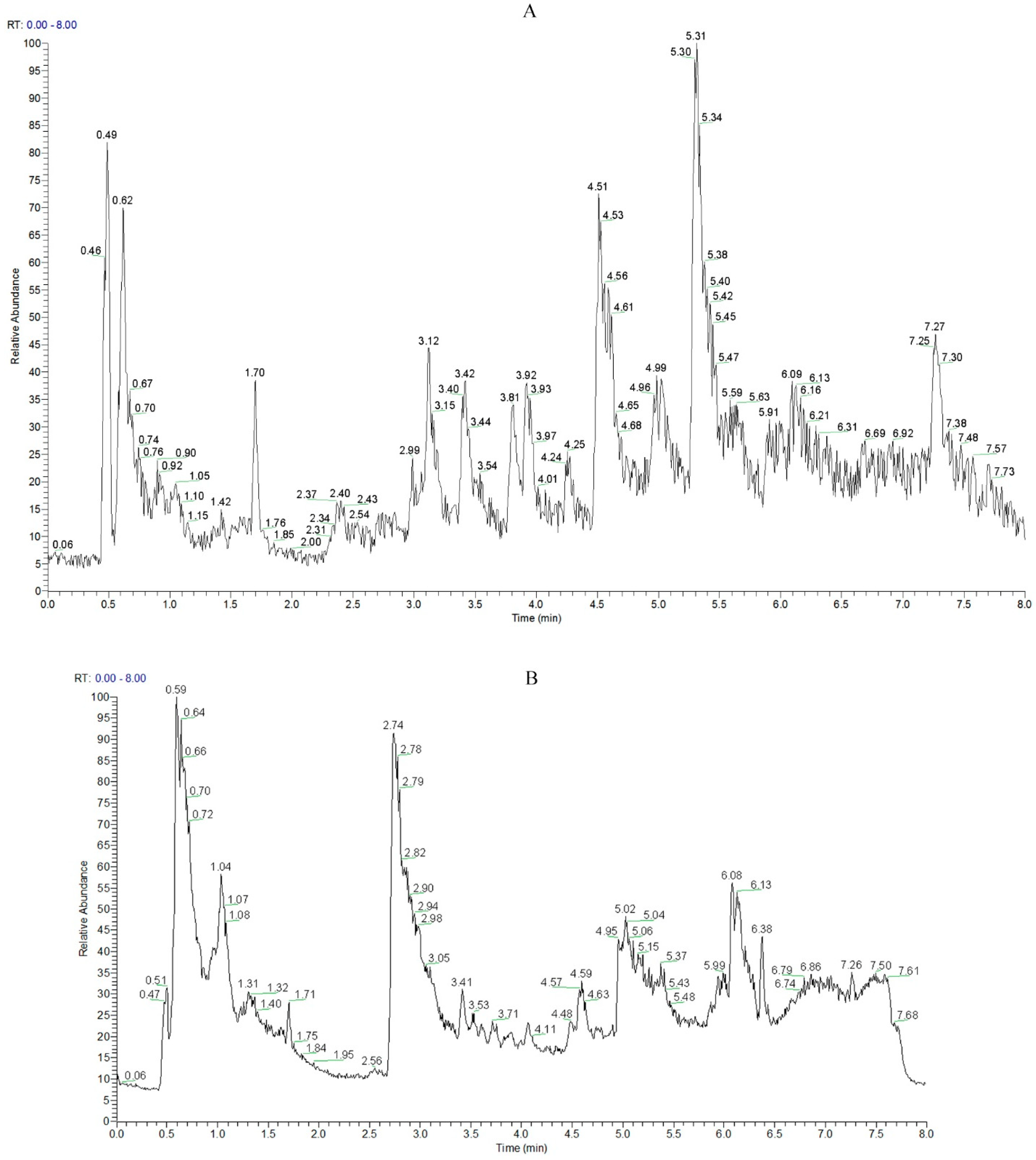
3.3. UHPLC–MS/MS Metabolic Profile Analysis
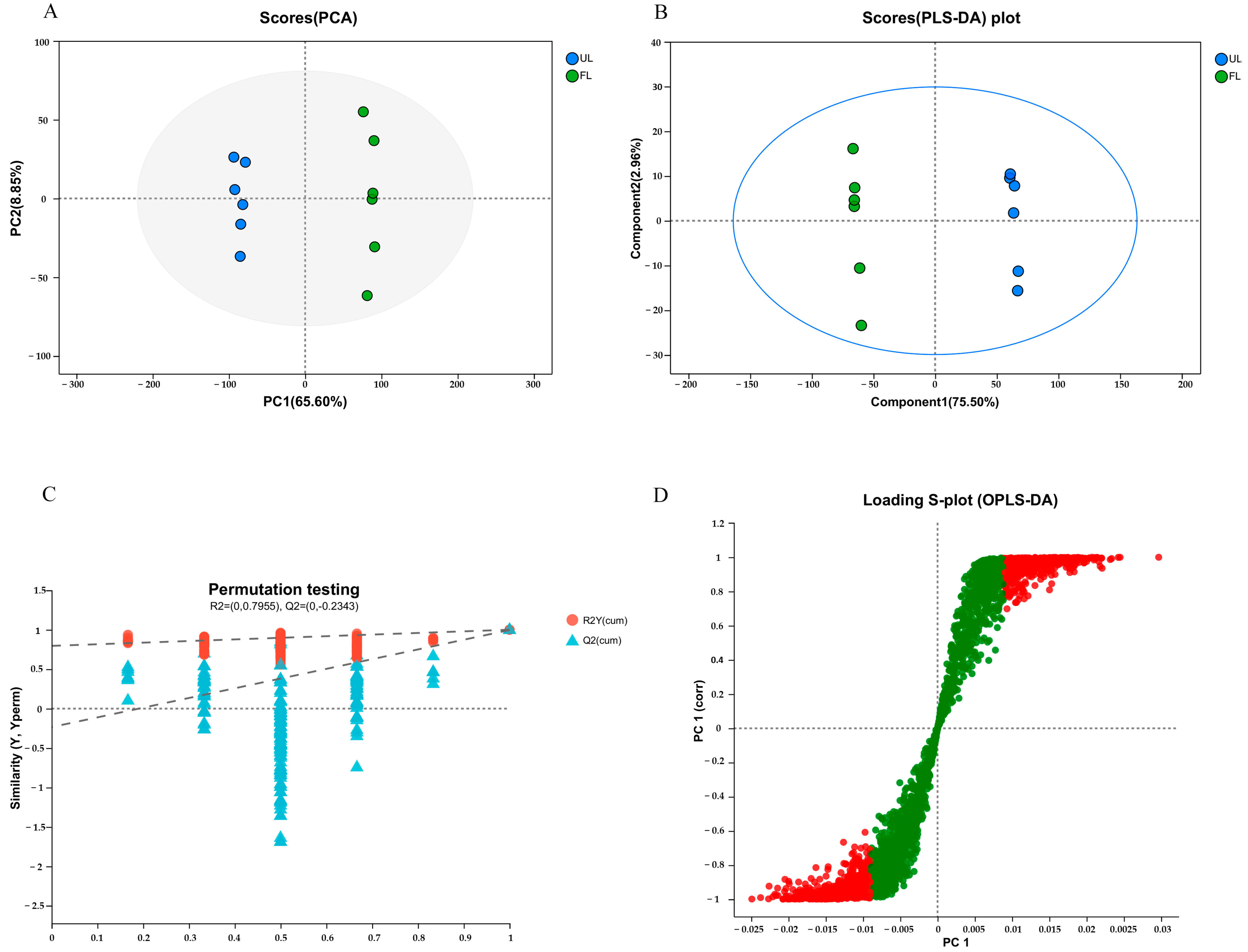
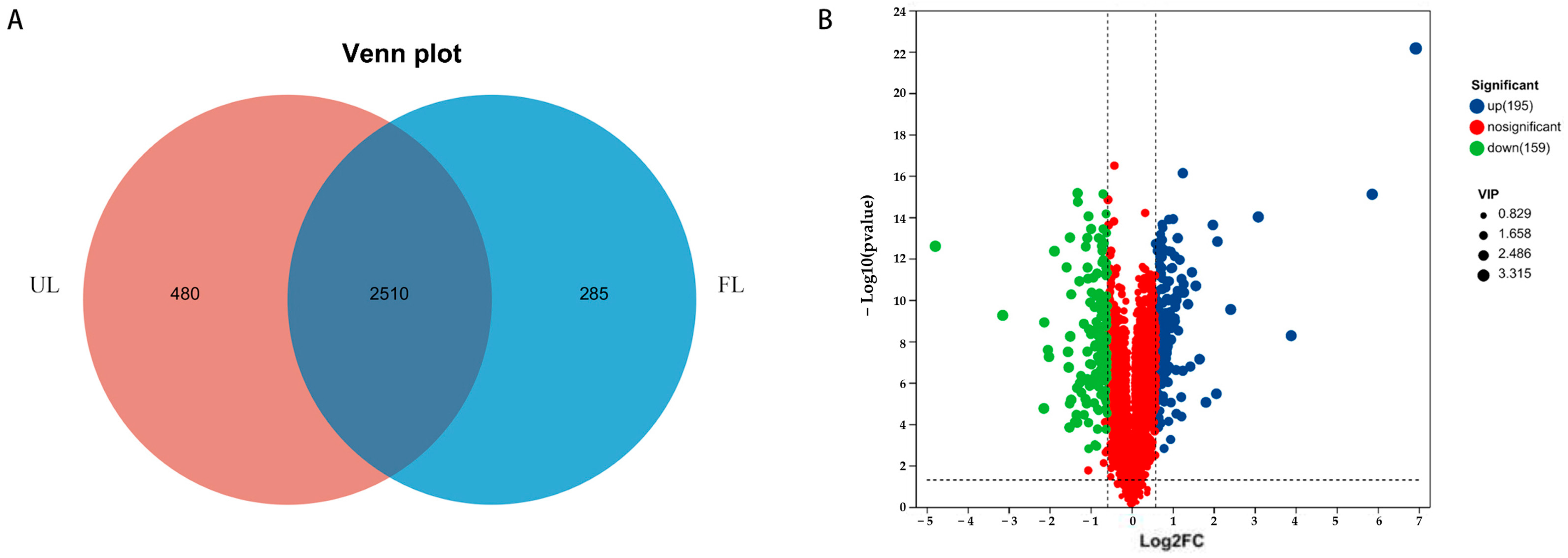
3.4. Multivariate Statistical Analysis
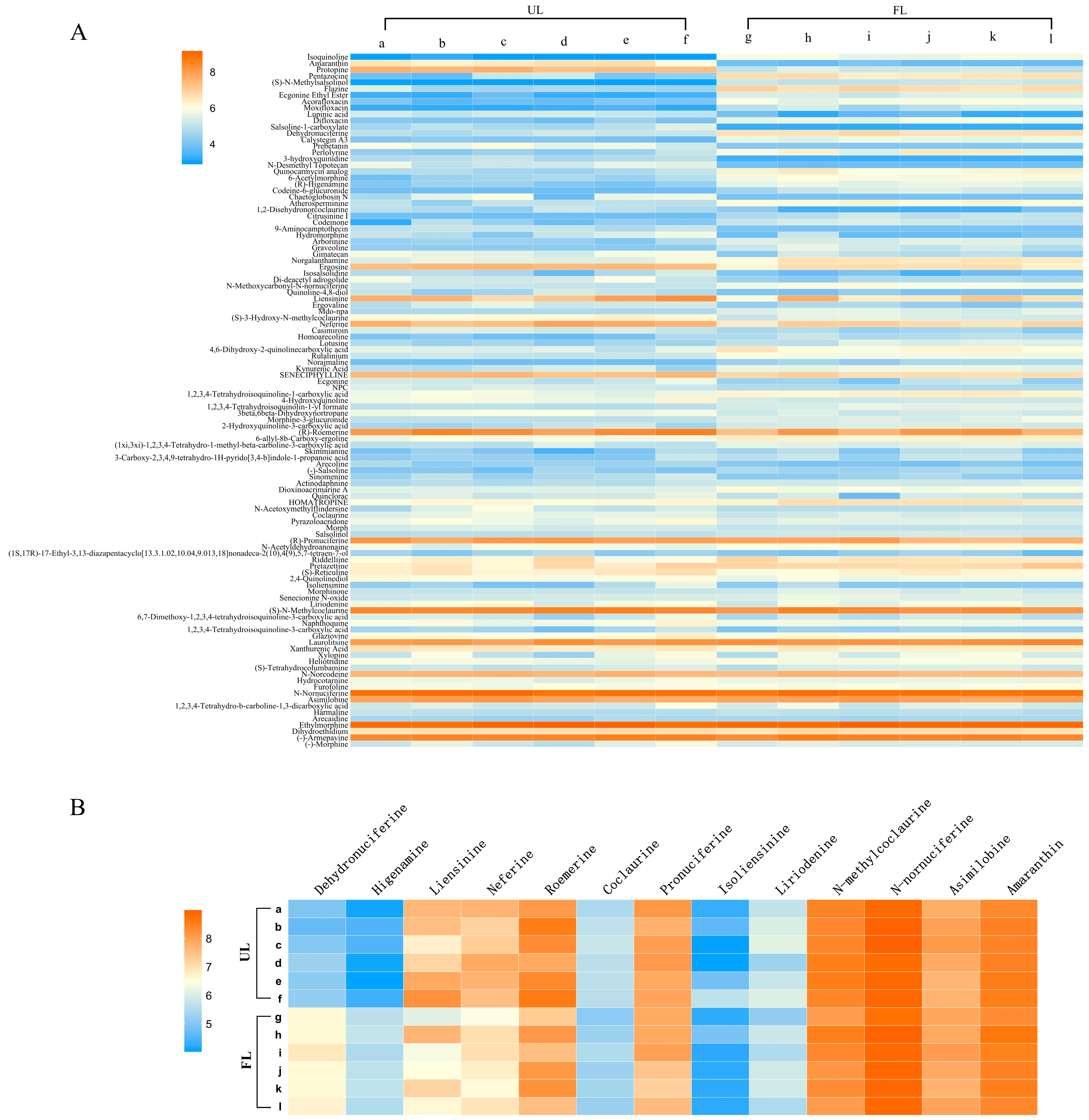
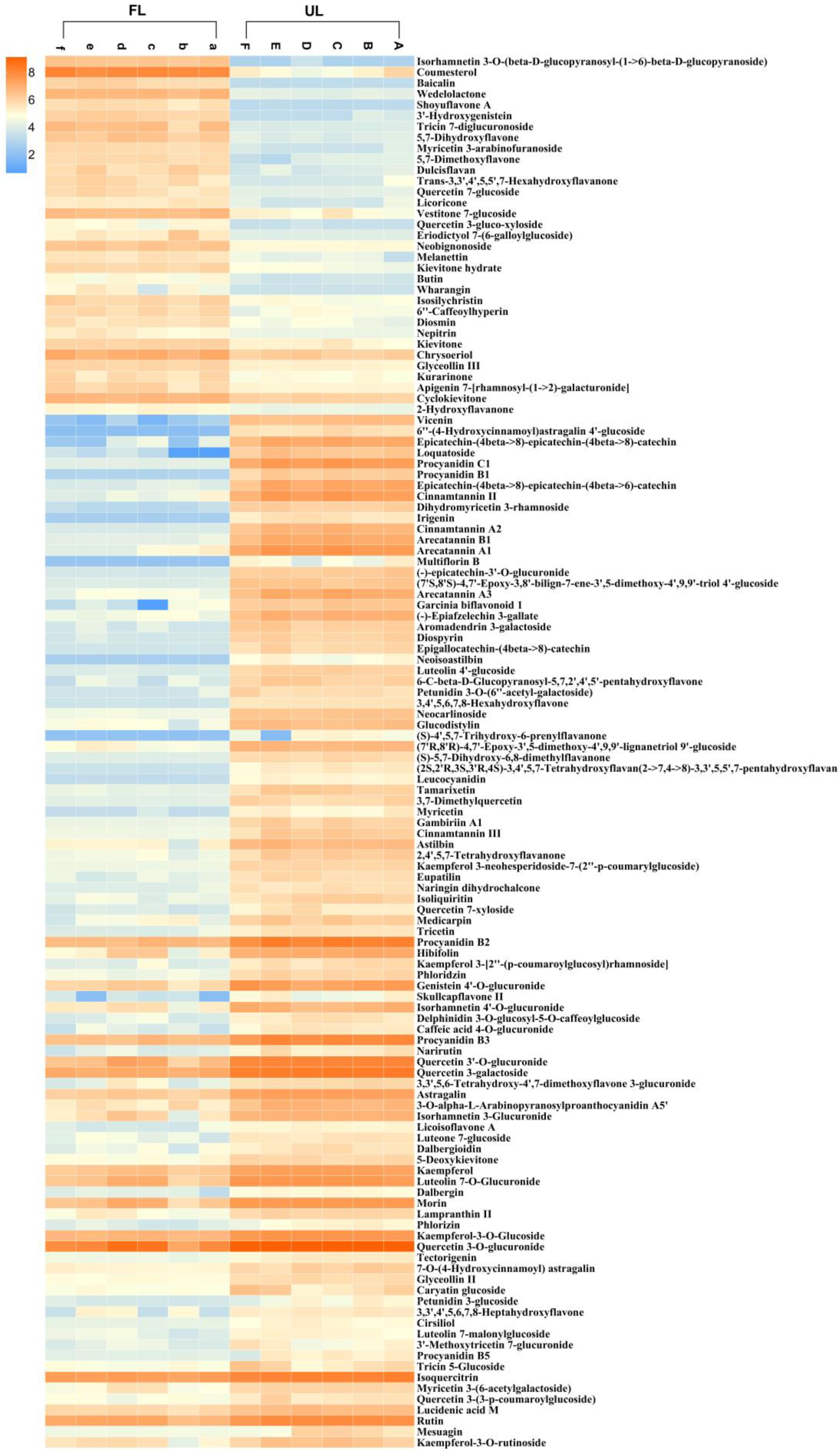
3.5. Analysis of Differential Metabolites
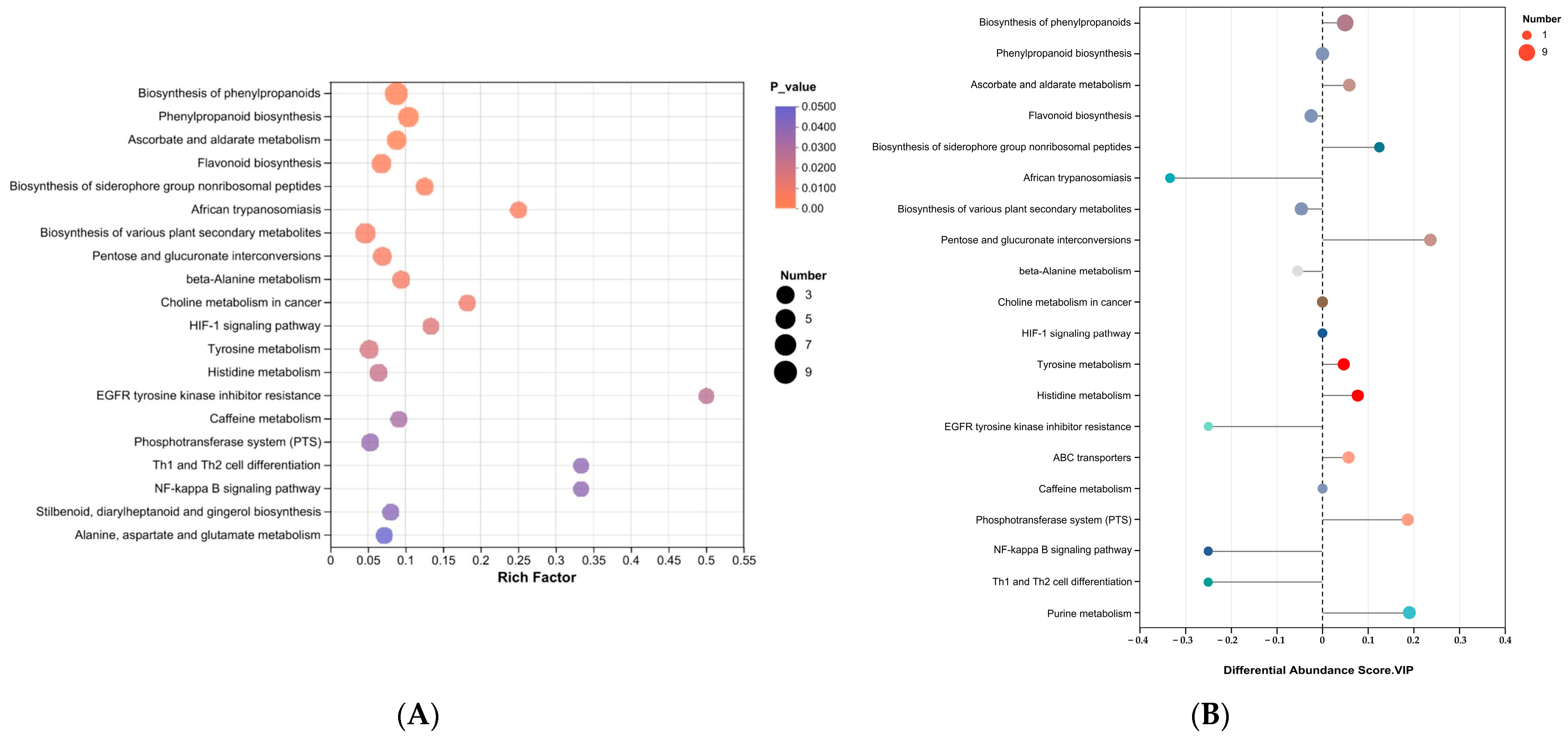
3.6. Metabolic Pathway Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, G.; Ma, F.; Xie, K.; Li, X.; Tan, X.; Xia, Y.; Wang, Y.; Dong, J. Liensinine alleviates mouse intestinal injury induced by sepsis through inhibition of oxidative stress, inflammation, and cell apoptosis. Int. Immunopharmacol. 2024, 127, 111335. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Cheng, Y.; Zeng, M.; Wang, Z.; Qin, F.; Wang, Y.; Chen, J.; He, Z. Lotus (Nelumbo nucifera Gaertn.) leaf: A narrative review of its Phytoconstituents, health benefits and food industry applications. Trends Food Sci. Technol. 2021, 112, 631–650. [Google Scholar] [CrossRef]
- Feng, J.; Wang, J.; Bu, T.; Ge, Z.; Yang, K.; Sun, P.; Wu, L.; Cai, M. Structural, in vitro digestion, and fermentation characteristics of lotus leaf flavonoids. Food Chem. 2023, 406, 135007. [Google Scholar] [CrossRef]
- Guo, Y.; Chen, X.; Qi, J.; Yu, B. Simultaneous qualitative and quantitative analysis of flavonoids and alkaloids from the leaves of Nelumbo nucifera Gaertn. using high-performance liquid chromatography with quadrupole time-of-flight mass spectrometry. J. Sep. Sci. 2016, 39, 2499–2507. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Xiao, W.; Liu, Z. Research progress of alkaloids in lotus leaves. Today Pharm. 2008, 3, 9–12+17. [Google Scholar]
- Huang, C.; Peng, X.; Pang, D.-J.; Li, J.; Paulsen, B.S.; Rise, F.; Chen, Y.-L.; Chen, Z.-L.; Jia, R.-Y.; Li, L.-X.; et al. Pectic polysaccharide from Nelumbo nucifera leaves promotes intestinal antioxidant defense in vitro and in vivo. Food Funct. 2021, 12, 10828–10841. [Google Scholar] [CrossRef]
- Qi, Q.; Li, R.; Li, H.-Y.; Cao, Y.-B.; Bai, M.; Fan, X.-J.; Wang, S.-Y.; Zhang, B.; Li, S. Identification of the anti-tumor activity and mechanisms of nuciferine through a network pharmacology approach. Acta Pharmacol. Sin. 2016, 37, 963–972. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Chen, G.; Zhang, Y.; Yang, M.; Chen, J.; Guo, M. Potential hypoglycemic, hypolipidemic, and anti-inflammatory bioactive components in Nelumbo nucifera leaves explored by bioaffinity ultrafiltration with multiple targets. Food Chem. 2022, 375, 131856. [Google Scholar] [CrossRef]
- Zhou, G.; Feng, X.; Tao, A. Explore the lipid-lowering and weight-reducing mechanism of lotus leaf based on network pharmacology and molecular docking. Evid.-Based Complement. Altern. Med. 2021, 2021, 1464027. [Google Scholar] [CrossRef]
- Wu, N.; Xie, H.; Fang, Y.; Liu, Y.; Xi, X.; Chu, Q.; Dong, G.; Lan, T.; Wei, Y. Isolation and purification of alkaloids from lotus leaves by ionic-liquid-modified high-speed countercurrent chromatography. J. Sep. Sci. 2018, 41, 571–577. [Google Scholar] [CrossRef]
- Wang, C.; Wang, L.; Yang, Y.; Hu, Y.; Cai, H.; Lu, Y. Research progress on alkaloid active ingredients in Nelumbinis Folium against non-alcoholic fatty liver disease. Shanghai J. Tradit. Chin. Med. 2023, 57, 76–82. [Google Scholar] [CrossRef]
- Zhu, X.; Hao, R.; Lv, X.; Zhou, X.; Li, D.; Zhang, C. Nuciferine ameliorates high-fat diet-induced disorders of glucose and lipid metabolism in obese mice based on the gut–liver axis. Food Front. 2023, 5, 188–201. [Google Scholar] [CrossRef]
- Yan, C.; Zhan, Y.; Yuan, S.; Cao, Y.; Chen, Y.; Dong, M.; Zhang, H.; Chen, L.; Jiang, R.; Liu, W.; et al. Nuciferine prevents obesity by activating brown adipose tissue. Food Funct. 2024, 15, 967–976. [Google Scholar] [CrossRef]
- Jiang, H.; Zhang, Q.; Yang, J.; Shu, Z.; Zhang, H. Research Progress on Extraction Technologies and Pharmacological Activities of Flavonoids from Lotus Leaf. Shandong Chem. Ind. 2022, 51, 79–81. [Google Scholar] [CrossRef]
- Wang, Z.; Xue, C.; Wang, X.; Zeng, M.; Wang, Z.; Chen, Q.; Chen, J.; Christian, M.; He, Z. Quercetin 3-O-glucuronide-rich lotus leaf extract promotes a Brown-fat-phenotype in C3H10T1/2 mesenchymal stem cells. Food Res. Int. 2023, 163, 112198. [Google Scholar] [CrossRef]
- Limwachiranon, J.; Huang, H.; Shi, Z.; Li, L.; Luo, Z. Lotus Flavonoids and Phenolic Acids: Health Promotion and Safe Consumption Dosages. Compr. Rev. Food Sci. Food Saf. 2018, 17, 458–471. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhou, Q.-M.; Xu, L.; Xie, X.; Wang, P.-X.; Xie, Z.-H.; Li, J.-L.; Tu, Z.-C. Extraction optimization and identification of four advanced glycation-end products inhibitors from lotus leaves and interaction mechanism analysis. Food Chem. 2023, 414, 135712. [Google Scholar] [CrossRef]
- Wang, Z.; Xue, C.; Yang, T.; Zeng, M.; Wang, Z.; Chen, Q.; Chen, J.; He, Z. Miquelianin, a main functional flavonoid of lotus leaf, induces thermogenic signature via p38-PINK1-PARKIN-mediated mitophagy and mimicking NRF2 signaling during brown adipocyte differentiation. Food Front. 2023, 4, 1831–1844. [Google Scholar] [CrossRef]
- Xie, Y.; Li, C.; Feng, H.; Jia, M.; Wei, X. Research progress on microbial fermentation of active ingredients of traditional Chinese medicine. Chin. Tradit. Pat. Med. 2025, 47, 182–187. [Google Scholar]
- He, Y.; Tao, Y.; Qiu, L.; Xu, W.; Huang, X.; Wei, H.; Tao, X. Lotus (Nelumbo nucifera Gaertn.) Leaf-Fermentation Supernatant Inhibits Adipogenesis in 3T3-L1 Preadipocytes and Suppresses Obesity in High-Fat Diet-Induced Obese Rats. Nutrients 2022, 14, 4348. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Huang, J.; Li, C.; Wang, J.; Jiang, X. Effects of shaking and fermentation on the quality of lotus leaf tea. Newsl. Seric. Tea 2023, 4, 1–7. [Google Scholar]
- Gao, X.; Kan, X.; Du, F.; Sun, L.; Li, X.; Liu, J.; Liu, X.; Yao, D. The Manufacturing Process of Lotus (Nelumbo Nucifera) Leaf Black Tea and Its Microbial Diversity Analysis. Foods 2025, 14, 519. [Google Scholar] [CrossRef]
- Li, Q.; Jin, Y.; Jiang, R.; Xu, Y.; Zhang, Y.; Luo, Y.; Huang, J.; Wang, K.; Liu, Z. Dynamic changes in the metabolite profile and taste characteristics of Fu brick tea during the manufacturing process. Food Chem. 2021, 344, 128576. [Google Scholar] [CrossRef]
- Kang, D.; Su, M.; Duan, Y.; Huang, Y. Eurotium cristatum, a potential probiotic fungus from Fuzhuan brick tea, alleviated obesity in mice by modulating gut microbiota. Food Funct. 2019, 10, 5032–5045. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Zhou, L.; Zhang, R.; Zhou, H.; Yang, Y. Material Composition Characteristics of Aspergillus cristatus under High Salt Stress through LC–MS Metabolomics. Molecules 2024, 29, 2513. [Google Scholar] [CrossRef]
- Deng, J.; Li, Y.; Yuan, Y.; Yin, F.; Chao, J.; Huang, J.; Liu, Z.; Wang, K.; Zhu, M. Secondary Metabolites from the Genus Eurotium and Their Biological Activities. Foods 2023, 12, 4452. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Qin, Z.; Liu, J.; Chen, L.; Xia, Y.; Li, X.; Liu, X.; Li, Y. Effect of Processing Technology on Quality and Functional Activity of Taxilli Herba Tea. Sci. Technol. Food Ind. 2023, 44, 149–157. [Google Scholar] [CrossRef]
- Zou, M.; Zhang, W.; Dong, Q.; Tang, C.; Cao, F.; Su, E. Submerged fermentation of Ginkgo biloba seed powder using Eurotium cristatum for the development of ginkgo seeds fermented products. J. Sci. Food Agric. 2021, 101, 1782–1791. [Google Scholar] [CrossRef]
- Zhang, D.; Huang, Y.; Fan, X.; Zeng, X. Effects of solid-state fermentation with Aspergillus cristatus (MK346334) on the dynamics changes in the chemical and flavor profile of dark tea by HS-SPME-GC–MS, HS-GC-IMS and electronic nose. Food Chem. 2024, 455, 139864. [Google Scholar] [CrossRef]
- GB/T 23776-2018; Methodology for Sensory Evaluation of Tea. China National Standardization Management Committee: Beijing, China, 2018.
- Shi, G.; Xiang, Q.; Fan, Y.; Zhao, W.; Liu, Y. Optimization of Process on Extracting Flavonoids from Dry Onion Skins by Alkaline Method. Open Chem. Eng. J. 2015, 9, 39–46. [Google Scholar] [CrossRef][Green Version]
- Kong, X.; Guo, Z.; Yao, Y.; Xia, L.; Liu, R.; Song, H.; Zhang, S. Acetic acid alters rhizosphere microbes and metabolic composition to improve willows drought resistance. Sci. Total Environ. 2022, 844, 157132. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Al-Dalali, S.; Zhou, H.; Xu, B. Influence of curing on the metabolite profile of water-boiled salted duck. Food Chem. 2022, 397, 133752. [Google Scholar] [CrossRef]
- Li, Z.; Wu, Y.; Hu, J.; Yang, G.; Wang, Z.; Sun, J. Dissection of the response mechanism of alfalfa under phosphite stress based on metabolomic and transcriptomic data. Plant Physiol. Biochem. 2022, 192, 35–49. [Google Scholar] [CrossRef]
- Du, Y.; Yang, W.; Yang, C.; Yang, X. A comprehensive review on microbiome, aromas and flavors, chemical composition, nutrition and future prospects of Fuzhuan brick tea. Trends Food Sci. Technol. 2022, 119, 452–466. [Google Scholar] [CrossRef]
- Xiang, Z.; Guan, H.; Zhao, X.; Xie, Q.; Xie, Z.; Cai, F.; Dang, R.; Li, M.; Wang, C. Dietary gallic acid as an antioxidant: A review of its food industry applications, health benefits, bioavailability, nano-delivery systems, and drug interactions. Food Res. Int. 2024, 180, 114068. [Google Scholar] [CrossRef]
- Sun, J.; Xia, P.; Liang, Z. Pharmacological efficacy of Nelumbinis folium and development of functional food products. J. Zhejiang Agric. Sci. 2021, 62, 1874–1881. [Google Scholar] [CrossRef]
- Bayazeid, O.; Nemutlu, E.; Eylem, C.C.; Yalçın, F.N. Neuroactivity of naturally occurring proaporphine alkaloid, pronuciferine. J. Biochem. Mol. Toxicol. 2020, 34, e22601. [Google Scholar] [CrossRef] [PubMed]
- Bayazeid, O.; Nemutlu, E.; Eylem, C.C.; İlhan, M.; Küpeli-Akkol, E.; Karahan, H.; Kelicen-Uğur, P.; Ersoz, T.; Yalçın, F.N. Neuroactivity of the naturally occurring aporphine alkaloid, roemerine. Nat. Prod. Res. 2021, 35, 6147–6152. [Google Scholar] [CrossRef]
- Ma, B.; Shi, S.; Guo, W.; Zhang, H.; Zhao, Z.; An, H. Liensinine, a Novel and Food-Derived Compound, Exerts Potent Antihepatoma Efficacy via Inhibiting the Kv10.1 Channel. J. Agric. Food Chem. 2024, 72, 4689–4702. [Google Scholar] [CrossRef]
- Manogaran, P.; Anandan, A.; Padma, V.V. Isoliensinine augments the therapeutic potential of paclitaxel in multidrug-resistant colon cancer stem cells and induced mitochondria-mediated cell death. J. Biochem. Mol. Toxicol. 2023, 37, e23395. [Google Scholar] [CrossRef]
- Levorato-Vinche, A.D.; Melhem, M.d.S.C.; Bonfietti, L.X.; De-La-Cruz-Chacón, I.; Boaro, C.S.F.; Fabro, A.T.; Ferreira, G.; Silva, J.d.F.d.; dos Santos, D.C.; Pereira, B.A.S.; et al. Antifungal activity of liriodenine on clinical strains of Cryptococcus neoformans and Cryptococcus gattii species complexes. J. Venom. Anim. Toxins Incl. Trop. Dis. 2022, 28, e20220006. [Google Scholar] [CrossRef] [PubMed]
- Weng, T.; Shen, C.; Chiu, Y.; Lin, Y.; Huang, Y. Effects of armepavine against hepatic fibrosis induced by thioacetamide in rats. Phytotherapy Res. 2012, 26, 344–353. [Google Scholar] [CrossRef] [PubMed]
- Al-Zahrani, M.H.; Alghamdi, R.A. In Silico Molecular Docking Analysis of the Potential role of Reticuline and Coclaurine as Anti-colorectal Cancer Alkaloids. J. Pharm. Res. Int. 2022, 34, 33–42. [Google Scholar] [CrossRef]
- Rodrigues, M.T.; Michelli, A.P.P.; Caso, G.F.; de Oliveira, P.R.; Rodrigues-Junior, D.M.; Morale, M.G.; Júnior, J.M.; Bortoluci, K.R.; Tamura, R.E.; da Silva, T.R.C.; et al. Lysicamine Reduces Protein Kinase B (AKT) Activation and Promotes Necrosis in Anaplastic Thyroid Cancer. Pharmaceuticals 2023, 16, 1687. [Google Scholar] [CrossRef]
- Nguyen, V.; Taine, E.G.; Meng, D.; Cui, T.; Tan, W. Chlorogenic Acid: A Systematic Review on the Biological Functions, Mechanistic Actions, and Therapeutic Potentials. Nutrients 2024, 16, 924. [Google Scholar] [CrossRef]
- Ren, F.; Hu, J.; Dang, Y.; Deng, H.; Ren, J.; Cheng, S.; Tan, M.; Zhang, H.; He, X.; Yu, H.; et al. Sphondin efficiently blocks HBsAg production and cccDNA transcription through promoting HBx degradation. J. Med. Virol. 2023, 95, e28578. [Google Scholar] [CrossRef]
- Zhou, L.; Liu, Q.; Hong, G.; Song, F.; Zhao, J.; Yuan, J.; Xu, J.; Tan, R.X.; Tickner, J.; Gu, Q.; et al. Cumambrin A prevents OVX-induced osteoporosis via the inhibition of osteoclastogenesis, bone resorption, and RANKL signaling pathways. FASEB J. 2019, 33, 6726–6735. [Google Scholar] [CrossRef]
- Gao, T.-H.; Liao, W.; Lin, L.-T.; Zhu, Z.-P.; Lu, M.-G.; Fu, C.-M.; Xie, T. Curcumae rhizoma and its major constituents against hepatobiliary disease: Pharmacotherapeutic properties and potential clinical applications. Phytomedicine 2022, 102, 154090. [Google Scholar] [CrossRef]
- Li, C.; Zhang, Z.; Wang, J. A Thermophilic Biofunctional Multienzyme Cascade Reaction for Cell-Free Synthesis of Salvianic Acid A and 3,4-Dihydroxymandelic Acid. ACS Sustain. Chem. Eng. 2019, 7, 18247–18253. [Google Scholar] [CrossRef]
- Reang, J.; Sharma, P.C.; Thakur, V.K.; Majeed, J. Understanding the Therapeutic Potential of Ascorbic Acid in the Battle to Overcome Cancer. Biomolecules 2021, 11, 1130. [Google Scholar] [CrossRef]
- Zou, M.-Y.; Nie, S.-P.; Yin, J.-Y.; Xie, M.-Y. Ascorbic acid induced degradation of polysaccharide from natural products: A review. Int. J. Biol. Macromol. 2020, 151, 483–491. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Chandel, S.; Ghosh, A.; Matta, T.; Gautam, A.; Bhattacharya, A.; Babu, S.S.; Sukla, S.; Nag, D.; Ravichandiran, V.; et al. Glucogallin Attenuates the LPS-Induced Signaling in Macrophages and Protects Mice against Sepsis. Int. J. Mol. Sci. 2022, 23, 11254. [Google Scholar] [CrossRef] [PubMed]
- Nomura, S.; Monobe, M.; Ema, K.; Yoshida, K.; Yamashita, S.; Ogino, A.; Nesumi, A. Effects of a Tea Cultivar “MK5601” on Behaviors and Hippocampal Neurotrophin-3 Levels in Middle-Aged Mice. J. Nutr. Sci. Vitaminol. 2021, 67, 170–179. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Liu, W.; Zhang, J.; Dong, Y.; Wu, J.; Dai, C.; Zhang, T.; Yang, G.; Zhang, Y.; Li, A. Bellidifolin ameliorates isoprenaline-induced cardiac hypertrophy by the Nox4/ROS signalling pathway through inhibiting BRD4. Cell Death Discov. 2023, 9, 279. [Google Scholar] [CrossRef]
- Woo, S.H.; Lee, S.-H.; Moon, S.-J.; Han, J.; Seo, K.-S.; Lee, H.; Lee, C.-H.; Hwang, J.H. Beta-lapachone ameliorates the progression of primary sclerosing cholangitis pathogenesis in rodent models. Life Sci. 2024, 337, 122342. [Google Scholar] [CrossRef]

| Project | Standard for Evaluation | Score (Points) |
|---|---|---|
| Appearance (15%) | Shade of yellow, with the surface covered with A. cristatus. | 11~15 |
| Shade of yellow, with less A. cristatus scattered on the surface. | 6~10 | |
| Jade green, without A. cristatus. | 0~5 | |
| Aroma (35%) | Unique A. cristatus aroma and fragrance of lotus leaf, with both exhibiting richness and potency. | 31~35 |
| The unique aroma of A. cristatus is mild, with a rich fragrance of lotus leaf. | 21~30 | |
| Mild A. cristatus and lotus leaf aroma. | 11~20 | |
| Only one aroma is smelled. | 0~10 | |
| Taste (35%) | Pronounced unique flavor of A. cristatus, providing a stimulating experience for the taste buds without any astringency. | 28~35 |
| Slightly unique flavor of A. cristatus, with a faintly bitter and astringent taste. | 15~27 | |
| Off-flavor, with a bitter and astringent taste. | 0~14 | |
| Infusion color (10%) | Orange-yellow, clear, and bright. | 6~10 |
| Pale yellow, clear and bright. | 0~5 | |
| Leaf residue (5%) | Reddish-brown, fairly uniform. | 3~5 |
| Green, not sufficiently uniform. | 0~2 |
| Group | Score | Total Score | ||||
|---|---|---|---|---|---|---|
| Appearance | Aroma | Taste | Infusion Color | Leaf Residue | ||
| UL | 4.90 ± 0.32 | 9.60 ± 0.84 | 12.20 ± 1.99 | 4.90 ± 0.32 | 2.00 ± 0.00 | 33.60 ± 2.12 |
| FL | 9.30 ± 1.70 * | 28.50 ± 5.68 * | 24.80 ± 3.62 * | 7.80 ± 0.79 * | 3.30 ± 0.48 * | 70.90 ± 7.81 * |
| Metabolite | Classification | Formula | VIP | Difference Multiple |
|---|---|---|---|---|
| Trans-Chlorogenic acid | phenylpropanoid | C16H18O9 | 3.3151 | 122.2079 |
| Sphondin | phenylpropanoid | C12H8O4 | 2.5886 | 14.848 |
| Cumambrin A | terpenes | C17H22O5 | 2.4607 | 2.4171 |
| Byssochlamic acid | phenylpropanoid | C18H20O6 | 2.3443 | 1.9447 |
| Furanodiene | terpenes | C15H20O | 2.3135 | 2.38 |
| Matricarin | lactones | C17H20O5 | 2.2349 | 1.7867 |
| Isorhamnetin 3-O-(beta-D-glucopyranosyl-(1->6) -beta-D-glucopyranoside) | phenols | C28H32O17 | 2.2183 | 2.1373 |
| Thiophene-4,5-epoxide | phenylpropanoid | C14H12O4S | 2.2001 | 2.0397 |
| 3-Oxoadipic acid | organic acids | C6H8O5 | 2.1892 | 1.8412 |
| 3,4-Dihydroxymandelic Acid | phenols | C8H8O5 | 2.1857 | 2.0714 |
| Cis-Caffeoyl tartaric acid | phenylpropanoid | C13H12O9 | 2.1702 | 2.0788 |
| Ascorbic acid | organic acids | C6H8O6 | 2.1698 | 2.3275 |
| D-Xylono-1,5-lactone | lactones | C5H8O5 | 2.1511 | 2.4134 |
| Beta-Glucogallin | phenylpropanoid | C13H16O10 | 2.1212 | 2.1199 |
| Gallic Acid | phenylpropanoid | C7H6O5 | 2.1197 | 1.5084 |
| Theogallin | phenylpropanoid | C14H16O10 | 2.1045 | 2.0177 |
| 4-Butylphenol | phenols | C10H14O | 2.1029 | 1.962 |
| Bellidifolin | quinones | C14H10O6 | 2.0952 | 1.793 |
| Beta-Lapachone | quinones | C15H14O3 | 2.0889 | 1.9392 |
| 10-Hydroxy-3-methoxy-1,3,5,7 -cadinatetraen-9-one | terpenes | C16H20O3 | 2.0854 | 1.8185 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dai, W.; Tan, Y.; He, S.; Zhou, L.; Ren, X.; Huang, Y.; Song, L.; Liu, Y. Non-Targeted Metabolomics Analysis Unravels Changes in Non-Volatile Metabolites in Folium nelumbinis (Lotus Leaf) Induced by Aspergillus cristatus-Mediated Fermentation. Fermentation 2025, 11, 279. https://doi.org/10.3390/fermentation11050279
Dai W, Tan Y, He S, Zhou L, Ren X, Huang Y, Song L, Liu Y. Non-Targeted Metabolomics Analysis Unravels Changes in Non-Volatile Metabolites in Folium nelumbinis (Lotus Leaf) Induced by Aspergillus cristatus-Mediated Fermentation. Fermentation. 2025; 11(5):279. https://doi.org/10.3390/fermentation11050279
Chicago/Turabian StyleDai, Wei, Yumei Tan, Shengling He, Luona Zhou, Xiyi Ren, Yonghui Huang, Li Song, and Yongxiang Liu. 2025. "Non-Targeted Metabolomics Analysis Unravels Changes in Non-Volatile Metabolites in Folium nelumbinis (Lotus Leaf) Induced by Aspergillus cristatus-Mediated Fermentation" Fermentation 11, no. 5: 279. https://doi.org/10.3390/fermentation11050279
APA StyleDai, W., Tan, Y., He, S., Zhou, L., Ren, X., Huang, Y., Song, L., & Liu, Y. (2025). Non-Targeted Metabolomics Analysis Unravels Changes in Non-Volatile Metabolites in Folium nelumbinis (Lotus Leaf) Induced by Aspergillus cristatus-Mediated Fermentation. Fermentation, 11(5), 279. https://doi.org/10.3390/fermentation11050279





