Fermented Chive (Allium schoenoprasum) with Lactobacillus plantarum: A Potential Antibiotic Alternative Feed Additive for Broilers Challenged with Escherichia coli
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Fermented Chive
2.2. Pathogenic Bacteria
2.3. Experimental Animals
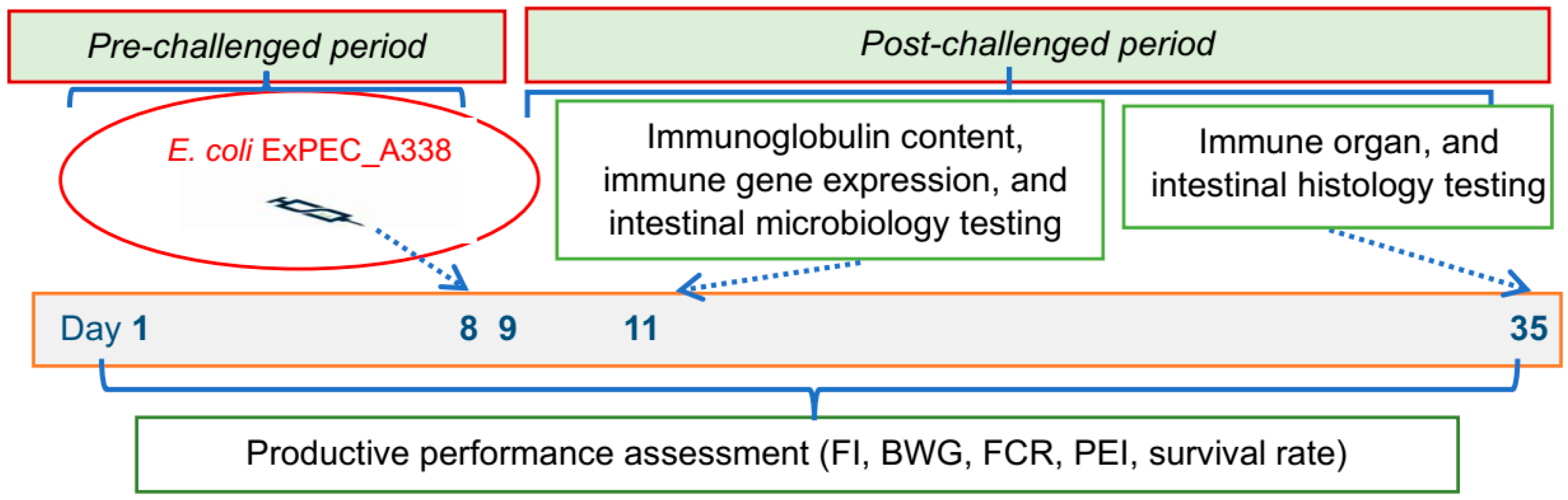
2.4. Experimental Design
2.5. Indicators and Research Methods
2.6. Statistical Analysis
3. Results
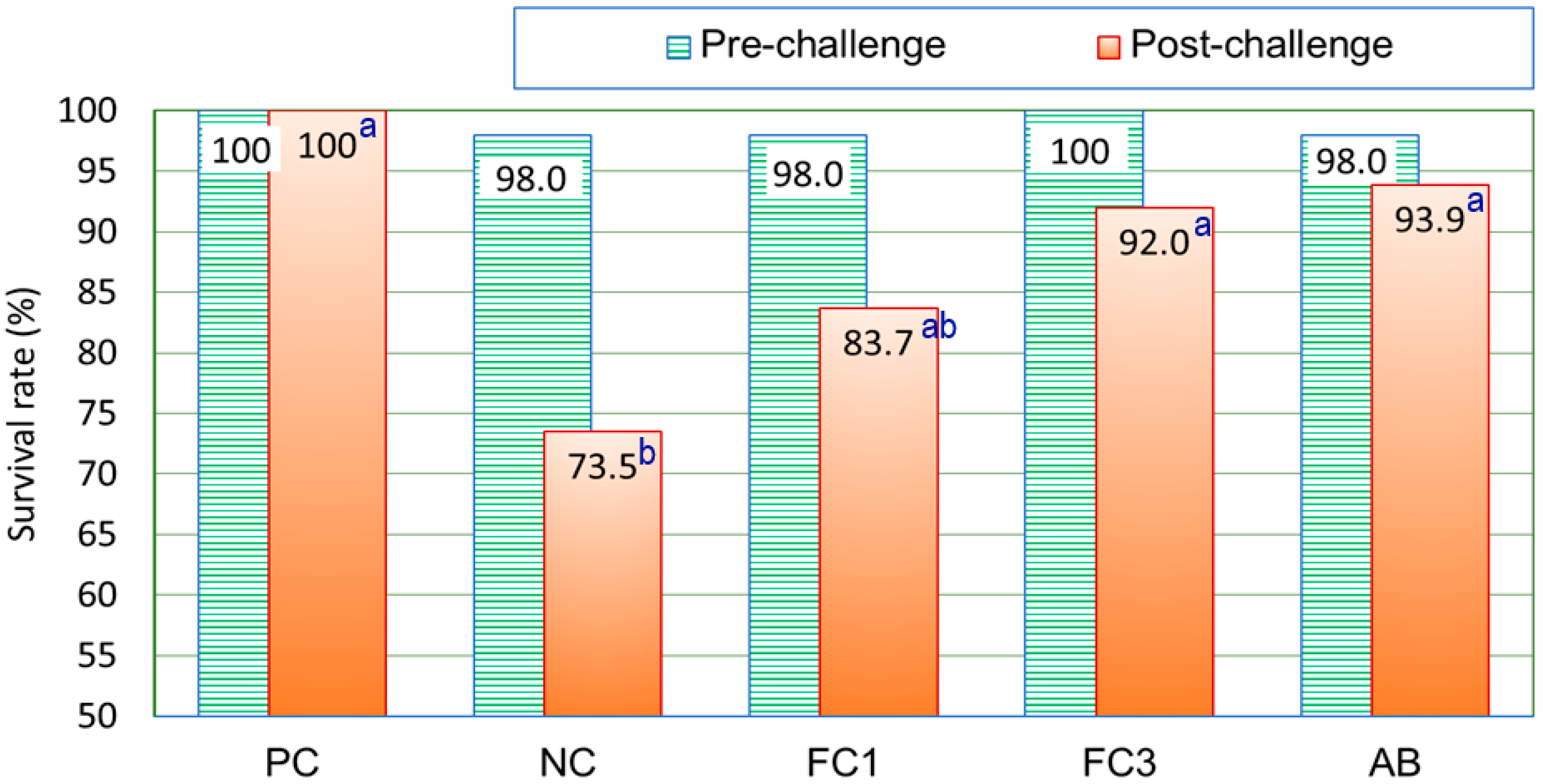
3.1. Effect of Supplementary Fermented Chive on the Productivity of Broiler Chickens
3.2. Effect of Fermented Chive Supplementation on Immune Function
4. Discussion
4.1. Effects of Supplementation on the Productivity of Experimental Broiler Chickens
4.2. Effects of Fermented Chive Supplementation on Immune Function
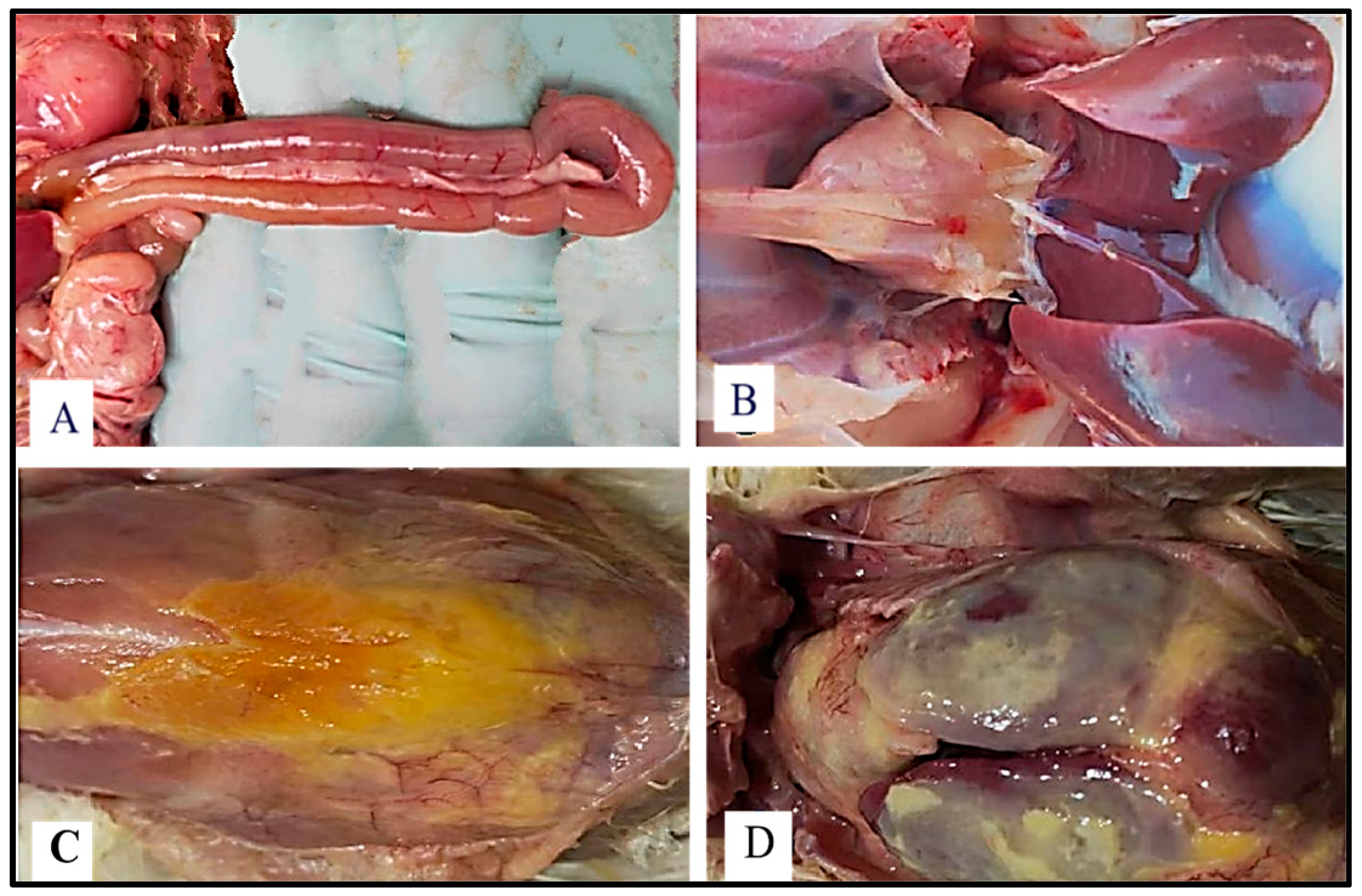
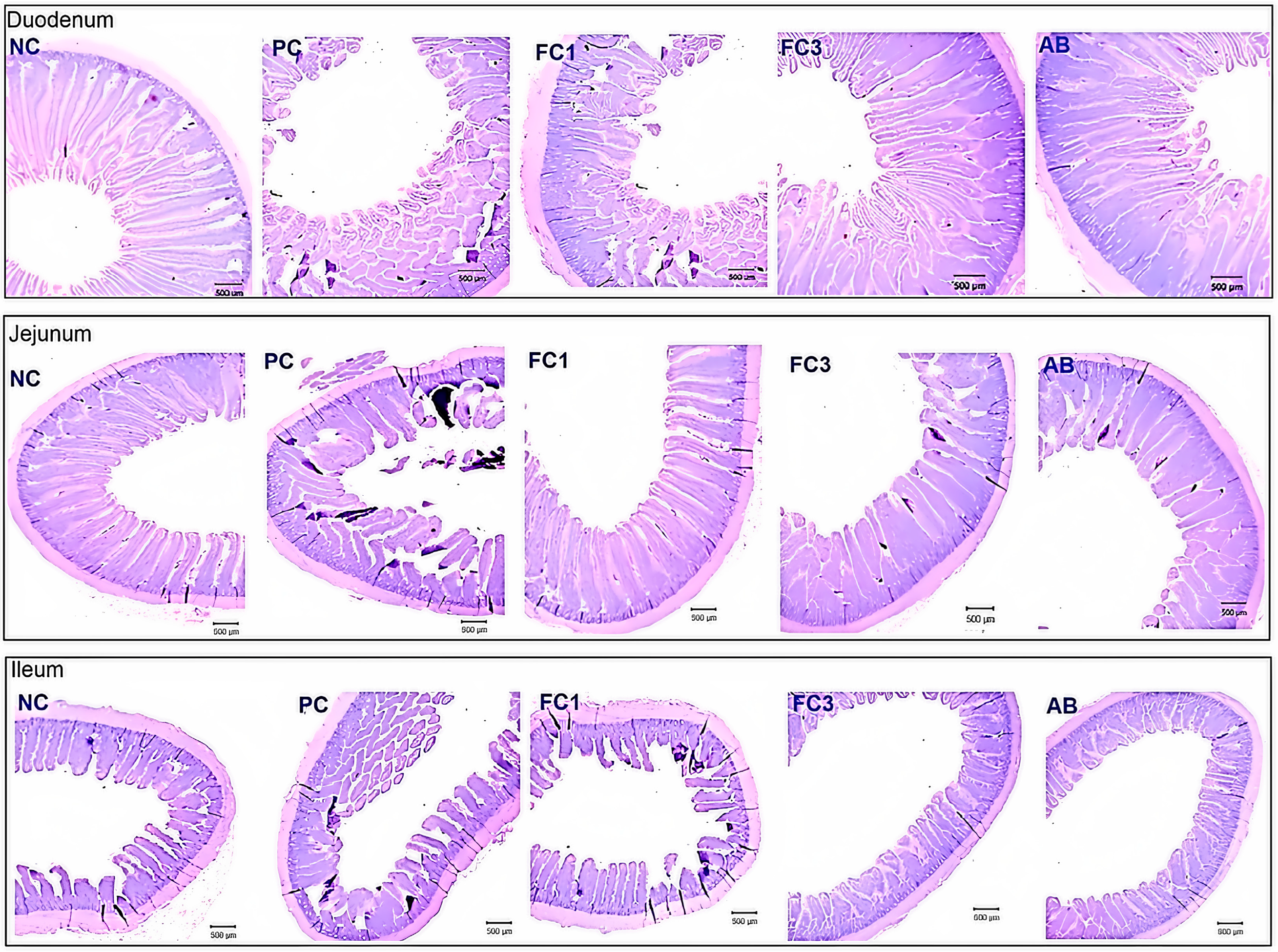
4.3. Effects of Fermented Chive Supplementation on Gut Health
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Watts, A.; Wigley, P. Avian pathogenic Escherichia coli: An overview of infection biology, antimicrobial resistance and vaccination. Antibiotics 2024, 13, 809. [Google Scholar] [CrossRef]
- Ronco, T.; Stegger, M.; Olsen, R.H.; Sekse, C.; Nordstoga, A.B.; Pohjanvirta, T.; Lilje, B.; Lyhs, U.; Andersen, P.S.; Pedersen, K. Spread of avian pathogenic Escherichia coli ST117 O78: H4 in Nordic broiler production. BMC Genom. 2017, 18, 1–8. [Google Scholar] [CrossRef]
- Hai, P.V.; Hoa, N.X. Effect of Allium schoenoprasum extract on immune status against Newcastle virus and growth performance of broiler chicken. Electron. J. Agric. Sci. Technol.—Hue Univ. Agric. For. 2020, 4, 2058–2064. [Google Scholar]
- Charen, E.; Harbord, N. Toxicity of herbs, vitamins, and supplements. Adv. Chronic Kidney Dis. 2020, 27, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Park, S.-L.; Lee, S.; Lee, S.-Y.; Ko, S.; Yoo, M. UPLC/ESI-MS/MS analysis of compositional changes for organosulfur compounds in garlic (Allium sativum L.) during fermentation. Food Chem. 2016, 211, 555–559. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Miao, Q.; Pan, C.; Yin, J.; Wang, L.; Qu, L.; Yin, Y.; Wei, Y. Research advances in probiotic fermentation of Chinese herbal medicines. iMeta 2023, 2, e93. [Google Scholar] [CrossRef] [PubMed]
- Xiang, H.; Sun-Waterhouse, D.; Waterhouse, G.I.N.; Cui, C.; Ruan, Z. Fermentation-enabled wellness foods: A fresh perspective. Food Sci. Hum. Wellness 2019, 8, 203–243. [Google Scholar] [CrossRef]
- Li, H.; Kang, Y.; Sun, Y.; Bian, C.; Fan, M.; Zhang, H.; Zhao, S.; Song, X.; Qiao, H. The role of Lactobacillus plantarum in solid-state fermentation of Astragalus membranaceus for broiler chicken feed. AMB Express 2025, 15, 26. [Google Scholar] [CrossRef]
- Hai, P.; Phuong, H.F.C.; Hung, P.H.S.; Na, T.T.; Lai, N.H.; Khuong, N.D.T.; Liem, T.N.; Hoa, N.X. Selection of Lactobacillus strains from native chicken feces for the fermentation of purple onion (Allium cepa L.) as an antibiotic alternative against Salmonella spp. in chickens. Open Vet. J. 2024, 14, 3525–3538. [Google Scholar] [CrossRef]
- TCVN 11892-1:2017; Good Agricultural Practice—Part 1: Crop Production. VietGAP: Hanoi, Vietnam, 2017.
- Complete Feed for Broilers—10 TCN 661-2005; Vietnam Ministry of Agriculture and Rural Development: Hanoi, Vietnam, 2025.
- Martins, J.M.S.; Carvalho, C.M.C.; Litz, F.; Silveira, M.M.; Moraes, C.; Silva, M.C.A.; Fagundes, N.; Fernandes, E. Productive and economic performance of broiler chickens subjected to different nutritional plans. Rev. Bras. Cienc. Avic. 2016, 18, 209–216. [Google Scholar] [CrossRef][Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Layton, C.; Bancroft, J.D.; Suvarna, S.K. 4—Fixation of tissues. In Bancroft’s Theory and Practice of Histological Techniques, 8th ed.; Suvarna, S.K., Layton, C., Bancroft, J.D., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 40–63. [Google Scholar]
- Kathayat, D.; Lokesh, D.; Ranjit, S.; Rajashekara, G. Avian pathogenic Escherichia coli (APEC): An overview of virulence and pathogenesis factors, zoonotic potential, and control strategies. Pathogens 2021, 10, 467. [Google Scholar] [CrossRef] [PubMed]
- Kothari, D.; Lee, W.-D.; Jung, E.; Niu, K.; Lee, C.; Kim, S.-K. Controlled fermentation using autochthonous Lactobacillus plantarum improves antimicrobial potential of Chinese chives against poultry pathogens. Antibiotics 2020, 9, 386. [Google Scholar] [CrossRef]
- Xu, F.; Wu, H.; Xie, J.; Zeng, T.; Hao, L.; Xu, W.; Lu, L. The effects of fermented feed on the growth performance, antioxidant activity, immune function, intestinal digestive enzyme activity, morphology, and microflora of yellow-feather chickens. Animals 2023, 13, 3545. [Google Scholar] [CrossRef] [PubMed]
- Alagawany, M.; Hegazy, M.I.; Farag, M.R.; El-Shall, N.A.; Sallam, S.M.; Dhama, K. Chapter 2—Probiotics, prebiotics, and synbiotics in animal and poultry nutrition. In Organic Feed Additives for Livestock; Elsevier: Amsterdam, The Netherlands, 2025; pp. 17–41. [Google Scholar]
- Richards, P.J.; Almutrafy, A.; Liang, L.; Flaujac Lafontaine, G.M.; King, E.; Fish, N.M.; Connerton, A.J.; Connerton, P.L.; Connerton, I.F. Prebiotic galactooligosaccharide feed modifies the chicken gut microbiota to efficiently clear Salmonella. mSystems 2024, 9, e0075424. [Google Scholar] [CrossRef]
- Adli, D.N.; Sholikin, M.M.; Ujilestari, T.; Ahmed, B.; Sadiqqua, A.; Harahap, M.A.; Sofyan, A.; Sugiharto, S. Effect of fermentation of herbal products on growth performance, breast meat quality, and intestinal morphology of broiler chickens: A meta-analysis. Ital. J. Anim. Sci. 2024, 23, 734–750. [Google Scholar] [CrossRef]
- Liu, J.; Gu, H.; Jia, R.; Li, S.; Chen, Z.; Zheng, A.; Chang, W.; Liu, G. Effects of Lactobacillus acidophilus on production performance and immunity of broiler chickens and their mechanism. Front. Vet. Sci. 2025, 12, 1554502. [Google Scholar] [CrossRef]
- Muratbayev, D.; Ygiyeva, A.; Bilyalov, Y.; Zaikovskaya, O.; Zhexenayeva, A. Morphogenesis of the spleen and cloacal bursa of a chicken embryo under the influence of “ligfolum” and “placenta denatured emulsified”. Int. J. Vet. Sci. 2023, 12, 847–852. [Google Scholar]
- Sattarova, R.; Shynybaev, K.; Bakiyeva, F.; Strochkov, V.; Boranbayeva, K.; Zhanserkenova, O.; Kassymbekova, S.; Ibadullayeva, A.; Khamzina, A. Metagenomic Analysis and identification of epizootic strains of the causative agent of infectious bovine Keratoconjunctivitis in Kazakhstan. Int. J. Vet. Sci. 2023, 12, 822–831. [Google Scholar]
- Malematja, E.; Manyelo, G.; Sebola, A.; Mabelebele, M. The role of insects in promoting the health and gut status of poultry. Comp. Clin. Pathol. 2023, 32, 501–513. [Google Scholar] [CrossRef]
- Sugiharto, S.; Ranjitkar, S. Recent advances in fermented feeds towards improved broiler chicken performance, gastrointestinal tract microecology and immune responses: A review. Anim. Nutr. 2019, 5, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Ismail, I.E.; Alagawany, M.; Taha, A.E.; Puvača, N.; Laudadio, V.; Tufarelli, V. Effect of dietary supplementation of garlic powder and phenyl acetic acid on productive performance, blood hematology, immunity and antioxidant status of broiler chickens. Anim. Biosci. 2021, 34, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Yang, K.; Zhang, A.; Chang, W.; Zheng, A.; Chen, Z.; Cai, H.; Liu, G. Effects of Lactobacillus acidophilus on the growth performance, immune response, and intestinal barrier function of broiler chickens challenged with Escherichia coli O157. Poult. Sci. 2021, 100, 101323. [Google Scholar] [CrossRef]
- Zhu, X.; Tao, L.; Liu, H.; Yang, G. Effects of fermented feed on growth performance, immune organ indices, serum biochemical parameters, cecal odorous compound production, and the microbiota community in broilers. Poult. Sci. 2023, 102, 102629. [Google Scholar] [CrossRef]
- Shang, Y.; Regassa, A.; Kim, J.H.; Kim, W.K. The effect of dietary fructooligosaccharide supplementation on growth performance, intestinal morphology, and immune responses in broiler chickens challenged with Salmonella enteritidis lipopolysaccharides. Poult. Sci. 2015, 94, 2887–2897. [Google Scholar] [CrossRef] [PubMed]
- Paraschivescu, C.; Barbosa, S.; Van Steenwinckel, J.; Gressens, P.; Glaichenhaus, N.; Davidovic, L. Early Life Exposure to Tumor Necrosis Factor Induces Precocious Sensorimotor Reflexes Acquisition and Increases Locomotor Activity During Mouse Postnatal Development. Front. Behav. Neurosci. 2022, 16, 845458. [Google Scholar] [CrossRef] [PubMed]
- Itoh, T.; Miyazono, D.; Sugata, H.; Mori, C.; Takahata, M. Anti-inflammatory Effects of heat-killed Lactiplantibacillus argentoratensis BBLB001 on a gut inflammation co-culture cell model and dextran sulfate sodium-induced colitis mouse model. Int. Immunopharmacol. 2024, 143, 113408. [Google Scholar] [CrossRef]
- De Vos, W.M.; Tilg, H.; Van Hul, M.; Cani, P.D. Gut microbiome and health: Mechanistic insights. Gut 2022, 71, 1020–1032. [Google Scholar] [CrossRef]
- Lin, T.-L.; Lu, C.-C.; Lai, W.-F.; Wu, T.-S.; Lu, J.-J.; Chen, Y.-M.; Tzeng, C.-M.; Liu, H.-T.; Wei, H.; Lai, H.-C. Role of gut microbiota in identification of novel TCM-derived active metabolites. Protein Cell 2021, 12, 394–410. [Google Scholar] [CrossRef]
- Celi, P.; Cowieson, A.; Fru-Nji, F.; Steinert, R.; Kluenter, A.-M.; Verlhac, V. Gastrointestinal functionality in animal nutrition and health: New opportunities for sustainable animal production. Anim. Feed. Sci. Technol. 2017, 234, 88–100. [Google Scholar] [CrossRef]
- Huang, R.; Wu, F.; Zhou, Q.; Wei, W.; Yue, J.; Xiao, B.; Luo, Z. Lactobacillus and intestinal diseases: Mechanisms of action and clinical applications. Microbiol. Res. 2022, 260, 127019. [Google Scholar] [CrossRef] [PubMed]
- Guan, X.; Li, W.; Meng, H. A double-edged sword: Role of butyrate in the oral cavity and the gut. Mol. Oral Microbiol. 2021, 36, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Teng, P.-Y.; Yadav, S.; de Souza Castro, F.L.; Tompkins, Y.H.; Fuller, A.L.; Kim, W.K. Graded Eimeria challenge linearly regulated growth performance, dynamic change of gastrointestinal permeability, apparent ileal digestibility, intestinal morphology, and tight junctions of broiler chickens. Poult. Sci. 2020, 99, 4203–4216. [Google Scholar] [CrossRef] [PubMed]
- Ceylan, A.; Saçaklı, P.; Özgenç Çınar, Ö.; Ramay, M.S.; Ahsan, U.; Harijaona, J.A.; Bayraktaroğlu, A.G.; Manghebati, F.; Calik, A. Effect of supplemental dietary phytogenic blends on growth performance, jejunal histomorphometry, and jejunal immunity of broiler chickens. Arch. Anim. Breed. 2025, 68, 13–26. [Google Scholar] [CrossRef]
| Component/Compound | Fresh Chive | Fermented Chive | Determination Method |
|---|---|---|---|
| Polyphenol (mg/g) | 10–15 | 15–20 | Folin-Ciocalteu |
| Quercetin (mg/g) | 2–4 | 3–6 | UV-Vis |
| Sulfur compounds | |||
| Allicin (mg/kg) | 1–1.5 | 1–1.5 | GC-MS |
| Thiosulfate (mg/g) | 5–7 | ~2–3 | GC |
| S-allyl cysteine (mg/g) | 0.1–0.3 | 1–3 | GC-MS |
| Organic acids | |||
| Lactic acid (%) | negligibility | 0.5–1.5 | HPLC |
| Acetic acid (%) | negligibility | 0.1–0.5 | HPLC |
| Citric acid (%) | ~0.2–0.5 | ~0.5–1 | HPLC |
| Crude protein (g/kg) | 16.3–20.1 | 16.2–22.3 | AOAC |
| Crude fat (g/kg) | 6–9 | 3–4 | AOAC |
| Crude fiber (g/kg) | 22.3–26.8 | 21.4–24.5 | AOAC |
| Metabolizable energy (kCal/100g) | 330–370 | 360–380 | AOAC |
| Nutrient | Starter Phase (<19 Days of Age) | Finisher Phase (≥19 Days of Age) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| NC | PC | AB | FC1 | FC3 | NC | PC | AB | FC1 | FC3 | |
| Ingredient composition | ||||||||||
| Fermented chive (FC) | 0 | 1 | 3 | 0 | 1 | 3 | ||||
| Yellow corn | 47 | 47.1 | 47.2 | 58.5 | 58.6 | 58.6 | ||||
| Soybean meal (36.7% CP) | 44.28 | 43.08 | 40.98 | 33.78 | 32.58 | 30.68 | ||||
| Fish meal | 5 | 5.1 | 5.1 | 4 | 4.1 | 4 | ||||
| CaCO3 (38%) | 2 | 2 | 2 | 2 | 2 | 2 | ||||
| CaHPO4 | 1 | 1 | 1 | 1 | 1 | 1 | ||||
| Sodium chloride | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | ||||
| Choline chloride (50%) | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | ||||
| DL-Methionine (99.5%) | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | ||||
| Vitamin premix 1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | ||||
| Mineral premix 2 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | ||||
| Calculated nutritional values | ||||||||||
| Crude protein | 22.2 | 21.3 | 22.2 | 18.0 | 18.1 | 18.1 | ||||
| Crude fat | 4.48 | 4.43 | 4.32 | 5.48 | 5.40 | 5.36 | ||||
| Crude fiber | 5.05 | 5.06 | 5.13 | 5.05 | 5.08 | 5.09 | ||||
| Calcium | 1.10 | 1.09 | 1.06 | 1.00 | 1.03 | 1.01 | ||||
| Phosphorus | 0.51 | 0.50 | 0.50 | 0.44 | 0.45 | 0.45 | ||||
| Lysine | 1.32 | 1.30 | 1.30 | 1.04 | 1.02 | 1.01 | ||||
| Metabolizable energy (kCal/kg) | 3000 | 3000 | 3000 | 3200 | 3200 | 3200 | ||||
| Gene | Sequence Primer (5′ to 3′) | Genbank ID | ||
|---|---|---|---|---|
| Forward Primer | Reverse Primer | Size (bp) | ||
| Tight-binding protein | ||||
| ZO-1 | CTTCAGGTGTTTCTCTTCCTCCTC | CTGTGGTTTCATGGCTGGAT | 121 | XM_413773.4 |
| Occludin | GCAGATGTCCAGCGGTFC1CFC1C | CGAAGAAGCAGATGAGGCAGAG | 89 | NM_205128.1 |
| Claudin-2 | CAFC1CTCCTGGGTCTGGTTGGT | GACAGCCATCCGCATCTTCT | 198 | NM_001013611.2 |
| Pro-inflammatory cytokines | ||||
| IL-4 | GTGCCCACGCT GTGCTFC1C | AGGAAACCTCT CCCTGGATGTC | 82 | GU119892.1 |
| IL-1β | GFC1CCGAGFC1C AACCCCTGC | AGCAACGGGAC GGT AATGAA | 204 | NM_204524.1 |
| TNF-α | CTCAGGACAGC CFC1TGCCAACA | CCACCACACGA CAG CCAAGT | 177 | XM_015294125.2 |
| IFN-γ | CCTCGCAACCT TCACCTCAC | CGCTGFC1ATCG TTG TCTTGGAG | 76 | FJ977575.1 |
| GAPDH | AACTTTGGCAT TGTGGAGGG | ACGCTGGGATG ATGTTCTGG | 130 | NM_204305.1 |
| Target | NC | PC | FC1 | FC3 | AB | Pooled SEM | p-Value |
|---|---|---|---|---|---|---|---|
| Pre-challenged period (1–7 days old) | |||||||
| BWG (g/head) | 25.5 | 26.9 | 26.5 | 25.6 | 26.6 | 0.25 | 0.195 |
| FI (g/head) | 45.5 | 45.5 | 45.5 | 45.6 | 45.6 | 0.29 | 0.341 |
| FCR | 1.78 | 1.74 | 1.76 | 1.81 | 1.75 | 0.16 | 0.166 |
| Post-challenged period (8–35 days old) | |||||||
| BWG (g/head) | 699.3 a | 633.7 b | 686.7 ab | 714.7 a | 699.4 a | 33.29 | <0.001 |
| FI (g/head) | 1588 b | 1471.3 c | 1623.2 ab | 1668.2 a | 1630.8 ab | 63.52 | <0.001 |
| FCR | 2.28 | 2.32 | 2.35 | 2.33 | 2.33 | 0.13 | 0.069 |
| Full time (1–35 days old) | |||||||
| BWG (g/head) | 724.4 ab | 659.4 c | 711.9 b | 739.5 a | 725.0 ab | 34.92 | <0.001 |
| FI (g/head) | 1634.4 ab | 1516.5 c | 1668.4 ab | 1713.5 a | 1576.2 bc | 89.51 | <0.001 |
| FCR | 2.26 ab | 2.30 ab | 2.34 a | 2.32 a | 2.17 b | 0.131 | <0.001 |
| PEI | 89.94 a | 57.37 c | 72.92 b | 83.91 a | 85.77 a | 3.57 | <0.001 |
| Treatment | Immune Organ Index (g/kg BW) | Immunoglobulin Concentration (g/L) | ||||
|---|---|---|---|---|---|---|
| Bursa of Fabricius | Spleen | Thymus | IgA | IgM | IgG | |
| NC | 2.01 b | 1.82 b | 3.44 | 3.23 b | 3.22 b | 1.93 |
| PC | 1.98 b | 1.79 b | 3.82 | 2.19 a | 2.18 a | 1.88 |
| FC1 | 2.04 b | 1.83 b | 3.74 | 3.22 b | 3.63 b | 2.02 |
| FC3 | 2.44 a | 2.25 a | 3.92 | 2.90 b | 3.32 b | 1.96 |
| AB | 1.99 b | 1.79 b | 3.51 | 2.46 ab | 2.69 ab | 1.98 |
| Pooled SEM | 0.02 | 0.02 | 0.06 | 0.04 | 0.04 | 0.03 |
| p-value | <0.01 | <0.01 | 0.262 | <0.01 | <0.01 | 0.618 |
| Gene | NC | PC | FC1 | FC3 | AB | Pooled SEM | p-Value |
|---|---|---|---|---|---|---|---|
| Tight-binding protein | |||||||
| ZO-1 | 0.93 b | 0.82 b | 0.95 b | 3.02 a | 0.96 b | 0.13 | <0.001 |
| Occludin | 1.12 | 1.16 | 0.97 | 1.08 | 1.19 | 0.13 | 0.146 |
| Claudin-2 | 0.91 bc | 0.42 d | 1.23 b | 2.75 a | 1.15 b | 0.14 | <0.001 |
| Pro-inflammatory cytokines | |||||||
| IL-4 | 2.33 b | 1.85 b | 3.12 a | 3.29 a | 3.38a | 0.19 | <0.001 |
| IL-1β | 1.24 | 1.27 | 1.37 | 1.34 | 1.34 | 0.24 | 0.949 |
| TNF-α | 1.14 c | 2.01 a | 1.62 b | 1.22 c | 1.19 c | 0.08 | <0.001 |
| IFN-γ | 1.84 c | 3.59 a | 2.88 b | 2.14 bc | 2.12 bc | 0.15 | <0.001 |
| Bacteria | NC | PC | FC1 | FC3 | AB | Pooled SEM | p-Value |
|---|---|---|---|---|---|---|---|
| Salmonella spp. | 4.61 b | 6.68 a | 4.63 b | 4.21 b | 4.54 b | 0.34 | <0.001 |
| E. coli | 6.67 ab | 7.1 a | 6.24 b | 5.51 c | 5.63 bc | 0.48 | <0.001 |
| Lactobacillus spp. | 4.84 bc | 5.49 b | 3.84 c | 7.22 a | 4.72 bc | 0.79 | <0.001 |
| Items | NC | PC | FC1 | FC3 | AB | Pooled SEM | p-Value |
|---|---|---|---|---|---|---|---|
| Duodenum | |||||||
| VH (µm) | 2489.3 a | 1817.5 b | 2150.5 ab | 2190.6 ab | 2244.6 ab | 110.8 | 0.005 |
| CD (µm) | 186.7 c | 379.6 a | 306.4 b | 276.4 b | 291.4 b | 21.09 | <0.001 |
| VH:CD | 13.33 a | 4.79 c | 7.02 b | 7.93 b | 7.70 b | 0.71 | <0.001 |
| Jejunum | |||||||
| VH (µm) | 1304.1 | 1150.2 | 1182.6 | 1223 | 1277 | 46.8 | 0.119 |
| CD (µm) | 164.8 b | 284.9 b | 271.8 a | 241.8 a | 256.8 a | 11.79 | <0.001 |
| VH:CD | 7.91 a | 4.04 c | 4.39b c | 5.06 b | 4.97b | 0.39 | <0.001 |
| Ileum | |||||||
| VH (µm) | 902.7 b | 786.9 c | 936.8 b | 1061.5 a | 907.2 b | 20.94 | 0.013 |
| CD (µm) | 152.8 c | 206.44 a | 184.69 ab | 182.54 ab | 171.65 bc | 7.48 | 0.023 |
| VH:CD | 5.91 a | 3.81 c | 5.07 ab | 5.82 a | 5.29 ab | 0.31 | 0.019 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hai, P.V.; Anh, L.X.; Hoa, N.X. Fermented Chive (Allium schoenoprasum) with Lactobacillus plantarum: A Potential Antibiotic Alternative Feed Additive for Broilers Challenged with Escherichia coli. Fermentation 2025, 11, 277. https://doi.org/10.3390/fermentation11050277
Hai PV, Anh LX, Hoa NX. Fermented Chive (Allium schoenoprasum) with Lactobacillus plantarum: A Potential Antibiotic Alternative Feed Additive for Broilers Challenged with Escherichia coli. Fermentation. 2025; 11(5):277. https://doi.org/10.3390/fermentation11050277
Chicago/Turabian StyleHai, Phan Vu, Le Xuan Anh, and Nguyen Xuan Hoa. 2025. "Fermented Chive (Allium schoenoprasum) with Lactobacillus plantarum: A Potential Antibiotic Alternative Feed Additive for Broilers Challenged with Escherichia coli" Fermentation 11, no. 5: 277. https://doi.org/10.3390/fermentation11050277
APA StyleHai, P. V., Anh, L. X., & Hoa, N. X. (2025). Fermented Chive (Allium schoenoprasum) with Lactobacillus plantarum: A Potential Antibiotic Alternative Feed Additive for Broilers Challenged with Escherichia coli. Fermentation, 11(5), 277. https://doi.org/10.3390/fermentation11050277









