Production of High-Value-Added Biomass by Saccharomyces cerevisiae Using Lignocellulosic Substrate
Abstract
1. Introduction
2. Materials and Methods
2.1. Material
2.2. Solid-State Fermentation
2.3. Chemical Composition
2.4. Minerals
2.5. Phenolic Compound Extraction and Quantification
2.6. Statisctical Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ali, M.M.; Hashim, N. Exploring Nutritional Compositions, Volatile Compounds, Health Benefits, Emerging Processing Technologies, and Potential Food Products of Glutinous Rice: A Review. Rice Sci. 2024, 31, 251–268. [Google Scholar] [CrossRef]
- Sharma, J.; Kumar, V.; Prasad, R.; Gaur, N.A. Engineering of Saccharomyces Cerevisiae as a Consolidated Bioprocessing Host to Produce Cellulosic Ethanol: Recent Advancements and Current Challenges. Biotechnol. Adv. 2022, 56, 107925. [Google Scholar] [CrossRef] [PubMed]
- Baptista, S.L.; Costa, C.E.; Cunha, J.T.; Soares, P.O.; Domingues, L. Metabolic Engineering of Saccharomyces Cerevisiae for the Production of Top Value Chemicals from Biorefinery Carbohydrates. Biotechnol. Adv. 2021, 47, 107697. [Google Scholar] [CrossRef]
- Ribeiro, A.C.; Graça, C.; Chiattoni, L.M.; Massarolo, K.C.; Duarte, F.; Mellado, M.; Soares, L.A. Fermentation Process in the Availability of Nutrients in Rice Bran. Res. Rev. J. Microbiol. Biotechnol. 2017, 6, 45–52. [Google Scholar]
- Khosravi, A.; Razavi, S.H. The Role of Bioconversion Processes to Enhance Bioaccessibility of Polyphenols in Rice. Food Biosci. 2020, 35, 100605. [Google Scholar] [CrossRef]
- Massarolo, K. Effect of Particle Size of Rice Bran on Gamma-Oryzanol Content and Compounds. J. Cereal Sci. 2017, 75, 54–60. [Google Scholar]
- Wang, W.; Guo, J.; Zhang, J.; Peng, J.; Liu, T.; Xin, Z. Isolation, Identification and Antioxidant Activity of Bound Phenolic Compounds Present in Rice Bran. Food Chem. 2015, 171, 40–49. [Google Scholar] [CrossRef]
- Sarkis, J.R.; Jaeschke, D.P.; Mercali, G.D.; Tessaro, I.C.; Marczak, L.D.F. Degradation Kinetics of Anthocyanins in Blackberry Pulp during Ohmic and Conventional Heating. Int. Food Res. J. 2019, 26, 87–97. [Google Scholar]
- Cai, S.; Wang, O.; Wu, W.; Zhu, S.; Zhou, F.; Ji, B.; Gao, F.; Zhang, D.; Liu, J.; Cheng, Q. Comparative Study of the Effects of Solid-State Fermentation with Three Filamentous Fungi on the Total Phenolics Content (TPC), Flavonoids, and Antioxidant Activities of Subfractions from Oats (Avena Sativa L.). J. Agric. Food Chem. 2012, 60, 507–513. [Google Scholar] [CrossRef]
- Christ-Ribeiro, A.; Graça, C.S.; Kupski, L.; Badiale-Furlong, E.; de Souza-Soares, L.A. Cytotoxicity, Antifungal and Anti Mycotoxins Effects of Phenolic Compounds from Fermented Rice Bran and Spirulina Sp. Process Biochem. 2019, 80, 190–196. [Google Scholar] [CrossRef]
- Massarolo, K.C.; de Souza, T.D.; Ribeiro, A.C.; Furlong, E.B.; de Souza Soares, L.A. Influence of Cultivation Rhizopus Oryzae on Rice Bran on Lipid Fraction: Fatty Acids and Phospholipids. Biocatal. Agric. Biotechnol 2016, 8, 204–208. [Google Scholar] [CrossRef]
- Xie, C.; Yuan, R.; Su, L.; Li, D.; Zhang, C.; Yin, Y.; Wang, P.; Yang, R. Improving Nutritional and Sensory Properties of Rice Bran by Germination and Solid-State Fermentation with Fungi. Food Biosci. 2024, 59, 103992. [Google Scholar] [CrossRef]
- Su, W.; Jiang, Z.; Wang, C.; Xu, B.; Lu, Z.; Wang, F.; Zong, X.; Jin, M.; Wang, Y. Dynamics of Defatted Rice Bran in Physicochemical Characteristics, Microbiota and Metabolic Functions during Two-Stage Co-Fermentation. Int. J. Food Microbiol. 2022, 362, 109489. [Google Scholar] [CrossRef] [PubMed]
- Christ-Ribeiro, A.; Chiattoni, L.M.; Mafaldo, C.R.F.; Badiale-Furlong, E.; Souza-Soares, L.A. de Fermented Rice-Bran by Saccharomyces Cerevisiae: Nutritious Ingredient in the Formulation of Gluten-Free Cookies. Food Biosci. 2021, 40, 100859. [Google Scholar] [CrossRef]
- AOAC International. Official Methods of Analysis of AOAC INTERNATIONAL; Latimer, G.W., Jr., Ed.; Oxford University Press: Oxford, UK, 2023. [Google Scholar]
- Benucci, I.; Fiorelli, V.; Lombardelli, C.; Liburdi, K.; Esti, M. Kinetic Characterization of Arginase from Saccharomyces Cerevisiae during Alcoholic Fermentation at Different Temperatures. LWT—Food Sci. Technol. 2017, 82, 268–273. [Google Scholar] [CrossRef]
- Dos, M.; Feddern, V.; Kupski, L.; Cipolatti, E.P.; Badiale-Furlong, E.; De Souza-Soares, L.A. Physico-Chemical Characterization of Fermented Rice Bran Biomass Fisico-Química de La Biomasa Del Salvado de Arroz Fermentado. CyTA—J. Food 2010, 8, 229–236. [Google Scholar] [CrossRef]
- Aruna, T.E.; Aworh, O.C.; Raji, A.O.; Olagunju, A.I. Protein Enrichment of Yam Peels by Fermentation with Saccharomyces Cerevisiae (BY4743). Ann. Agric. Sci. 2017, 62, 33–37. [Google Scholar] [CrossRef]
- Xu, Z.; Ying, C.; Bai, P.; Demberel, S.; Tumenjargal, B.; Yang, L.; Liu, D. Microbial Dynamics, Metabolite Profiles, and Chemical Composition in Saccharomyces Cerevisiae and Kluyveromyces Marxianus Co-Culture during Solid-State Fermentation. Food Biosci. 2025, 64, 105849. [Google Scholar] [CrossRef]
- Olukomaiya, O.O.; Adiamo, O.Q.; Fernando, W.C.; Mereddy, R.; Li, X.; Sultanbawa, Y. Effect of Solid-State Fermentation on Proximate Composition, Anti-Nutritional Factor, Microbiological and Functional Properties of Lupin Flour. Food Chem. 2020, 315, 126238. [Google Scholar] [CrossRef]
- da Silva, I.J.S.; Paim, A.P.S.; da Silva, M.J. Composition and Estimate of Daily Mineral Intake from Samples of Brazilian Rice. Microchem. J. 2018, 137, 131–138. [Google Scholar] [CrossRef]
- Schmidt, C.G.; Gonçalves, L.M.; Prietto, L.; Hackbart, H.S.; Furlong, E.B. Antioxidant Activity and Enzyme Inhibition of Phenolic Acids from Fermented Rice Bran with Fungus Rizhopus Oryzae. Food Chem. 2014, 146, 371–377. [Google Scholar] [CrossRef] [PubMed]
- Oduguwa, O.O.; Edema, M.O.; Ayeni, A.O. Physico-Chemical and Microbiological Analyses of Fermented Corn Cob, Rice Bran and Cowpea Husk for Use in Composite Rabbit Feed. Bioresour. Technol. 2008, 99, 1816–1820. [Google Scholar] [CrossRef]
- Orlean, P. Architecture and Biosynthesis of the Saccharomyces Cerevisiae Cell Wall. Genetics 2012, 192, 775–818. [Google Scholar] [CrossRef] [PubMed]
- Abduh, M.Y.; Alyssa, S.; Butar, R.A.; Pane, I.S.S.; Melani, L.; Puad, N.I.M. Effects of Solid-State Fermentation Using Aspergillus Niger on Yield, Total Phenolic Content, and Antioxidant Activity of Defatted Rice Bran Extract. Food Chem. Adv. 2025, 7, 100957. [Google Scholar] [CrossRef]
- Dey, P.; Banerjee, J.; Maiti, M.K. Comparative Lipid Profiling of Two Endophytic Fungal Isolates—Colletotrichum Sp. and Alternaria Sp. Having Potential Utilities as Biodiesel Feedstock. Bioresour. Technol. 2011, 102, 5815–5823. [Google Scholar] [CrossRef] [PubMed]
- Hui, L.; Wan, C.; Hai-Tao, D.; Xue-Jiao, C.; Qi-Fa, Z.; Yu-Hua, Z. Direct Microbial Conversion of Wheat Straw into Lipid by a Cellulolytic Fungus of Aspergillus Oryzae A-4 in Solid-State Fermentation. Bioresour. Technol. 2010, 101, 7556–7562. [Google Scholar] [CrossRef]
- Phongthai, S.; Lim, S.T.; Rawdkuen, S. Optimization of Microwave-Assisted Extraction of Rice Bran Protein and Its Hydrolysates Properties. J. Cereal. Sci. 2016, 70, 146–154. [Google Scholar] [CrossRef]
- Chauhan, P.S. Role of Various Bacterial Enzymes in Complete Depolymerization of Lignin: A Review. Biocatal. Agric. Biotechnol. 2020, 23, 101498. [Google Scholar] [CrossRef]
- Yoo, C.G.; Meng, X.; Pu, Y.; Ragauskas, A.J. The Critical Role of Lignin in Lignocellulosic Biomass Conversion and Recent Pretreatment Strategies: A Comprehensive Review. Bioresour. Technol. 2020, 301, 122784. [Google Scholar] [CrossRef]
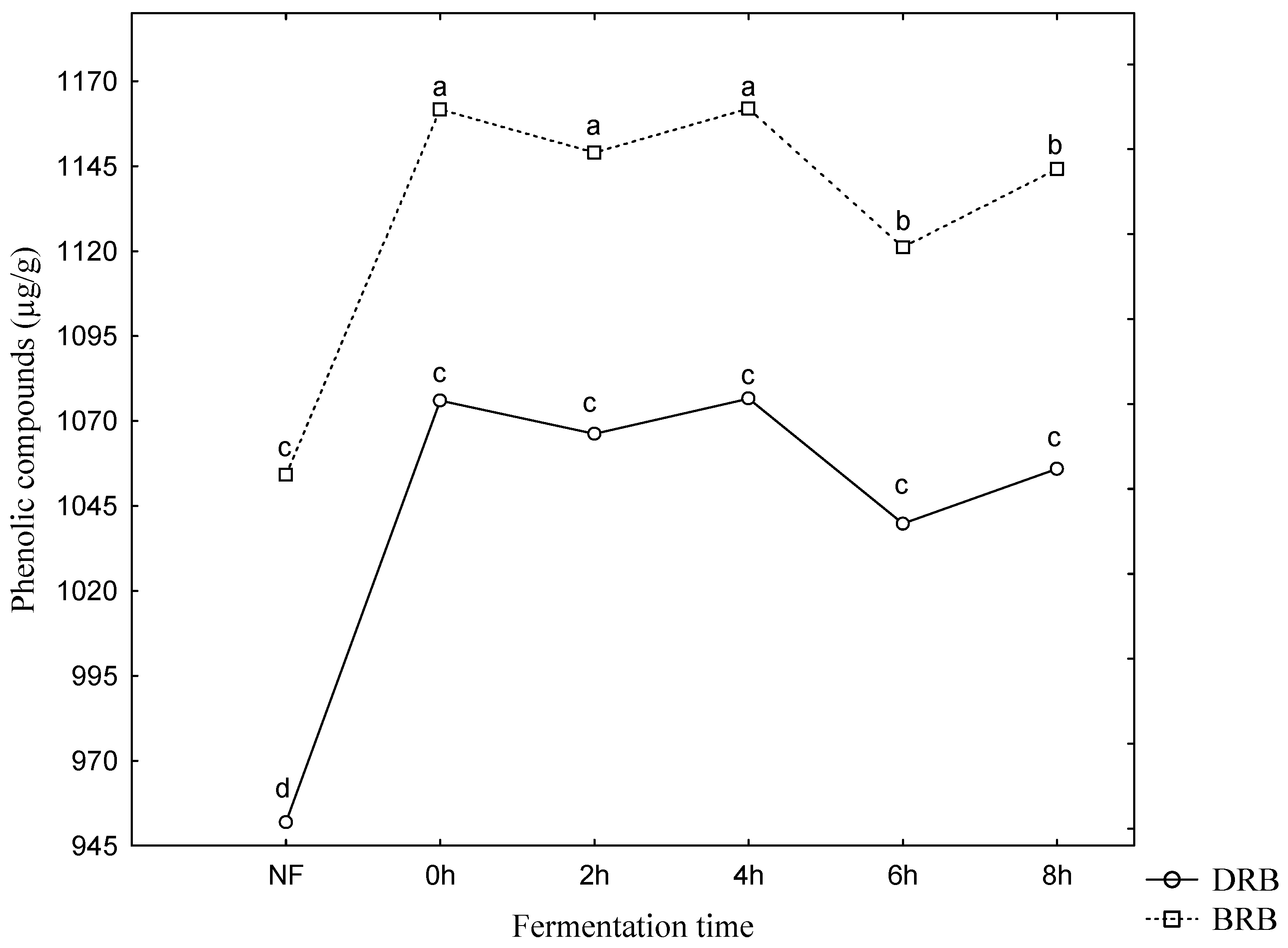
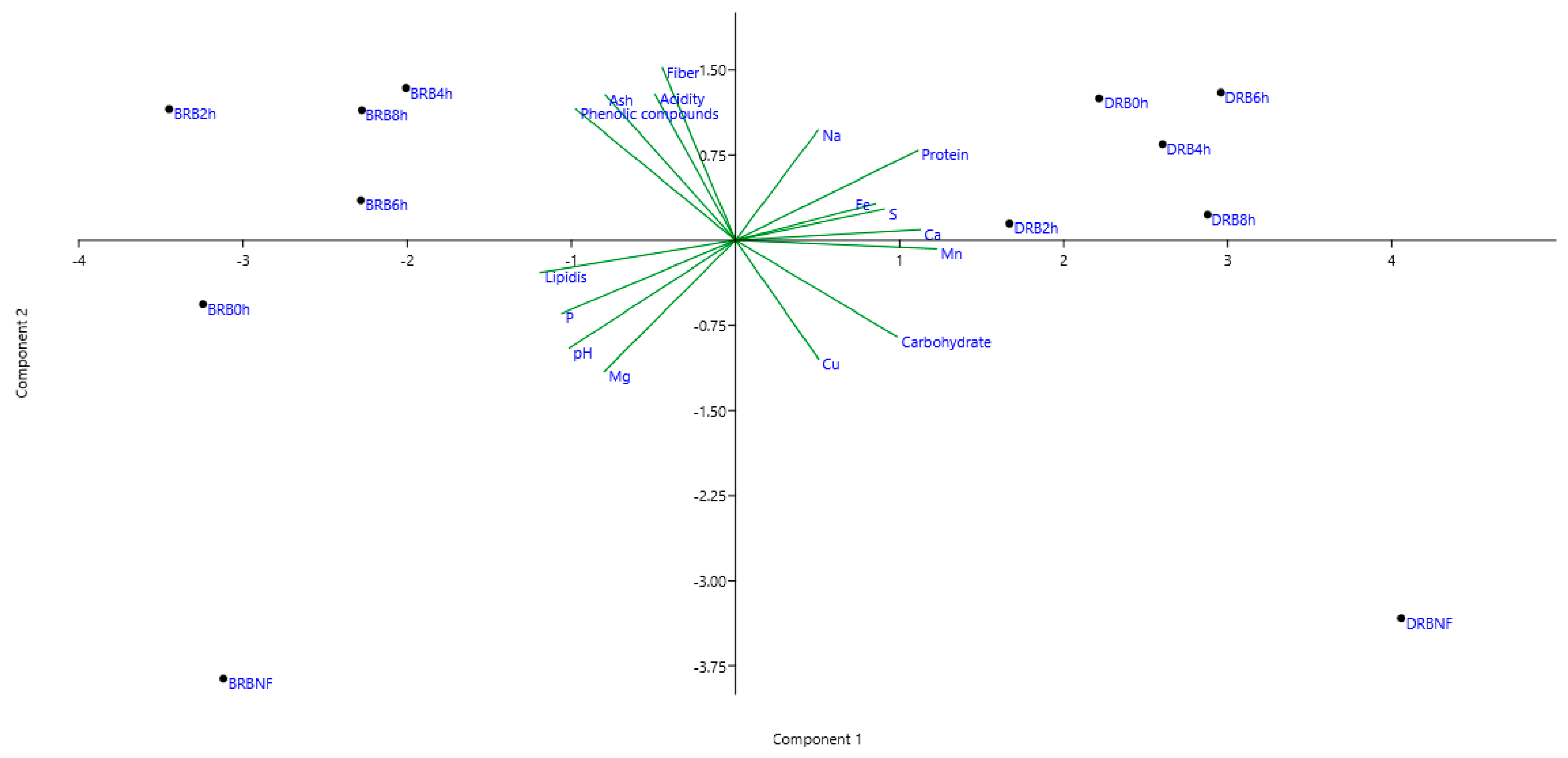
| Steps | Time (min) | T (°C) | Length of Stay (min) | Pressure (bar) |
|---|---|---|---|---|
| 1 | 20 | 170 | 10 | 35 |
| 2 | 5 | 200 | 25 | 35 |
| 3 | 5 | 50 | 20 | 35 |
| Parameters | Conditions |
|---|---|
| Potency (W) | 1400 |
| Main gas flow (L min−1) | 15 |
| Auxiliary gas flow (L min−1) | 0.2 |
| Neutralization gas flow (L min−1) | 0.7 |
| Wave-length (nm) | Ca—315.887 Fe—238.204 Mg—279.077 Na—589.592 P—214.914 S—181.975 Cr—267.717 Cu—324.759 Mn—259.374 Zn—213.857 |
| Ash (%) | Protein (%) | Lipids (%) | Fiber (%) | Carbohydrate (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| BRB | DRB | BRB | DRB | BRB | DRB | BRB | DRB | BRB | DRB | |
| NF | 15.6 cA | 14.1 bB | 13.9 cB | 33.2 cdA | 40.3 aA | 3.2 aB | 6.1 eA | 5.3 bA | 23.9 aB | 44.1 aA |
| 0 h | 20.2 bcA | 18.1 aB | 17.9 bcB | 35.9 bcA | 28.8 bA | 4.1 aB | 7.4 dA | 8.1 aA | 25.4 aB | 33.7 bA |
| 2 h | 25.7 aA | 18.1 aB | 18.1 abcB | 31.9 dA | 31.6 abA | 4.5 aB | 7.8 dA | 8.2 aA | 16.6 abB | 37.1 abA |
| 4 h | 20.0 bcA | 17.7 aB | 29.5 aB | 38.3 bA | 28.6 abA | 5.6 aB | 7.9 cdA | 7.6 aA | 12.8 bB | 31.9 bA |
| 6 h | 21.3 abA | 17.9 aB | 24.3 abcB | 42.5 aA | 26.6 bA | 5.8 aB | 9.3 aA | 7.8 aB | 18.4 abA | 25.8 bA |
| 8 h | 20.7 abcA | 17.7 aB | 25.8 bB | 38.5 bA | 34.5 abA | 5.1 aB | 8.9 bcA | 6.2 bB | 9.9 bB | 32.3 bA |
| BRB | ||||||
|---|---|---|---|---|---|---|
| NF | 0 h | 2 h | 4 h | 6 h | 8 h | |
| pH | 6.57 aA | 6.44 bA | 6.42 cA | 6.38 dA | 6.31 eA | 6.24 fA |
| Acidity (%) | 0.18 eA | 0.29 aA | 0.26 bA | 0.24 cA | 0.22 dA | 0.22 dA |
| DRB | ||||||
| NF | 0 h | 2 h | 4 h | 6 h | 8 h | |
| pH | 6.27 aB | 6.17 bB | 6.10 cB | 6.06 dB | 6.06 dB | 6.02 eB |
| Acidity (%) | 0.17 bB | 0.23 aB | 0.22 aB | 0.24 aA | 0.23 aA | 0.21 aA |
| Analyte | BRB | DRB | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NF | 0 h | 2 h | 4 h | 6 h | 8 h | NF | 0 h | 2 h | 4 h | 6 h | 8 h | |
| Ca | 559 aB | 545 aB | 479 aB | 553 aB | 575 aB | 607 aB | 858 aA | 746 aC | 794 aB | 784 aA | 1053 aA | 991 aA |
| Fe | 134 bA | 251 abA | 161 abA | 271 aA | 184 abB | 189 abA | 309 aA | 218 aA | 243 aA | 398 aA | 290 aA | 259 aA |
| Mg | 14,086 aA | 13,793 abA | 13,407 bA | 13,231 bA | 13,418 bA | 13,543 abA | 13,261 aB | 12,840 aB | 13,228 aA | 13,450 aA | 13,010 aA | 13,304 aA |
| Na | 80.5 cB | 116 cB | 136 bA | 128 cA | 140 aA | 135 bB | 116 bA | 144 bA | 125bA | 131bA | 145bA | 229 aA |
| P | 25,569 aA | 25,607 aA | 24,456 cA | 24,456 cA | 25,543 abA | 24,455 bcA | 23,427 abB | 22,846 bB | 23,661 abB | 23,500 abB | 23,020 abB | 23,966 aA |
| S | 1889 aA | 1777 aB | 1893 aA | 1973 aA | 1923 aA | 2018 aA | 2142 aA | 2151 aA | 1966 aA | 2227 aA | 2047 aA | 1938 aA |
| Cr | <0.46 | <0.46 | <0.46 | <0.46 | <0.46 | <0.46 | <0.46 | <0.46 | <0.46 | <0.46 | <0.46 | <0.46 |
| Cu | 7.50 aA | 8.00 aA | 7.50 aA | 7.20 aA | 8.00 aA | 7.40 aA | 8.5 aA | 7.60 aA | 7.80 aA | 7.60 aA | 7.60 aA | 7.90 aA |
| Mn | 194 aB | 191 aB | 192 aB | 192 aB | 192 aB | 195 aB | 276 aA | 258 aA | 260 aA | 262 aA | 260 aA | 264 aA |
| Zn | 111 aA | 115 aA | 84.2 aA | 105 aA | 84.6 aB | 95.3 aB | 117 aA | 111 aA | 106 aA | 121 aA | 136 abA | 116 aA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Christ-Ribeiro, A.; da Silva Graça, C.; Massarolo, K.C.; Jaeschke, D.P.; de Souza Soares, L.A. Production of High-Value-Added Biomass by Saccharomyces cerevisiae Using Lignocellulosic Substrate. Fermentation 2025, 11, 257. https://doi.org/10.3390/fermentation11050257
Christ-Ribeiro A, da Silva Graça C, Massarolo KC, Jaeschke DP, de Souza Soares LA. Production of High-Value-Added Biomass by Saccharomyces cerevisiae Using Lignocellulosic Substrate. Fermentation. 2025; 11(5):257. https://doi.org/10.3390/fermentation11050257
Chicago/Turabian StyleChrist-Ribeiro, Anelise, Carolina da Silva Graça, Kelly Cristina Massarolo, Débora Pez Jaeschke, and Leonor Almeida de Souza Soares. 2025. "Production of High-Value-Added Biomass by Saccharomyces cerevisiae Using Lignocellulosic Substrate" Fermentation 11, no. 5: 257. https://doi.org/10.3390/fermentation11050257
APA StyleChrist-Ribeiro, A., da Silva Graça, C., Massarolo, K. C., Jaeschke, D. P., & de Souza Soares, L. A. (2025). Production of High-Value-Added Biomass by Saccharomyces cerevisiae Using Lignocellulosic Substrate. Fermentation, 11(5), 257. https://doi.org/10.3390/fermentation11050257







