The Effect of Skin Contact, β-Lyase and Fermentation Gradient Temperature on Fermentation Esters and Free Volatile Thiols in Oregon Chardonnay Wine
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Winemaking Protocol
- Tank 1: set at a constant 13 °C for the FG0 schedule;
- Tank 2: set at 20 °C for 96 h and dropped to 13 °C for the FG1 schedule;
- Tank 3: set at a constant 20 °C for the FG2 schedule;
- Tank 4: set at a constant 13 °C for the FG2 schedule.
2.3. Basic Wine Chemistry
2.4. Ester Analysis
2.4.1. Chemicals
2.4.2. Preparation of Standards
2.4.3. Sample Preparation
2.4.4. Headspace Solid-Phase Microextraction Gas Chromatography Mass Spectrometry (HS-SPME-GC-MS)
- Method 1: Nineteen ester compounds were analyzed by adapting a method for the quantitative determination of wine esters by (HS-SPME)-GC-MS [29]. Briefly, samples were analyzed in a Shimadzu QP2010 GC-MS instrument (Shimadzu Scientific Instruments, Kyoto, Japan) equipped with a CTC Combi-Pal autosampler (CTC-Analytics) and with a split injector. The chromatography configuration contained two columns connected in sequence, Stablewax (30 m × 0.25 mm ID × 0.25 µm film thickness, Restek Corp., Bellefonte, PA, USA) connected to an Rxi column (15 m × 0.25 mm ID × 0.25 µm film thickness, Restek Corp., Bellefonte, PA, USA) using a universal press-tight connector (Restek Corp., Bellefonte, PA, USA). The method parameters for the oven were as follows: the injector temperature was set at 250 °C. The column oven was held at 35 °C for 10 min and then increased to 250 °C at 4 °C/min and held at this temperature for 10 min. The flow control mode was set using the pressure mode at a constant 32.2 kPa. The prepared diluted (tenfold) samples were incubated and agitated for 10 min at 60 °C. The GC used helium as the carrier gas, set at a linear velocity of 21.5 cm/s. The ion source and interface temperatures were set at 200 °C and 250 °C, respectively. The MS spectra were operated in electron impact (EI) mode at an ionization energy of 70 eV, switching between full scan mode and selective ion mode (SIM), with variable gain factors for SIM mode (Tables S3 and S4). The total run time was 73.75 min per sample. The MS acquisition mode was set to full scan for all 41 compounds. The NIST05 (National Institute of Standards and Technology) mass spectral library and reference standards, composed of pure compounds, were used to confirm the identities of all standards used. Quantitative parameters for all compounds and labelled standards are shown in Table S1.
- Method 2. Nineteen ester compounds were analyzed using a similar procedure to method 1. Quantitative parameters for all compounds and labelled standards are shown in Table S2. The oven ramp and MS spectra mode for full scan and SIM modes were altered from method 1 based on the different compounds analyzed (Tables S5 and S6). The column oven was held at 35 °C for 10 min, and then increased to 200 °C at 4 °C/min and held at this temperature for 1 min, then increased to 250 °C at a rate of 10 °C/min and held at that temperature for 5 min.
2.5. Volatile Thiol Analysis
2.5.1. Chemicals
2.5.2. Internal Standard Synthesis
2.5.3. Volatile Thiol Derivatization and Concentration
2.5.4. Liquid Chromatography-Tandem Mass Spectrometry (LC-MS/MS) Analysis
2.6. Limit of Detection (LOD), Limit of Quantitation (LOQ), and Method Validation
2.7. Repeatability, Reproducibility, and Accuracy
2.8. Data Analysis
3. Results
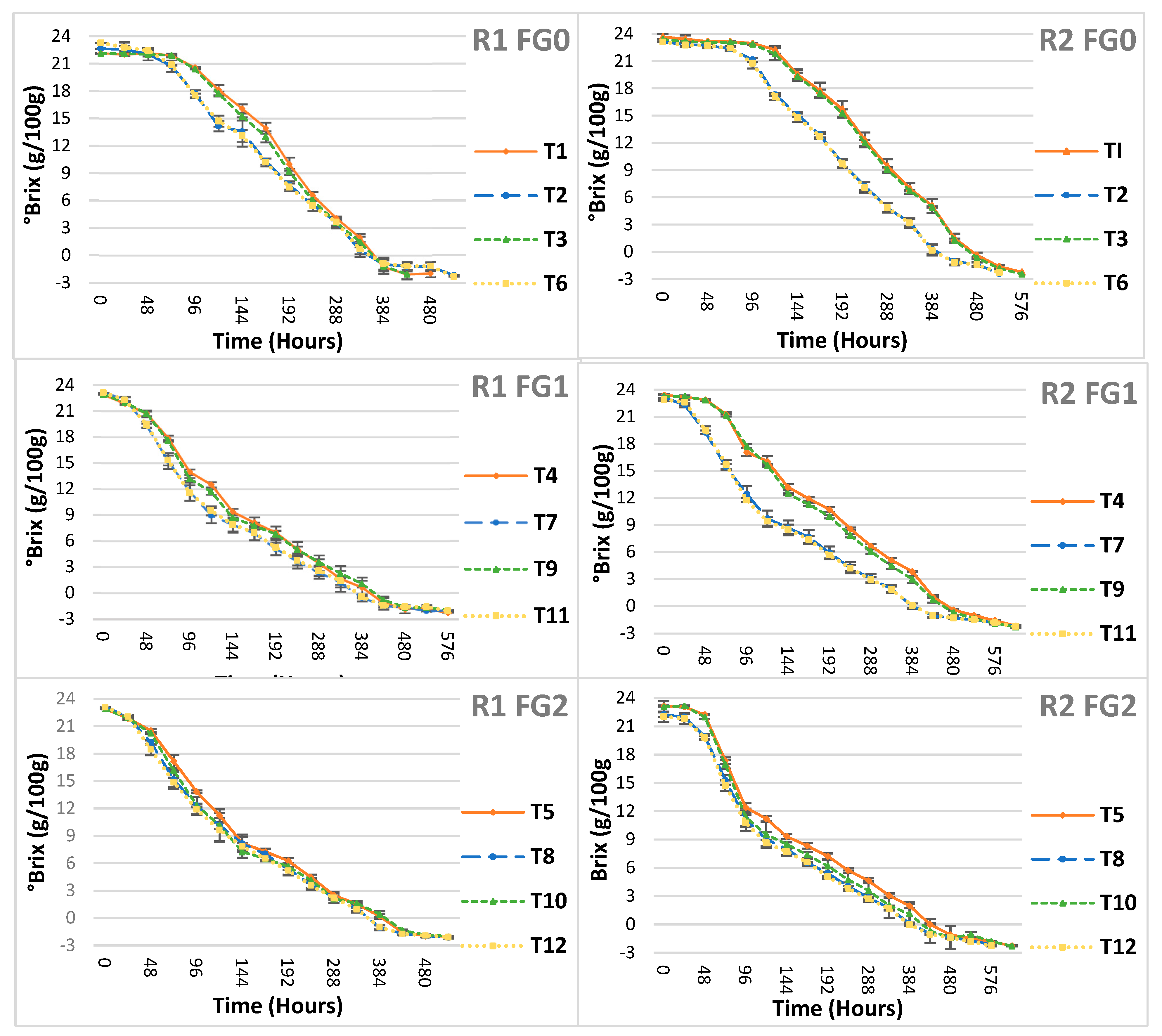
3.1. Experimental Repetitions
3.2. Wine Basic Chemistry
3.3. Fermentation Gradient Schedules
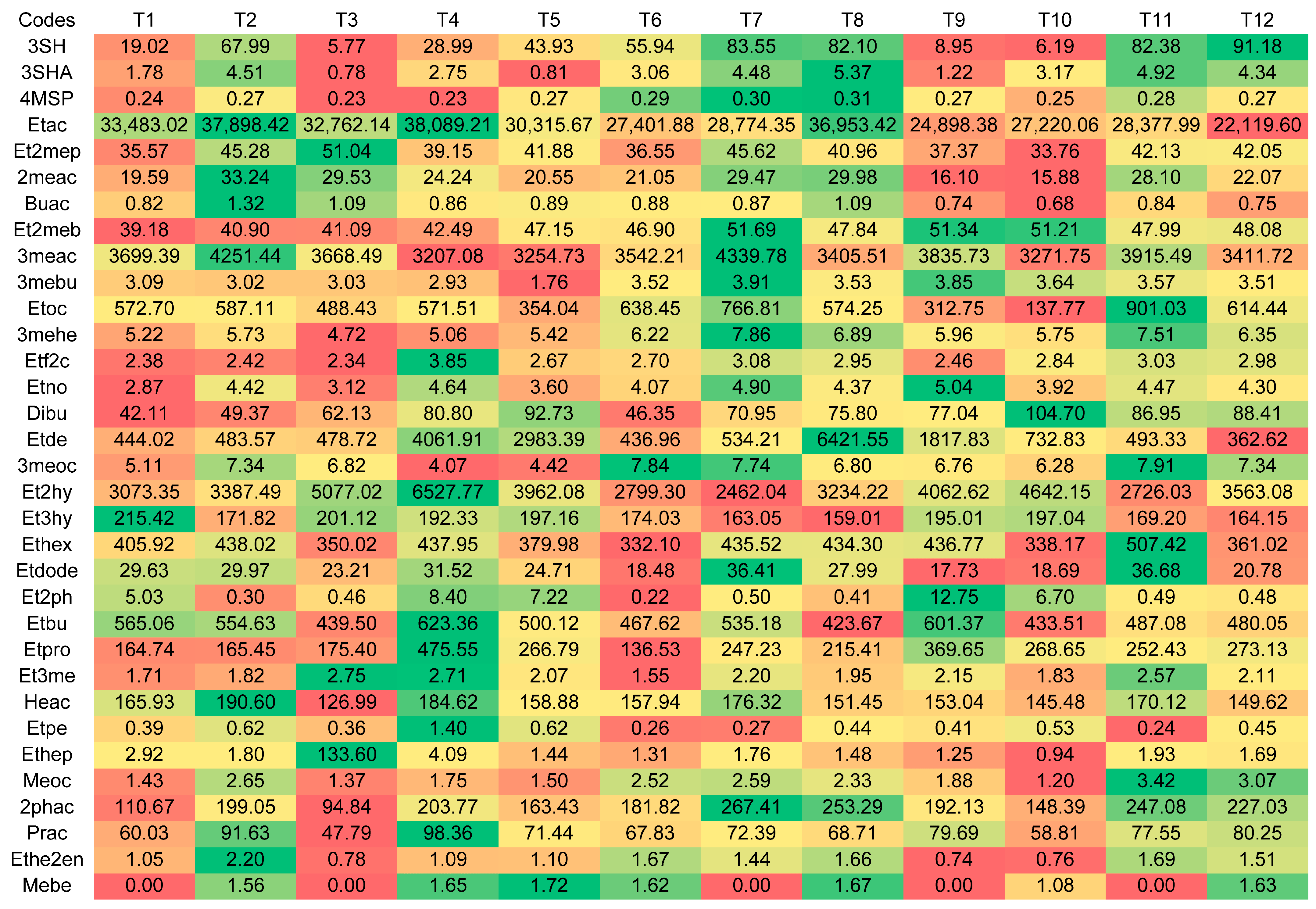
3.4. Ester Concentrations
3.5. Thiol Concentrations
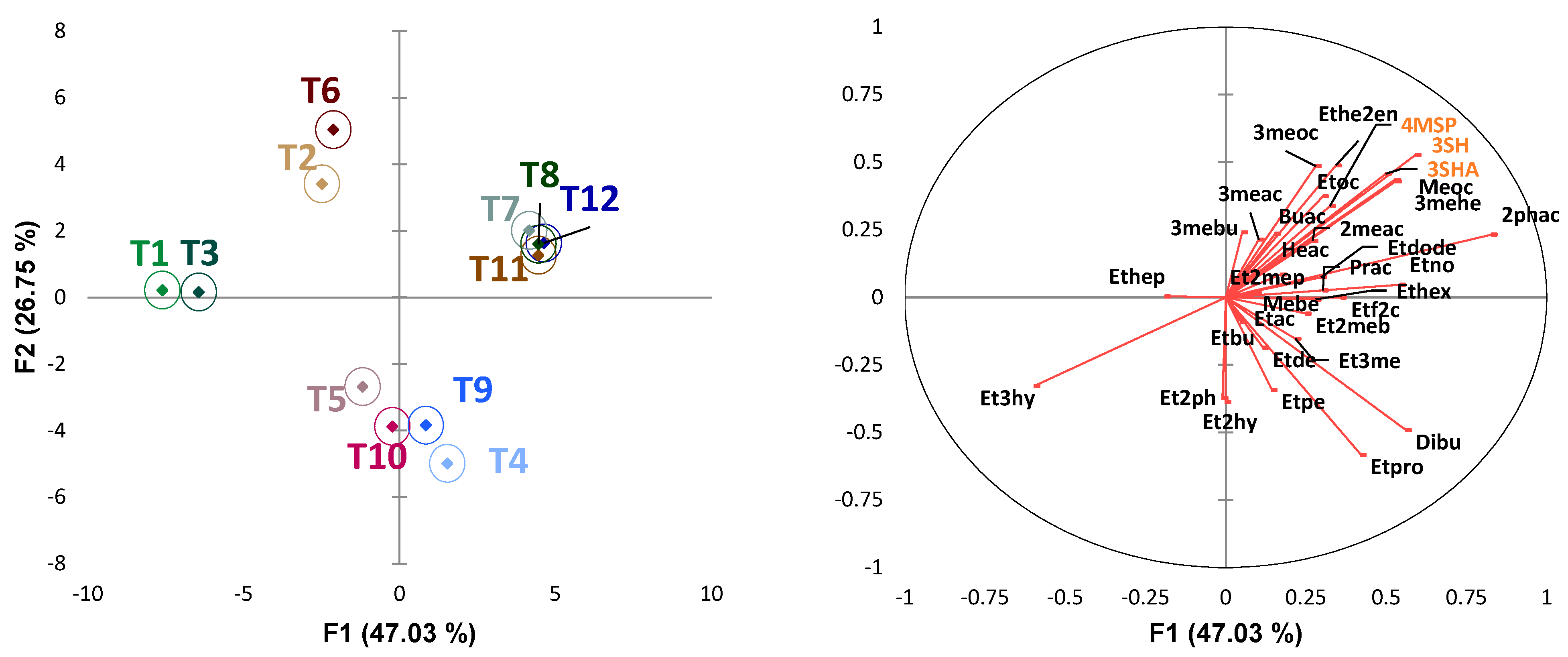
3.6. Assessing the Contribution of Esters and Thiols to the Wine Treatments
4. Discussion
4.1. Effect of Winemaking Process on Basic Wine Chemistry and Fermentation Rate
4.2. Effect of Skin Contact on Volatile Thiols and Esters Concentrations
4.3. Effect of β-Lyase on Volatile Thiols and Esters Concentrations
4.4. Effect of Fermentation Gradient Temperature on Thiols and Esters Concentrations
4.5. The Effect of Combining SC and FG on Thiols and Esters Concentrations
4.6. Feasibility of FG in Large-Scale Production in the Winery
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 3SH | 3-sulfanylhexan-1-ol |
| 3SHA | 3-sulfanylhexyl acetate |
| 4MSP | 4-methyl-4-sulfanylpentan-2-one |
| HS-SPME-GC-MS | Headspace solid-phase microextraction gas chromatography mass spectrometry |
| LC-MS/MS | Liquid chromatography-tandem mass spectrometry |
| FG | Fermentation gradient |
| SC | Skin contact |
| BL | β-lyase |
| HPLC | High-performance liquid chromatography |
| SIDA | Stable isotope dilution assay |
| DVB/CAR/PDMS | Stableflex Divinylbenzene/Carboxen/Polydimethylsiloxane |
| SIM | Single ion mode |
| LOD | Limit of detection |
| LOQ | Limit of quantification |
| HSD | Honest significant difference |
| CVA | Canonical variate analysis |
| LAB | Lactic acid bacteria |
| FAEE | Fatty acid ethyl esters |
References
- Gambetta, J.M.; Bastian, S.E.P.; Cozzolino, D.; Jeffery, D.W. Factors Influencing the Aroma Composition of Chardonnay Wines. J. Agric. Food Chem. 2014, 62, 6512–6534. [Google Scholar] [CrossRef] [PubMed]
- Maggu, M.; Winz, R.; Kilmartin, P.A.; Trought, M.C.T.; Nicolau, L. Effect of Skin Contact and Pressure on the Composition of Sauvignon Blanc Must. J. Agric. Food Chem. 2007, 55, 10281–10288. [Google Scholar] [CrossRef] [PubMed]
- Torija, M.J.; Beltran, G.; Novo, M.; Poblet, M.; Guillamón, J.M.; Mas, A.; Rozès, N. Effects of Fermentation Temperature and Saccharomyces Species on the Cell Fatty Acid Composition and Presence of Volatile Compounds in Wine. Int. J. Food Microbiol. 2003, 85, 127–136. [Google Scholar] [CrossRef]
- Belda, I.; Ruiz, J.; Esteban-Fernández, A.; Navascués, E.; Marquina, D.; Santos, A.; Moreno-Arribas, M. Microbial Contribution to Wine Aroma and Its Intended Use for Wine Quality Improvement. Molecules 2017, 22, 189. [Google Scholar] [CrossRef] [PubMed]
- Tian, T.; Ruppel, M.; Osborne, J.; Tomasino, E.; Schreiner, R.P. Fertilize or Supplement: The Impact of Nitrogen on Vine Productivity and Wine Sensory Properties in Chardonnay. Am. J. Enol. Vitic. 2022, 73, 148–161. [Google Scholar] [CrossRef]
- Garde-Cerdán, T.; Ancín-Azpilicueta, C. Review of Quality Factors on Wine Ageing in Oak Barrels. Trends Food Sci. Technol. 2006, 17, 438–447. [Google Scholar] [CrossRef]
- Tomasino, E.; Song, M.; Fuentes, C. Odor Perception Interactions between Free Monoterpene Isomers and Wine Composition of Pinot Gris Wines. J. Agric. Food Chem. 2020, 68, 3220–3227. [Google Scholar] [CrossRef]
- Chen, L.; Capone, D.L.; Jeffery, D.W. Analysis of Potent Odour-Active Volatile Thiols in Foods and Beverages with a Focus on Wine. Molecules 2019, 24, 2472. [Google Scholar] [CrossRef]
- University of Oregon. 2020 Oregon Vineyard and Winery Report. Available online: https://industry.oregonwine.org/wp-content/uploads/sites/2/2020-Winery-Vineyard-Report-8-31-21-Rev.pdf (accessed on 21 August 2022).
- Vaudour, E. The quality of grapes and wine in relation to geography: Notions of terroir at various scales. J. Wine Res. 2002, 13, 117–141. [Google Scholar] [CrossRef]
- Capone, D.; Francis, L.; Williamson, P.; Herderich, M.; Johnson, D. Struck match, freshness and tropical fruit: Thiols and Chardonnay flavor. Wine Vitic. J. 2017, 32, 31–35. [Google Scholar]
- Jeffery, D.W. Spotlight on varietal thiols and precursors in grapes and wines. Aust. J. Chem. 2016, 69, 1323–1330. [Google Scholar] [CrossRef]
- Grant-Preece, P.A.; Pardon, K.H.; Capone, D.L.; Cordente, A.G.; Sefton, M.A.; Jeffery, D.W.; Eslsey, G.M. Synthesis of wine thiol conjugates and labeled analogues: Fermentation of glutathione conjugate 3-mercaptohexan-1-ol yields the corresponding cysteine conjugate and free thiol. J. Agric. Food Chem. 2010, 58, 1383–1389. [Google Scholar] [CrossRef]
- Capone, D.L.; Barker, A.; Williamson, P.O.; Francis, I.L. The Role of Potent Thiols in Chardonnay Wine Aroma: Potent Thiols in Chardonnay Wine. Aust. J. Grape Wine Res. 2018, 24, 38–50. [Google Scholar] [CrossRef]
- Iobbi, A.; Tomasino, E. Adapting Polarized Projective Mapping to Investigate Fruitiness Aroma Perception of White Wines from Oregon. Beverages 2021, 7, 46. [Google Scholar] [CrossRef]
- Tominaga, T.; Furrer, A.; Henry, R.; Dubourdieu, D. Identification of New Volatile Thiols in the Aroma of Vitis vinifera L. Var. Sauvignon Blanc Wines. Flavour Fragr. J. 1998, 13, 4. [Google Scholar] [CrossRef]
- Coetzee, C.; du Toit, W.J. A Comprehensive Review on Sauvignon Blanc Aroma with a Focus on Certain Positive Volatile Thiols. Food Res. Int. 2012, 45, 287–298. [Google Scholar] [CrossRef]
- Roland, A.; Schneider, R.; Razungles, A.; Cavelier, F. Varietal Thiols in Wine: Discovery, Analysis and Applications. Chem. Rev. 2011, 111, 7355–7376. [Google Scholar] [CrossRef] [PubMed]
- King, E.S.; Osidacz, P.; Curtin, C.; Bastian, S.E.P.; Francis, I.L. Assessing Desirable Levels of Sensory Properties in Sauvignon Blanc Wines—Consumer Preferences and Contribution of Key Aroma Compounds: Consumer Preference of Sauvignon Blanc Flavours. Aust. J. Grape Wine Res. 2011, 17, 169–180. [Google Scholar] [CrossRef]
- Benkwitz, F.; Tominaga, T.; Kilmartin, P.A.; Lund, C.; Wohlers, M.; Nicolau, L. Identifying the Chemical Composition Related to the Distinct Aroma Characteristics of New Zealand Sauvignon Blanc Wines. Am. J. Enol. Vitic. 2012, 63, 62–72. [Google Scholar] [CrossRef]
- Green, J.A.; Parr, W.V.; Breitmeyer, J.; Valentin, D.; Sherlock, R. Sensory and Chemical Characterization of Sauvignon Blanc Wine: Influence of Source of Origin. Food Res. Int. 2011, 44, 2788–2797. [Google Scholar] [CrossRef]
- Iobbi, A.; Di, Y.; Tomasino, E. Revealing the sensory impact of different levels and combinations of esters and volatile thiols in Chardonnay wines. Heliyon 2023, 9, e12862. [Google Scholar] [CrossRef] [PubMed]
- Swiegers, J.H.; Pretorius, I.S. Modulation of Volatile Sulfur Compounds by Wine Yeast. Appl. Microbiol. Biotechnol. 2007, 74, 954–960. [Google Scholar] [CrossRef]
- Killian, E.; Ough, C.S. Fermentation Esters: Formation and Retention as Affected by Fermentation Temperature. Am. J. Enol. Vitic. 1979, 30, 5. [Google Scholar] [CrossRef]
- Masneufpomarede, I.; Mansour, C.; Murat, M.; Tominaga, T.; Dubourdieu, D. Influence of Fermentation Temperature on Volatile Thiols Concentrations in Sauvignon Blanc Wines. Int. J. Food Microbiol. 2006, 108, 385–390. [Google Scholar] [CrossRef]
- Swiegers, J.H.; Francis, I.L.; Herderich, M.J.; Pretorius, I.S. Meeting Consumer Expectations through Management in Vineyard and Winery. Wine Ind. J. 2006, 21, 10. [Google Scholar]
- Acquaviva, V.; D’Auria, M.; Racioppi, R. Changes in Aliphatic Ester Composition of White Wines during Exposition to Light. An HS-SPME-GC-MS Study. J. Wine Res. 2014, 25, 63–74. [Google Scholar] [CrossRef]
- Siebert, T.E.; Smyth, H.E.; Capone, D.L.; Neuwöhner, C.; Pardon, K.H.; Skouroumounis, G.K.; Herderich, M.J.; Sefton, M.A.; Pollnitz, A.P. Stable Isotope Dilution Analysis of Wine Fermentation Products by HS-SPME-GC-MS. Anal. Bioanal. Chem. 2005, 381, 937–947. [Google Scholar] [CrossRef] [PubMed]
- Antalick, G.; Perello, M.-C.; de Revel, G. Development, Validation and Application of a Specific Method for the Quantitative Determination of Wine Esters by Headspace-Solid-Phase Microextraction-Gas Chromatography–Mass Spectrometry. Food Chem. 2010, 121, 1236–1245. [Google Scholar] [CrossRef]
- Capone, D.L.; Ristic, R.; Pardon, K.H.; Jeffery, D.W. Simple Quantitative Determination of Potent Thiols at Ultratrace Levels in Wine by Derivatization and High-Performance Liquid Chromatography–Tandem Mass Spectrometry (HPLC-MS/MS) Analysis. Anal. Chem. 2015, 87, 1226–1231. [Google Scholar] [CrossRef]
- Miller, J.; Miller, J.C. Statistics and chemometrics for analytical chemistry. Pearson education 2018.
- Callejόn, R.M.; González, A.G.; Troncoso, A.M.; Morales, M.L. Optimization and validation of headspace sorptive extraction for the analysis of volatile compounds in wine vinegars. J. Chromatogr. A 2008, 1204, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, A.; Gupta, V.B. Methods for the Determination of Limit of Detection and Limit of Quantitation of the Analytical Methods. Chron Young Sci. 2011, 2, 21–25. [Google Scholar] [CrossRef]
- Song, M.; Xia, Y.; Tomasino, E. Investigation of a Quantitative Method for the Analysis of Chiral Monoterpenes in White Wine by HS-SPME-MDGC-MS of Different Wine Matrices. Molecules 2015, 20, 7359–7378. [Google Scholar] [CrossRef]
- Tomasino, E.; Harrison, R.; Breitmeyer, J.; Sedcole, R.; Sherlock, R.; Frost, A. Aroma Composition of 2-Year-Old New Zealand Pinot Noir Wine and Its Relationship to Sensory Characteristics Using Canonical Correlation Analysis and Addition/Omission Tests: Chemistry and Sensory of New Zealand Pinot Noir. Aust. J. Grape Wine Res. 2015, 21, 376–388. [Google Scholar] [CrossRef]
- Olejar, K.J.; Fedrizzi, B.; Kilmartin, P.A. Enhancement of Chardonnay Antioxidant Activity and Sensory Perception through Maceration Technique. LWT—Food Sci. Technol. 2016, 65, 152–157. [Google Scholar] [CrossRef]
- Valero, E.; Millan, M.C.; Mauricio, J.C.; Ortega, J.M. Effect of Grape Skin Maceration on Sterol, Phospholipid, and Fatty Acid Contents of Saccharomyces Cerevisiae During Alcoholic Fermentation. Am. J. Enol. Vitic. 1998, 49, 119–124. [Google Scholar] [CrossRef]
- Patel, P.; Herbst-Johnstone, M.; Lee, S.A.; Gardner, R.C.; Weaver, R.; Nicolau, L.; Kilmartin, P.A. Influence of Juice Pressing Conditions on Polyphenols, Antioxidants, and Varietal Aroma of Sauvignon Blanc Microferments. J. Agric. Food Chem. 2010, 58, 7280–7288. [Google Scholar] [CrossRef]
- Sumby, K.M.; Grbin, P.R.; Jiranek, V. Microbial Modulation of Aromatic Esters in Wine: Current Knowledge and Future Prospects. Food Chem. 2010, 121, 1–16. [Google Scholar] [CrossRef]
- Waterhouse, A.L.; Sacks, G.L.; Jeffery, D.W. Understanding Wine Chemistry; John Wiley & Sons: Hoboken, NJ, USA, 2016. [Google Scholar]
- Aleixandre-Tudo, J.L.; Weightman, C.; Panzeri, V.; Nieuwoudt, H.; du Toit, W.J. Effect of Skin Contact before and during Alcoholic Fermentation on the Chemical and Sensory Profile of South African Chenin Blanc White Wines. S. Afr. J. Enol. Vitic. 2015, 36, 366–377. [Google Scholar] [CrossRef]
- Burin, V.M.; Caliari, V.; Bordignon-Luiz, M.T. Nitrogen Compounds in Must and Volatile Profile of White Wine: Influence of Clarification Process before Alcoholic Fermentation. Food Chem. 2016, 202, 417–425. [Google Scholar] [CrossRef]
- Molina, A.M.; Swiegers, J.H.; Varela, C.; Pretorius, I.S.; Agosin, E. Influence of Wine Fermentation Temperature on the Synthesis of Yeast-Derived Volatile Aroma Compounds. Appl. Microbiol. Biotechnol. 2007, 77, 675–687. [Google Scholar] [CrossRef] [PubMed]
- Bonnaffoux, H.; Roland, A.; Schneider, R.; Cavelier, F. Spotlight on release mechanisms of volatile thiols in beverages. Food Chem 2021, 339, 127628. [Google Scholar] [CrossRef]
- Tondini, F.; Gambetta, J.; Chen, L. The Effects of Pre-Fermentative Additions on Yeast Volatile Aromas and Thiols in Sauvignon Blanc and Chardonnay. 17th Australian Wine Technical Conference Proceedings. Available online: https://chrome-extension://efaidnbmnnnibpcajpcglclefindmkaj/https://static1.squarespace.com/static/6552af6c68af6b453550c54c/t/65b9a86b282daf6754629043/1706666182338/17th+AWITC+Proceedings.pdf (accessed on 21 August 2022).
- Swiegers, J.H.; Pretorius, I.S. Yeast Modulation of Wine Flavor. In Advances in Applied Microbiology; Elsevier: Amsterdam, The Netherlands, 2005; Volume 57, pp. 131–175. [Google Scholar] [CrossRef]
- Morakul, S.; Mouret, J.-R.; Nicolle, P.; Aguera, E.; Sablayrolles, J.-M.; Athès, V. A Dynamic Analysis of Higher Alcohol and Ester Release During Winemaking Fermentations. Food Bioprocess Technol. 2013, 6, 818–827. [Google Scholar] [CrossRef]
- Saerens, S.M.G.; Delvaux, F.; Verstrepen, K.J.; Van Dijck, P.; Thevelein, J.M.; Delvaux, F.R. Parameters Affecting Ethyl Ester Production by Saccharomyces Cerevisiae during Fermentation. Appl. Environ. Microbiol. 2008, 74, 454–461. [Google Scholar] [CrossRef]
- Bene, Z.S.; Kállay, M. Polyphenol contents of skin-contact fermented white wines. Acta Aliment. 2019, 48, 515–524. [Google Scholar] [CrossRef]
- García-Romero, E.; Pérez-Coello, M.; Cabezudo, M.D.; Sánchez-Muñoz, G.; Martín-Alvarez, P.J. Fruity flavor increase of Spanish Airén white wines made by brief fermentation skin contact/Aumento del aroma afrutado de los vinos blancos Airén fermentados en presencia de hollejos. Food Sci. Technol. Int. 1999, 5, 149–157. [Google Scholar] [CrossRef]
- Sokolowsky, M.; Rosenberger, A.; Fischer, U. Sensory impact of skin contact on white wines characterized by descriptive analysis, time–intensity analysis and temporal dominance of sensations analysis. Food Qual. Prefer. 2015, 39, 285–297. [Google Scholar] [CrossRef]
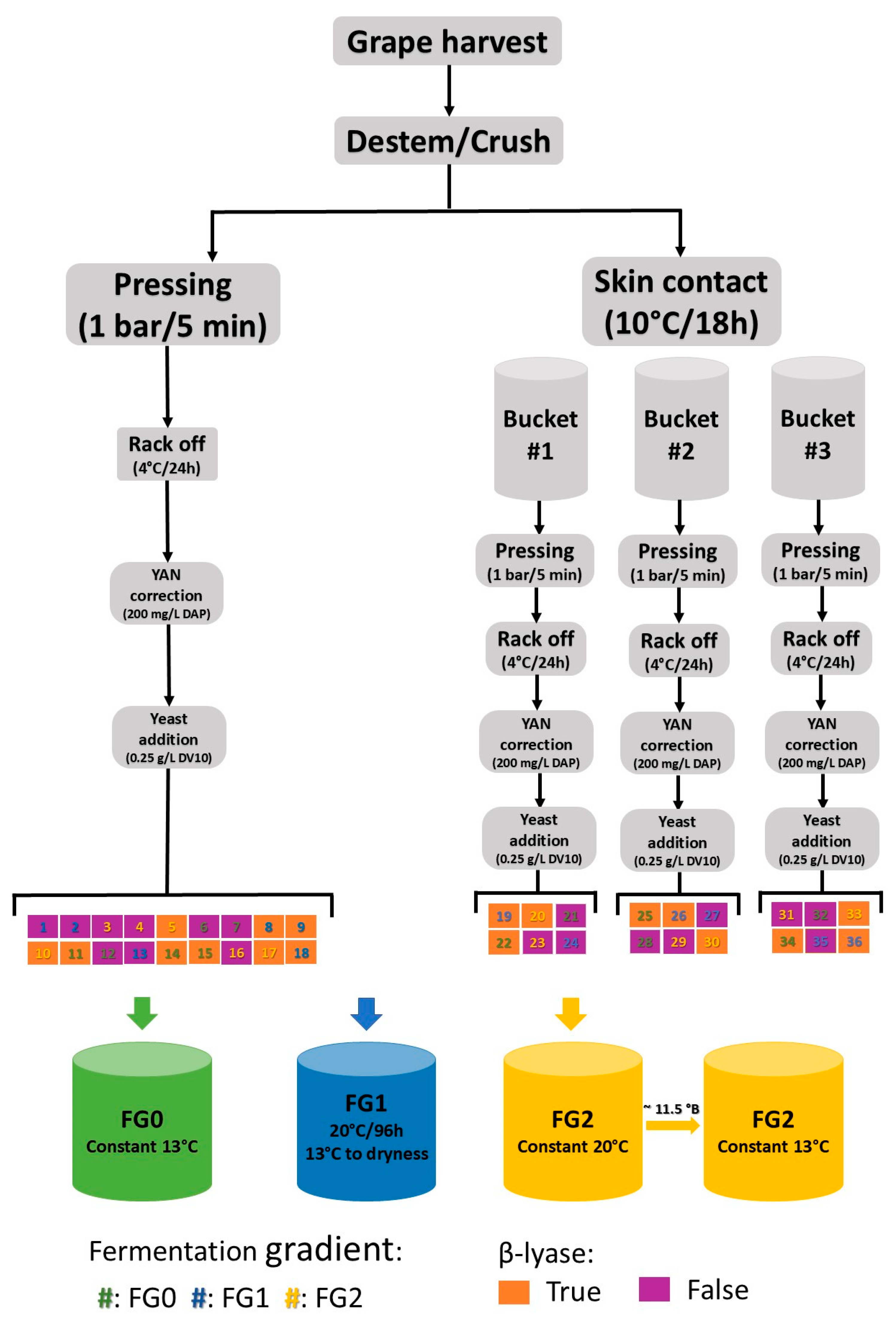
| Treatment | SC | BL | FG |
|---|---|---|---|
| T1 (Control) | No | No | 0 |
| T2 | Yes | No | 0 |
| T3 | No | Yes | 0 |
| T4 | No | No | 1 |
| T5 | No | No | 2 |
| T6 | Yes | Yes | 0 |
| T7 | Yes | No | 1 |
| T8 | Yes | No | 2 |
| T9 | No | Yes | 1 |
| T10 | No | Yes | 2 |
| T11 | Yes | Yes | 1 |
| T12 | Yes | Yes | 2 |
| Parameters * | SC | BL | FG | ||||
|---|---|---|---|---|---|---|---|
| No | Yes | No | Yes | 0 | 1 | 2 | |
| pH | 3.02 (±0.08) a | 3.15 (±0.03) b | 3.09 (±0.08) a | 3.08 (±0.09) a | 3.1 (±0.08) a | 3.08 (±0.09) a | 3.07 (±0.08) a |
| TA (g/L) | 8.2 (±0.86) a | 6.5 (±0.28) b | 7.3 (±1.08) a | 7.4 (±1.05) a | 7.13 (±1.21) a | 7.39 (±0.9) b | 7.53 (±1.04) b |
| RS (g/L) | 0.14 (±0.06) a | 0.22 (±0.08) a | 0.17 (±0.08) a | 0.19 (±0.08) a | 0.16 (±0.07) a | 0.19 (±0.09) ab | 0.19 (±0.08) b |
| AA (g/L) | 0.05 (±0.03) a | 0.05 (±0.03) a | 0.05 (±0.03) a | 0.05 (±0.03) a | 0.07 (±0.04) a | 0.04 (±0.02) b | 0.04 (±0.02) b |
| MA (g/L) | 2.67 (±0.42) a | 2.34 (±0.16) b | 2.5 (±0.35) a | 2.52 (±0.36) a | 2.48 (±0.34) a | 2.52 (±0.36) a | 2.51 (±0.37) a |
| EtOH (% v/v) | 13.37 (±0.43) a | 13.44 (±0.18) a | 13.4 (±0.3) a | 13.41 (±0.37) a | 13.46 (±0.34) a | 13.4 (±0.33) a | 13.36 (±0.33) a |
| Compounds | Codes | Skin Contact | β-Lyase | Fermentation Gradient | ||||
|---|---|---|---|---|---|---|---|---|
| Yes | No | Yes | No | 0 | 1 | 2 | ||
| 3-sulfanylhexan-1-ol (ng/L) | 3SH | 139.12 (±72.33) a | 19.59 (±38.04) a | 79.52 (±82.83) a | 79.20 (±83.43) a | 51.88 (±56.79) a | 93.14 (±89.50) b | 93.06 (±91.46) b |
| 3-sulfanylhexyl acetate (ng/L) | 3SHA | 8.06 (±4.46) a | 1.53 (±3.50) a | 4.56 (±5.06) a | 5.03 (±5.26) a | 3.5 (±3.47) a | 5.66 (±5.64) b | 5.23 (±5.80) b |
| 4-methyl-4-sulfanylpentan-2-one (ng/L) | 4MSP | <2.2 a | <2.2 a | <2.2 a | <2.2 a | <2.2 a | <2.2 a | <2.2 a |
| Ethyl acetate (μg/L) | Etac | 53,992.06 (±24,104.11) a | 55,957.90 (±36,536.01) a | 52,952.61 (±34,850.53) a | 56,997.35 (±26,364.40) a | 51,962.41 (±17,742.49) a | 58,419.29 (±39,913.79) a | 54,543.24 (±30,784.72) a |
| Ethyl propanoate (μg/L) | Etpro | 370.40 (±122.32) a | 454.71 (±222.80) b | 412.41 (±155.84) a | 412.70 (±209.46) a | 257.83 (±79.92) a | 534.62 (±193.23) c | 445.22 (±136.30) b |
| Ethyl butanoate (μg/L) | Etbu | 841.14 (±192.58) a | 847.41 (±269.79) a | 810.65 (±186.30) a | 877.90 (±270.04) a | 833.42 (±256.81) ab | 905.06 (±172.22) a | 794.35 (±250.85) b |
| Ethyl pentanoate (μg/L) | Etpe | 0.72 (±0.63) a | 0.95 (±0.74) a | 0.70 (±0.51) a | 0.97 (±0.81) b | 0.58 (±0.35) a | 0.94 (±0.89) b | 0.9915 (±0.65) b |
| Ethyl hexanoate (μg/L) | Ethex | 732.02 (±248.65) a | 651.37 (±278.09) a | 690.03 (±285.6) a | 693.37 (±246.66) a | 601.64 (±208.53) a | 784.68 (±283.89) b | 688.78 (±269.76) ab |
| Ethyl heptanoate (μg/L) | Ethep | 2.79 (±0.95) a | 16.22 (±80.88) a | 15.98 (±80.90) a | 3.04 (±2.26) a | 22.72 (±98.40) a | 3.34 (±2.10) a | 2.47 (±1.11) a |
| Ethyl octanoate (μg/L) | Etoc | 1202.16 (±495.14) a | 694.93 (±510.05) b | 990.06 (±618.82) a | 907.03 (±497.57) a | 899.71 (±430.42) ab | 1107.89 (±632.96) a | 838.04 (±570.14) b |
| Ethyl nonanoate (μg/L) | Etno | 7.38 (±1.11) a | 6.52 (±1.24) a | 6.95 (±1.08) a | 6.95 (±1.40) a | 6.34 (±1.21) a | 7.77 (±1.05) b | 6.75 (±1.02) a |
| Ethyl decanoate (μg/L) | Etde | 1633.05 (±5119.57) a | 2518.55 (±4306.41) a | 1549.59 (±2879.39) a | 2602.02 (±6025.17) a | 749.82 (±246.89) a | 2361.92 (±4157.42) ab | 3115.67 (±6888.71) b |
| Ethyl dodecanoate (μg/L) | Etdode | 48.58 (±22.08) a | 40.18 (±14.36) a | 39.60 (±18.72) a | 49.16 (±18.25) b | 38.17 (±16.85) a | 53.61 (±22.48) b | 41.36 (±13.11) a |
| Ethyl 2-methylpropanoate (μg/L) | Et2mep | 70.46 (±11.90) a | 67.92 (±19.31) a | 68.62 (±16.82) a | 69.76 (±15.30) a | 68.52 (±17.39) a | 70 (±14.50) a | 69.05 (±16.20) a |
| Ethyl 2-methylbutanoate (μg/L) | Et2meb | 79.57 (±2.51) a | 79.12 (±2.53) a | 79.35 (±2.25) a | 79.34 (±2.78) a | 78.65 (±2.16) a | 80.20 (±3.12) b | 79.18 (±1.88) ab |
| Ethyl 3-methylbutanoate (μg/L) | Et3me | 3.57 (±1.20) a | 3.60 (±1.28) a | 3.67 (±1.35) a | 3.49 (±1.11) a | 3.14 (±1.20) a | 4.012 (±1.17) b | 3.60 (±1.18) ab |
| Ethyl (E)-hex-2-enoate (μg/L) | Ethe2en | 2.84 (±0.99) a | 1.64 (±0.60) a | 2.18 (±1.03) a | 2.31 (±1) a | 2.21 (±1.17) a | 2.24 (±0.91) a | 2.28 (±0.94) a |
| Ethyl furan-2-carboxylate (μg/L) | Etf2c | 4.91 (±0.59) a | 4.54 (±1.09) a | 4.61 (±0.63) a | 4.84 (±1.09) a | 4.40 (±0.46) a | 5 (±1.33) b | 4.78 (±0.48) b |
| Ethyl 2-phenylacetate (μg/L) | Et2ph | 0.69 (±0.31) a | 8.73 (±17.86) b | 5.81 (±16.65) a | 3.60 (±8.48) a | 1.19 (±3.58) a | 6.766125 (±18.37) a | 6.16 (±12.58) a |
| Ethyl 2-hydroxypropanoate (μg/L) | Et2hy | 5408.59 (±2344.33) a | 7196.76 (±3149.32) b | 6565.87 (±2972.79) a | 6039.48 (±2834.94) a | 5560.11 (±2402.33) a | 6345.68 (±3255.87) a | 7002.23 (±2846.91) a |
| Ethyl 3-hydroxybutanoate (μg/L) | Et3hy | 279.68 (±30.97) a | 339.63 (±51.43) b | 308.39 (±46.01) a | 310.91 (±57.28) a | 331.72 (±57.83) a | 295.88 (±45.38) b | 301.36 (±44.18) b |
| Methyl octanoate (μg/L) | Meoc | 4.72 (±1.42) a | 2.68 (±1.03) b | 3.98 (±1.89) a | 3.44 (±1.20) b | 3.25 (±1.36) a | 4.13 (±1.71) b | 3.73 (±1.61) ab |
| Methyl benzoate (μg/L) | Mebe | 1.25 (±2.55) a | 0.95 (±2.28) a | 1.03 (±2.35) a | 1.17 (±2.50) a | 0.78 (±2.07) a | 0.25 (±1.22) a | 2.27 (±3.11) b |
| Diethyl butanedioate (μg/L) | Dibu | 117.25 (±35.74) a | 124.67 (±37.91) a | 126.42 (±38.43) a | 115.49 (±34.71) a | 81.68 (±20.53) a | 130.40 (±23.18) a | 150.79 (±25.12) a |
| 3-Methylbutyl butanoate (μg/L) | 3mebu | 5.92 (±0.15) a | 5.43 (±1.42) b | 5.85 (±0.18) a | 5.50 (±1.43) b | 5.92 (±0.17) a | 5.91 (±0.19) a | 5.18 (±1.67) b |
| 3-Methylbutyl hexanoate (μg/L) | 3mehe | 11.47 (±1.61) a | 9.27 (±1.05) b | 10.27 (±1.93) a | 10.48 (±1.53) a | 9.82 (±1.48) a | 11.07 (±1.94) b | 10.22 (±1.55) a |
| 3-Methylbutyl octanoate (μg/L) | 3meoc | 12.65 (±3.22) a | 9.41 (±2.17) b | 12.13 (±2.70) a | 9.94 (±3.26) b | 11.13 (±3.31) a | 11.41 (±3.18) a | 10.56 (±3.01) a |
| Propyl acetate (μg/L) | Prac | 132.75 (±45.56) a | 112.42 (±49.50) a | 118.87 (±44.89) a | 126.30 (±51.86) a | 108.83 (±49.76) a | 135.62 (±41.26) b | 123.30 (±50.61) ab |
| Butyl acetate (μg/L) | Buac | 1.69 (±0.64) a | 1.26 (±0.68) a | 1.33 (±0.62) a | 1.60 (±0.73) b | 1.46 (±0.80) a | 1.41 (±0.49) a | 1.53 (±0.74) a |
| Hexyl acetate (μg/L) | Heac | 283.48 (±66.04) a | 254.61 (±82.19) a | 258.09 (±68.10) a | 280 (±81.59) a | 260.51 (±83.55) a | 285.60 (±68.99) a | 261.02 (±71.77) a |
| 2-Methylpropyl acetate (μg/L) | 2meac | 48.29 (±18.55) a | 34.34 (±21.21) b | 38.39 (±19.94) a | 44.23 (±21.84) a | 38.24 (±19.50) a | 44.52 (±18.73) a | 41.18 (±24.22) a |
| 3-Methylbutyl acetate (μg/L) | 3meac | 6544.29 (±1396.71) a | 5795.03 (±1481.85) b | 6073.77 (±1312.52) a | 6265.55 (±1638.98) a | 6264.56 (±1561.82) ab | 6472.76 (±1209.87) a | 5771.66 (±1574.38) b |
| 2-Phenylethyl acetate (μg/L) | 2phac | 383.98 (±68.31) a | 255.47 (±84.97) b | 307.88 (±99.02) a | 331.57 (±100.29) b | 245.15 (±95.82) a | 381.30 (±69.10) c | 332.74 (±82.09) b |
| Thiols total (ng/L) | - | 147.67 (±75.40) a | 21.55 (±39.05) a | 84.53 (±86.50) a | 84.70 (±87.64) a | 55.83 (±59.16) a | 99.26 (±94.58) b | 98.75 (±95.16) b |
| Ester total (mg/L) | - | 72.23 (±28.32) a | 75.50 (±39.36) a | 71.47 (±37.34) a | 76.26 (±30.83) a | 68.45 (±20.14) a | 78.47 (±42.50) a | 74.66 (±35.66) a |
| Codes † | SC | BL | FG | SC×BL | SC×FG | BL×FG | SC×BL×FG |
|---|---|---|---|---|---|---|---|
| 3SH | ns | ns | *** | ns | *** | ns | * |
| 3SHA | ns | ns | ** | ns | ns | ns | ns |
| 4MSP | * | ns | ns | ns | ns | * | ns |
| Etac | ns | ns | ns | ** | ns | * | ns |
| Et2mep | ns | ns | ns | ns | ns | ns | * |
| 2meac | *** | ns | ns | * | ns | ns | ns |
| Buac | ns | ** | ns | ns | ns | ns | ns |
| Et2meb | ns | ns | ** | ns | ns | ns | * |
| 3meac | ** | ns | * | * | ns | ns | ns |
| 3mebu | *** | ** | *** | *** | *** | *** | *** |
| Etoc | * | ns | * | ns | ns | ns | ns |
| 3mehe | *** | ns | *** | ns | ** | ns | ns |
| Etf2c | * | ns | *** | ns | ns | ** | * |
| Etno | ns | ns | *** | ns | *** | ns | ns |
| Dibu | * | ** | *** | * | * | ns | *** |
| Etde | ns | ns | * | ns | ns | * | ns |
| 3meoc | ** | *** | ns | ns | * | ns | ns |
| Et2hy | * | ns | ns | ns | ns | * | ns |
| Et3hy | *** | ns | ** | ns | ns | ns | ns |
| Ethex | ns | ns | ** | ns | ns | * | ns |
| Etdode | ns | *** | *** | ns | *** | ns | ns |
| Et2ph | *** | ns | * | ns | ns | ns | ns |
| Etbu | ns | ns | * | ns | * | ns | ns |
| Etpro | *** | ns | *** | ns | *** | ns | ns |
| Et3me | ns | ns | *** | ns | ns | ns | ns |
| Heac | ns | * | ns | ns | ns | ns | ns |
| Etpe | ns | ** | ** | ns | * | * | ns |
| Ethep | ns | ns | ns | ns | ns | ns | ns |
| Meoc | ** | ** | *** | * | ns | ns | ns |
| 2phac | *** | ** | *** | ns | *** | ns | ns |
| Prac | ns | ns | ** | ns | ** | ns | ns |
| Ethe2en | ns | ns | ns | ns | ns | ns | ns |
| Mebe | ns | ns | *** | ns | * | ns | ns |
| Total thiols (ng/L) | ns | ns | *** | ns | *** | ns | * |
| Total esters (mg/L) | ns | ns | ns | * | ns | * | ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iobbi, A.; Osborne, J.; Di, Y.; Tomasino, E. The Effect of Skin Contact, β-Lyase and Fermentation Gradient Temperature on Fermentation Esters and Free Volatile Thiols in Oregon Chardonnay Wine. Fermentation 2025, 11, 250. https://doi.org/10.3390/fermentation11050250
Iobbi A, Osborne J, Di Y, Tomasino E. The Effect of Skin Contact, β-Lyase and Fermentation Gradient Temperature on Fermentation Esters and Free Volatile Thiols in Oregon Chardonnay Wine. Fermentation. 2025; 11(5):250. https://doi.org/10.3390/fermentation11050250
Chicago/Turabian StyleIobbi, Angelica, James Osborne, Yanming Di, and Elizabeth Tomasino. 2025. "The Effect of Skin Contact, β-Lyase and Fermentation Gradient Temperature on Fermentation Esters and Free Volatile Thiols in Oregon Chardonnay Wine" Fermentation 11, no. 5: 250. https://doi.org/10.3390/fermentation11050250
APA StyleIobbi, A., Osborne, J., Di, Y., & Tomasino, E. (2025). The Effect of Skin Contact, β-Lyase and Fermentation Gradient Temperature on Fermentation Esters and Free Volatile Thiols in Oregon Chardonnay Wine. Fermentation, 11(5), 250. https://doi.org/10.3390/fermentation11050250





