Efficient Synthesis of High-Active Myoglobin and Hemoglobin by Reconstructing the Mitochondrial Heme Synthetic Pathway in Engineered Saccharomyces cerevisiae
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Strains and Cultural Conditions
2.3. Plasmids and the Cassettes for Gene Knocking-In/Out Construction
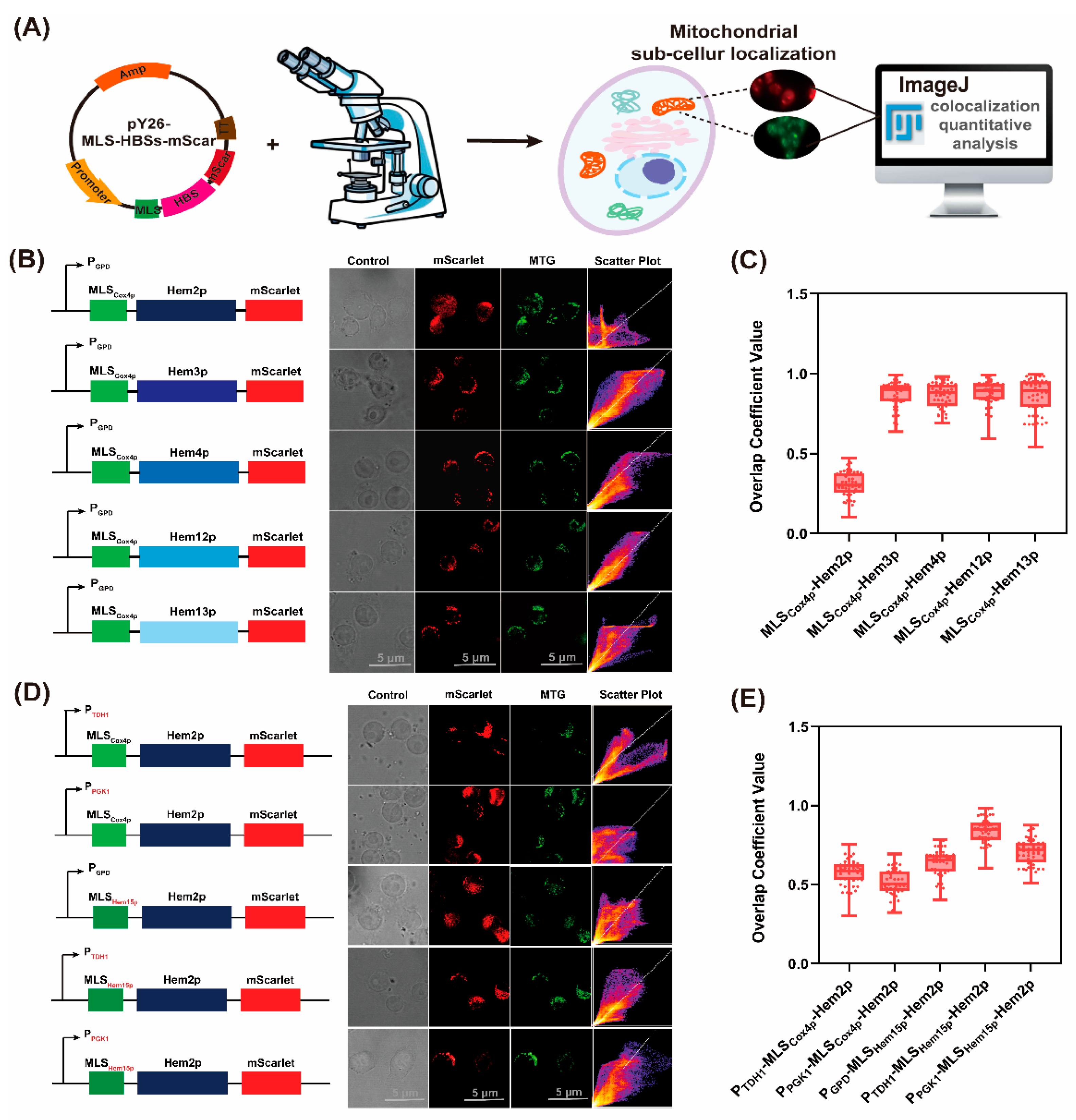
2.4. Fluorescent Visualization and Analysis by Microscopy
2.5. Qualitative and Quantitative Analysis of Fluorescent Localization
2.6. Intracellular Heme Measurements
2.7. Isolation of Mitochondria
2.8. The Measurements of ALA in Mitochondria and Cytoplasm
2.9. Expression and Purification of Myoglobin and Hemoglobin
2.10. Determination of Heme-Binding Ratios in Purified Myoglobin and Hemoglobin
2.11. Determination of Functional Activities for Synthesized Myoglobin and Hemoglobin
3. Results and Discussion
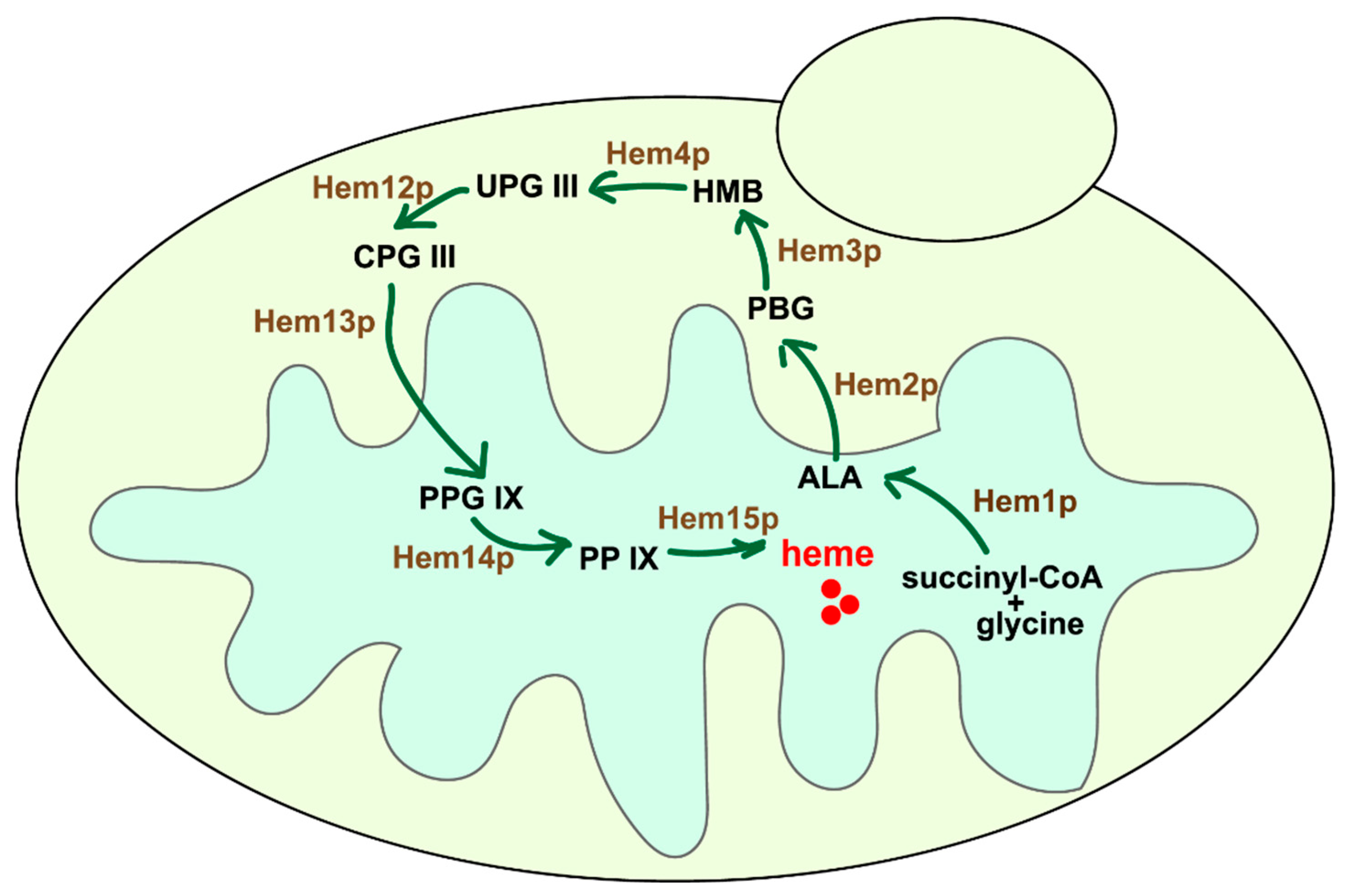
3.1. The Effective Mitochondrial Localization of Cytoplasmic Heme Synthetic Enzymes in S. cerevisiae
3.2. The Reconstruction of Heme Synthetic Pathway in the Mitochondria
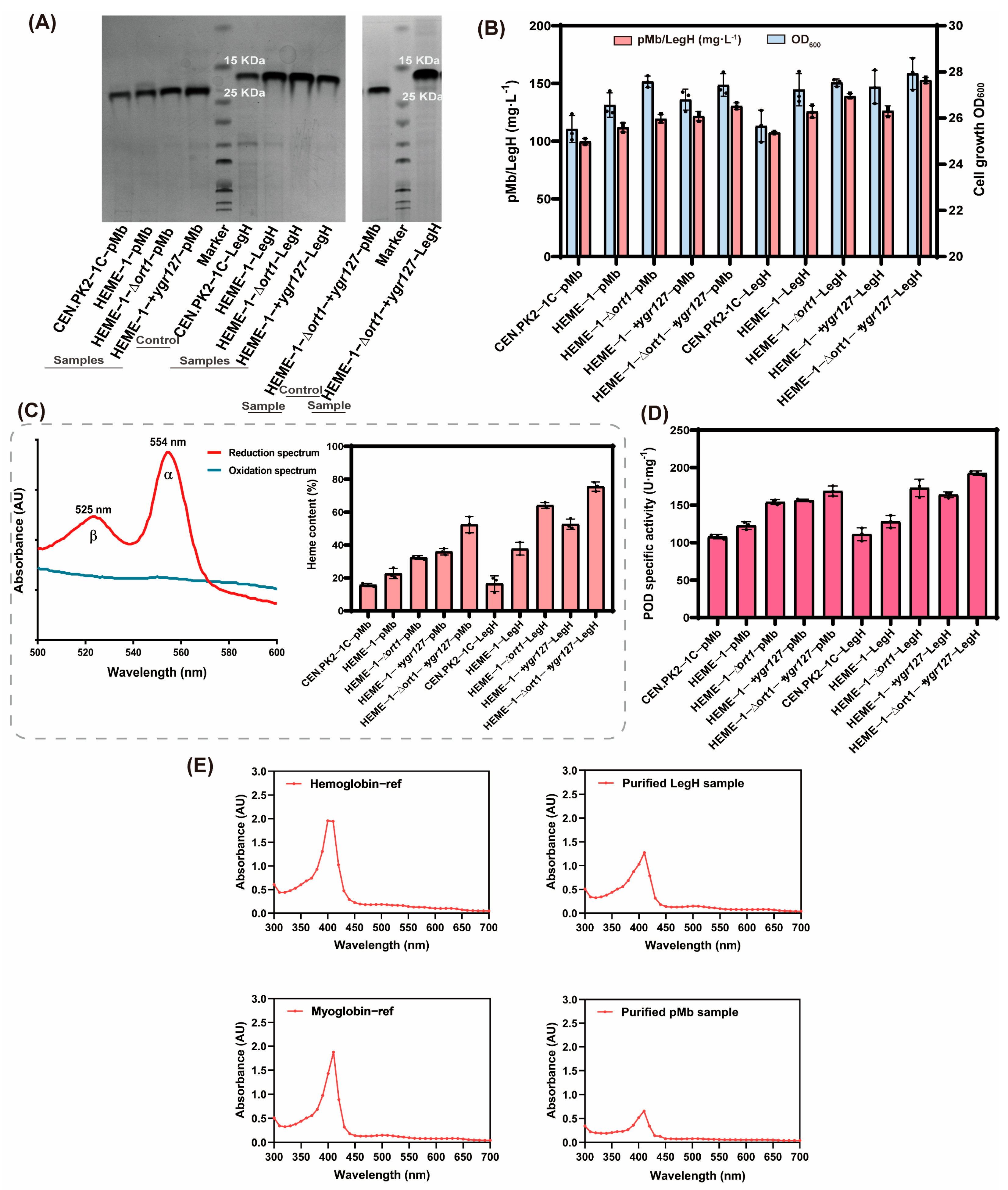
3.3. The Mining and Modifications of Mitochondrial ALA and Heme Transporters to Enhance Heme Supply
3.4. The Biochemical Properties of pMb and LegH Synthesized in the Engineered S. cerevisiae
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xing, Y.; Gao, S.; Zhang, X.; Zang, J. Dietary heme-containing proteins: Structures, applications, and challenges. Foods 2022, 11, 3594. [Google Scholar] [CrossRef] [PubMed]
- Foley, E.L.; Hvitved, A.N.; Eich, R.F.; Olson, J.S. Mechanisms of nitric oxide reactions with globins using mammalian myoglobin as a model system. J. Inorg. Biochem. 2022, 233, 111839. [Google Scholar] [CrossRef] [PubMed]
- Giardina, B. Hemoglobin: Multiple molecular interactions and multiple functions. An example of energy optimization and global molecular organization. Mol. Asp. Med. 2022, 84, 101040. [Google Scholar] [CrossRef]
- Ahmad, M.I.; Farooq, S.; Alhamoud, Y.; Li, C.B.; Zhang, H. Soy Leghemoglobin: A review of its structure, production, safety aspects, and food applications. Trends Food Sci. Technol. 2023, 141, 104199. [Google Scholar] [CrossRef]
- Simsa, R.; Yuen, J.; Stout, A.; Rubio, N.; Fogelstrand, P.; Kaplan, D.L. Extracellular heme proteins influence bovine myosatellite cell proliferation and the color of cell-based meat. Foods 2019, 8, 521. [Google Scholar] [CrossRef] [PubMed]
- Jolien, D.; Ann De, W.; Lore, D.; Ilse, F.; Irena, S.; Elsa, L.; Andy de, J.; Hermes, S. Improving the aromatic profile of plant-based meat alternatives: Effect of myoglobin addition on volatiles. Foods 2022, 11, 1985. [Google Scholar] [CrossRef]
- Zhao, X.; Zhou, J.; Du, G.; Chen, J. Recent advances in the microbial synthesis of hemoglobin. Trends Biotechnol. 2021, 39, 286–297. [Google Scholar] [CrossRef]
- Guirimand, G.; Kulagina, N.; Papon, N.; Hasunuma, T.; Courdavault, V. Innovative tools and strategies for optimizing yeast cell factories. Trends Biotechnol. 2021, 39, 488–504. [Google Scholar] [CrossRef]
- Swenson, S.A.; Moore, C.M.; Marcero, J.R.; Medlock, A.E.; Reddi, A.R.; Khalimonchuk, O. From synthesis to utilization: The ins and outs of mitochondrial heme. Cells 2020, 9, 579. [Google Scholar] [CrossRef]
- Shao, Y.; Xue, C.; Liu, W.; Zuo, S.; Wei, P.; Huang, L.; Lian, J.; Xu, Z. High-level secretory production of leghemoglobin in Pichia pastoris through enhanced globin expression and heme biosynthesis. Bioresour. Technol. 2022, 363, 127884. [Google Scholar] [CrossRef]
- Ge, J.Z.; Wang, X.L.; Bai, Y.G.; Wang, Y.R.; Wang, Y.; Tu, T.; Qin, X.; Su, X.Y.; Luo, H.Y.; Yao, B.; et al. Engineering Escherichia coli for efficient assembly of heme proteins. Microb. Cell Factories 2023, 22, 59. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.F.; Martinez, J.L.; Liu, Z.H.; Petranovic, D.; Nielsen, J. Balanced globin protein expression and heme biosynthesis improve production of human hemoglobin in Saccharomyces cerevisiae. Metab. Eng. 2014, 21, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Zhou, J.; Li, J.; Du, G.; Chen, J.; Wang, M.; Zhao, X. Systematic engineering of Saccharomyces cerevisiae for efficient synthesis of hemoglobins and myoglobins. Bioresour. Technol. 2023, 370, 128556. [Google Scholar] [CrossRef]
- Malina, C.; Larsson, C.; Nielsen, J. Yeast mitochondria: An overview of mitochondrial biology and the potential of mitochondrial systems biology. FEMS Yeast Res. 2018, 18, foy040. [Google Scholar] [CrossRef]
- Chiabrando, D.; Marro, S.; Mercurio, S.; Giorgi, C.; Petrillo, S.; Vinchi, F.; Fiorito, V.; Fagoonee, S.; Camporeale, A.; Turco, E.; et al. The mitochondrial heme exporter FLVCR1b mediates erythroid differentiation. J. Clin. Investig. 2012, 122, 4569–4579. [Google Scholar] [CrossRef] [PubMed]
- Yee, D.A.; DeNicola, A.B.; Billingsley, J.M.; Creso, J.G.; Subrahmanyam, V.; Tang, Y. Engineered mitochondrial production of monoterpenes in Saccharomyces cerevisiae. Metab. Eng. 2019, 55, 76–84. [Google Scholar] [CrossRef]
- Jia, H.J.; Chen, T.H.; Qu, J.Z.; Yao, M.D.; Xiao, W.H.; Wang, Y.; Li, C.; Yuan, Y.J. Collaborative subcellular compartmentalization to improve GPP utilization and boost sabinene accumulation in Saccharomyces cerevisiae. Biochem. Eng. J. 2020, 164, 107768. [Google Scholar] [CrossRef]
- Guo, Q.D.; Xu, J.Q.; Li, J.C.; Tang, S.Y.; Cheng, Y.H.; Gao, B.; Xiong, L.B.; Xiong, J.; Wang, F.Q.; Wei, D.Z. Synergistic increase in coproporphyrin III biosynthesis by mitochondrial compartmentalization in engineered Saccharomyces cerevisiae. Synth. Syst. Biotechnol. 2024, 9, 834–841. [Google Scholar] [CrossRef]
- Zinchuk, V.; Wu, Y.; Grossenbacher-Zinchuk, O. Bridging the gap between qualitative and quantitative colocalization results in fluorescence microscopy studies. Sci. Rep. 2013, 3, 1365. [Google Scholar] [CrossRef]
- Michener, J.K.; Nielsen, J.; Smolke, C.D. Identification and treatment of heme depletion attributed to overexpression of a lineage of evolved P450 monooxygenases. Proc. Natl. Acad. Sci. USA 2012, 109, 19504–19509. [Google Scholar] [CrossRef]
- Meisinger, C.; Pfanner, N.; Truscott, K.N. Isolation of yeast mitochondria. Methods Mol. Biol. 2006, 313, 33–39. [Google Scholar] [PubMed]
- Corcelli, A.; Saponetti, M.S.; Zaccagnino, P.; Lopalco, P.; Mastrodonato, M.; Liquori, G.E.; Lorusso, M. Mitochondria isolated in nearly isotonic KCl buffer: Focus on cardiolipin and organelle morphology. Biochim. Biophys. Acta-Biomembr. 2010, 1798, 681–687. [Google Scholar] [CrossRef] [PubMed]
- Pan, D.Q.; Lindau, C.; Lagies, S.; Wiedemann, N.; Kammerer, B. Metabolic profiling of isolated mitochondria and cytoplasm reveals compartment-specific metabolic responses. Metabolomics 2018, 14, 59. [Google Scholar] [CrossRef]
- Bragagni, M.; Scozzafava, A.; Mastrolorenzo, A.; Supura, C.T.; Mura, P. Development and ex vivo evaluation of 5-aminolevulinic acid-loaded niosomal formulations for topical photodynamic therapy. Int. J. Pharm. 2015, 494, 258–263. [Google Scholar] [CrossRef]
- Tomokuni, K.; Hirai, Y. Factors affecting determination of delta-aminolevulinate by use of Ehrlichs reagent. Clin. Chem. 1986, 32, 192–193. [Google Scholar] [CrossRef]
- Barr, I.; Guo, F. Pyridine hemochromagen assay for determining the concentration of heme in purified protein solutions. Bio-Protoc. 2015, 5, e1594. [Google Scholar] [CrossRef]
- Hu, J.J.; Dong, L.X.; Outten, C.E. The redox environment in the mitochondrial intermembrane space is maintained separately from the cytosol and matrix. J. Biol. Chem. 2008, 283, 29126–29134. [Google Scholar] [CrossRef]
- Bindels, D.S.; Postma, M.; Haarbosch, L.; van Weeren, L.; Gadella, T.W.J. Multiparameter screening method for developing optimized red-fluorescent proteins. Nat. Protoc. 2020, 15, 450–478. [Google Scholar] [CrossRef] [PubMed]
- Dunn, K.W.; Kamocka, M.M.; McDonald, J.H. A practical guide to evaluating colocalization in biological microscopy. Am. J. Physiol.-Cell Physiol. 2011, 300, C723–C742. [Google Scholar] [CrossRef]
- Gao, S.; Zhou, H.R.; Zhou, J.W.; Chen, J. Promoter-library-based pathway optimization for efficient (2S)-naringenin production from p-coumaric acid in Saccharomyces cerevisiae. J. Agric. Food Chem. 2020, 68, 6884–6891. [Google Scholar] [CrossRef]
- Savojardo, C.; Martelli, P.L.; Fariselli, P.; Casadio, R. TPpred3 detects and discriminates mitochondrial and chloroplastic targeting peptides in eukaryotic proteins. Bioinformatics 2015, 31, 3269–3275. [Google Scholar] [CrossRef] [PubMed]
- Apel, A.R.; d’Espaux, L.; Wehrs, M.; Sachs, D.; Li, R.A.; Tong, G.J.; Garber, M.; Nnadi, O.; Zhuang, W.; Hillson, N.J.; et al. A Cas9-based toolkit to program gene expression in Saccharomyces cerevisiae. Nucleic Acids Res. 2017, 45, 496–508. [Google Scholar]
- Shaw, W.M.; Khalil, A.S.; Ellis, T. A multiplex moclo toolkit for extensive and flexible engineering of Saccharomyces cerevisiae. ACS Synth. Biol. 2023, 12, 3393–3405. [Google Scholar] [CrossRef] [PubMed]
- Vögtle, F.N.; Burkhart, J.M.; Gonczarowska-Jorge, H.; Kücükköse, C.; Taskin, A.A.; Kopczynski, D.; Ahrends, R.; Mossmann, D.; Sickmann, A.; Zahedi, R.P.; et al. Landscape of submitochondrial protein distribution. Nat. Commun. 2017, 8, 290. [Google Scholar] [CrossRef] [PubMed]
- Palmieri, F.; Agrimi, G.; Blanco, E.; Castegna, A.; Di Noia, M.A.; Iacobazzi, V.; Lasorsa, F.M.; Marobbio, C.M.T.; Palmieri, L.; Scarcia, P.; et al. Identification of mitochondrial carriers in Saccharomyces cerevisiae by transport assay of reconstituted recombinant proteins. Biochim. Biophys. Acta-Bioenerg. 2006, 1757, 1249–1262. [Google Scholar] [CrossRef]
- Ferramosca, A.; Zara, V. Mitochondrial carriers and substrates transport network: A lesson from Saccharomyces cerevisiae. Int. J. Mol. Sci. 2021, 22, 8496. [Google Scholar] [CrossRef]
- Luzzani, C.; Cardillo, S.B.; Moretti, M.B.; García, S.C. New insights into the regulation of the Saccharomyces cerevisiae UGA4 gene: Two parallel pathways participate in carbon-regulated transcription. Microbiol. Soc. 2007, 153, 3677–3684. [Google Scholar] [CrossRef]
- Guernsey, D.L.; Jiang, H.; Campagna, D.R.; Evans, S.C.; Ferguson, M.; Kellogg, M.D.; Lachance, M.; Matsuoka, M.; Nightingale, M.; Rideout, A.; et al. Mutations in mitochondrial carrier family gene SLC25A38 cause nonsyndromic autosomal recessive congenital sideroblastic anemia. Nat. Genet. 2009, 41, 651–653. [Google Scholar] [CrossRef]
- Lunetti, P.; Damiano, F.; De Benedetto, G.; Siculella, L.; Pennetta, A.; Muto, L.; Paradies, E.; Marobbio, C.M.T.; Dolce, V.; Capobianco, L. Characterization of human and yeast mitochondrial glycine carriers with implications for heme biosynthesis and anemia. J. Biol. Chem. 2016, 291, 19746–19759. [Google Scholar] [CrossRef]
- Li, Y.X.; Han, S.R.; Gao, H.C. Heme homeostasis and its regulation by hemoproteins in bacteria. mLife 2024, 3, 327–342. [Google Scholar] [CrossRef]
- Sun, F.X.; Zhao, Z.Z.; Willoughby, M.M.; Shen, S.Q.; Zhou, Y.; Shao, Y.Y.; Kang, J.; Chen, Y.T.; Chen, M.Y.; Yuan, X.J.; et al. HRG-9 homologues regulate haem trafficking from haem-enriched compartments. Nature 2022, 610, 768–774. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.D.; Yu, H.B.; Zhou, J.W.; Li, J.H.; Chen, J.; Du, G.C.; Lee, S.Y.; Zhao, X.R. Whole-Cell P450 biocatalysis using engineered Escherichia coli with fine-tuned heme biosynthesis. Adv. Sci. 2023, 10, e2205580. [Google Scholar] [CrossRef] [PubMed]
- Kosmachevskaya, O.V.; Nasybullina, E.I.; Shumaev, K.B.; Topunov, A.F. Expressed soybean leghemoglobin: Effect on Escherichia coli at oxidative and nitrosative stress. Molecules 2021, 26, 7207. [Google Scholar] [CrossRef]
- Yu, F.; Zhao, X.R.; Zhou, J.W.; Lu, W.; Li, J.H.; Chen, J.; Du, G.C. Biosynthesis of high-active hemoproteins by the efficient heme-supply Pichia Pastoris chassis. Adv. Sci. 2023, 10, 2302826. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, X.; Wang, Y.; Wang, Y.; Zhou, J.; Li, J.; Chen, J.; Du, G.; Zhao, X. Efficient Synthesis of High-Active Myoglobin and Hemoglobin by Reconstructing the Mitochondrial Heme Synthetic Pathway in Engineered Saccharomyces cerevisiae. Fermentation 2025, 11, 246. https://doi.org/10.3390/fermentation11050246
Sun X, Wang Y, Wang Y, Zhou J, Li J, Chen J, Du G, Zhao X. Efficient Synthesis of High-Active Myoglobin and Hemoglobin by Reconstructing the Mitochondrial Heme Synthetic Pathway in Engineered Saccharomyces cerevisiae. Fermentation. 2025; 11(5):246. https://doi.org/10.3390/fermentation11050246
Chicago/Turabian StyleSun, Xiaoyan, Yunpeng Wang, Yijie Wang, Jingwen Zhou, Jianghua Li, Jian Chen, Guocheng Du, and Xinrui Zhao. 2025. "Efficient Synthesis of High-Active Myoglobin and Hemoglobin by Reconstructing the Mitochondrial Heme Synthetic Pathway in Engineered Saccharomyces cerevisiae" Fermentation 11, no. 5: 246. https://doi.org/10.3390/fermentation11050246
APA StyleSun, X., Wang, Y., Wang, Y., Zhou, J., Li, J., Chen, J., Du, G., & Zhao, X. (2025). Efficient Synthesis of High-Active Myoglobin and Hemoglobin by Reconstructing the Mitochondrial Heme Synthetic Pathway in Engineered Saccharomyces cerevisiae. Fermentation, 11(5), 246. https://doi.org/10.3390/fermentation11050246






