Abstract
This study investigates the potential of utilising the proteolytic activity of two different strains, Levilactobacillus brevis FST140 and Pediococcus pentosaceus FST22, to assess their impact on wheat gluten proteins. A high-power ultrasound (US) treatment (850 kHz; 500 W/cm2; 35 °C) was used to activate the proteolytic system of LAB to promote gliadin-like protein degradation in wheat wholemeal-based sourdough. The proteolytic activity of L. brevis and P. pentosaceus increased two-fold with 10 and 20 min US stimulation, respectively, compared to fermentation without ultrasonication. Regarding the impact of proteolysis and sonication on gliadin proteins, fermentation with both strains reduced gliadin content in commercial gluten by an average of 77.4% compared to the untreated sample, and additional US treatment further enhanced gliadin degradation efficiency to an average of 83.5%. The combined application of US and lactic acid fermentation initiated a seven-fold decrease in wheat wholemeal flour (WF) gliadin levels compared to the untreated sample (47.2 mg/g). Furthermore, the synergistic application of US, LAB, and yeast fermentation allowed us to reduce gliadin content up to 1.6 mg/g, as well as to reduce gluten content in the sourdough up to 3 mg/g. Despite complete hydrolysis of the gliadin fraction under the combined effects of US and fermentation, glutenins were less affected by the applied treatments in all cases. The technology presented in this study offers a promising approach for producing gluten-free or low-gluten fermented products in the bread-making industry.
1. Introduction
Gluten is a complex network of storage proteins, primarily composed of gliadin and glutenin, which are abundant in wheat (Triticum aestivum) and related cereals such as barley and rye [1]. While gluten plays a crucial role in providing the viscoelastic properties essential for dough functionality, it also poses significant health risks for individuals with gluten-related disorders, including celiac disease, gluten sensitivity, and wheat allergies [2].
Gliadin proteins constitute approximately half of the total prolamins in gluten and are traditionally categorised into α-, β-, γ-, and ω-gliadins based on their electrophoretic mobility at acidic pH. Meanwhile, glutenin proteins are subdivided into high molecular weight (HMW) and low molecular weight (LMW) subunits [3]. Among these, ω-gliadin has been identified as a major allergen in wheat-dependent exercise-induced anaphylaxis, whereas immunogenic T-cell stimulatory epitopes are also present in α- and γ-gliadins [4], and HMW glutenin proteins can also induce a toxic response in co-celiac patients [5].
Despite the rising consumer demand for gluten-free products, replicating the structural and sensory attributes of traditional wheat-based foods remains a major challenge. The elimination of gluten from cereal-based baked products has a significantly detrimental effect on the bread-making process, dough rheology, and bread quality. Gluten-free doughs, which cannot develop a network similar to traditional breads due to differences in protein properties, are less elastic and stickier, and gluten-free bread also tends to have a poor texture characteristics, a low nutritional value, reduced flavour, as well as a shorter shelf-life [6]. As a result, there is a growing need for innovative strategies to mitigate gluten immunogenicity, preserving the desirable qualities of wheat-based products.
Microbial fermentation, particularly using lactic acid bacteria (LAB), has emerged as a promising approach for gluten degradation. Previous studies have demonstrated that sourdough fermentation of wheat and nontoxic flours with selected LAB strains can significantly reduce gluten levels in wheat-based products, making them safer for individuals with gluten sensitivities, as was evidenced by sourdough protein analysis as well as agglutination and intestinal permeability tests [7,8]. This eco-friendly, natural process not only enhances the nutritional profile of fermented products but also improves flavour, texture, and shelf stability [9].
During the last decade, novel sourdough-based biotechnological applications have been proposed to meet consumers’ demand for healthier and more natural foods. One of the most promising and relatively easy methods of reducing gluten immunoreactivity is the use of probiotic LAB strains with specific hydrolysing properties. For example, the LAB strains, such as Lacticaseibacillus casei LC130, Lacticaseibacillus paracasei LPC100, and Streptococcus thermophilus ST250, and especially their mixture, were found to effectively hydrolyse immunoreactive gliadin peptides [10].
Recent findings suggest that sourdough yeast–LAB interactions further contribute to reduced gluten immunogenicity by promoting gluten depolymerisation and hydrolysis, primarily by weakening glutenin polymerisation and breaking down glutenin peptides [11]. Mixed inocula, such as Pediococcus acidilactici XZ31 and Saccharomyces cerevisiae JM4, have demonstrated superior efficacy in degrading toxic/immunogenic peptides compared to monocultures [11].
Furthermore, emerging technologies in food processing have the potential to enhance microbial fermentation efficiency and improve the quality of gluten-free and low-gluten products. Novel approaches, including ultrasound (US) treatment, have been explored for their ability to optimise food industry processes, ensuring sustainable production [12]. Ultrasound processing, being used alone or in combination with other processing methods, has been shown to significantly enhance food quality, and is therefore regarded as an effective technique [13]. Low-frequency, high-intensity US induces cavitation effects that disrupt protein structures, enhance mass transfer, and stimulate microbial enzymatic activity [14,15]. Studies have shown that combining US with microbial fermentation can accelerate proteolysis, thereby facilitating more efficient gluten degradation [16]. The effect of US on microbial growth and enzymatic activity is highly dependent on the processing parameters, with high-intensity US (16–100 kHz, 10–100 W/cm2) being particularly effective in modulating fermentation kinetics [17,18].
This study aimed to develop a sustainable and effective strategy for producing low-gluten fermented products that cater to individuals with gluten sensitivity while maintaining product quality. This research focuses on the synergistic application of microbial fermentation and high-frequency, low-power US treatment to hydrolyse toxic gliadin subunits in wheat-wholemeal-flour dough. Specifically, the study evaluates the influence of the US treatment on the proteolytic activity of Levilactobacillus brevis FST140 and Pediococcus pentosaceus FST22 strains, as well as the qualitative and quantitative composition of gliadins. The results of this study are expected to be of significant interest to the food industry, providing new insights and establishing a theoretical foundation for future studies.
2. Materials and Methods
2.1. Materials
A commercial wholemeal wheat flour (WF) produced by SC “Malsena Plus” (Vievis, Lithuania) with an appropriate chemical composition (moisture 12.8%, protein 10.5%, wet gluten 21.4%, soluble protein 2.11% total protein, fat 1.8%, ash 0.6%, fibre 11.2%) was purchased from a local market, and commercial gluten (moisture 6.6%, protein 77.4%) was provided by the SC “Roquette Amilina” (Panevėžys, Lithuania).
2.2. Microorganisms
Lactic acid bacteria Lactobacillus brevis (ATCC 367) and Pediococcus pentosaceus (ATCC 25745), previously isolated from a spontaneously fermented wheat flour media [19], were used in the fermentation experiments. Before the experiment, the LAB strains stored at –80 °C (Microbank system, Pro-Lab Diagnostics, Birkenhead, UK) were multiplied in a Man–Rogosa–Sharpe (MRS) broth with Tween80 (Biolife, Milan, Italy) at 35 °C for 48 h under anaerobic conditions (cell count ~9 log CFU/mL).
2.3. Ultrasound-Assisted Fermentation
Ultrasound (US) with a frequency of 850 kHz (power 500 W/cm2) was applied for the intensification of the LAB fermentation process and the degradation of gluten proteins. For the US treatment, the sample of gluten (5 g) or WF (10 g) was mixed with 20 mL of distilled water in a plastic container (layer thickness 15 mm). After adding 2% (v/v) of pure bacterial suspension to the resulting medium, the samples after mixing were placed in an ultrasonic high-power bath (type 5/1575, Meinhardt Ultraschalltechnik, Leipzig, Germany) and sonicated at maximum intensity and 35 °C temperature for different time periods (10, 20, and 30 min). Untreated gluten and the WF sample were analysed as a control. All samples were lyophilised and used for the gliadin and glutenin analysis. Each experiment was repeated four times.
2.4. Fermentation Experiments
The WF sample (100 g) was mixed with distilled water to achieve a moisture content of 65%. Additionally, commercial gluten (10 g) was mixed with 50 mL of distilled water. The samples were then supplemented with 2% (v/v) of a bacterial suspension containing approximately 109 CFU/mL of pure cells. The WF fermentation was conducted in duplicate for 96 h at 35 °C. Sub-samples (each of 10 g) of the WF sourdough were collected after mixing and every 24 h thereafter to determine the pH, soluble protein content, and proteolytic activity. Gluten was fermented separately for 24 h under the same conditions. For the yeast fermentation experiment, WF was first fermented for 24 h with selected LAB. After, 2.5 g/100 g of baker’s yeast were added and fermentation continued for an additional hour at 35 °C. Untreated WF was analysed as a control sample. For gliadin and glutenin protein analysis, all samples were lyophilised.
2.5. Proteolytic Activity Determination
The mode of action of LAB proteases was determined by Sigma-Aldrich non-specific protease assay using casein as a substrate [20]. For the assay, the fermented sample (5 g) was mixed with 20 mL of 100 mM lactic acid buffer (pH 3). The mixture was centrifuged (5500 rpm; 15 min), and the obtained supernatant was used as an enzyme solution to assess LAB proteolytic activity. The sample (1 mL) was added to 1 mL of 1.5% (w/v) casein solution and the mixture was incubated at 37 °C for 60 min. Then, 2 mL of 400 mM TCA solution was added to stop the enzyme action. The samples were centrifuged (5500 rpm; 15 min), and 1 mL of supernatant was mixed with 5 mL of 400 mM Na2CO3 and 1 mL of 2N Folin–Ciocalteu’s phenol reagent diluted with distilled water (1:10 v/v). The absorbance of obtained solutions was measured with spectrophotometer at 670 nm wavelength. For the control, the same steps are carried out as in the test sample, except that the TCA solution was added prior to sample extraction. One unit of enzyme activity was defined as the amount of enzyme that liberates an equivalent of 1 μg of tyrosine per minute from the substrate (casein) under the assay conditions (37 °C, 30 min, pH 3) and was reported in terms of protease activity per gram of fermented sample.
2.6. Determination of Free Amino Acids
The concentrations of free amino acids in the wheat flour and fermented samples were determined using ion-exchange chromatography and measured photometrically by reacting with ninhydrin at a wavelength of 570 nm. The lyophilized sample (1 g) was extracted with 100 mL of 0.1M HCl with 2% thiodiglycol solution. The mixture was stirred for 60 min using a magnetic stirrer and was left for the precipitate to form. The 10 mL of the liquid was mixed with 5 mL of 6% sulfosalicylic acid solution and stirred with a magnetic stirrer for 5 min, centrifuged, and the supernatant was separated. The 10 mL of the resulting solution was diluted into 100 mL with citrate buffer (0.2M, pH 2.2, prepared by dissolving 19.61 g sodium citrate, 5 mL thiodiglycol, 1 g phenol and 16.5 mL HCl), adjusting the pH to 2.2 with a 1M sodium hydroxide solution. Amino acids were analysed using a Ultrafast Liquid Chromatography (UFLC) (Shimadzu, Kyoto, Japan) with automated o-phthalaldehyde (OPA)/9-fluorenylmethyl chloroformate (FMOC) and coupled with a YMC-Triart C18 column (1.9 pm, YMC Co., Ltd., Kyoto, Japan) and the fluorescence detector RF-20Axs. The separation was performed according to the conditions described by Jukonyte et al. [18]. The amino acid standards (A9781 Sigma-Aldrich, Hamburg, Germany) of 0.5 μmol/mL concentration, except for L-cystine at 0.25 μmol/mL in 0.2M sodium citrate pH 2.2, were analysed. For the quantitative evaluation of the amino acid profile, the calibration curve was built in the range of 10 µMol/L–200 µMol/L.
2.7. Gluten Protein Extraction
Osborne fractionation of gliadin and glutenin fractions from untreated or fermented WF or pure gluten was performed following the method as described by Dhaka ir Khatkar [21]. Five grams of powdered gluten or lyophilised fermented or ultrasonicated flour was mixed with 50 mL of 70% (v/v) ethanol and mixed for 60 min at room temperature (25 °C) on a magnetic stirrer. The mixture was then centrifuged at 5500 rpm for 30 min at 4 °C. The obtained supernatant was collected as the gliadin fraction. For the isolation of glutenin, 50 mL of 50% 1-propanol + 1% DTT solution was added to the precipitate obtained in the first step and stirred for 30 min, inverting every 10 min. The samples were kept in a water bath at 60 °C for 90 min, inverting every 15 min. After extraction, the samples were centrifuged, the supernatant was discarded, and the precipitated glutenin was dissolved in 5 mL of 50% 2-propanol + 0.08 M Tris-HCl (pH 7.5) + 1% DTT + 2 M urea solution. All extractions were performed in triplicate. Gliadin and glutenin contents (%) were calculated on a dry-matter basis after the freeze-drying of extracts.
2.8. Capillary Electrophoresis
The capillary electrophoresis of protein was performed on an Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA, USA). The sample buffer consisted of 0.4 mL of 2 M urea, 50% 1-propanol, 0.1 M DDT (dithiothreitoyl), and 0.082 M Tris-HCl (buffer pH 8.0). The gliadin extract (100 µL) was mixed in the test tubes with the sample preparation buffer (2 M urea, 15% glycerol, 0.1 M DDT, and 0.1 M Tris-HCl (pH 8.0), 150 µL of the buffer solution, and 75 µL of distilled water by vigorous shaking. The samples were kept in a 25 °C ultrasonic bath for 15 min, then centrifuged (12,200 rpm, 5 min) in a microcentrifuge (Benchmark Scientific, Sayreville, NJ, USA). The 4 µL of resulting centrifugate was mixed with 2 µL of denaturing solution (200 µL of sample buffer and 7 µL of mercaptoethanol), centrifuged, and was subsequently kept in a boiling water bath for 5 min. After heating, 84 µL of distilled water was added to each sample. A special microplate was filled with the gel staining solution, containing Coomassie Brilliant Blue G-250 dye, then the wells were filled with test samples and protein standards solution (10 samples per chip, sample volume 4 µL, protein sizing range 5–230 kDa). The proteins analysis data were processed using Agilent 2100 Expert (Agilent Technologies, USA) software.
2.9. High-Performance Liquid Chromatography
Gliadins were analysed by a Thermo Ultimate 3000 HPLC system (Thermo Fisher Scientific, JAV, Waltham, MA, USA) equipped with an ESA Corona Ultra CAD detector (Thermo Scientific Dionex, Waltham, MA, USA) and an integrated Waters BEH200 SEC 125A column (4.6 × 300 mm; 1.7 µm) (Waters Corporation, Milford, MA, USA) thermostated at 30 °C. Separation was carried out with a flow rate of 0.3 mL/min and an injection volume of 5 μL of the protein solution (1 mg/mL), which had been previously filtered with a 0.22 µm pore-size filter. The mobile-phase system consisted of solvent A (70% water/0.1% trifluoroacetic acid (TFA), v/v) and solvent B (30% acetonitrile/0.1% TFA, v/v). The gradient for protein separation was as follows: 0 min-0% B, 0.5 min-24% B, 20 min-56% B, 24 min-90% B, 30 min-0% B, detection: UV absorbance at 210 nm.
2.10. Protein Electrophoresis
The SDS-PAGE of glutenins was performed according to Siddiqi et al. [22]. The freeze-dried gliutenin sample (1 mg) was re-suspended in 1 mL 2X Laemmli sample buffer solution of pH 6.8 containing 62.5 mM Tris-HCl, 2% SDS, 25% glycerol, and 0.01% bromophenol blue. Proteins were separated using 4% stacking gel and 12% resolving gel. Samples were treated by 5% ß-mercaptoethanol, and obtained dispersions were heated in boiling water for 5 min. After centrifugation at 11,000× g for 20 min at 4 °C, the supernatant (10 μL for each lane) was used for SDS-PAGE analysis (Mini-Protean 3, Bio-Rad Laboratories, Hercules, CA, USA). The stabilised and stained gels were scanned and analysed.
2.11. Analysis
The moisture content in the raw material was determined according to the AOAC approved Method 930.15, and the crude protein content was determined by the Kjeldahl nitrogen (method 920.152, N × 5.7) [23]. The pH of fermented samples was measured directly by a pH-metre (PP-15; Sartorius, Goettingen, Germany). The concentration of soluble proteins in fermented or sonicated sample extracts was determined according to the Bradford assay [24]. The soluble protein was expressed as a mg of soluble protein per 1 g of fermentation medium.
2.12. Texture Analysis
Dough samples were subjected to extension testing on a TA.XT2i Texture Analyser (Stable Micro Systems, Godalming, UK). A cylindrical aluminium probe 20 mm in diameter was used for dough compression (compression force of 1 kg) at a pre-test speed of 2 mm/s, test speed 10 mm/s, and penetration distance 10 mm. For analysis, 50 g dough samples were placed in a plastic measurement vessel (diameter of 25 mm and height of 50 mm). The dough hardness, consistency, index of viscosity, and cohesiveness were measured. Dough hardness (firmness) was defined as the maximum positive force exerted during compression. Consistency was defined as the positive area of the compression peak. Dough cohesiveness was defined as the maximum negative force, which represents the work necessary to pull the compressing plunger away from the sample. The index of viscosity was defined as the negative area of the force peak.
2.13. Statistical Analysis
Fermentation experiments and gluten protein isolation were performed in duplicates. All analyses were performed at least in triplicates, presenting mean values. Analysis of variance (ANOVA) was performed using a statistical package of SPSS version 25 (IBM SPSS, Armonk, NY, USA). Duncan’s multiple range test was used to determine the differences between means and was considered as significant at p ≤ 0.05.
3. Results and Discussion
3.1. Proteolytic Activity of Lactic Acid Bacteria Strains in Wheat Wholemeal Fermentation Medium
The changes in the proteolytic activities of tested LABs during fermentation of wheat wholemeal (WF) are presented in Table 1. Data revealed that proteolytic activities in WF fermented at 35 °C with both LAB strains significantly increased (p ≤ 0.05) with the increase in fermentation time. The highest proteolytic activity of 26.57 PU/100 g was determined after 48 h of fermentation for L. brevis, and P. pentosaceus produced the highest proteolytic activity (15.53 PU/100 g) after 72 h of fermentation. The activation of LAB strains was confirmed by a significant (p ≤ 0.05) reduction in the pH of the medium during the 24–48 h fermentation period for L. brevis and the 48–72 h period for P. pentosaceus, depending on their adaptation to the medium and the proteolytic activities of the tested strains (Table 1).

Table 1.
The changes in pH, soluble protein (SP), and proteolytic activity (PA) of tested lactic acid bacteria during fermentation of wheat wholemeal sourdough.
The changes in soluble protein (SP) content in the fermentation medium demonstrated that L. brevis (Lb) adapted to the WF medium much faster than P. pentosaceus (Pp). During the first 24 h, the SP content increased by 34 and 53%, respectively, followed by an additional increase on average by 20.5% over the 48–72 h period compared to the initial concentrations (102.12 and 94.89 mg/100 g, respectively). However, prolonged fermentation reduced the SP content in the medium. Since the proteolytic activities of LAB strains increased with fermentation time, the SP contents initially rose due to enhanced protein degradation [25]. Variations in the SP contents are likely influenced by the adaptation of LABs to the medium and their capacity to break down protein-based components, leading to an increased release of soluble proteins [26,27].
Protein degradation during WF-sourdough fermentation was also estimated by the changes in the concentrations of free amino acids (Table 2). Fermentation by selected lactobacilli resulted in a significantly higher (p ≤ 0.05) total concentration (1.85 to 2.12 g/kg d.w.) of free amino acids compared to the control dough (0.87 g/kg d.w.), with some differences in the amino acid patterns.

Table 2.
Free amino acid concentrations (mg/kg) in wheat sourdoughs before (UWF) and after 24 h fermentation with P. pentosaceus and L. brevis strains.
In comparison to unprocessed dough, both Lactobacillus strains significantly (p ≤ 0.05) increased the concentrations of most amino acids, with L. brevis particularly enhancing Asp and Met levels. P. pentosaceus exhibited a lower capacity for amino acid release during the first 24 h of fermentation. Additionally, in both cases, sourdough fermentation led to a considerable reduction in Ala and His content and increased the levels of branched-chain amino acids, Leu and Ile.
According to the literature, the rise in the total free amino acid (FAA) content during fermentation aligns well with the peak aminopeptidase and dipeptidase activities observed in the early stationary phase [28]. The steady and pronounced increase in the FAA levels in both fermented sourdoughs suggests a more efficient and accelerated proteolytic activity, likely facilitated by the optimal initial pH (5.4–5.5).
Proteolysis during sourdough fermentation remains a complex and not yet fully understood process, largely due to the intertwined effects of acidification and enzymatic activity, as well as the involvement of both endogenous wheat-flour proteolytic enzymes and microbial proteases. Although existing studies have primarily focused on the accumulation of free amino acids and the adaptation of LAB enzymes to the dough environment [26], the present study further highlights the crucial role of lactic acid bacteria in protein degradation. These findings underscore their technological relevance in sourdough fermentation and reveal new potential benefits for nutritional applications.
3.2. The Use of Ultrasound Technology to Activate Lactic Acid Bacteria for Sourdough Fermentation
The ultrasonic cavitation was used to increase the hydrolytic activity of the selected LABs in order to increase WF gluten protein degradation. The results of the effect of the US treatment of the WF fermentation medium (850 kHz, 100% intensity, temperature 35 °C) on the proteolytic activity of L. brevis and P. pentosaceus strains are presented in Figure 1.
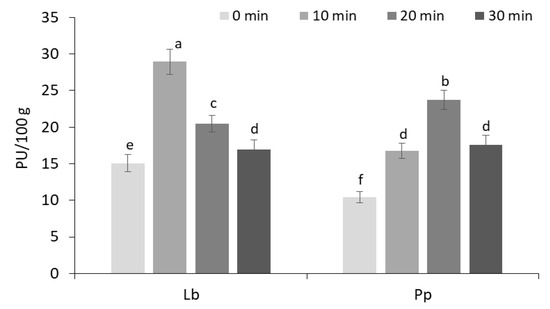
Figure 1.
The effect of ultrasonication (10, 20, 30 min) on proteolytic activities (PU) of L. brevis FST140 (Lb) and P. pentosaceus FST22 (Pp) after 24 h sourdough fermentation at 35 °C temperature. The different letters a–f indicate significant differences between values (p ≤ 0.05).
The results showed that the ultrasonication time significantly (p ≤ 0.05) affected the proteolytic activity of both LABs, depending on the fermentation time. With 10 and 20 min US treatment, the proteolytic activities of L. brevis and P. pentosaceus in 24 h fermented sourdoughs reached on average 2-fold increase compared to the unsonicated samples (15.09 and 10.42 PU/100 g, respectively), and longer sonication (20 and 30 min, respectively) reduced the proteolytic activities of tested LABs on average up to 17 PU/100 g (Figure 1).
The literature suggests that the cell walls of Gram-positive bacteria, such as pediococci and lactobacilli, exhibit greater resistance to the US waves, while their enzymatic activities can be enhanced under ultrasonic treatment [29]. Additionally, US has been shown to accelerate the transport of oxygen and nutrients essential for microbial growth, thereby promoting a faster microbial proliferation rate [30].
In summary, the use of US-assisted LAB fermentation of WF causes the proteolysis of gluten proteins to be significantly increased during the 24 h fermentation period. By selecting microorganisms and the US treatment conditions, the efficiency of the entire microbial fermentation can be successfully regulated (Figure 2).

Figure 2.
The effect of the US treatment [20 min—for P. pentosaceus FST22 (Pp) and 10 min—for L. brevis FST140 (Lb)] on the changes in proteolytic activity (A) and pH (B) during 96 h sourdough fermentation at 35 °C temperature. The different letters a–f indicate significant differences between values (p ≤ 0.05).
The longer US-induced sourdough fermentation initiated ambiguous changes in the proteolytic activity of both tested strains (Figure 2A). With 20 min-US treatment, P. pentosaceus proteolytic activity increased from 24.14 PU/100 g to 26.17 PU/100 g during the 24–48 h period but reduced up to 12.13 PU/100 g after 72 h of fermentation. In the case of L. brevis, 10 min ultrasonicated bacteria showed proteolytic activity lower by 24–65% after 48–72 h fermentation period compared to the 24 h fermented sample. After 96 h of fermentation, proteolytic activity in the ultrasonicated medium was determined at a low level for both strains (on average 3.72 PU/100 g), showing a reduction in the degradation intensity of proteins.
The changes in acidity occurred more intensively in the US-treated WF medium than in the untreated medium (Figure 2B). The acidity of sourdough fermented by L. brevis and P. pentosaceus increased throughout the fermentation period; the pH decreased by 17 and 23%, respectively, after 24 h of fermentation and by an average of 34 and 37%, respectively, during the 72–96 h period compared to the initial value (pH 5.4) (Figure 2B). Meanwhile, in the untreated WF medium, the pH change was 5.6–31.0 and 7.3–34.0% for L. brevis and P. pentosaceus samples, respectively (Table 1).
Recent studies have shown that fermentation efficiency can be significantly enhanced due to the ultrasound-induced stimulation of microorganisms, which improves their metabolism and enzyme activity [31,32,33]. In most cases, short-term US treatment (20–37 kHz; ≤30 min) has been used to pre-treat microorganisms before fermentation, as prolonged exposure to cavitation can inhibit bacterial enzyme activity [32,34]. A study by Hashemi et al. demonstrated that US treatment (30 kHz, 6 min) enhanced the efficiency of Limosilactobacillus reuteri PTCC 1655 fermentation (37 °C, 30 h) in Bakraei juice. However, extending sonication time reduced processing efficiency and led to a decline in microbial population [35]. Similarly, Tseng et al. reported a 16.5% increase in α-glucosidase activity in L. plantarum BCRC 10357 under ultrasonic stimulation (40 kHz; 300 W; 20 min) compared to untreated samples (22.89 U/mL) after 36 h of fermentation in black soymilk at 37 °C. This indicates that encapsulated bacteria exhibit high adaptability to ultrasonic stress [29].
Although wheat flour contains endogenous proteolytic enzymes active at an acidic pH (3.5−4.0) [36], the proteolytic activity in whole-wheat flour, as determined in our study, was relatively low (0.512 PU/100 g) and did not significantly affect the proteolytic activity of LABs during fermentation. Therefore, it can be concluded that a combined approach—fermentation with specific LAB strains and enhancement of their enzymatic activity via ultrasonic cavitation—can effectively degrade the gliadin fraction of wheat gluten.
In summary, integrating the US technology into controlled fermentation processes can improve fermentation efficiency. Additionally, ultrasound can alter protein properties by promoting the breakdown of proteins into free amino acids and peptides [37].
3.3. The Effect of Ultrasound and Lactic Acid Fermentation on Pure Gluten Degradation
The results of the effect of sonication and fermentation of commercial gluten with P. pentosaceus and L. brevis strains on the gliadin content and gliadin subunits distribution are presented in Figure 3. The results of the experiments indicated both types of tested LAB strains, as well as ultrasonic cavitation, having a distinct impact on the gliadins. The gliadin content was significantly decreased (up to 25%) in 30 min-ultrasonicated gluten compared to the untreated gluten sample (518 mg/g protein) (Figure 3B).
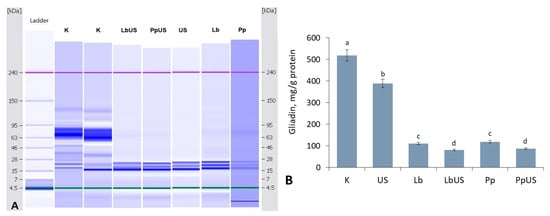
Figure 3.
The effect of sonication and LAB fermentation of commercial gluten with P. pentosaceus and L. brevis strains on gliadin subunit distribution (A) and content (B). Samples: K—untreated gluten; Lb, Pp—gluten fermented with L. brevis or P. pentosaceus; US—ultrasound-treated gluten sample. The different letters a–d within the columns indicate significant differences between values (p ≤ 0.05).
According to Marcuzzo et al., sonication (24 kHz, 200 W, 3–12 min) did not cause significant alterations in various gluten fractions, suggesting minimal protein breakdown at the molecular level [38]. Similarly, Cheng et al. reported that US treatment (20–28 kHz, 10 min) induced denaturation of whey proteins and better exhibited their viscoelastic properties and the microstructure of gels [39]. The differences observed in our study may stem from the use of a higher ultrasound frequency and power (850 kHz, 500 W/cm2), which could have enhanced gliadin degradation.
When assessing the impact of microbial fermentation on gliadin degradation, significant differences (p > 0.05) were not found between the two tested strains; fermentation with L. brevis or P. pentosaceus efficiently hydrolysed the gliadin fraction, depending on the strain’s characteristics. On average, gliadin content was reduced by 77.9% following fermentation with both strains (Figure 3B). The effect of combined treatments included breaking down gliadin macromolecules of a molecular weight (MW) in the range of 30–120 kDa (Figure 3A), and only subunits with a MW of approximately 17–25 kDa have been identified (Figure 3B).
The combination of US and fermentation allowed the degradation of 76 kDa gliadin subunits and the reduction in gliadin content to 81.5–87.4 mg/g protein (Figure 3A), i.e., the degradation efficiency reached an average of 83.7% (Figure 3B).
The electropherogram of gliadins of fermented samples revealed MWs of 17, 21, and 25 kDa and a negligible amount of 76 kDa protein (Figure 3A). In our study, smaller MW protein molecules remained, i.e., 18, 20, and 24 kDa, after treatment of gluten, but they are not the causes of celiac disease [4,40]. Similarly, in the study of Zotta et al. [41], proteins of molecular weight in a range of 20–27 kDa were identified after 24 h of fermentation with different Lactobacillus sp. bacteria.
According to the literature, the protein components that exhibit celiac activity are primarily gliadin proteins with molecular masses between 31 and 50 kDa, whereas glutenins are generally considered non-toxic [42]. Specifically, ω-gliadins have the highest molecular weights, ranging from 46 to 74 kDa, while α- and γ-gliadins fall within the range of 30–45 kDa [43].
The changes in the gluten proteins related to the presence of LAB were due to the appearance of new protein fragments from gliadins and the degradation of the HMW glutenin subunits (peaks between 10.5–13 min) (Figure 4).
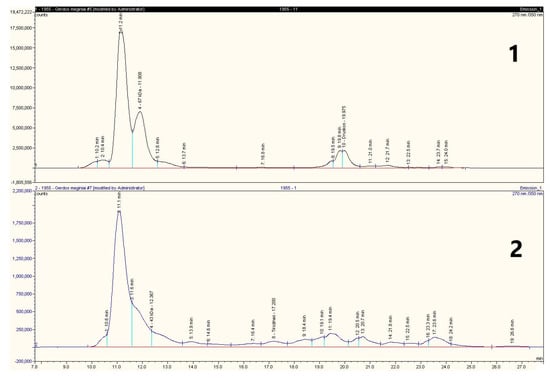
Figure 4.
The chromatograms of gluten gliadin fraction. 1—gliadins of untreated gluten; 2—gliadins of the US treated and fermented with L. brevis FST140 gluten.
According to the literature data, every type of LAB had distinct impacts on the composition of gluten proteins. These effects include breaking down gluten macromolecules depending on unique acidification kinetics of each strain [44,45,46]. In our experiment, the amount of gliadins determined after fermentation and sonication did not differ significantly between L. brevis and P. pentosaceus, but gliadins were degraded slightly more efficiently by L. brevis, which may be due to the higher degradation efficiency of the latter strain (78.8%) compared to P. pentosaceus (77.0%) (Figure 3B).
In the case of glutenins, the combined treatment of wheat wholemeal with US and fermentation for 24 h led to a degraded glutenin fraction of the pure gluten. In the electrophoretic gel of glutenins (Figure S4), no changes were noticed in the fermented or ultrasonicated gluten samples, while after combined treatment the higher molecular weight glutenins were broken down, leaving subunits between 36 and 45 kDa. The electrophoresis results showed that, unlike gliadins, glutenins were not as strongly affected by the applied measures when comparing all samples with the control (Figure S4).
Dvořáček et al. determined that the typical electropherogram peaks characteristic of glutenins were assigned to proteins with the following molecular weights: 44, 45, 57, 100, 106, and 112 kDa [47]. In contrast, gliadins, which are primarily responsible for celiac immunotoxicity, exhibit molecular weights typically ranging from 30 kDa to 50 kDa, with α- and γ-gliadins in the 30–45 kDa range and ω-gliadins reaching 46–74 kDa [43]. It was reported that during 24 h of fermentation with LABs, the HMW glutenins, which form the basis of the glutenin macropolymer, were broken down into smaller subunits, disrupting the gluten network, and thus, increasing the solubility of all gluten proteins [46]. The acidic conditions that are present in sourdough not only increase the solubility of glutenins but also create an ideal environment for the activation of cereal aspartic proteinases, which were the most active proteinase group in sourdoughs. Because the degradation of gluten proteins in wheat sourdough is most probably catalysed mainly by aspartic proteinases that are active under acidic conditions, it should be possible to regulate the degree of proteolysis by regulating the fermentation conditions that affect the proteolytic activity of sourdough LABs [46].
The literature reports that during lactic acid fermentation at least 20% of glutenins remain unaffected, which is crucial for bread quality control as glutenins are responsible for dough elasticity [48]. The complete removal of gluten proteins from flour would compromise the structural integrity of the dough, underscoring the importance of maintaining an optimal balance between gluten protein fractions.
3.4. The Effect of Ultrasound on Wheat Flour Gluten Protein Degradation During Sourdough Fermentation
The possibility of using the US technology and fermentation with L. brevis FST140 and S. cerevisiae yeast for the elimination of gluten protein toxicity in wholemeal wheat-flour sourdoughs was evaluated with the prospect of using these fermented products for the production of gluten-free or low-gluten baked goods. The gliadin contents in differently treated WF products are presented in Figure 5.

Figure 5.
The effect of ultrasound (US) and microbial fermentation of wheat wholemeal treatment on gliadin content. Samples: K—untreated WF; USY—sonicated and fermented with S. cerevisiae yeast sourdough; USF—sonicated and fermented with L. brevis sourdough; USFY—sonicated and fermented with L. brevis and yeast sourdough. The different letters a–d within the columns indicate significant differences between values (p ≤ 0.05).
It was found that all the technologies used significantly (p ≤ 0.05) reduced the gliadin content in treated flour sourdoughs. The combination of US and lactic acid fermentation reduced the gliadin content by approximately 7-fold compared to untreated wheat flour (WF) (4.72 g/100 g) (Figure 5). The combination of sonication and 24 h fermentation with L. brevis, followed by yeast fermentation, was highly effective, reducing gliadin content in sourdough to 0.16 g/100 g. In contrast, the US-initiated yeast fermentation was less effective, reducing gliadin content by 2.2-fold.
In our study, gliadin isolated from wheat flour accounted for about 45% of the total protein (Figure 5). According to the literature, wheat gluten typically contains gliadin at around 30–50% of its total protein content, resulting in a gliadin/glutenin ratio of 1.3 to 2.5 [49]. Fu et al. reported that P. acidilactici XZ31 significantly degraded gluten peptides and reduced gluten immunogenicity, while a mixed inoculum of P. acidilactici XZ31 and yeast showed superior efficiency in breaking down toxic and immunogenic peptides compared to monoculture fermentation [11]. Similarly, Rizzello et al. demonstrated that a bacterial complex containing L. alimentarius 15M, L. brevis 14G, L. sanfranciscensis 7A, L. hilgardii 51B, and baker’s yeast effectively reduced gluten content in flour to 12 mg/kg after 48 h fermentation [48].
The proteins of the gliadin fraction extracted from the ultrasonicated and LAB-treated WF samples were analysed by molecular sieve chromatography to identify changes in the components constituting the fraction. The results are presented in Figure 6.
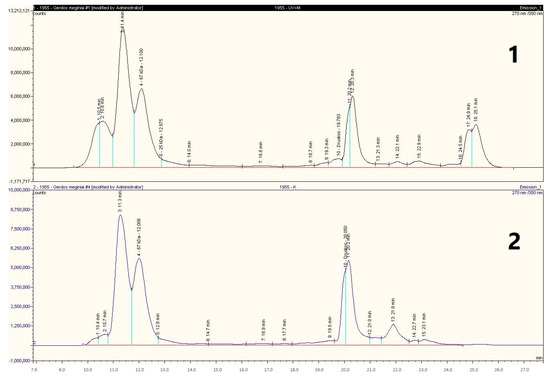
Figure 6.
The chromatograms of gluten gliadins from raw and processed wholemeal wheat flour (WF). Samples: 1—WF treated with L. brevis FST140, ultrasound, and S. cerevisiae yeast; 2—gliadin fraction isolated from raw WF.
Based on the results of the chromatographic analysis, the longest retention time and the largest molecular mass distribution of proteins were noticed in the sample treated by US-assisted fermentation with L. brevis and yeast (Figure 6). The results showed that by combining biotechnological tools and ultrasound, it was possible to significantly break down the HMW proteins (detected at 10–11,16–17, and 20 min). However, it was not possible to completely break down the HMW proteins detected at 11–12 min, which may have been extracted together with gliadin as degraded glutenin subunits. This is because in commercial gluten treated with L. brevis FST140 and US the HMW protein residues were not detected, indicating that the entire gliadin fraction may was hydrolysed (Figure 4).
3.5. Influence of Biotechnological Tools and Ultrasound on Sourdough Texture Properties
The results of the study of the influence of biotechnological tools and US treatment on dough texture are presented in Figure 7. It was found that the hardest dough with the strongest consistency values (4.27 N and 14.1 N·s, respectively) was that made from the unfermented WF. The samples treated under various conditions were significantly softer after fermentation compared to the control. The lowest hardness (0.15 N) and consistency (1.1 N·s) were determined in the sourdough produced from the ultrasonicated and fermented with LAB and yeast wheat wholemeal, indicating a high degree of hydrolysis since peptide bonds are broken and the consistency of the sourdough becomes weak.
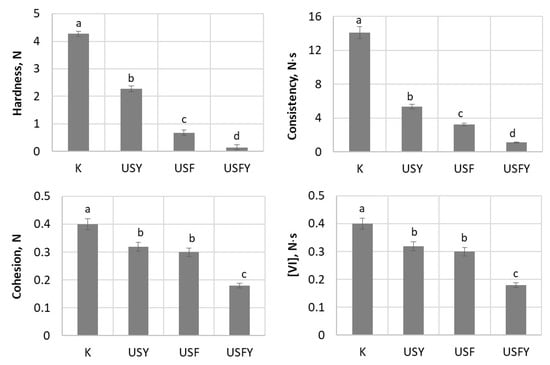
Figure 7.
The effect of ultrasound (US)-assisted microbial fermentation dough texture properties. Samples: K—untreated WF dough; USY—dough prepared by US-assisted fermentation with S. cerevisiae yeast; USF—dough prepared by US-assisted fermentation with L. brevis; USFY—dough prepared by US-assisted fermentation with L. brevis and yeast. VI—viscosity index. The different letters a–d within the columns indicate significant differences between values (p ≤ 0.05).
When analysing the values of the cohesion and viscosity index (VI) of the samples, it was found that the lowest values of these parameters were the USY sample compared to the control. The application of complex biotools reduced the dough cohesion by 2.2-fold and the VI value by 3-fold (Figure 7). The lowest values of these parameters, 0.18 N and 0.063 N∙s, were determined in the dough treated with US, L. brevis, and yeast.
During the LAB–yeast sourdough fermentation, various amino acids, such as phenylalanine, leucine, or cysteine, can be formed, which are responsible for specific bread flavours [50]. Additionally, Luo et al., investigating the effects of US-assisted dough fermentation on steamed bread quality, revealed that US treatment (40 kHz; 36–38 °C, 20–50 min) improved the bread’s specific volume and reduced crumb hardness [51].
Studies have shown that gliadins act as a plasticizer during the formation of dough and improve the viscosity and extensibility of the developed dough [52,53]. The elasticity and extensibility are two essential properties required in a balanced proportion for the development of good quality of wheat products like bread, chapatti, cookies, biscuits, and noodles. Consequently, variation in the gluten microstructure and optimised dough properties due to addition of individual gliadin demonstrates their key role in preparation of quality wheat products [54].
4. Conclusions
This study provides a new insight into the role of selected microbial strains in wheat gluten protein hydrolysis and a possible reduction in immunogenicity during sourdough fermentation. The Lactobacillus brevis and Pediococcus pentosaceus fermentations resulted the degradation of pure gluten, as well as wheat wholemeal-flour gluten, significantly reducing the content of toxic gluten protein subunits in the fermented products. As a result of US pretreatment, proteolytic enzyme activities and the metabolic performance of tested microorganisms were promoted. The interaction between sourdough yeast and LABs more effectively degraded gluten, primarily by breaking down gliadin and hydrolysing glutenin peptides. A comprehensive approach to gluten detoxification in wholemeal-wheat flour products—combining US treatment with a 24 h fermentation using proteolytic Lactobacillus brevis FST140 strain and S. cerevisiae yeast—effectively reduced gliadin content up to 1.6 mg/g and lowered the overall gluten levels, meeting the regulatory threshold for low-gluten products. Up to 30% of this fermented product can be incorporated into gluten-free or low-gluten baked goods made from millet, corn, rice, buckwheat, or oat flour.
These findings underscore the impact of ultrasound-assisted sourdough fermentation on whole-wheat flour gluten, providing a foundation for the development of hypoallergenic, low-gluten products using a multi-strain starter culture. The results are expected to be of significant interest to both the broader research community and the food industry, providing new insights and establishing a theoretical foundation for future studies. The future efforts should be focused on targeted processes and optimally selected sourdough lactic acid bacteria and yeast, depending on the functional/nutritional characteristics of the raw material and those desired in the food product.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/fermentation11050238/s1, Figure S1: The effect of ultrasonication on gluten gliadin fraction molecular mass distribution; Figure S2: The effect of LAB fermentation on gluten gliadin fraction molecular mass distribution; Figure S3: The effect of ultrasound and LAB fermentation on gluten gliadin fraction molecular mass distribution; Figure S4: The electrophoretic gel of glutenins.
Author Contributions
Conceptualization, D.Z. and D.C.; methodology, validation, D.Z. and D.C.; formal analysis, investigation, K.C.; data curation, D.Z.; writing—original draft preparation, D.Z.; writing—review and editing, D.Z. and D.C.; supervision, D.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Materials. Further inquiries can be directed to the corresponding author.
Acknowledgments
During the preparation of this manuscript, the authors used ChatGPT (https://chatgpt.com/) for the checking of English style. The authors have reviewed and edited the output and take full responsibility for the content of this publication.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Gasparre, N.; Rosell, C.M. Wheat gluten: A functional protein still challenging to replace in gluten-free cereal-based foods. Cereal Chem. 2023, 100, 243–255. [Google Scholar] [CrossRef]
- Fasano, A.; Berti, I.; Gerarduzzi, T.; Not, T.; Colletti, R.B.; Drago, S.; Elitsur, Y.; Green, P.H.; Guandalini, S.; Hill, I.D.; et al. Prevalence of celiac disease in at-risk and not-at-risk groups in the United States: A large multicenter study. Arch. Intern. Med. 2012, 163, 286–292. [Google Scholar] [CrossRef] [PubMed]
- Siddiqi, R.A.; Singh, T.P.; Rani, M.; Sogi, D.S. Electrophoretic characterization and proportion of different protein fractions in wheat cultivars of North-India. J. Agric. Food Res. 2021, 4, 100137. [Google Scholar] [CrossRef]
- Lee, J.; Kim, S.R.; Park, J.H.; Park, K.H.; Jeong, K.Y.; Lee, J.H.; Kang, C.S.; Kim, K.H.; Park, J.W. Evaluation of allergenicity on a ω-5 gliadin-deficient cultivar in wheat-dependent exercise-induced anaphylaxis. Allergy Asthma Immunol. Res. 2022, 14, 379–392. [Google Scholar] [CrossRef] [PubMed]
- Dewar, D.H.; Amato, M.; Ellis, H.J.; Pollock, E.L.; Gonzalez-Cinca, N.; Wieser, H.; Ciclitira, P.J. The toxicity of high molecular weight glutenin subunits of wheat to patients with coceliac disease. Eur. J. Gastroenterol. Hepatol. 2006, 18, 483–491. [Google Scholar] [CrossRef]
- Conte, P.; Fadda, C.; Drabińska, N.; Krupa-Kozak, U. Technological and nutritional challenges, and novelty in gluten-free breadmaking: A review. Pol. J. Food Nutr. Sci. 2019, 69, 5–21. [Google Scholar] [CrossRef]
- Di Cagno, R.; De Angelis, M.; Auricchio, S.; Greco, L.; Clarke, C.; De Vincenzi, M.; Giovannini, C.; D’Archivio, M.; Landolfo, F.; Parrilli, G.; et al. Sourdough bread made from wheat and nontoxic flours and started with selected lactobacilli is tolerated in celiac sprue patients. Appl. Environ. Microbiol. 2010, 76, 1088–1096. [Google Scholar] [CrossRef]
- Rizzello, C.G.; Curiel, J.A.; Nionelli, L.; Vincentini, O.; Di Cagno, R.; Silano, M.; Gobbetti, M.; Coda, R. Use of fungal proteases and selected sourdough lactic acid bacteria for making wheat bread with an intermediate content of gluten. Food Microbiol. 2014, 37, 59–68. [Google Scholar] [CrossRef]
- Arora, K.; Ameur, H.; Polo, A.; Di Cagno, R.; Rizzello, C.G.; Gobbetti, M. Thirty years of knowledge on sourdough fermentation: A systematic review. Trends Food Sci. Technol. 2021, 108, 71–83. [Google Scholar] [CrossRef]
- Leszczyńska, J.; Szczepankowska, A.K.; Majak, I.; Mańkowska, D.; Smolińska, B.; Ścieszka, S.; Diowksz, A.; Cukrowska, B.; Aleksandrzak-Piekarczyk, T. Reducing immunoreactivity of gluten peptides by probiotic lactic acid bacteria for dietary management of gluten-related diseases. Nutrients 2024, 16, 976. [Google Scholar] [CrossRef]
- Fu, W.; Jia, X.; Liu, C.; Meng, X.; Zhang, K.; Tao, S.; Xue, W. Sourdough yeast-bacteria interactions results in reduced immunogenicity by increasing depolymerization and hydrolysis of gluten. Innov. Food Sci. Emerg. Technol. 2023, 84, 103281. [Google Scholar] [CrossRef]
- Režek Jambrak, A.; Da Cruz, A.G.; Chen, J. Ultrasonic food processing as a green and sustainable technology. Ultrason. Sonochem. 2024, 106, 106880. [Google Scholar] [CrossRef] [PubMed]
- Singla, M.; Sit, N. Application of ultrasound in combination with other technologies in food processing: A review. Ultrason. Sonochem. 2021, 73, 105506. [Google Scholar] [CrossRef] [PubMed]
- Ojha, K.S.; Mason, T.J.; O’Donnell, C.P.; Kerry, J.P.; Tiwari, B.K. Ultrasound technology for food fermentation applications. Ultrason. Sonochem. 2017, 34, 410–417. [Google Scholar] [CrossRef]
- Gallo, M.; Ferrara, L.; Naviglio, D. Application of ultrasound in food science and technology: A perspective. Foods 2018, 7, 164. [Google Scholar] [CrossRef]
- Bai, J.W.; Li, Z.J.; Wang, L.F.; He, J. Ultrasonic effect on the structure and allergenicity of wheat gluten. J. Cereal Sci. 2016, 69, 138–144. [Google Scholar]
- Huang, G.; Chen, S.; Dai, C.; Sun, L.; Sun, W.; Tang, Y.; Xiong, F.; He, R.; Ma, H. Effects of ultrasound on microbial growth and enzyme activity. Ultrason. Sonochem. 2017, 37, 144–149. [Google Scholar] [CrossRef]
- Chavan, P.; Sharma, P.; Sharma, S.R.; Mittal, T.C.; Jaiswal, A.K. Application of high-intensity ultrasound to improve food processing efficiency: A review. Foods 2022, 11, 122. [Google Scholar] [CrossRef]
- Jukonyte, R.; Zadeike, D.; Bartkiene, E.; Lele, V.; Cernauskas, D.; Suproniene, S.; Juodeikiene, G. A potential of brown rice polish as a substrate for the lactic acid and bioactive compounds production by the lactic acid bacteria newly isolated from cereal-based fermented products. LWT 2018, 97, 323–331. [Google Scholar] [CrossRef]
- Cupp-Enyard, C. Sigma’s Non-specific Protease Activity Assay—Casein as a Substrate. J. Vis. Exp. 2008, 19, e899. [Google Scholar]
- Dhaka, V.; Khatkar, B. Effects of gliadin/glutenin and HMW-GS/LMW-GS ratio on dough rheological properties and bread-making potential of wheat varieties: Gluten proteins, dough rheology and bread quality. J. Food Qual. 2015, 38, 71–82. [Google Scholar] [CrossRef]
- Siddiqi, R.A.; Sogi, D.S.; Sehajpal, P.K. Effect of short-term sourdough fermentation on wheat protein. Cogent Food Agric. 2016, 2, 1132983. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 17th ed.; The Association of Official Analytical Chemists: Gaithersburg, MD, USA, 2018. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Islam, S.; Miah, M.A.S.; Islam, M.F.; Bhuiyan, M.N.I.; Tisa, K.J.; Naim, M.R. Exploring the effects of spontaneous and solid-state fermentation on the physicochemical, functional and structural properties of whole wheat flour (Triticum aestivum L.). Innov. Food Sci. Emerg. Technol. 2024, 97, 103798. [Google Scholar] [CrossRef]
- Fraberger, V.; Ladurner, M.; Nemec, A.; Grunwald-Gruber, C.; Call, L.M.; Hochegger, R.; Domig, K.J.; D’Amico, S. Insights into the potential of sourdough-related lactic acid bacteria to degrade proteins in wheat. Microorganisms 2020, 8, 1689. [Google Scholar] [CrossRef]
- Canesin, M.R.; Cazarin, C.B.B. Nutritional quality and nutrient bioaccessibility in sourdough bread. Curr. Opin. Food Sci. 2021, 40, 81–86. [Google Scholar] [CrossRef]
- Collar, C.; Mascaros, A.F.; Prieto, J.A.; De Barber, C.B. Changes in free amino acids during fermentation of wheat doughs started with pure culture of lactic acid bacteria. Cereal Chem. 1991, 68, 66–72. [Google Scholar]
- Tseng, H.-C.; Yang, C.-Y. Assessment of ultrasonic stress on survival and β-glucosidase activity of encapsulated Lactiplantibacillus plantarum BCRC 10357 in fermentation of black soymilk. Foods 2022, 11, 1234. [Google Scholar] [CrossRef]
- Pitt, W.G.; Ross, S.A. Ultrasound increases the rate of bacterial cell growth. Biotechnol. Prog. 2003, 19, 1038–1044. [Google Scholar] [CrossRef]
- Bolívar-Jacobo, N.A.; Reyes-Villagrana, R.A.; Espino-Solís, G.P.; Rentería-Monterrubio, A.L.; Arévalos-Sánchez, M.M.; Sánchez-Vega, R.; Santellano-Estrada, E.; Chávez-Flores, D.; Chávez-Martínez, A. The Effects of a High-Intensity Ultrasound on the Fermentative Activity and Kinetic Growth of Lactobacillus acidophilus and Lactobacillus helveticus. Fermentation 2023, 9, 356. [Google Scholar] [CrossRef]
- Shokri, S.; Shekarforoush, S.S.; Hosseinzadeh, S. Stimulatory effects of low-intensity ultrasound on the growth kinetics and metabolic activity of Lactococcus lactis subsp. lactis. Process Biochem. 2020, 89, 1–8. [Google Scholar] [CrossRef]
- Yu, Z.; Su, Y.; Zhang, Y.; Zhu, P.; Mei, Z.; Zhou, X.; Yu, H. Potential use of ultrasound to promote fermentation, maturation, and properties of fermented foods: A review. Food Chem. 2021, 357, 129805. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Chen, S.; Tang, Y.; Dai, C.; Sun, L.; Ma, H.; He, R. Stimulation of low-intensity ultrasound on fermentation of skim milk medium for yield of yoghurt peptides by Lactobacillus paracasei. Ultrason. Sonochem. 2019, 51, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, S.M.B.; Jafarpour, D.; Soto, E.R.; Barba, F.J. Ultrasound-assisted lactic acid fermentation of Bakraei (Citrus reticulata cv. Bakraei) juice: Physicochemical and bioactive properties. Fermentation 2023, 9, 37. [Google Scholar] [CrossRef]
- Bradauskiene, V.; Vaiciulyte-Funk, L.; Cernauskas, D.; Dzingeleviciene, R.; Lima, J.P.M.; Bradauskaite, A.; Tita, M.A. The efficacy of plant enzymes bromelain and papain as a tool for reducing gluten immunogenicity from wheat bran. Processes 2022, 10, 1948. [Google Scholar] [CrossRef]
- Zhang, Y.; Ma, H.; Wang, B.; Qu, W.; Li, Y.; He, R.; Wali, A. Effects of ultrasound pretreatment on the enzymolysis and structural characterization of wheat gluten. Food Biophys. 2015, 10, 385–395. [Google Scholar] [CrossRef]
- Marcuzzo, E.; Peressini, D.; Debeaufort, F.; Sensidoni, A. Effect of ultrasound treatment on properties of gluten-based film. Innov. Food Sci. Emerg. Technol. 2010, 11, 451–457. [Google Scholar] [CrossRef]
- Cheng, Y.; Donkor, P.O.; Yeboah, G.B.; Ayim, I.; Wu, J.; Ma, H. Modulating the in vitro digestion of heat-set whey protein emulsion gels via gelling properties modification with sequential ultrasound pretreatment. LWT 2021, 149, 111856. [Google Scholar] [CrossRef]
- Urade, R.; Sato, N.; Masaaki, S. Gliadins from wheat grain: An overview, from primary structure to nanostructures of aggregates. Biophys. Rev. 2018, 10, 435–443. [Google Scholar] [CrossRef]
- Zotta, T.; Piraino, P.; Ricciardi, A.; McSweeney, P.L.; Parente, E. Proteolysis in model sourdough fermentations. J. Agric. Food Chem. 2006, 54, 2567–2574. [Google Scholar] [CrossRef]
- Scherf, K.A.; Koehler, P.; Wieser, H. Gluten and wheat sensitivities. J. Cereal Sci. 2016, 67, 2–11. [Google Scholar] [CrossRef]
- Wieser, H.; Koehler, P.; Scherf, K.A. Chemistry of gluten proteins: Quantitative composition. Cereal Chem. 2023, 100, 36–55. [Google Scholar] [CrossRef]
- Nutter, J.; Saiz, A.I.; Iurlina, M.O. Microstructural and conformational changes of gluten proteins in wheat-rye sourdough. J. Cereal Sci. 2019, 87, 91–97. [Google Scholar] [CrossRef]
- Silva, N.H.C.S.; Vilela, C.; Marrucho, I.M.; Freire, C.S.R.; Neto, C.P.; Silvestre, A.J.D. Protein-based materials: From sources to innovative sustainable materials for biomedical applications. J. Mater. Chem. B 2014, 2, 3715–3740. [Google Scholar] [CrossRef]
- Loponen, J.; Mikola, M.; Katina, K.; Sontag-Strohm, T.; Salovaara, H. Degradation of HMW glutenins during wheat sourdough fermentations. Cereal Chem. 2004, 81, 87–93. [Google Scholar] [CrossRef]
- Dvoraček, V.; Čurn, V. Evaluation of protein fractions as biochemical markers for identification of spelt wheat cultivars (Triticum spelta L.). Plant Soil Environ. 2003, 49, 99–105. [Google Scholar] [CrossRef]
- Rizzello, C.G.; De Angelis, M.; Di Cagno, R.; Camarca, A.; Silano, M.; Losito, I.; De Vincenzi, M.; De Bari, M.D.; Palmisano, F.; Maurano, F.; et al. Highly efficient gluten degradation by lactobacilli and fungal proteases during food processing: New perspectives for celiac disease. Appl. Environ. Microbiol. 2007, 73, 4499–4507. [Google Scholar] [CrossRef]
- Pronin, D.; Geisslitz, S.; Börner, A.; Scherf, K.A. Fingerprinting of wheat protein profiles for improved distinction between wheat cultivars and species. Cereal Chem. 2020, 97, 999–1009. [Google Scholar] [CrossRef]
- Xu, D.; Zhang, Y.; Tang, K.; Hu, Y.; Xu, X.; Gänzle, M.G. Effect of mixed cultures of yeast and lactobacilli on the quality of wheat sourdough bread. Front. Microbiol. 2019, 10, 2113. [Google Scholar] [CrossRef]
- Luo, D.; Wu, R.; Zhang, J.; Zhang, K.; Xu, B.; Li, P.; Yuan, Y.; Li, X. Effects of ultrasound-assisted dough fermentation on the quality of steamed bread. J. Cereal Sci. 2018, 83, 147–152. [Google Scholar] [CrossRef]
- Kłosok, K.; Welc, R.; Fornal, E.; Nawrocka, A. Effects of physical and chemical factors on the structure of gluten, gliadins and glutenins as studied with spectroscopic methods. Molecules 2021, 26, 508. [Google Scholar] [CrossRef] [PubMed]
- Dangi, P.; Chaudhary, N.; Khatkar, B.S. Rheological and microstructural characteristics of low molecular weight glutenin subunits of commercial wheats. Food Chem. 2019, 297, 124989. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, J.; Li, L.; Xiang, L.; Zhao, L.; Liu, J.; Liu, S.; Yang, Q.; Wu, J.; Chen, X. Effect of gliadin from Psathrostachys huashanica on dough rheological properties and biscuit quality. Food Chem. 2023, 425, 136537. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).