Purification and Functional Characterization of a New Endoglucanase from Pleurotus djamor PLO13 Produced by Solid-State Fermentation of Agro-Industrial Waste
Abstract
1. Introduction
2. Materials and Methods
2.1. Obtaining and Preparing Waste
2.2. Preparing the Inoculum
2.3. Production of Endoglucanase by Solid State Fermentation
2.4. Enzyme Extraction
2.5. Endoglucanase Activity
2.6. Purification of Endoglucanase
2.7. Protein Concentration
2.8. Electrophoresis (SDS-PAGE)
2.9. Characterization of Endoglucanase
3. Results and Discussion
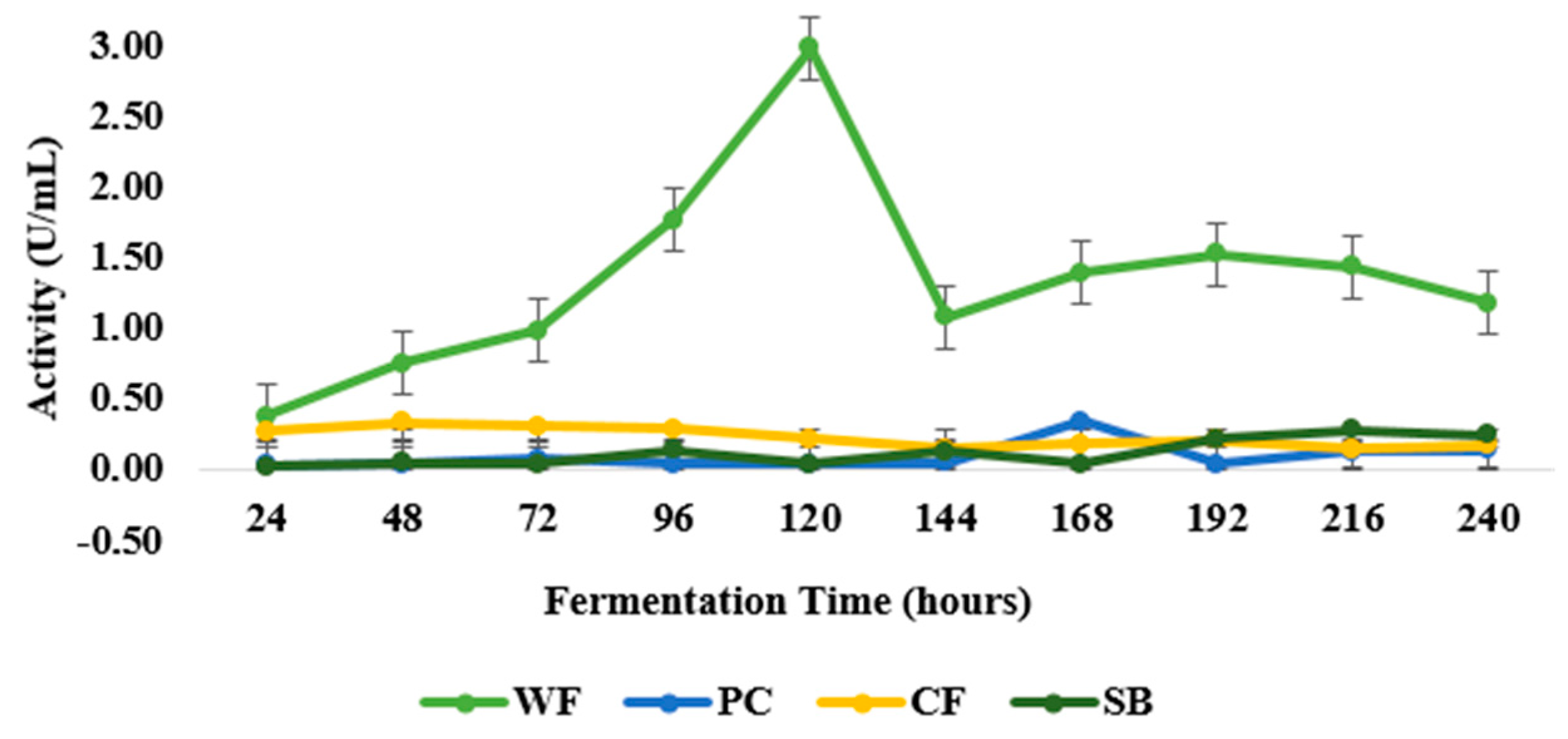
3.1. Physicochemical Analysis, Selection of Lignocellulosic Waste, and Fermentation Profile
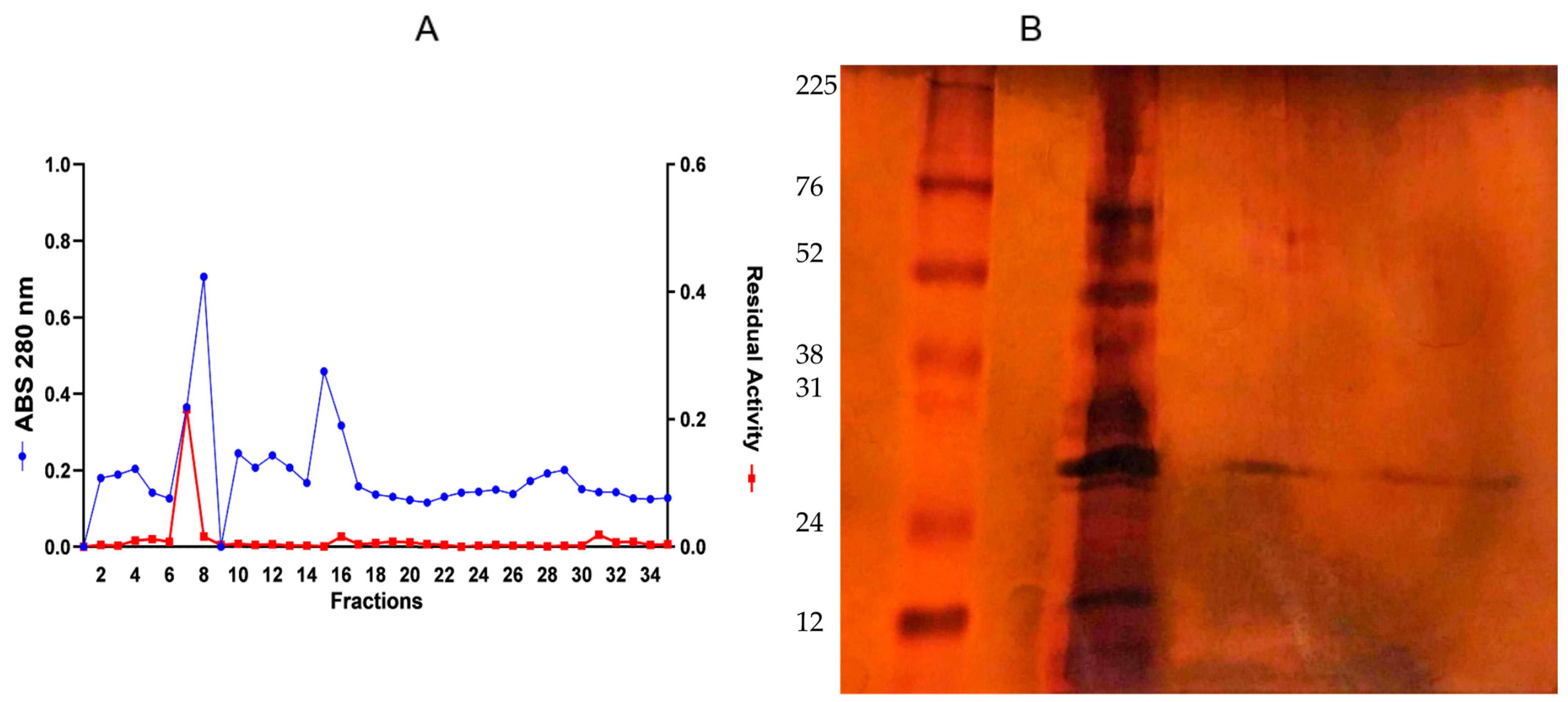
3.2. Ethanol Precipitation of Endoglucanase
3.3. Enzyme Purification: Ion-Exchange Chromatography on DEAE-Sepharose
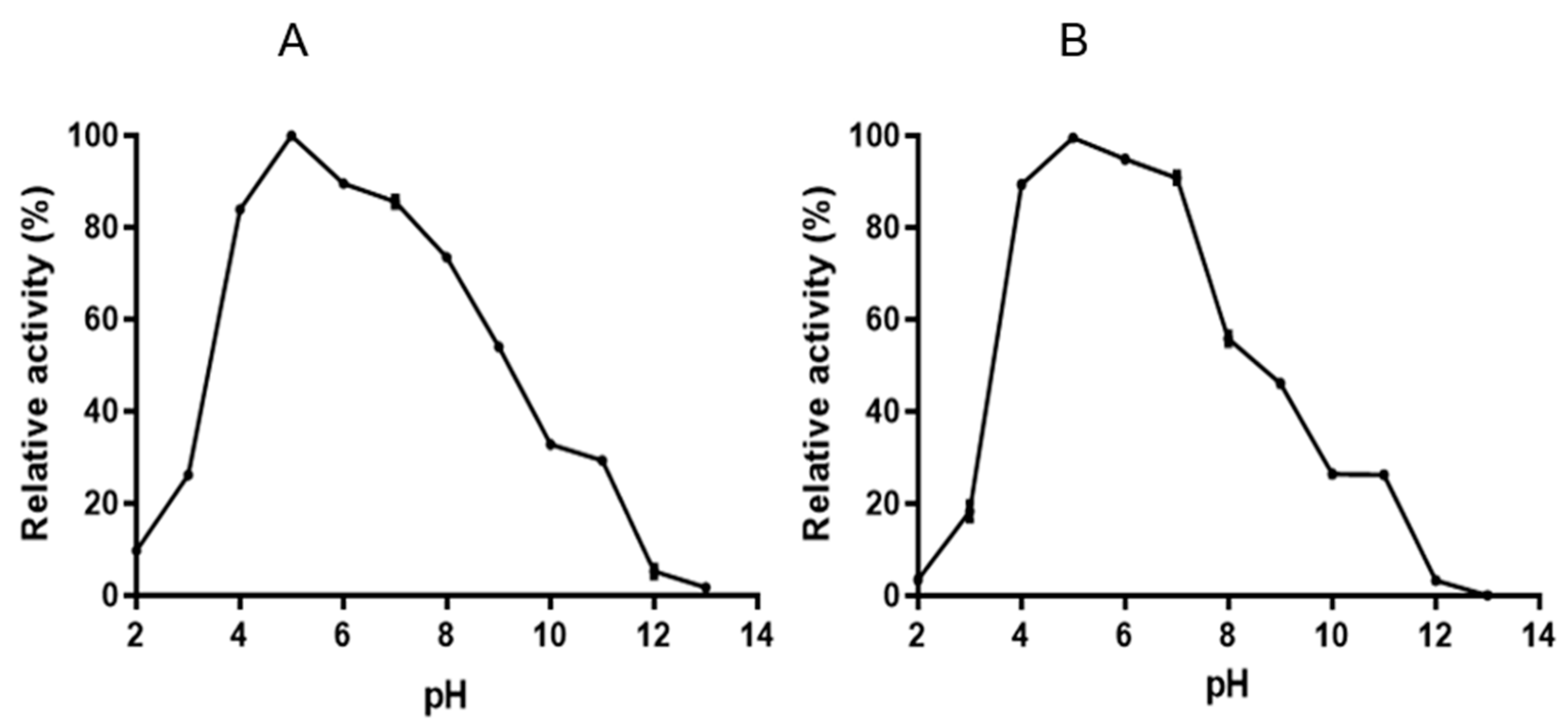
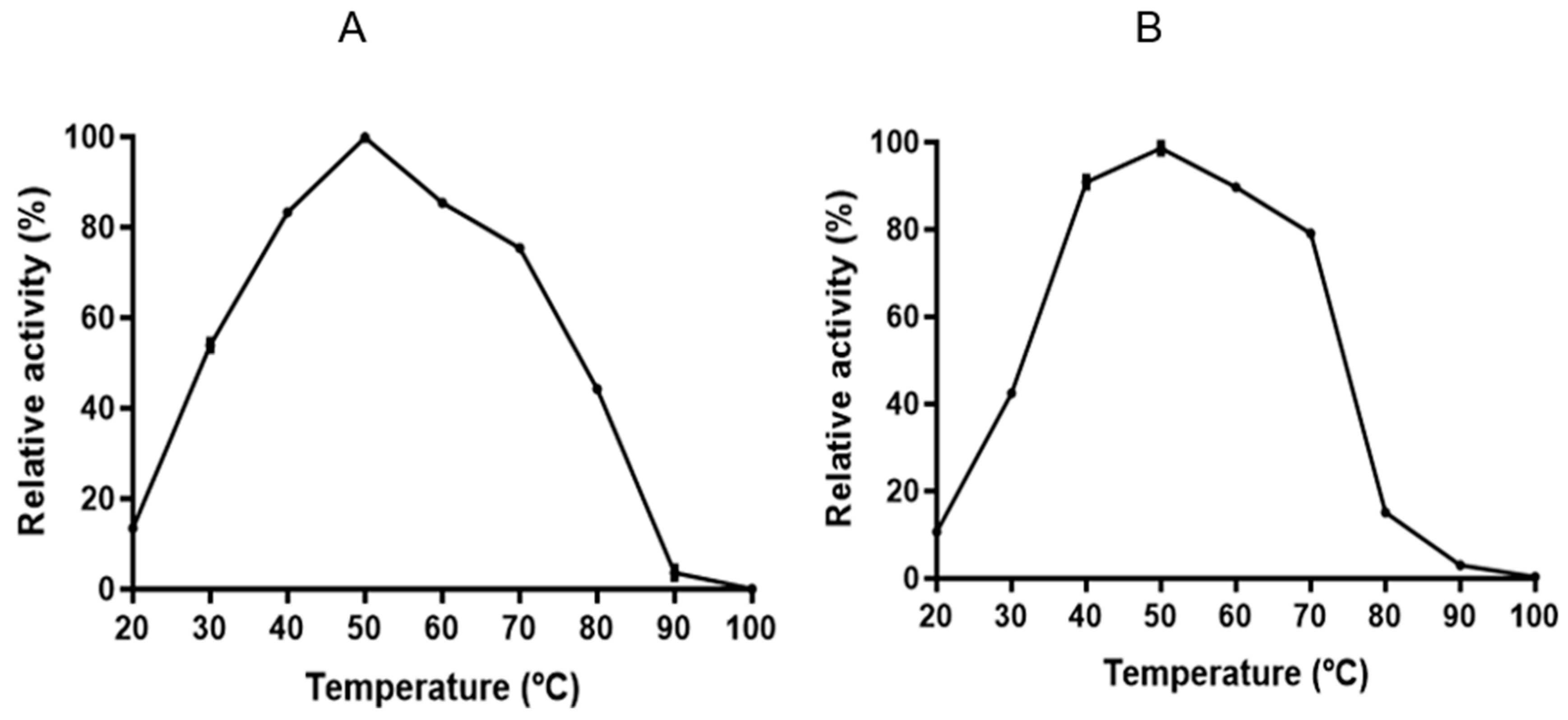
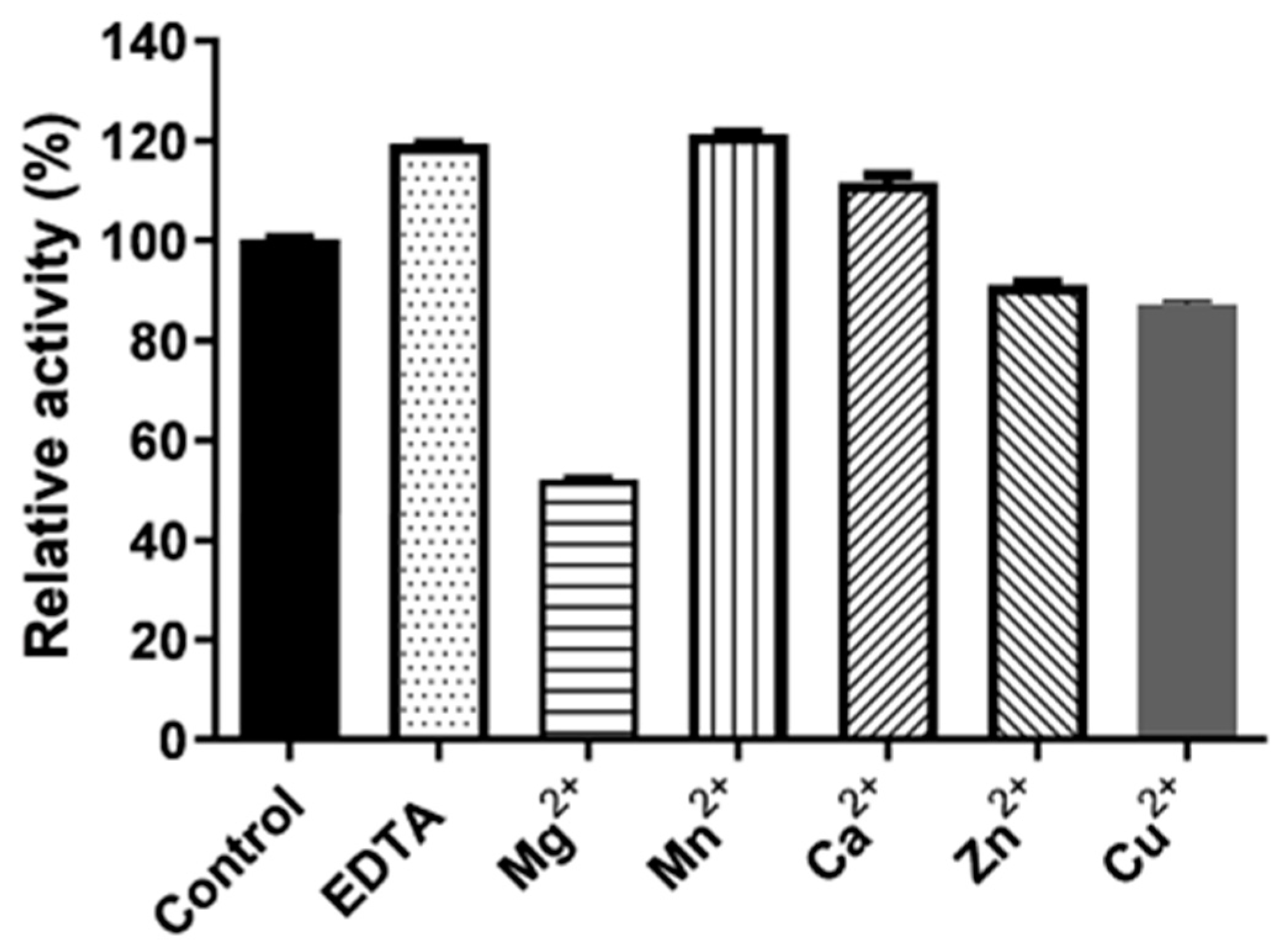
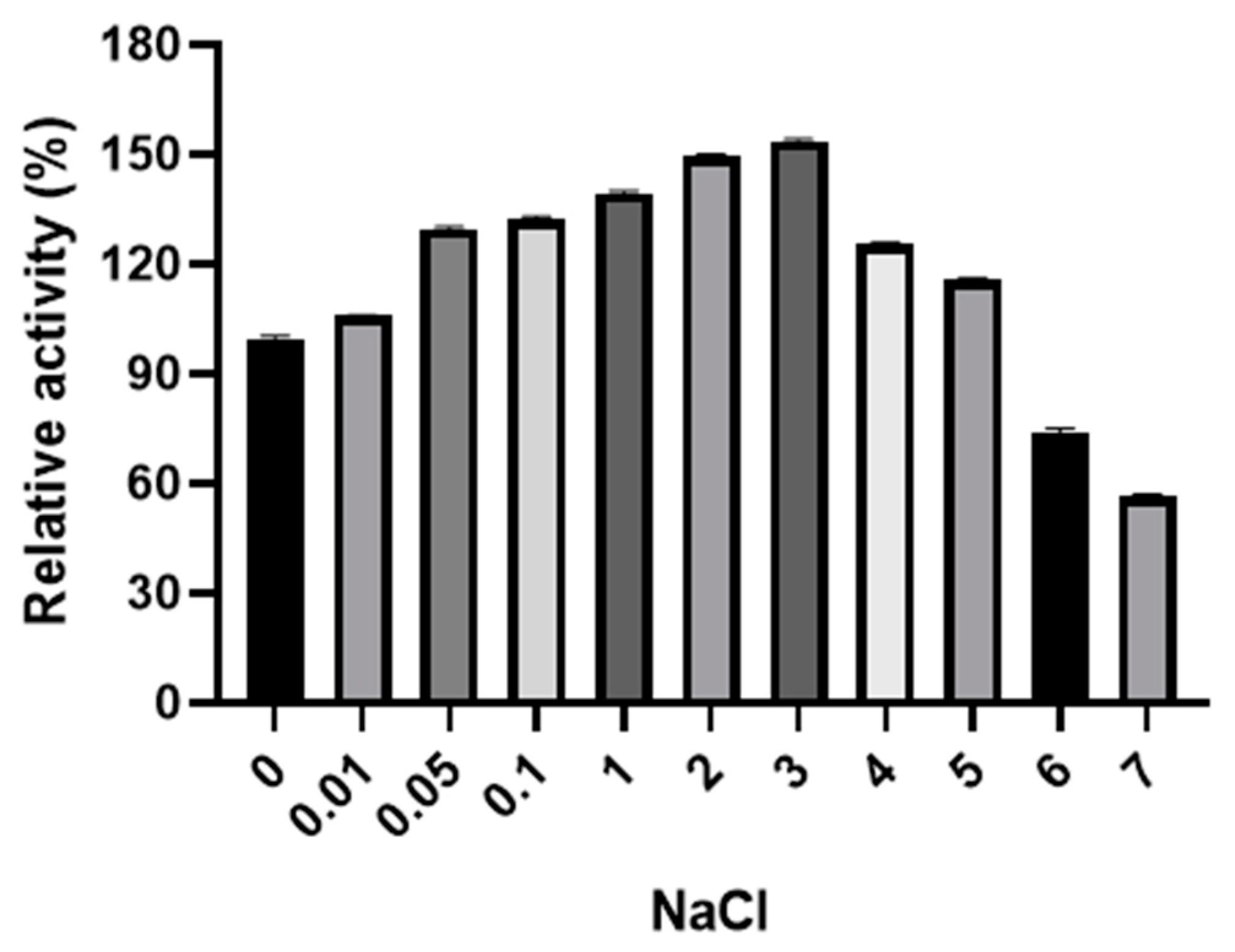
3.4. Characterization of Endoglucanase
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- de Carvalho, M.S.; de Menezes, L.H.S.; Pimentel, A.B.; Costa, F.S.; Oliveira, P.C.; dos Santos, M.M.O.; de Carvalho Tavares, I.M.; Irfan, M.; Bilal, M.; Dias, J.C.T.; et al. Application of chemometric methods for the optimization secretion of xylanase by Aspergillus oryzae in solid state fermentation and its application in the saccharification of agro-industrial waste. Waste Biomass Valorization 2023, 14, 3183–3193. [Google Scholar] [CrossRef]
- Leite, P.; Sousa, D.; Fernandes, H.; Ferreira, M.; Costa, A.R.; Filipe, D.; Gonçalves, M.; Peres, H.; Belo, I.; Salgado, J.M. Recent advances in production of lignocellulolytic enzymes by solid-state fermentation of agro-industrial wastes. Curr. Opin. Green Sustain. Chem. 2021, 27, 100407. [Google Scholar]
- Cavalcante, P.A.W.; Coêlho, D.F.; Silva, C.F.; de Souza Abud, A.K.; Souza, R.R. Utilização de resíduos lignocelulósicos na produção de celulases por Aspergillus niger em fermentação em estado sólido. Sci. Plena 2018, 14. [Google Scholar] [CrossRef]
- Ferreira, A.N.; Da Silva, A.T.; Nascimento, J.S.D.; Souza, C.B.D.; Silva, M.D.C.; Grillo, L.A.M.; da Luz, J.M.R.; Pereira, H.J.V. Production, characterization, and application of a new chymotrypsin-like protease from Pycnoporus sanguineus. Biocatal. Biotransform. 2023, 42, 324–333. [Google Scholar]
- dos Santos Gomes, M.M.O.; Nicodemos, I.S.; da Costa Silva, M.; dos Santos Martins, T.V.; de FreitasGonçalves, J.M.D.M.S.; Meneghetti, S.M.P.; Gomes, F.S.; Pereira, H.J.V. Re-recycling agro-industrial waste: Exploiting activated carbon from cocoa shells after solid-state fermentation as a support for endoglucanase immobilization. Biomass Convers. Biorefin. 2024. [Google Scholar] [CrossRef]
- Prasad, B.R.; Padhi, R.K.; Ghosh, G. A review on key pretreatment approaches for lignocellulosic biomass to produce biofuel and value-added products. Int. J. Environ. Sci. Technol. 2023, 20, 6929–6944. [Google Scholar]
- Durmaz, E.; Sertkaya, S.; Yilmaz, H.; Olgun, C.; Ozcelik, O.; Tozluoglu, A.; Candan, Z. Lignocellulosic bionanomaterials for biosensor applications. Micromachines 2023, 14, 1450. [Google Scholar] [CrossRef]
- dos Santos, D.M.R.C.; Albuquerque, F.; Silva, T.P.; Ferreira, A.N.; Machado, S.S.; da Luze, J.M.R.; Pereira, H.J.V. Production, Purification, Characterization, and Application of Halotolerant and Thermostable Endoglucanase Isolated from Pycnoporus sanguineus. Waste Biomass Valorization 2023, 14, 3211–3222. [Google Scholar]
- Sohail, M.; Barzkar, N.; Michaud, P.; Jahromi, S.T.; Babich, O.; Sukhikh, S.; Das, R.; Nahavandi, R. Cellulolytic and xylanolytic enzymes from yeasts: Properties and industrial applications. Molecules 2022, 27, 3783. [Google Scholar] [CrossRef]
- Silva, T.P.; de Albuquerque, F.S.; Ferreira, A.N.; dos Santos, D.M.R.C.; dos Santos, T.V.; Meneghetti, S.M.P.; Franco, M.; da Luz, J.M.R.; Pereira, H.J.V. for enhancing the enzymatic saccharification of agro-residues using a Botrytis ricini endoglucanase. Biotechnol. Appl. Biochem. 2022, 70, 184–192. [Google Scholar]
- Fernandes, L.M.G.; de Carvalho-Silva, J.; Ferreira-Santos, P.; Porto, A.L.F.; Converti, A.; da Cunha, M.N.C.; Porto, T.S. Valorization of agro-industrial residues using Aspergillus heteromorphus URM0269 for protease production: Characterization and purification. Int. J. Biol. Macromol. 2024, 273, 133199. [Google Scholar]
- Bahkali, A.H.; Syed, A.; Elgorban, A.M.; Abdel-Wahab, M.A.; Srivastava, N.; Gupta, V.K. Novel strategy to elevate solid state fermentation to produce alkilophilic endoglucanase using date waste feedstocks and peapod extract based nutrient media and expired probiotic strain: Application in fermentable sugar production. Process Saf. Environ. Prot. 2024, 183, 580–586. [Google Scholar] [CrossRef]
- Costa Silva, M.D.; Costa, R.B.; do Nascimento, J.S.; Gomes, M.M.O.D.S.; Ferreira, A.N.; Grillo, L.A.M.; da Luz, J.M.R.; Pereira, H.J.V. Production of milk-coagulating protease by fungus Pleurotus djamor through solid state fermentation using wheat bran as the low-cost substrate. Prep. Biochem. Biotechnol. 2024, 55, 278–284. [Google Scholar] [CrossRef]
- Liu, X.; Kokare, C. Microbial enzymes of use in industry. In Biotechnology of Microbial Enzymes; Academic Press: New York, NY, USA, 2023; pp. 405–444. [Google Scholar]
- Rigo, D.; Gayeski, L.; Tres, G.A.; Camera, F.D.; Zeni, J.; Valduga, E.; Backes, G.T. Produção microbiológica de enzimas: Uma revisão. Braz. J. Dev. 2021, 7, 9232–9254. [Google Scholar] [CrossRef]
- Dulf, F.V.; Vodnar, D.C.; Dulf, E.-H. Solid-state fermentation with Zygomycetes fungi as a tool for biofortification of apple pomace with γ-linolenic acid, carotenoid pigments and phenolic antioxidants. Food Res. Int. 2023, 173, 113448. [Google Scholar] [CrossRef]
- Lopes, L.S.; Vieira, N.; da Luz, J.M.R.; Marliane de Cássia, S.S.; Cardoso, W.S.; Kasuya, M.C.M. Production of fungal enzymes in Macaúba coconut and enzymatic degradation of textile dye. Biocatal. Agric. Biotechnol. 2020, 26, 101651. [Google Scholar] [CrossRef]
- Velez, M.E.V.; da Luz, J.M.R.; Silva, M.d.C.S.d.; Cardoso, W.S.; Lopes, L.d.S.; Vieira, N.A.; Kasuya, M.C.M. Production of bioactive compounds by the mycelial growth of Pleurotus djamor in whey powder enriched with selenium. LWT 2019, 114, 108376. [Google Scholar] [CrossRef]
- Yang, S.; Arslan-Tontul, S.; Fogliano, V.; Casertano, M.; Fan, W.; Xu, Y.; Nie, Y.; Vilas-Franquesa, A. Upcycling of melanoidin-rich Chinese distilled spent grain through solid-state fermentation by Aspergillus awamori. Bioresour. Technol. 2025, 416, 131817. [Google Scholar] [CrossRef]
- Kant, S.; Das, S.; Roy, S.; Tripathy, S. Fungal cellulases: A comprehensive review. Nucleus 2024, 416, 131817. [Google Scholar] [CrossRef]
- Silva, T.P.; Ferreira, A.N.; de Albuquerque, F.S.; Barros, A.C.d.A.; da Luz, J.M.R.; Gomes, F.S.; Pereira, H.J.V. Box–Behnken experimental design for the optimization of enzymatic saccharification of wheat bran. Biomass Convers. Biorefin. 2021, 12, 5597–5604. [Google Scholar] [CrossRef]
- Liu, D.; Zhang, R.; Yang, X.; Xu, Y.; Tang, Z.; Tian, W.; Shen, Q. Expression, purification and characterization of two thermostable endoglucanases cloned from a lignocellulosic decomposing fungi Aspergillus fumigatus Z5 isolated from compost. Protein Expr. Purif. 2011, 79, 176–186. [Google Scholar] [PubMed]
- Detoni, B.; Delai, V.M.; da Silva, W.J.; Kadowaki, M.K.; Silva, J.L.d.C.; Simão, R.d.C.G.; Bifano, T.D.; Simões, M.R.; Maller, A. Production and partial characterization of endoglucanase by Thermothelomyces heterothallicus PA2S4T and its application in biopolishing of denim jeans. Cellulose 2024, 31, 6989–7001. [Google Scholar]
- Sutaoney, P.; Rai, S.N.; Sinha, S.; Choudhary, R.; Gupta, A.; Singh, S.K.; Banerjee, P. Current perspective in research and industrial applications of microbial cellulases. Int. J. Biol. Macromol. 2024, 264, 130639. [Google Scholar]
- Zheng, F.; Basit, A.; Wang, J.; Zhuang, H.; Chen, J.; Zhang, J. Characterization of a novel acidophilic, ethanol tolerant and halophilic GH12 β-1, 4-endoglucanase from Trichoderma asperellum ND-1 and its synergistic hydrolysis of lignocellulosic biomass. Int. J. Biol. Macromol. 2024, 254, 127650. [Google Scholar]
- Dixit, M.; Shukla, P. Multi-efficient endoglucanase from Aspergillus niger MPS25 and its potential applications in saccharification of wheat straw and waste paper deinking. Chemosphere 2023, 313, 137298. [Google Scholar]
- Arantes, V.; Las-Casas, B.; Dias, I.K.; Yupanqui-Mendoza, S.L.; Nogueira, C.F.; Marcondes, W.F. Enzymatic approaches for diversifying bioproducts from cellulosic biomass. Chem. Commun. 2024, 60, 9704–9732. [Google Scholar]
- Singh, G.; Sahu, S.; Bharti, S.; Arya, S.K. Significance of enzymes for the recycling of wasted non-food biomass to value added products: A sustainable stewardship towards the cleaner environment. Process Saf. Environ. Prot. 2024, 190, 395–412. [Google Scholar]
- Ibrahim, A.M.; Hamouda, R.A.; El-Naggar, N.E.-A.; Al-Shakankery, F.M. Bioprocess development for enhanced endoglucanase production by newly isolated bacteria, purification, characterization and in-vitro efficacy as anti-biofilm of Pseudomonas aeruginosa. Sci. Rep. 2021, 11, 9754. [Google Scholar]
- Chaudhary, N.; Grover, M. Bioindustrial applications of thermostable Endoglucanase purified from Trichoderma viride towards the conversion of agrowastes to value-added products. Protein Expr. Purif. 2023, 211, 106324. [Google Scholar]
- Nargotra, P.; Sharma, V.; Sharma, S.; Bangotra, R.; Bajaj, B.K. Purification of an ionic liquid stable cellulase from Aspergillus aculeatus PN14 with potential for biomass refining. Environ. Sustain. 2022, 5, 313–323. [Google Scholar]
- AOAC—Association of Official Analytical Chemistry. Official Methods of Analysis, 16th ed.; AOAC International: Arlington, MA, USA, 1995. [Google Scholar]
- Miller, G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar]
- Ázar, R.I.L.; Morgan, T.; Barbosa, M.H.; Guimarães, V.M.; Ximenes, E.; Ladisch, M. Impact of protein blocking on enzymatic saccharification of bagasse from sugarcane clones. Biotechnol. Bioeng. 2019, 116, 1584–1593. [Google Scholar]
- Mamo, J.; Getachew, P.; Kuria, M.S.; Assefa, F. Application of Milk-Clotting Protease from Aspergillus oryzae DRDFS13 MN726447 and Bacillus subtilis SMDFS 2B MN715837 for Danbo Cheese Production. J. Food Qual. 2020, 2020, 8869010. [Google Scholar]
- Tanasković, S.J.; Šekuljica, N.; Jovanović, J.; Gazikalović, I.; Grbavčić, S.; Đorđević, N.; Sekulić, M.V.; Hao, J.; Luković, N.; Knežević-Jugović, Z. Upgrading of valuable food component contents and anti-nutritional factors depletion by solid-state fermentation: A way to valorize wheat bran for nutrition. J. Cereal Sci. 2021, 99, 103159. [Google Scholar]
- Gomathi, D.; Muthulakshmi, C.; Kumar, D.G.; Ravikumar, G.; Kalaiselvi, M.; Uma, C. Submerged fermentation of wheat bran by Aspergillus flavus for production and characterization of carboxy methyl cellulase. Asian Pac. J. Trop. Biomed. 2012, 2, S67–S73. [Google Scholar]
- Houfani, A.A.; Anders, N.; Spiess, A.C.; Baldrian, P.; Benallaoua, S. Insights from enzymatic degradation of cellulose and hemicellulose to fermentable sugars—A review. Biomass Bioenergy 2020, 134, 105481. [Google Scholar]
- AlGhanimi, A.A.J.; AlEbadi, S.M.A.; Al-Ethari, A.Y.H. Partial purification and characterization of protease from local isolate of Beuveria bassiana. Sci. J. Med. Res. 2020, 4, 17–22. [Google Scholar]
- Paul, T.; Jana, A.; Mandal, A.K.; Mandal, A.; Mohpatra, P.K.D.; Mondal, K.C. Bacterial keratinolytic protease, imminent starter for Next Gen leather and detergent industries. Sustain. Chem. Pharm. 2016, 3, 8–22. [Google Scholar]
- Farinas, C.S.; Scarpelini, L.M.; Miranda, E.A.; Neto, V.B. Evaluation of operational parameters on the precipitation of endoglucanase and xylanase produced by solid state fermentation of Aspergillus niger. Braz. J. Chem. Eng. 2011, 28, 17–26. [Google Scholar] [CrossRef]
- Narra, M.; Dixit, G.; Divecha, J.; Kumar, K.; Madamwar, D.; Shah, A.R. Production, purification and characterization of a novel GH 12 family endoglucanase from Aspergillus terreus and its application in enzymatic degradation of delignified rice straw. Int. Biodeterior. Biodegrad. 2014, 88, 150–161. [Google Scholar]
- Silva, T.P.; de Albuquerque, F.S.; Dos Santos, C.W.V.; Franco, M.; Caetano, L.C.; Pereira, H.J.V. Production, purification, characterization and application of a new halotolerant and thermostable endoglucanase of Botrytis ricini URM 5627. Bioresour. Technol. 2018, 270, 263–269. [Google Scholar]
- Liang, L.; Xue, D. Kinetics of cellulose hydrolysis by halostable cellulase from a marine Aspergillus niger at different salinities. Process Biochem. 2017, 63, 163–168. [Google Scholar]
- Patel, A.; Shah, A. Purification and characterization of novel, thermostable and non-processive GH5 family endoglucanase from Fomitopsis meliae CFA. Int. J. Biol. Macromol. 2021, 182, 1161–1169. [Google Scholar]
- Ding, S.; Ge, W.; Buswell, J.A. Cloning of multiple cellulase cDNAs from Volvariella volvacea and their differential expression during substrate colonization and fruiting. FEMS Microbiol. Lett. 2006, 263, 207–213. [Google Scholar]
- Ben Hmad, I.; Boudabbous, M.; Belghith, H.; Gargouri, A. A novel ionic liquid-stable halophilic endoglucanase from Stachybotrys microspora. Process Biochem. 2017, 54, 59–66. [Google Scholar]
- Asha, P.; Divya, J.; Singh, I.B. Purification and characterisation of processive-type endoglucanase and β-glucosidase from Aspergillus ochraceus MTCC 1810 through saccharification of delignified coir pith to glucose. Bioresour. Technol. 2016, 213, 245–248. [Google Scholar]
- Ain, Q.U.; Hussain, M.A.; Mahmood, R.T.; Muzaffar, S.; Islam, A.; Khan, J. Production, Purification and Characterization of Endoglucanase from Locally Isolated Aspergillus flavus: Production of Endoglucanase by Aspergillus flavus. Biol. Sci. PJSIR 2022, 65, 235–245. [Google Scholar]
- Akram, F.; Haq, I.U.; Imran, W.; Mukhtar, H. Insight perspectives of thermostable endoglucanases for bioethanol production: A review. Renew. Energy 2018, 122, 225–238. [Google Scholar]
- Haq, I.U.; Akram, F.; Khan, M.A.; Hussain, Z.; Nawaz, A.; Iqbal, K.; Shah, A.J. CenC, a multidomain thermostable GH9 processive endoglucanase from Clostridium thermocellum: Cloning, characterization and saccharification studies. World J. Microbiol. Biotechnol. 2015, 31, 1699–1710. [Google Scholar]
- Wolf, K.; Gilbert, A. EDTA—Ethylenediaminetetraacetic acid. In Detergents; Springer: Berlin/Heidelberg, Germany, 1992; pp. 243–259. [Google Scholar]
- Pham, T.H.; Quyen, D.T.; Nghiem, N.M. Purification and properties of an endoglucanase from Aspergillus niger VTCC-F021. Turk. J. Biol. 2012, 36, 694–701. [Google Scholar]
- Naika, G.S.; Tiku, P.K. Influence of ethylenediaminetetraacetic acid (EDTA) on the structural stability of endoglucanase from Aspergillus aculeatus. J. Agric. Food Chem. 2011, 59, 7341–7345. [Google Scholar] [PubMed]
- Marques, G.L.; Reis, N.d.S.; Silva, T.P.; Ferreira, M.L.O.; Aguiar-Oliveira, E.; de Oliveira, J.R.; Franco, M. Production and characterisation of xylanase and endoglucanases produced by Penicillium roqueforti ATCC 10110 through the solid-state fermentation of rice husk residue. Waste Biomass Valorization 2018, 9, 2061–2069. [Google Scholar]
- Gunde-Cimerman, N.; Zalar, P. Extremely halotolerant and halophilic fungi inhabit brine in solar salterns around the globe. Food Technol. Biotechnol. 2014, 52, 170–179. [Google Scholar]
- Xu, J.; He, B.; Wu, B.; Wang, B.; Wang, C.; Hu, L. An ionic liquid tolerant cellulase derived from chemically polluted microhabitats and its application in in situ saccharification of rice straw. Bioresour. Technol. 2014, 157, 166–173. [Google Scholar]
- Hua, C.; Li, W.; Han, W.; Wang, Q.; Bi, P.; Han, C.; Zhu, L. Characterization of a novel thermostable GH7 endoglucanase from Chaetomium thermophilum capable of xylan hydrolysis. Int. J. Biol. Macromol. 2018, 117, 342–349. [Google Scholar]
- Bettache, A.; Estelle, C.; Azzouz, Z.; Boucherba, N.; Bouiche, C.; Hamma, S.; Maibeche, R.; Duchiron, F.; Benallaoua, S. Purification and characterization of an endoglucanase produced from Streptomyces sp. strainbpng23. J. Microbiol. Biotechnol. Food Sci. 2020, 10, 284–288. [Google Scholar]
| Components | Wheat Bran (WF) | Coconut Fiber (CF) | Sugarcane Bagasse (SB) | Pineapple Crown (PC) |
|---|---|---|---|---|
| Crude protein | 19.46 ± 0.53 | 3.98 ± 0.62 | 2.67 ± 0.09 | 7.77 ± 0.21 |
| NDF | 51.93 ± 1.94 | 79.09 ± 0.49 | 95.8 ± 0.12 | 89.38 ± 0.17 |
| FDA | 13.32 ± 0.89 | 71.37 ± 0.37 | 63.17 ± 0.20 | 44.38 ± 0.32 |
| Lignin | 3.37 ± 0.46 | 30.24 ± 0.19 | 10.24 ± 1.08 | 4.96 ± 0.24 |
| Cellulose | 9.94 ± 0.98 | 41.12 ± 0.24 | 52.92 ± 0.15 | 39.42 ± 0.09 |
| Hemicellulose | 38.86 ± 1.65 | 7.72 ± 0.61 | 32.63 ± 0.08 | 44.9 ± 0.19 |
| Purification Steps | Total Activity (U) | Total Protein (mg) | Specific Activity (U/mg of Protein) | Recovery (%) |
|---|---|---|---|---|
| Crude extract | 7.19 | 6.9 | 1.04 | 100 |
| Fraction 80–100% | 6.75 | 0.95 | 7.10 | 94 |
| DEAE-Sepharose | 7.91 | 0.60 | 13.18 | 110 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, M.d.C.; Costa, R.B.; Gomes, M.M.O.d.S.; Nascimento, J.S.d.; Gonçalves, A.H.d.S.; Nunes, J.A.; Santos, M.A.d.; Gomes, F.S.; Luz, J.M.R.d.; Grillo, L.A.M.; et al. Purification and Functional Characterization of a New Endoglucanase from Pleurotus djamor PLO13 Produced by Solid-State Fermentation of Agro-Industrial Waste. Fermentation 2025, 11, 182. https://doi.org/10.3390/fermentation11040182
Silva MdC, Costa RB, Gomes MMOdS, Nascimento JSd, Gonçalves AHdS, Nunes JA, Santos MAd, Gomes FS, Luz JMRd, Grillo LAM, et al. Purification and Functional Characterization of a New Endoglucanase from Pleurotus djamor PLO13 Produced by Solid-State Fermentation of Agro-Industrial Waste. Fermentation. 2025; 11(4):182. https://doi.org/10.3390/fermentation11040182
Chicago/Turabian StyleSilva, Monizy da Costa, Ricardo Bezerra Costa, Marta Maria Oliveira dos Santos Gomes, Josiel Santos do Nascimento, Andreza Heloiza da Silva Gonçalves, Jéssica Alves Nunes, Marta Angelo dos Santos, Francis Soares Gomes, José Maria Rodrigues da Luz, Luciano Aparecido Meireles Grillo, and et al. 2025. "Purification and Functional Characterization of a New Endoglucanase from Pleurotus djamor PLO13 Produced by Solid-State Fermentation of Agro-Industrial Waste" Fermentation 11, no. 4: 182. https://doi.org/10.3390/fermentation11040182
APA StyleSilva, M. d. C., Costa, R. B., Gomes, M. M. O. d. S., Nascimento, J. S. d., Gonçalves, A. H. d. S., Nunes, J. A., Santos, M. A. d., Gomes, F. S., Luz, J. M. R. d., Grillo, L. A. M., & Pereira, H. J. V. (2025). Purification and Functional Characterization of a New Endoglucanase from Pleurotus djamor PLO13 Produced by Solid-State Fermentation of Agro-Industrial Waste. Fermentation, 11(4), 182. https://doi.org/10.3390/fermentation11040182






