Microbial Synthesis of Neo-Allo-Ocimene by Celery-Derived Neo-Allo-Ocimene Synthase
Abstract
1. Introduction
2. Materials and Methods
2.1. Strains and Reagents
2.2. Plasmid Construction
2.3. Bioinformatics Analysis of Putative Terpene Synthase
2.4. Shake Flask Fermentation
2.5. Characterization and Quantification of Neo-Allo-Ocimene
2.6. Optimization of Fermentation Conditions
2.7. Scanning Electron Microscopy (SEM) Observation
2.8. Statistical Analysis
3. Results
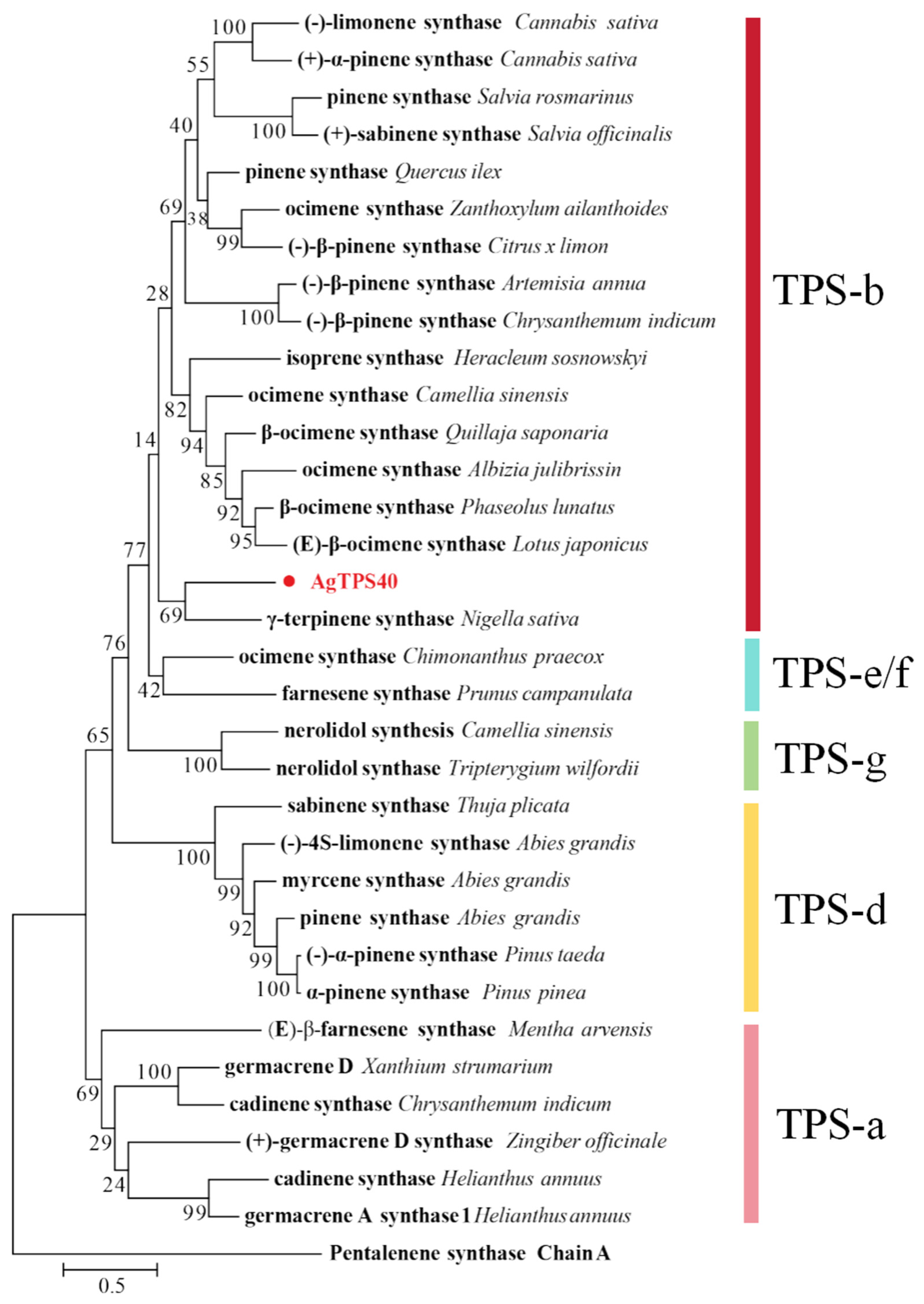
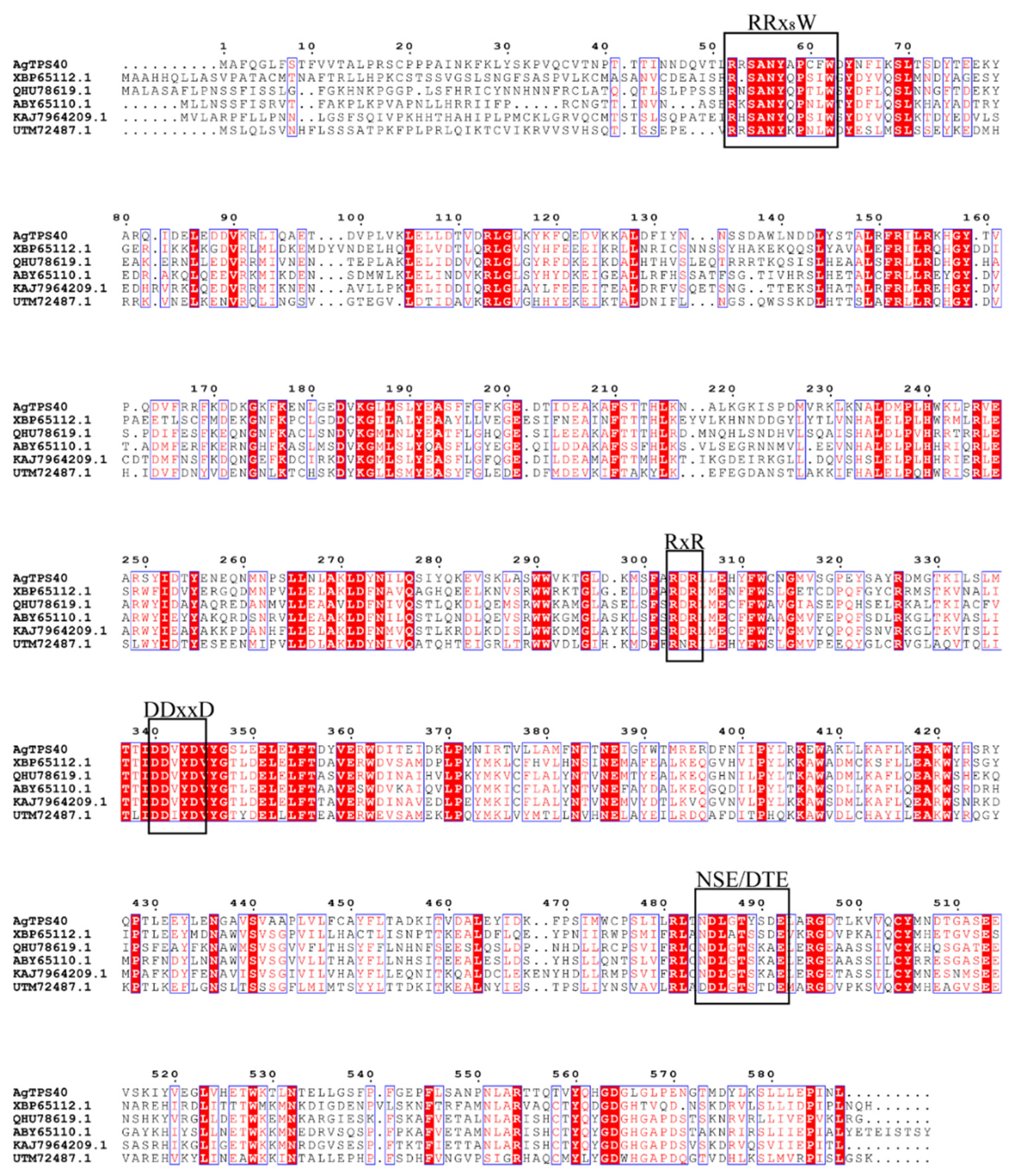
3.1. Sequence and Phylogenetic Analysis of Putative Terpene Synthase
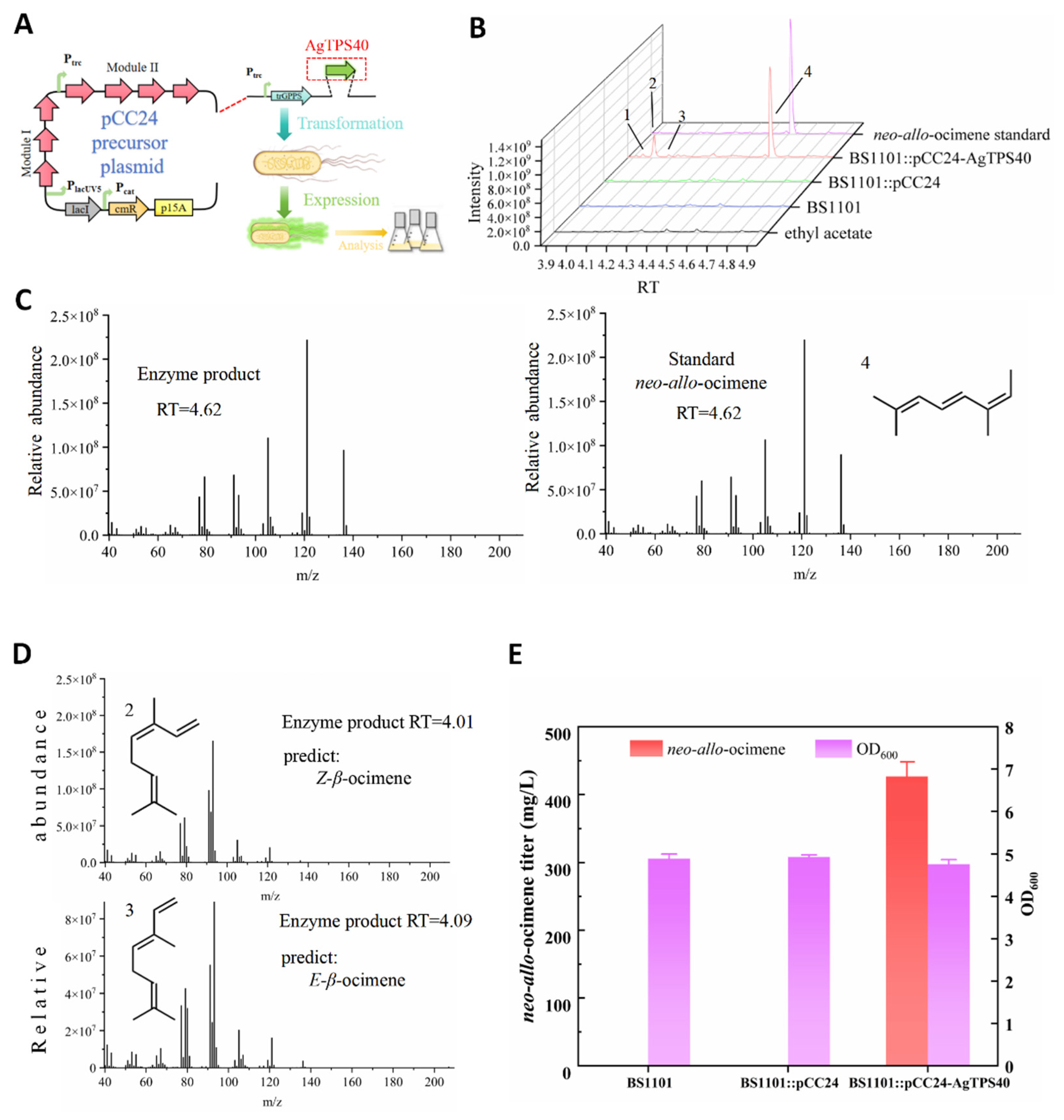
3.2. Function Characterization of AgTPS40 In Vivo
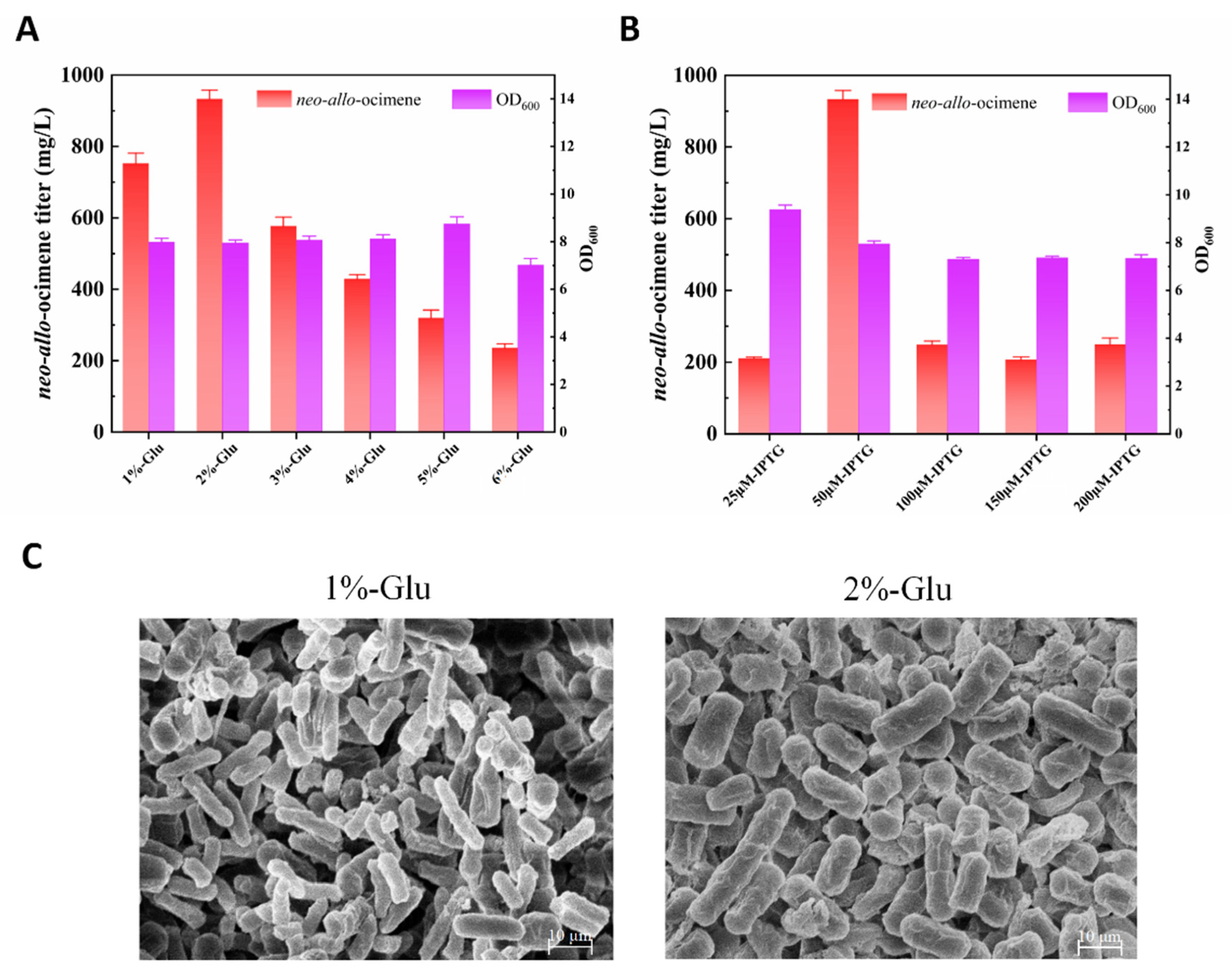
3.3. Optimization of the Production Process
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, C.; Yao, G.; Wang, F.; Bao, S.; Wan, X.; Han, P.; Wang, K.; Song, T.; Jiang, H. Identification of a (+)-Cubenene Synthase from Filamentous Fungi Acremonium chrysogenum. Biochem. Biophys. Res. Commun. 2023, 677, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Jia, Q.; Brown, R.; Köllner, T.G.; Fu, J.; Chen, X.; Wong, G.K.-S.; Gershenzon, J.; Peters, R.J.; Chen, F. Origin and Early Evolution of the Plant Terpene Synthase Family. Proc. Natl. Acad. Sci. USA 2022, 119, e2100361119. [Google Scholar] [CrossRef]
- Ashour, M.; Wink, M.; Gershenzon, J. Biochemistry of Terpenoids: Monoterpenes, Sesquiterpenes and Diterpenes. In Biochemistry of Plant Secondary Metabolism; Wink, M., Ed.; Wiley-Blackwell: Oxford, UK, 2010; pp. 258–303. ISBN 978-1-4443-2050-3. [Google Scholar]
- De Alvarenga, J.F.R.; Genaro, B.; Costa, B.L.; Purgatto, E.; Manach, C.; Fiamoncini, J. Monoterpenes: Current Knowledge on Food Source, Metabolism, and Health Effects. Crit. Rev. Food Sci. Nutr. 2023, 63, 1352–1389. [Google Scholar] [CrossRef]
- Liu, Z.; Li, Q.X.; Song, B. Pesticidal Activity and Mode of Action of Monoterpenes. J. Agric. Food Chem. 2022, 70, 4556–4571. [Google Scholar] [CrossRef]
- Mendez-Perez, D.; Alonso-Gutierrez, J.; Hu, Q.; Molinas, M.; Baidoo, E.E.K.; Wang, G.; Chan, L.J.G.; Adams, P.D.; Petzold, C.J.; Keasling, J.D.; et al. Production of Jet Fuel Precursor Monoterpenoids from Engineered Escherichia coli. Biotechnol. Bioeng. 2017, 114, 1703–1712. [Google Scholar] [CrossRef]
- Ding, G.; Zhang, S.; Ma, B.; Liang, J.; Li, H.; Luo, Y.; He, N. Origin and Functional Differentiation of (E)-β-Ocimene Synthases Reflect the Expansion of Monoterpenes in Angiosperms. J. Exp. Bot. 2020, 71, 6571–6586. [Google Scholar] [CrossRef] [PubMed]
- Behr, A.; Johnen, L. Myrcene as a Natural Base Chemical in Sustainable Chemistry: A Critical Review. ChemSusChem 2009, 2, 1072–1095. [Google Scholar] [CrossRef] [PubMed]
- Anandakumar, P.; Kamaraj, S.; Vanitha, M.K. D-limonene: A Multifunctional Compound with Potent Therapeutic Effects. J. Food Biochem. 2021, 45, e13566. [Google Scholar] [CrossRef]
- Farré-Armengol, G.; Filella, I.; Llusià, J.; Peñuelas, J. β-Ocimene, a Key Floral and Foliar Volatile Involved in Multiple Interactions between Plants and Other Organisms. Molecules 2017, 22, 1148. [Google Scholar] [CrossRef]
- Chen, H.; Yue, Y.; Yu, R.; Fan, Y. A Hedychium coronarium Short Chain Alcohol Dehydrogenase Is a Player in Allo-Ocimene Biosynthesis. Plant Mol. Biol. 2019, 101, 297–313. [Google Scholar] [CrossRef]
- Xiao, Y.; Qian, J.; Hou, X.; Zeng, L.; Liu, X.; Mei, G.; Liao, Y. Diurnal Emission of Herbivore-Induced (Z)-3-Hexenyl Acetate and Allo-Ocimene Activates Sweet Potato Defense Responses to Sweet Potato Weevils. J. Integr. Agric. 2023, 22, 1782–1796. [Google Scholar] [CrossRef]
- Kishimoto, K.; Matsui, K.; Ozawa, R.; Takabayashi, J. Analysis of Defensive Responses Activated by Volatile Allo-Ocimene Treatment in Arabidopsis thaliana. Phytochemistry 2006, 67, 1520–1529. [Google Scholar] [CrossRef]
- Benelli, G.; Govindarajan, M.; Rajeswary, M.; Senthilmurugan, S.; Vijayan, P.; Alharbi, N.S.; Kadaikunnan, S.; Khaled, J.M. Larvicidal Activity of Blumea Eriantha Essential Oil and Its Components against Six Mosquito Species, Including Zika Virus Vectors: The Promising Potential of (4E,6Z)-Allo-Ocimene, Carvotanacetone and Dodecyl Acetate. Parasitol. Res. 2017, 116, 1175–1188. [Google Scholar] [CrossRef]
- Sahu, P.; Sarkar, P.; Bhowmick, A.K. Synthesis and Characterization of a Terpene-Based Sustainable Polymer: Poly-Alloocimene. ACS Sustain. Chem. Eng. 2017, 5, 7659–7669. [Google Scholar] [CrossRef]
- Chen, C.; Liu, J.; Yao, G.; Bao, S.; Wan, X.; Wang, F.; Wang, K.; Song, T.; Han, P.; Liu, T.; et al. A Novel, Genetically Encoded Whole-Cell Biosensor for Directed Evolution of Myrcene Synthase in Escherichia coli. Biosens. Bioelectron. 2023, 228, 115176. [Google Scholar] [CrossRef]
- Zeng, W.; Jiang, Y.; Shan, X.; Zhou, J. Engineering Saccharomyces cerevisiae for Synthesis of β-Myrcene and (E)-β-Ocimene. 3 Biotech 2023, 13, 384. [Google Scholar] [CrossRef] [PubMed]
- Nagegowda, D.A.; Gupta, P. Advances in Biosynthesis, Regulation, and Metabolic Engineering of Plant Specialized Terpenoids. Plant Sci. 2020, 294, 110457. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Q.; Wang, P.; Li, T.; Liu, J.; Zhang, Y.; Wang, Y.; Sun, L.; Shen, H. Complete Mitochondrial Genome Sequence and Identification of a Candidate Gene Responsible for Cytoplasmic Male Sterility in Celery (Apium graveolens L.). Int. J. Mol. Sci. 2021, 22, 8584. [Google Scholar] [CrossRef]
- Saleh, M.M.; Zwaving, J.H.; Malingré, T.M.; Bos, R. The Essential Oil of Apium graveolens var. secalinum and Its Cercaricidal Activity. Pharm. Weekbl. Sci. Ed. 1985, 7, 277–279. [Google Scholar] [CrossRef]
- Li, M.; Li, X.; Zhou, J.; Sun, Y.; Du, J.; Wang, Z.; Luo, Y.; Zhang, Y.; Chen, Q.; Wang, Y.; et al. Genome-Wide Identification and Analysis of Terpene Synthase (TPS) Genes in Celery Reveals Their Regulatory Roles in Terpenoid Biosynthesis. Front. Plant Sci. 2022, 13, 1010780. [Google Scholar] [CrossRef]
- Lange, B.M.; Rujan, T.; Martin, W.; Croteau, R. Isoprenoid Biosynthesis: The Evolution of Two Ancient and Distinct Pathways across Genomes. Proc. Natl. Acad. Sci. USA 2000, 97, 13172–13177. [Google Scholar] [CrossRef] [PubMed]
- Perez-Gil, J.; Behrendorff, J.; Douw, A.; Vickers, C.E. The Methylerythritol Phosphate Pathway as an Oxidative Stress Sense and Response System. Nat. Commun. 2024, 15, 5303. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Cheng, S.; Cao, J.; Qiao, J.; Zhao, G.-R. Systematic Optimization of Limonene Production in Engineered Escherichia coli. J. Agric. Food Chem. 2019, 67, 7087–7097. [Google Scholar] [CrossRef]
- Yang, J.; Nie, Q.; Ren, M.; Feng, H.; Jiang, X.; Zheng, Y.; Liu, M.; Zhang, H.; Xian, M. Metabolic Engineering of Escherichia coli for the Biosynthesis of Alpha-Pinene. Biotechnol. Biofuels 2013, 6, 60. [Google Scholar] [CrossRef]
- Bao, S.-H.; Jiang, H.; Zhu, L.-Y.; Yao, G.; Han, P.-G.; Wan, X.-K.; Wang, K.; Song, T.-Y.; Liu, C.-J.; Wang, S.; et al. A Dynamic and Multilocus Metabolic Regulation Strategy Using Quorum-Sensing-Controlled Bacterial Small RNA. Cell Rep. 2021, 36, 109413. [Google Scholar] [CrossRef]
- Kim, E.-M.; Eom, J.-H.; Um, Y.; Kim, Y.; Woo, H.M. Microbial Synthesis of Myrcene by Metabolically Engineered Escherichia coli. J. Agric. Food Chem. 2015, 63, 4606–4612. [Google Scholar] [CrossRef]
- Jones, D.T.; Taylor, W.R.; Thornton, J.M. The Rapid Generation of Mutation Data Matrices from Protein Sequences. Bioinformatics 1992, 8, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Sarria, S.; Wong, B.; Martín, H.G.; Keasling, J.D.; Peralta-Yahya, P. Microbial Synthesis of Pinene. ACS Synth. Biol. 2014, 3, 466–475. [Google Scholar] [CrossRef]
- Chen, F.; Tholl, D.; Bohlmann, J.; Pichersky, E. The Family of Terpene Synthases in Plants: A Mid-Size Family of Genes for Specialized Metabolism That Is Highly Diversified throughout the Kingdom. Plant J. 2011, 66, 212–229. [Google Scholar] [CrossRef]
- Christianson, D.W. Structural Biology and Chemistry of the Terpenoid Cyclases. Chem. Rev. 2006, 106, 3412–3442. [Google Scholar] [CrossRef] [PubMed]
- Rudolf, J.D.; Chang, C.-Y. Terpene Synthases in Disguise: Enzymology, Structure, and Opportunities of Non-Canonical Terpene Synthases. Nat. Prod. Rep. 2020, 37, 425–463. [Google Scholar] [CrossRef]
- Li, Y.; Xian, H.; Xu, Y.; Zhu, Y.; Sun, Z.; Wang, Q.; Qi, Q. Fine Tuning the Glycolytic Flux Ratio of EP-Bifido Pathway for Mevalonate Production by Enhancing Glucose-6-Phosphate Dehydrogenase (Zwf) and CRISPRi Suppressing 6-Phosphofructose Kinase (PfkA) in Escherichia coli. Microb. Cell Factories 2021, 20, 32. [Google Scholar] [CrossRef]
- Zhang, J.; Yuan, Y.; Wang, Z.; Chen, T. Metabolic Engineering of Halomonas bluephagenesis for High-Level Mevalonate Production from Glucose and Acetate Mixture. Metab. Eng. 2023, 79, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Fäldt, J.; Arimura, G.; Gershenzon, J.; Takabayashi, J.; Bohlmann, J. Functional Identification of AtTPS03 as (E)-β-Ocimene Synthase: A Monoterpene Synthase Catalyzing Jasmonate- and Wound-Induced Volatile Formation in Arabidopsis thaliana. Planta 2003, 216, 745–751. [Google Scholar] [CrossRef]
- Sparkman, O.D. Identification of Essential Oil Components by Gas Chromatography/Quadrupole Mass Spectroscopy Robert P. Adams. J. Am. Soc. Mass Spectrom. 2005, 16, 1902–1903. [Google Scholar] [CrossRef]
- Lin, C.-H.; Chao, L.K.; Lin, L.-Y.; Wu, C.-S.; Chu, L.-P.; Huang, C.-H.; Chen, H.-C. Analysis of Volatile Compounds from Different Parts of Houttuynia cordata Thunb. Molecules 2022, 27, 8893. [Google Scholar] [CrossRef] [PubMed]
- Bao, S.-H.; Zhang, D.-Y.; Meng, E. Improving biosynthetic production of pinene through plasmid recombination elimination and pathway optimization. Plasmid 2019, 105, 102431. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; Gao, T.; Bao, S.; Han, P.; Yao, G.; Song, T.; Zhu, L.; Chen, C.; Jiang, H. Microbial Synthesis of Neo-Allo-Ocimene by Celery-Derived Neo-Allo-Ocimene Synthase. Fermentation 2025, 11, 153. https://doi.org/10.3390/fermentation11030153
Liu Z, Gao T, Bao S, Han P, Yao G, Song T, Zhu L, Chen C, Jiang H. Microbial Synthesis of Neo-Allo-Ocimene by Celery-Derived Neo-Allo-Ocimene Synthase. Fermentation. 2025; 11(3):153. https://doi.org/10.3390/fermentation11030153
Chicago/Turabian StyleLiu, Zheng, Ting Gao, Shaoheng Bao, Penggang Han, Ge Yao, Tianyu Song, Longbao Zhu, Chang Chen, and Hui Jiang. 2025. "Microbial Synthesis of Neo-Allo-Ocimene by Celery-Derived Neo-Allo-Ocimene Synthase" Fermentation 11, no. 3: 153. https://doi.org/10.3390/fermentation11030153
APA StyleLiu, Z., Gao, T., Bao, S., Han, P., Yao, G., Song, T., Zhu, L., Chen, C., & Jiang, H. (2025). Microbial Synthesis of Neo-Allo-Ocimene by Celery-Derived Neo-Allo-Ocimene Synthase. Fermentation, 11(3), 153. https://doi.org/10.3390/fermentation11030153




