Carnosic Acid Production from Sugarcane Syrup by Engineered Yeast in Fed-Batch Fermentation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Strain
2.2. Fermentations
2.3. Extraction of Metabolites
2.4. Quantification of Metabolites
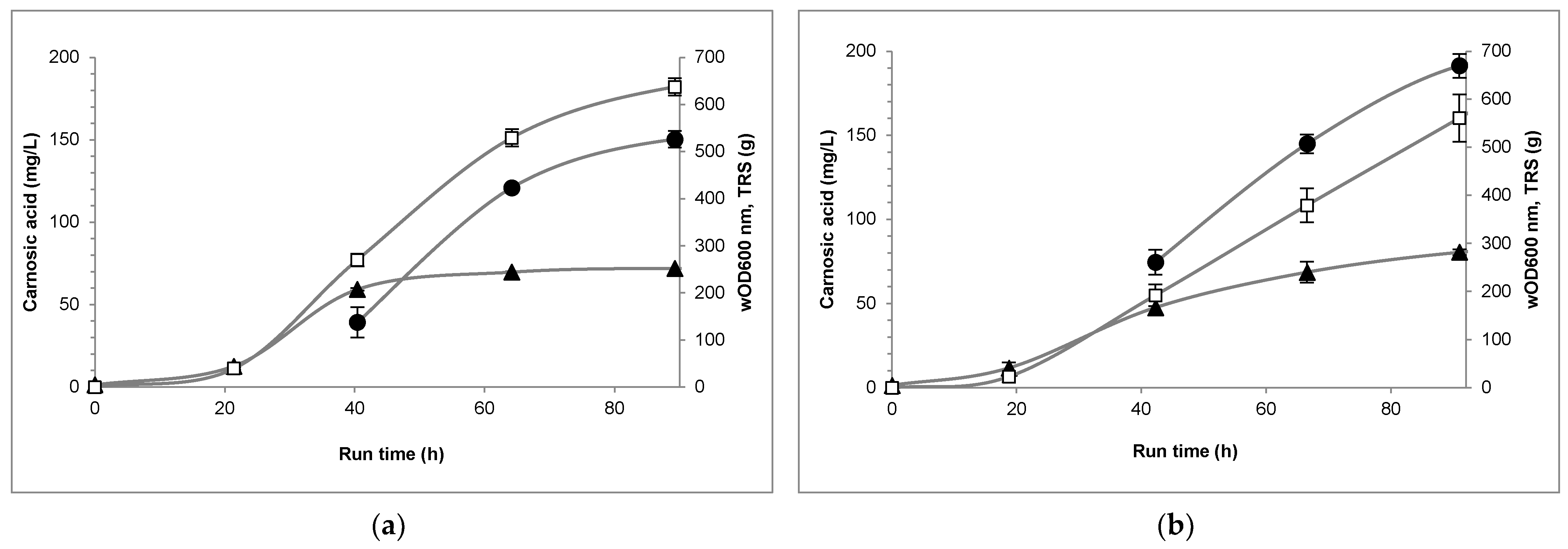
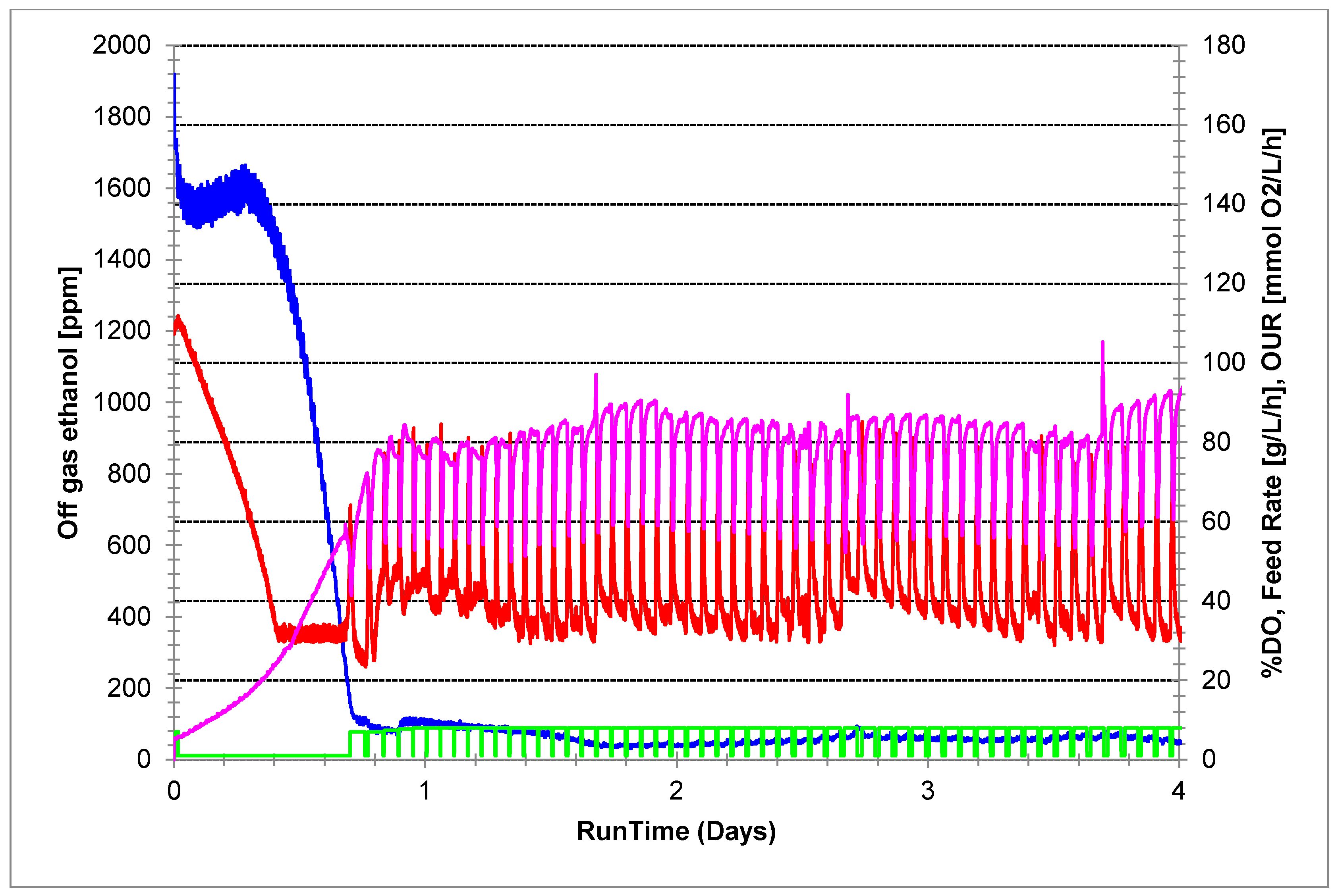
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hill, R.A.; Connolly, J.D. Triterpenoids. Nat. Prod. Rep. 2013, 30, 1028–1065. [Google Scholar] [CrossRef]
- Birtić, S.; Dussort, P.; Pierre, F.X.; Bily, A.C.; Roller, M. Carnosic acid. Phytochemistry 2015, 115, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Scheler, U.; Brandt, W.; Porzel, A.; Rothe, K.; Manzano, D.; Božić, D.; Papaefthimiou, D.; Balcke, G.U.; Henning, A.; Lohse, S.; et al. Elucidation of the biosynthesis of carnosic acid and its reconstitution in yeast. Nat. Commun. 2016, 7, 12942. [Google Scholar] [CrossRef]
- Hu, T.; Zhou, J.; Tong, Y.; Su, P.; Li, X.; Liu, Y.; Liu, N.; Wu, X.; Zhang, Y.; Wang, J.; et al. Engineering chimeric diterpene synthases and isoprenoid biosynthetic pathways enables high-level production of miltiradiene in yeast. Metab. Eng. 2020, 60, 87–96. [Google Scholar] [CrossRef]
- Ignea, C.; Athanasakoglou, A.; Ioannou, E.; Georgantea, P.; Trikka, F.A.; Loupassaki, S.; Roussis, V.; Makris, A.M.; Kampranis, S.C. Carnosic acid biosynthesis elucidated by a synthetic biology platform. Proc. Natl. Acad. Sci. USA 2016, 113, 3681–3686. [Google Scholar] [CrossRef] [PubMed]
- Ignea, C.; Athanasakoglou, A.; Andreadelli, A.; Apostolaki, M.; Iakovides, M.; Stephanou, E.G.; Makris, A.M.; Kampranis, S.C. Overcoming the plasticity of plant specialized metabolism for selective diterpene production in yeast. Sci. Rep. 2017, 7, 8855. [Google Scholar] [CrossRef] [PubMed]
- Wei, P.; Zhang, C.; Bian, X.; Lu, W. Metabolic engineering of Saccharomyces cerevisiae for heterologous carnosic acid production. Front. Bioeng. Biotechnol. 2022, 10, 916605. [Google Scholar] [CrossRef]
- Carsanba, E.; Pintado, M.; Oliveira, C. Fermentation strategies for production of pharmaceutical terpenoids in engineered yeast. Pharmaceuticals 2021, 14, 295. [Google Scholar] [CrossRef]
- Lopes, A.; Azevedo-Silva, J.; Carsanba, E.; Pintado, M.; Oliveira, A.S.; Ferreira, C.; Pereira, J.O.; Carvalho, A.P.; Oliveira, C. Peptide extract from spent yeast improves resistance of Saccharomyces cerevisiae to oxidative stress. Appl. Microbiol. Biotechnol. 2023, 107, 3405–3417. [Google Scholar] [CrossRef]
- Carvalho, L.C.; Oliveira, A.L.S.; Carsanba, E.; Pintado, M.; Oliveira, C. Phenolic compounds modulation in β-farnesene fed-batch fermentation using sugarcane syrup as feedstock. Ind. Crops Prod. 2022, 188, 115721. [Google Scholar] [CrossRef]
- Carvalho, L.C.; Oliveira, A.L.S.; Carsanba, E.; Lopes, A.; Leal, T.; Ribeiro, M.; Fernandes, S.; Pintado, M.; Oliveira, C. Removal of phenolic compounds from sugarcane syrup and impact on Saccharomyces cerevisiae fermentation for β-farnesene production. Biotechnol. J. 2024, 19, e2300465. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.; Gomes, M.H.; Alexandre, E.M.C.; Poças, F.; Almeida, D.P.F.; Pintado, M. Phytochemicals preservation in strawberry as affected by pH modulation. Food Chem. 2015, 170, 74–83. [Google Scholar] [CrossRef]
- Moreno, S.; Scheyer, T.; Romano, C.S.; Vojnov, A.A. Antioxidant and antimicrobial activities of rosemary extracts linked to their polyphenol composition. Free Radic. Res. 2006, 40, 223–231. [Google Scholar] [CrossRef]
- Nieto, G.; Ros, G.; Castillo, J. Antioxidant and antimicrobial properties of rosemary (Rosmarinus officinalis, L.): A review. Medicines 2018, 5, 98. [Google Scholar] [CrossRef]
- Argüelles, A.; Sánchez-Fresneda, R.; Guirao-Abad, J.P.; Belda, C.; Lozano, J.A.; Solano, F.; Argüelles, J.C. Novel bi-factorial strategy against Candida albicans viability using Carnosic acid and Propolis: Synergistic antifungal action. Microorganisms 2020, 8, 749. [Google Scholar] [CrossRef] [PubMed]
- Alavi, M.S.; Fanoudi, S.; Ghasemzadeh, R.M.; Mehri, S.; Hosseinzadeh, H. An updated review of protective effects of rosemary and its active constituents against natural and chemical toxicities. Phytother. Res. 2021, 35, 1313–1328. [Google Scholar] [CrossRef]
- Bahri, S.; Jameleddine, S.; Shlyonsky, V. Relevance of carnosic acid to the treatment of several health disorders: Molecular targets and mechanisms. Biomed. Pharmacother. 2016, 84, 569–582. [Google Scholar] [CrossRef] [PubMed]
- Meadows, A.L.; Hawkins, K.M.; Tsegaye, Y.; Antipov, E.; Kim, Y.; Raetz, L.; Dahl, R.H.; Tai, A.; Mahatdejkul-Meadows, T.; Xu, L.; et al. Rewriting yeast central carbon metabolism for industrial isoprenoid production. Nat. Cell Biol. 2016, 537, 694–697. [Google Scholar] [CrossRef]
- Paddon, C.J.; Westfall, P.J.; Pitera, D.J.; Benjamin, K.; Fisher, K.; McPhee, D.J.; Leavell, M.D.; Tai, A.; Main, A.; Eng, D.; et al. High-level semi-synthetic production of the potent antimalarial artemisinin. Nat. Cell Biol. 2013, 496, 528–532. [Google Scholar] [CrossRef]
- Hill, P.; Benjamin, K.; Bhattacharjee, B.; Garcia, F.; Leng, J.; Liu, C.-L.; Murarka, A.; Pitera, D.; Porcel, E.M.R.; Da Silva, I.; et al. Clean manufacturing powered by biology: How Amyris has deployed technology and aims to do it better. J. Ind. Microbiol. Biotechnol. 2020, 47, 965–975. [Google Scholar] [CrossRef]
- Huo, D.; Chen, X.Z.; Yang, H.Q.; Cao, Y. Metabolic engineering of Candida tropicalis for carnosic acid production. Food Ferment. Ind. 2024, 50, 45–51. [Google Scholar]
- Li, T.; Liu, X.; Xiang, H.; Zhu, H.; Lu, X.; Feng, B. Two-phase fermentation systems for microbial production of plant-derived terpenes. Molecules 2024, 29, 1127. [Google Scholar] [CrossRef] [PubMed]




| Growth Kinetics | |
|---|---|
| Growth rate (h−1) | 0.197 ± 0.009 |
| Doubling time (h) | 3.52 ± 0.16 |
| TRS consumed (g/L) | 26.1 |
| Final cell viability (%) | 92.1 |
| Feed Rate 12–26 g/L/h Fixed DO Trigger | Feed Rate 7–8 g/L/h Dynamic DO Trigger | |
|---|---|---|
| Titer (mg/L) | 150.4 ± 5.0 | 191.4 ± 5.0 |
| Yield (mg/g TRS) | 0.236 | 0.341 |
| Productivity (mg/L/h) | 1.6 | 2.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carsanba, E.; Fernandes, S.; Beato, F.; Carvalho, L.C.; Pintado, A.; Lopes, A.; Ribeiro, M.; Leal, T.; Pintado, M.; Oliveira, C. Carnosic Acid Production from Sugarcane Syrup by Engineered Yeast in Fed-Batch Fermentation. Fermentation 2025, 11, 147. https://doi.org/10.3390/fermentation11030147
Carsanba E, Fernandes S, Beato F, Carvalho LC, Pintado A, Lopes A, Ribeiro M, Leal T, Pintado M, Oliveira C. Carnosic Acid Production from Sugarcane Syrup by Engineered Yeast in Fed-Batch Fermentation. Fermentation. 2025; 11(3):147. https://doi.org/10.3390/fermentation11030147
Chicago/Turabian StyleCarsanba, Erdem, Sara Fernandes, Felipe Beato, Luís Carlos Carvalho, Ana Pintado, Ana Lopes, Mónica Ribeiro, Tânia Leal, Manuela Pintado, and Carla Oliveira. 2025. "Carnosic Acid Production from Sugarcane Syrup by Engineered Yeast in Fed-Batch Fermentation" Fermentation 11, no. 3: 147. https://doi.org/10.3390/fermentation11030147
APA StyleCarsanba, E., Fernandes, S., Beato, F., Carvalho, L. C., Pintado, A., Lopes, A., Ribeiro, M., Leal, T., Pintado, M., & Oliveira, C. (2025). Carnosic Acid Production from Sugarcane Syrup by Engineered Yeast in Fed-Batch Fermentation. Fermentation, 11(3), 147. https://doi.org/10.3390/fermentation11030147






