Characterization of Low pH and Inhibitor Tolerance Capacity of Candida krusei Strains
Abstract
1. Introduction
2. Materials and Methods
2.1. Yeast Strains and Media
2.2. Spot Assay
2.3. Aerobic Growth Experiments
2.4. Anaerobic Fermentation Experiments
2.5. Pathway Analysis to Elucidate Low pH Tolerance Capacity
2.6. Comparative Sequence Analysis to Elucidate Inhibitor Tolerance Capacity
3. Results and Discussion
3.1. Effect of Low pH, Acids and Inhibitors on Growth
3.2. Ethanol Production Under Low pH Stress Conditions
3.3. Elucidation of the Low pH and Inhibitor Tolerance Capacity of C. krusei NBRC1664
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, Z.; Deng, Z.; Davis, S.; Ciais, P. Monitoring global carbon emissions in 2022. Nat. Rev. Earth Environ. 2023, 4, 205–206. [Google Scholar] [CrossRef] [PubMed]
- Bolan, S.; Padhye, L.P.; Jasemizad, T.; Govarthanan, M.; Karmegam, N.; Wijesekara, H.; Amarasiri, D.; Hou, D.; Zhou, P.; Biswal, B.K.; et al. Impacts of climate change on the fate of contaminants through extreme weather events. Sci. Total Environ. 2024, 909, 168388. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, C.J. The 2015 Paris Climate Change Conference: COP21. Sci. Prog. 2016, 99, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Renewables 2019. 2019. Available online: https://www.iea.org/reports/renewables-2019 (accessed on 16 January 2025).
- Akita, H.; Matsushika, A. A Short Review of second-generation isobutanol production by SHF and SSF. Appl. Biosci. 2024, 3, 296–309. [Google Scholar] [CrossRef]
- Bušić, A.; Marđetko, N.; Kundas, S.; Morzak, G.; Belskaya, H.; Ivančić Šantek, M.; Komes, D.; Novak, S.; Šantek, B. Bioethanol production from renewable raw materials and its separation and purification: A review. Food Technol. Biotechnol. 2018, 56, 289–311. [Google Scholar] [CrossRef]
- Zhao, Y.; Damgaard, A.; Christensen, T.H. Bioethanol from corn stover—A review and technical assessment of alternative biotechnologies. Prog. Energy Combust. Sci. 2018, 67, 275–291. [Google Scholar] [CrossRef]
- Offei, F.; Mensah, M.; Thygesen, A.; Kemausuor, F. Seaweed bioethanol production: A process selection review on hydrolysis and fermentation. Fermentation 2018, 4, 99. [Google Scholar] [CrossRef]
- Akita, H.; Goshima, T.; Suzuki, T.; Itoiri, Y.; Kimura, Z.-I.; Matsushika, A. Application of Pichia kudriavzevii NBRC1279 and NBRC1664 to simultaneous saccharification and fermentation for bioethanol production. Fermentation 2021, 7, 83. [Google Scholar] [CrossRef]
- Carlotti, A.; Couble, A.; Domingo, J.; Miroy, K.; Villard, J. Species-specific identification of Candida krusei by hybridization with the CkF1,2 DNA probe. J. Clin. Microbiol. 1996, 34, 1726–1731. [Google Scholar] [CrossRef]
- Kurtzman, C.P.; Smiley, M.J.; Johnson, C.J. Emendation of the genus Issatchenkia Kudriavzev and comparison of species by deoxyribonucleic acid reassociation, mating reaction, and ascospore ultrastructure. Int. J. Syst. Bacteriol. 1980, 30, 503–513. [Google Scholar] [CrossRef]
- Akita, H.; Matsushika, A. Transcription analysis of the acid tolerance mechanism of Pichia kudriavzevii NBRC1279 and NBRC1664. Fermentation 2023, 9, 559. [Google Scholar] [CrossRef]
- Akita, H.; Matsushika, A. Inhibitor tolerance capacity of Pichia kudriavzevii NBRC1279 and NBRC1664. Fermentation 2024, 10, 331. [Google Scholar] [CrossRef]
- Thompson, J.D.; Higgins, D.G.; Gibson, T.J. CLUSTAL W: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994, 22, 4673–4680. [Google Scholar] [CrossRef]
- Gouet, P.; Courcelle, E.; Stuart, D.I.; Métoz, F. ESPript: Analysis of multiple sequence alignments in PostScript. Bioinformatics 1999, 15, 305–308. [Google Scholar] [CrossRef]
- Ndubuisi, I.A.; Qin, Q.; Liao, G.; Wang, B.; Moneke, A.N.; Ogbonna, J.C.; Jin, C.; Fang, W. Effects of various inhibitory substances and immobilization on ethanol production efficiency of a thermotolerant Pichia kudriavzevii. Biotechnol. Biofuels 2020, 13, 91. [Google Scholar] [CrossRef]
- Ji, H.; Xu, K.; Dong, X.; Sun, D.; Jin, L. Sequential production of D-xylonate and ethanol from non-detoxified corncob at low-pH by Pichia kudriavzevii via a two-stage fermentation strategy. J. Fungi 2021, 7, 1038. [Google Scholar] [CrossRef]
- Hoppert, L.; Kölling, R.; Einfalt, D. Investigation of stress tolerance of Pichia kudriavzevii for high gravity bioethanol production from steam-exploded wheat straw hydrolysate. Bioresour. Technol. 2022, 364, 128079. [Google Scholar] [CrossRef]
- Pongcharoen, P.; Tawong, W.; Pathaichindachote, W.; Rod-In, W. Physiological responses contributing to multiple stress tolerance in Pichia kudriavzevii with potential enhancement for ethanol fermentation. J. Biosci. Bioeng. 2024, 138, 314–323. [Google Scholar] [CrossRef]
- Avchar, R.; Lanjekar, V.; Dhakephalkar, P.K.; Dagar, S.S.; Baghela, A. Compost as an untapped niche for thermotolerant yeasts capable of high-temperature ethanol production. Lett. Appl. Microbiol. 2022, 74, 109–121. [Google Scholar] [CrossRef]
- Li, C.; Liu, Q.; Wang, Y.; Yang, X.; Chen, S.; Zhao, Y.; Wu, Y.; Li, L. Salt stress improves thermotolerance and high-temperature bioethanol production of multi-stress-tolerant Pichia kudriavzevii by stimulating intracellular metabolism and inhibiting oxidative damage. Biotechnol. Biofuels 2021, 14, 222. [Google Scholar] [CrossRef]
- De Nadal, E.; Posas, F. The HOG pathway and the regulation of osmoadaptive responses in yeast. FEMS Yeast Res. 2022, 22, foac013. [Google Scholar] [CrossRef]
- Babazadeh, R.; Furukawa, T.; Hohmann, S.; Furukawa, K. Rewiring yeast osmostress signalling through the MAPK network reveals essential and non-essential roles of Hog1 in osmoadaptation. Sci. Rep. 2014, 4, 4697. [Google Scholar] [CrossRef] [PubMed]
- Tatebayashi, K.; Yamamoto, K.; Tomida, T.; Nishimura, A.; Takayama, T.; Oyama, M.; Kozuka-Hata, H.; Adachi-Akahane, S.; Tokunaga, Y.; Saito, H. Osmostress enhances activating phosphorylation of Hog1 MAP kinase by mono-phosphorylated Pbs2 MAP2K. EMBO J. 2020, 39, e103444. [Google Scholar] [CrossRef] [PubMed]
- Salat-Canela, C.; Pérez, P.; Ayté, J.; Hidalgo, E. Stress-induced cell depolarization through the MAP kinase-Cdc42 axis. Trends Cell Biol. 2022, 33, 124–137. [Google Scholar] [CrossRef]
- Raitt, D.C.; Posas, F.; Saito, H. Yeast Cdc42 GTPase and Ste20 PAK-like kinase regulate Sho1-dependent activation of the Hog1 MAPK pathway. EMBO J. 2000, 19, 4623–4631. [Google Scholar] [CrossRef]
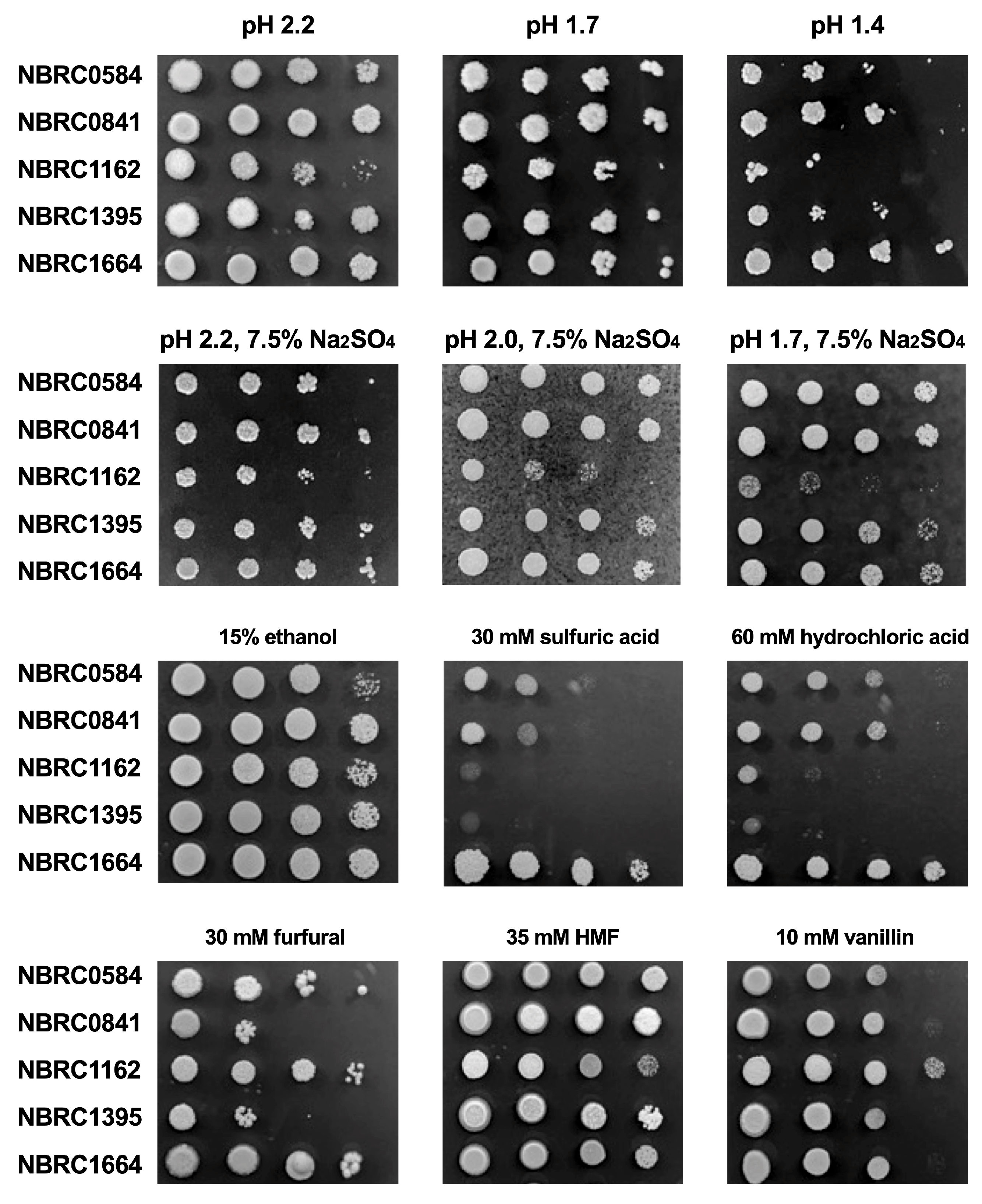
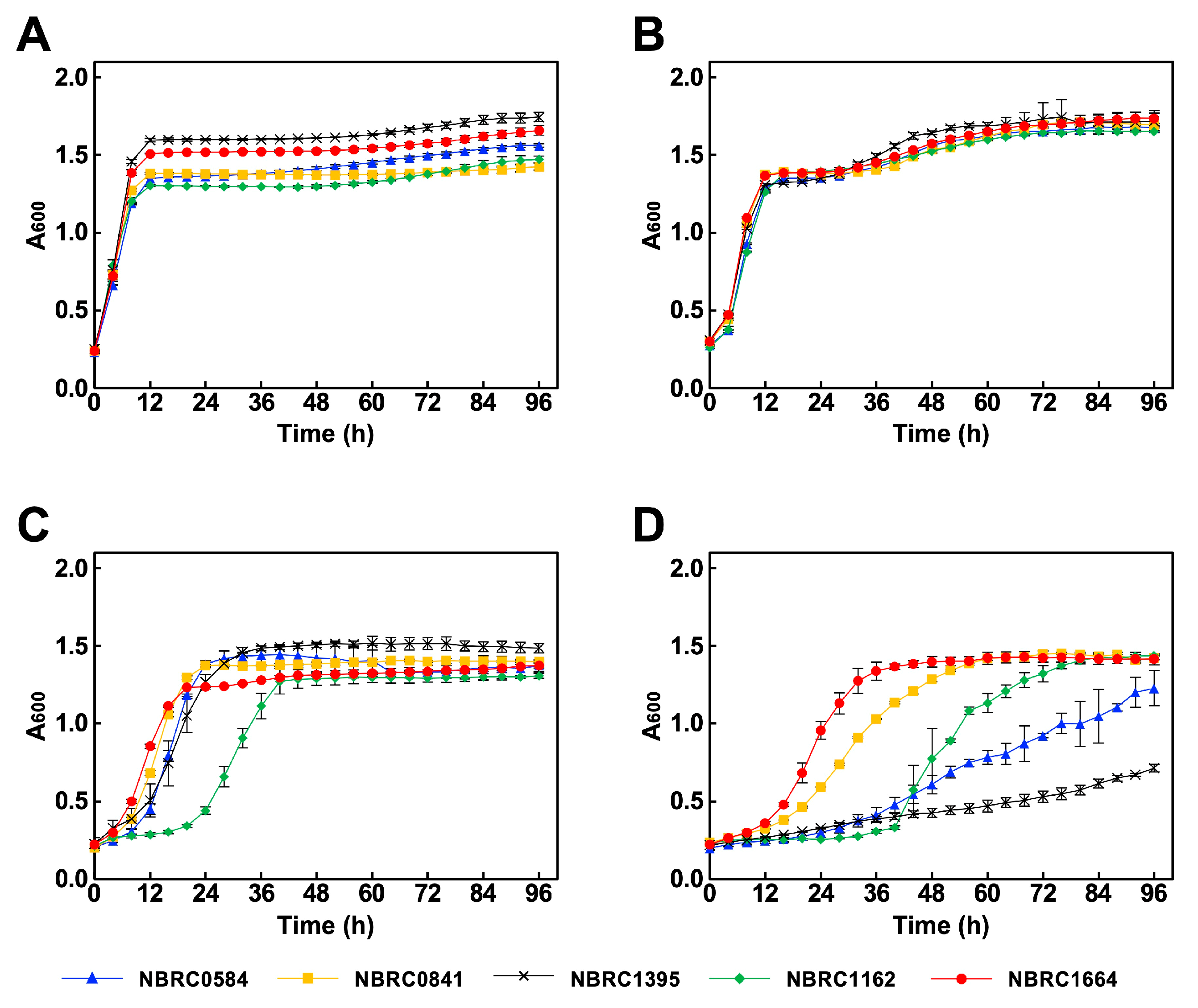

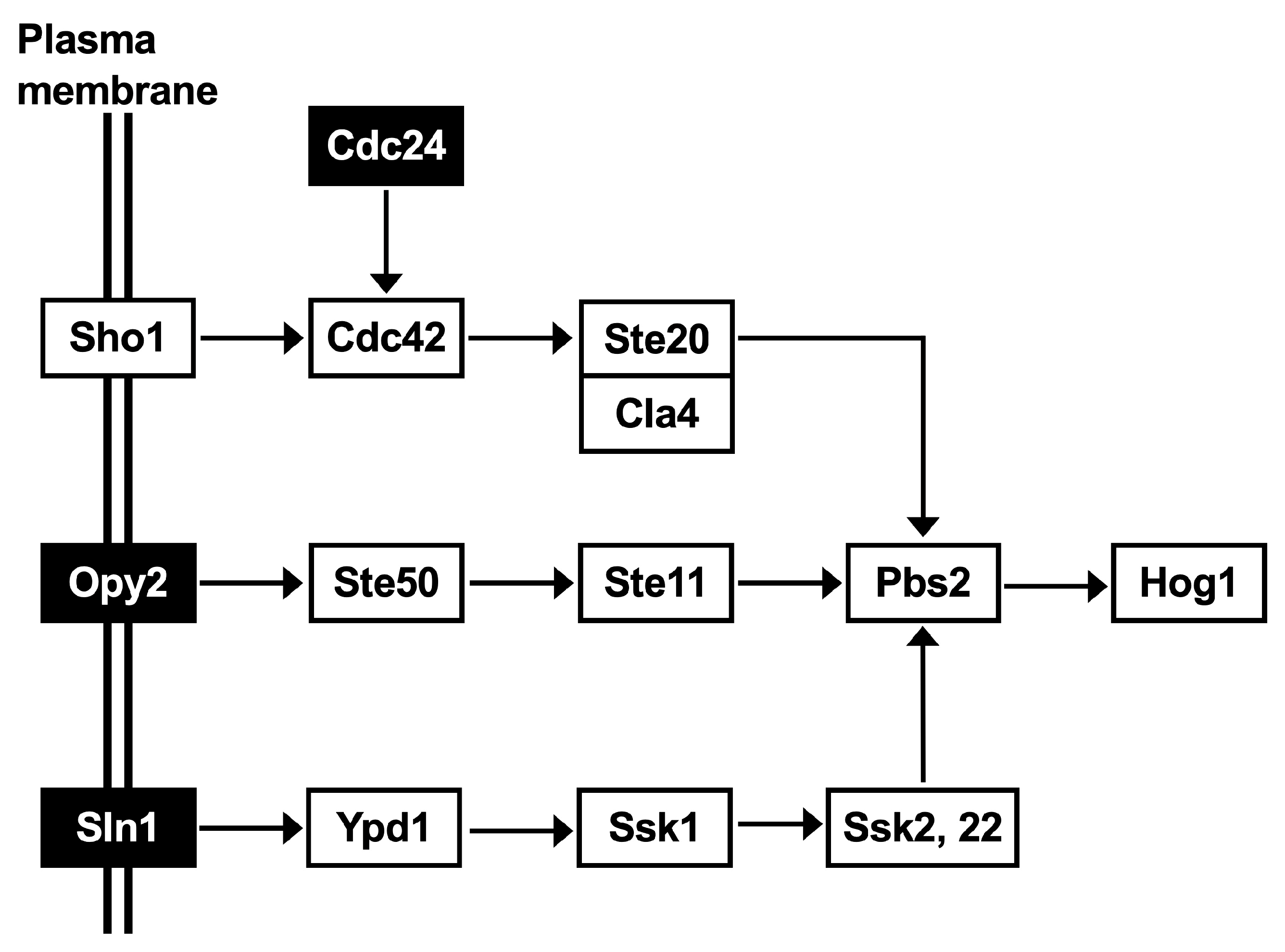

| Strain | pH 5.8 | pH 2.0 | pH 1.7 | pH 1.7 with 2.5% Na2SO4 |
|---|---|---|---|---|
| NBRC0584 | 0.147 ± 0.001 | 0.153 ± 0.002 | 0.0655 ± 0.0032 | 0.0258 ± 0.0018 |
| NBRC0841 | 0.144 ± 0.003 | 0.156 ± 0.001 | 0.101 ± 0.002 | 0.0406 ± 0.0050 |
| NBRC1162 | 0.139 ± 0.002 | 0.152 ± 0.002 | 0.0230 ± 0.0021 | 0.0145 ± 0.0010 |
| NBRC1395 | 0.155 ± 0.011 | 0.150 ± 0.003 | 0.0646 ± 0.0044 | 0.0249 ± 0.0047 |
| NBRC1664 | 0.154 ± 0.002 | 0.158 ± 0.002 | 0.114 ± 0.002 | 0.0456 ± 0.0051 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akita, H.; Moriguchi, D.; Matsushika, A. Characterization of Low pH and Inhibitor Tolerance Capacity of Candida krusei Strains. Fermentation 2025, 11, 146. https://doi.org/10.3390/fermentation11030146
Akita H, Moriguchi D, Matsushika A. Characterization of Low pH and Inhibitor Tolerance Capacity of Candida krusei Strains. Fermentation. 2025; 11(3):146. https://doi.org/10.3390/fermentation11030146
Chicago/Turabian StyleAkita, Hironaga, Daisuke Moriguchi, and Akinori Matsushika. 2025. "Characterization of Low pH and Inhibitor Tolerance Capacity of Candida krusei Strains" Fermentation 11, no. 3: 146. https://doi.org/10.3390/fermentation11030146
APA StyleAkita, H., Moriguchi, D., & Matsushika, A. (2025). Characterization of Low pH and Inhibitor Tolerance Capacity of Candida krusei Strains. Fermentation, 11(3), 146. https://doi.org/10.3390/fermentation11030146





