Abstract
Xylitol is a sugar–alcohol compound with broad applications in fields such as the food, dental, and pharmaceutical sectors. Although xylitol biosynthesis has gained attention, the current strategy for industrial xylitol production majorly relies on the chemical hydrogenation of xylose, which is energy-intensive and environmentally harmful. In this study, the toxicity of xylitol toward Escherichia coli was first examined, and the result demonstrated that Escherichia coli is robust against xylitol at 150 g/L. Genes encoding xylose reductases from different microorganisms were then selected and compared for xylitol production in different E. coli strains. The introduction of xylose reductase of Zymomonas mobilis, driven by the constitutive strong promoter Pgap or Pgap-6M into E. coli, resulted in the accumulation of xylitol at a titer of 64.1 g/L. The increase in NADPH by overexpressing the soluble pyridine nucleotide transhydrogenase encoded by sthA improved the xylitol titer to 83.5 g/L. Seven genes encoding xylose transporters, such as XylE and XylFGH, as well as five mutants of the xylose symporter Glf were then overexpressed and compared for xylitol production. Mutant glfL445I exhibited the highest improvement in xylitol production at a titer of 88.4 ± 0.7 g/L and a yield of 0.95 g/g. Our study thus demonstrated that xylose reductase derived from Z. mobilis is the best one for xylitol production in E. coli, and xylitol production can be further improved by combining diverse metabolic engineering strategies. Our study, thus, provides efficient xylose reductase and a recombinant strain for future industrial xylitol production.
1. Introduction
Xylitol is a pentose sugar alcohol with important application prospects in fields such as the food, dental, feed, and pharmaceutical sectors because of its strong sweetening properties, low calorie content, and lack of carcinogenic and cariostatic properties [,,]. In addition, xylitol is considered as one of the twelve major high-value intermediates as a building block for a variety of basic chemicals []. As a mainstream functional sugar alcohol product, global xylitol is expected to expand further, especially in the health sector, with a demand of 200 billion from 2021 to 2026 [,]. Currently, the industrial production of xylitol relies on the chemical hydrogenation of xylose, which is energy-intensive and environmentally harmful [,,]. Therefore, a biological process with high selectivity may be an alternative environmentally friendly process with a low energy demand []. For example, xylitol production by microorganisms uses the sustainable lignocellulosic biomass material containing abundant sugars of glucose and xylose [].
Various microorganisms, including bacteria, fungi, and yeasts, possess the intrinsic ability to synthesize xylitol through xylose metabolism, and have been applied for xylitol production []. Yeast strains such as Candida, Kluyveromyces and Debaromyces are naturally able to convert xylose into xylitol with high efficiency [,,]. In addition, metabolic engineering strategies, including overexpressing key enzymes, improving cofactor regeneration, and removing competing pathways, have been applied to improve xylose utilization and xylitol production in other microorganisms, such as E. coli, Corynebacterium glutamicum, and Bacillus subtilis [,,,]. The introduction of efficient xylose transporters into the host can further boost xylitol production [,,,,,,,,,].
Since xylitol biosynthesis pathway involves the reduction of xylose to xylitol by xylose reductase, which uses cofactors like NADPH [], it is necessary to explore efficient and stable xylose reductases (XR), as well as an effective system for cofactor regeneration [], to improve xylitol production. The model bacterial species E. coli is often used as a chassis for efficient bulk chemicals production, such as L-alanine [], 1,3-PDO [], D-lactic acid [], succinic acid [], and 1,4-butanediol [] due to its rapid growth, simple nutritional requirements, ease of genetic manipulation, and well-understood metabolic pathways []. There has been considerable research and progress regarding the production of xylitol from xylose and lignocellulosic hydrolysates with recombinant E. coli []. For example, an effective recombinant strain was constructed and used for xylitol production from hemicellulosic hydrolysates with a titer of 150 g/L at a productivity of 1.40 g/L/h [].
In this study, an integrated strategy was developed to improve xylitol production, including the introduction and comparison of xylose reductases from different species, increase in cofactor NADPH supply, and improvement of xylose transportation by examining and selecting efficient xylose transporters (Figure 1).
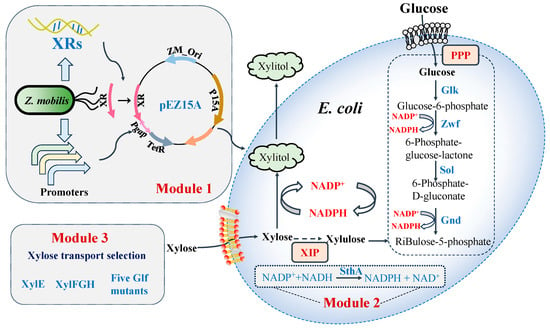
Figure 1.
Metabolic pathway and engineering strategy for xylitol production in E. coli. Glk: glucokinase, Gnd: 6-phosphogluconate dehydrogenase, Sol: 6-phosphogluconolactonase, SthA: pyridine nucleotide transhydrogenase, XIP: xylose isomerase pathway, XR, xylose reductase, Zwf: NADP+-dependent glucose-6-phosphate dehydrogenase.
2. Materials and Methods
2.1. Strains, Media, and Cultural Condition
E. coli DH5α was used for plasmid construction and E. coli trans110 was used as the host for plasmid demethylation. All E. coli strains were cultured in a Luria–Bertani medium (LB, 10 g/L tryptone, 5 g/L yeast extract, 10 g/L NaCl and 1.5% agar for solid) at 37 °C, 250 rpm []. Z. mobilis ZM4 was used as the parental strain, and cultured in rich medium (RMG2, 10 g/L yeast extract, 20 g/L glucose, 2 g/L KH2PO4 and 1.5% agar for solid) at 30 °C. For xylitol production in E. coli, xylose was supplemented LB medium as a fermentation medium (LBX). When necessary, 100 µg/mL spectinomycin was added to select recombinant strains and maintain plasmid stability during the process of xylitol production.
For xylitol toxicity investigation, seed cultures of E. coli were incubated into LB medium with different xylitol concentration (0, 50, 100, 125, 150 g/L) addition. During fermentation, cell growth in terms of the absorbance value (OD600nm) was determined at different time points. The specific growth rate was calculated using four time point including 2, 4, 6, and 8 h.
2.2. DNA Manipulation Techniques
All plasmids and strains used in this study are listed in Table 1. The shuttle plasmid pEZ15A was used for gene overexpression in E. coli []. Gene xyrA and promoter Pgap and Pgap-6M were amplificated from the genome of Z. mobilis. Overlap PCR was used for the connection between promoters and gene fragments. For all plasmid constructions, primers ordered from TsingKe (Beijing, China) containing 15~20 nucleotides overlapping regions for polymerase chain reaction (PCR) to obtain linearized gene fragments. After purification with a gel purification kit (TsingKe, Beijing, China), fragments and vectors were assembled using the T5 exonuclease (NEB, USA) method, as previously described [].

Table 1.
Plasmids and strains used in this work.
Competent cells were plated on LB agar plates containing appropriate antibiotics. Correct recombinants were selected by colony PCR and confirmed by Sanger sequencing (Sangon Biotech Co., Ltd., Shanghai, China). The universal primers 15A-fwd/rev were used for colony PCR to check the insert size. Colonies containing correct plasmids with expected PCR product length through colony PCR screenings were cultivated in an LB medium containing appropriate antibiotics for plasmid extraction, which was further confirmed by Sanger sequencing (Sangon Biotech Co., Ltd., Shanghai, China).
2.3. SDS-PAGE and Protein Expression
Total protein samples were prepared using a 4 × SDS sample buffer at the proper dilution. The mixture was vortexed and heated using a thermo-block for 10 min at 100 °C. The solutions were then cooled and centrifuged. Protein samples with a volume of 5 μL were loaded onto a 5–12% polyacrylamide gel and electrophoresis was performed with a constant current of 110 V (Bio-Rad Laboratories, Hercules, CA, USA). The proteins were stained with Coomassie Brilliant Blue to visualize proteins.
2.4. Batch Fermentation in Shake Flasks
Shake-flask cultures of E. coli for xylitol production contained 20 mL LB medium with 100 g/L xylose supplementation. Strains were pre-cultivated in LB medium overnight and then inoculated directly in a shake-flask with an initial OD600nm of 0.1 at 37 °C, 250 rpm. In terms of the absorbance value, cell growth was measured spectrobolometrically at 600nm (UV-1800, AOE, Shanghai, China) at different time points during fermentation. Samples were then collected and centrifuged at 13,000× g for 1 min, filtered through 0.22 μm filters, and stored at −80 °C before metabolites were quantified using high-performance liquid chromatograph (HPLC). Three replicates were used for each condition.
2.5. Analytical Procedures
Concentrations of xylose, xylitol, glucose, and ethanol in the supernatant were then determined by an HPLC system (HPLC Prominence, Shimadzu, Kyoto, Japan) equipped with a refractive index detector (RID) and a Bio-Rad Aminex HPX-87H column (Bio-Rad, USA), as described previously [,]. The column temperature was set at 60 °C and 5 mM H2SO4 solution was used as the mobile phase, with a flow rate of 0.5 mL/min.
2.6. Statistical Analyses
Data presented in the graphs are the mean ± SD of three replications calculated by the GraphPad Prism statistical software (version 8.0.1, GraphPad, La Jolla, CA, USA). One-way ANOVA analysis was performed as needed, and only a p-value ≤ 0.05 was considered as statistically significant.
3. Results
3.1. Investigation of Xylitol Toxicity to E. coli
The capability to tolerate biochemicals was considered as the major limiting factor for host microorganism selection and industrial applications. In this study, the cytotoxicity of xylitol to E. coli was first evaluated as shown in Figure 2. The cell biomass of bacteria in terms of OD600nm value decreased by nearly half, from 12.2 to 6.9, along with an increase in xylitol concentration from 0 to 150 g/L (Figure 2A). Compared to growth without xylitol, the growth rate of E. coli also decreased from 0.47 to 0.11 h−1 with 150 g/L xylitol supplementation (Figure 2B).
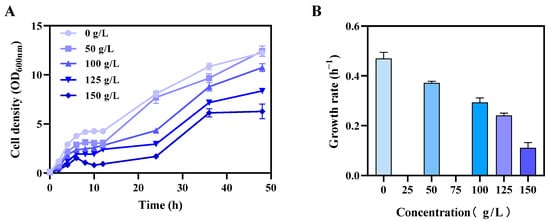
Figure 2.
Effects of xylitol on cell growth of E. coli. Cell growth curve (A) and growth rate (B) with xylitol supplementation at concentrations of 0, 50, 100, 125, 150 g/L. E. coli was cultured in LB medium at 37 °C, 250 rpm. Values are the means of experiments with three technical replicates, and bars are standard deviations.
These results, in general, indicate that E. coli could be an ideal host microorganism for xylitol production due to their high tolerance to xylitol. However, a high concentration of xylitol still affected the cell growth of E. coli, which could potentially be optimized for xylitol tolerance through adaptative laboratory evolution and metabolic engineering to increase robustness and productivity [].
3.2. Selection and Comparison of Xylose Reductases from Different Microorganisms for Efficient Xylitol Producers of E. coli
Xylose reductase (XR) is an oxidoreductase that plays a critical role in the microbial fermentation of xylose to produce xylitol. A xylose reductase from Z. mobilis based on the cell extract activity using NADPH as electron donor has been discovered and characterized [], but has not yet been applied for xylitol production. Xylose reductase from Candida tropicalis encoded by GRA1 is mostly used for xylitol production []. In this study, comparing for fermentation efficiency of the gene GRA1, recombinant strains with xyrA driven by constitutive strong promoters were expressed in E. coli (Supplementary Figure S1). The results showed that the recombinant strains containing xyrA gene from Z. mobilis exhibited good xylitol production efficiency with 14.9 ± 0.8 g/L xylitol produced 36 h post-fermentation in an RMG2X2 medium and no xylitol production in the strain containing the GRA1 gene (Supplementary Figure S1), while a small portion of xylose was utilized for endogenous metabolism, including the xylose isomerization pathway (XI) (Figure 1). Therefore, all subsequent experiments will use the xylose reductase (XR) gene xyrA from Z. mobilis. Furthermore, four different E. coli strains, BL21, Trans110, DH5α, T1, were evaluated as host stains for xylitol production because promoter compatibility and metabolite production capability can be significantly different among different host strains. Among these strains examined, BL21 containing pX1 plasmids showed the highest xylitol titer of 11.3 ± 0.80 g/L within 30 h in a medium containing initial xylose concentration of 20 g/L (Figure 3A). This may be due to the BL21 strain knocking out the two proteases Lon and OmpT, which can preferentially avoid the degradation of the XyrA protein []. Additionally, the methylation-deficient strain Ttans110 exhibited lower xylitol production, which may be due to the methylation modification of plasmids affecting the expression of foreign genes. Thus, BL21 was chosen as a host for subsequent xylitol production.
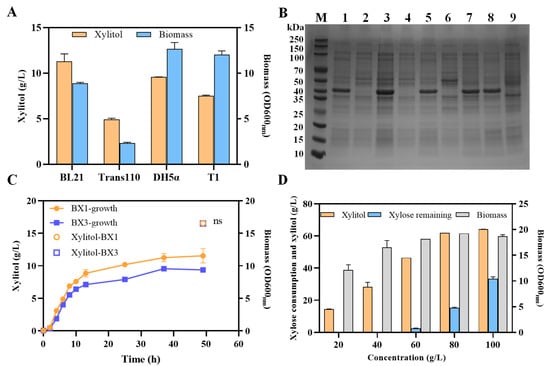
Figure 3.
The selection and application of xylose reductases, promoters, and host strains for xylose production. Xylitol titer and final biomass obtained with xylose reductase xyrA in different E. coli strains in RMG2X2 medium (A). SDS-PAGE analysis and purified XyrA driven by Pgap promoter in E coli BL21 (B). Cell growth and xylitol production with xyrA gene overexpression driven by strong promoter Pgap or Pgap-6M in E. coli in RMG2X2 medium (C). Effect of xylose concentration on xylitol production using the recombinant strain BX1 (D). Lane M: protein marker, Lane 1: T1 containing pX1 plasmid, Lane 2: T1 containing empty vector, Lane 3: DH5α containing pX1 plasmid, Lane 4: DH5α containing empty vector, Lane 5: Trans110 containing pX1 plasmid, Lane 6: Trans110 containing empty vector, Lane 7: BL21 containing pX1 plasmid, Lane 8: BL21 containing pX3 plasmid, Lane 9: BL21 containing empty vector. Error bars represent the standard deviation values obtained in triplicate experiments.
In addition, the strength and compatibility of promoters plays a crucial role in controlling the expression of target gene. The strength of the Pgap promoter from Z. mobilis and mutant Pgap-6M has been identified using GFP as a reporter gene in E. coli []. To confirm the application of the above two promoters for xylitol production in E. coli, the xyrA gene was driven by Pgap or mutant Pgap-6M promoter with the conducted fermentation tests in a medium containing 20 g/L of xylose. Previous study has confirmed that mutant Pgap-6M has higher intensity in E. coli, while its intensity in Z. mobilis is lower than that of the wild-type Pgap.
The xyrA gene codes for xylose reductase (about 38 kDa) whose expression level in E. coli was analyzed by SDS-PAGE (Figure 3B), which revealed that xyrA gene driven by Z. mobilis-derived Pgap and Pgap-6M promoter was expressed successfully in different E. coli strains. The results showed that there was not any improvement in xylitol production with a tire of 16.5 g/L with the stronger promoter Pgap-6M, and the cell biomass of BX1 was higher than BX3 (Figure 3C). This may be because the XR in E. coli was already highly expressed under the original Pgap promoter, and the stronger promoter Pgap-6M could not further increase the expression level of the already highly expressed XR (Figure 3B).
To further improve xylitol production, the effect of xylose concentration on xylitol production using the recombinant strain BX1 was verified (Figure 3D, Table S1). The biomass increases with the increase in xylose concentration due to the endogenous xylose isomerase pathway, reaching its maximum at a xylose concentration of 80 g/L (Table S1). Additionally, although the concentration of xylitol at a titer of 62.2 ± 2.9 is the highest one when the initial xylose concentration reaches 100 g/L, its productivity of 1.03 g/g remains similar to that using 80 g/L xylose. The yield is lower than that of using 80 g/L xylose with a significant amount of xylose remaining (Figure 3D).
3.3. Improvement of Xylitol Production by Enhancing NADPH Supply and Xylose Transport
To further improve the xylose utilization and xylitol production, two metabolic engineering strategies including cofactor supply and xylose transport enhancement have been implemented (Figure 1). Since the xylose reductase encoded by xyrA from Z. mobilis is an NADPH-dependent enzyme, more NADPH is required to meet the needs for xylitol production []. Previous studies have demonstrated that the enhancement of cofactor NADPH contributes to elevating dehydrogenase activity and PHB production [,]. To enhance the supply of the NADPH in the heterologous xylitol synthesis pathway by increasing the expression of glucose-6-phosphate dehydrogenase encoded by the zwf gene and soluble pyridine nucleotide transhydrogenase encoded by the sthA gene, thereby promoting an increase in xylitol titer (Figure 4A). The results showed that compared to the recombinant strain BX4 overexpressing the zwf gene, the strain BX5 overexpressing the sthA gene exhibited slightly better performance in both xylitol production and xylose consumption (Figure 4B). Subsequent experiments will adopt the strategy of overexpressing the sthA gene to supply NADPH in the xylitol production pathway, further optimizing the fermentation process and improving xylitol titer.
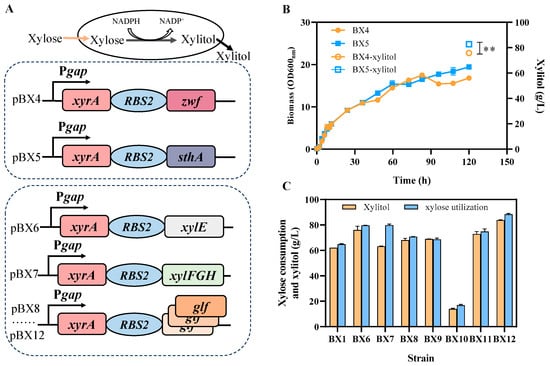
Figure 4.
Improvement of xylitol production by enhancing NADPH supply and xylose transport. Schematic of gene overexpression for enhancing NADPH supply or xylose transport to improve xylitol production, different mutants of glf are represented by different colors (A). Cell growth and xylitol production of recombinant strains of BX4 and BX5 overexpressing zwf or sthA, respectively (B). Xylose consumption and xylitol production by E. coli strains expressing different xylose transporter encoding by following gene: xylE, XylFGH, glfA18T, glfV275F, glfRD5, glf1028+T, and glfL445I (C). One-way ANOVA analysis was conducted for titer of xylitol between strain BX4 and BX5, ** represents p-value < 0.01. Error bars represent the standard deviation values obtained in triplicate experiments.
The uptake of xylose into cells is also a critical step in the entire metabolic system that converts substrate xylose into target xylitol product. Seven xylose transporters including major facilitator superfamily XylE, a high-affinity xylose ABC transporter XylFGH, and five mutants of the glucose-facilitated diffusion protein Glf (glfA18T, glfV275F, glfRD5, glf1028+T, and glfL445I) with enhanced xylose transport capabilities [] were selected and overexpressed to enhance the xylose uptake (Figure 4A). The results exhibited that the recombinant strains overexpressing the two endogenous xylose transporters XylE or XylFGH produced more xylitol, indicating that it is an effective strategy to enhance xylitol production by enhancing xylose transport (Figure 4C). Furthermore, compared with XylE, the effect of XylFGH on boosting xylitol titer was more pronounced, and three Glf mutants glfV275F, glf1028+T, and glfL445I produced higher xylitol titer than the BX1 strain, indicating that these glf mutants can enhance xylose transport efficiency in E. coli. Among the all-xylose transporters, mutant glfL445I exhibited the highest improvement in xylitol production with a titer of 88.4 ± 0.7 g/L at a yield of 0.95 g/g within 120 h.
To investigate whether co-expression of the three genes xyrA, sthA, and glfL445I can further improve xylitol production in E. coli, a recombinant expression plasmid pBX13 was constructed and transformed into E. coli to obtain strain BX13 (Supplementary Figure S2). The results indicated that the titer of xylitol from the co-expression of these three genes was actually lower than expected, which could be due to the energy demand to express three genes related to energy metabolism of cofactor balance and transport. The recombinant strain BX12 that co-expresses xyrA and glfL445I in BL21 is, thus, the one with the highest xylitol titer, which could be the chassis for further engineering to improve xylitol titer.
3.4. Effect of Oxygen to Xylitol Production
Fermentation process is a key parameter for cell growth and product biosynthesis. Although significant process has been made in the biosynthesis of xylitol through metabolic engineering in various microorganisms []. Recent study has found that low oxygenation promotes xylitol production in yeast []. Therefore, the effect of oxygen was then investigated in recombinant strain BX12 in this study (Figure 5). The results exhibited that xylitol titer was affected by the oxygen level in shake flasks. As the fermentation liquid volume decreased from 80% to 20%, the increase in oxygen content leads to an increase in xylitol production. The highest xylitol titer of 83.8 ± 0.3 g/L was achieved 120 h post-inoculation, with 20% working volume with a yield of 0.95 g/g.
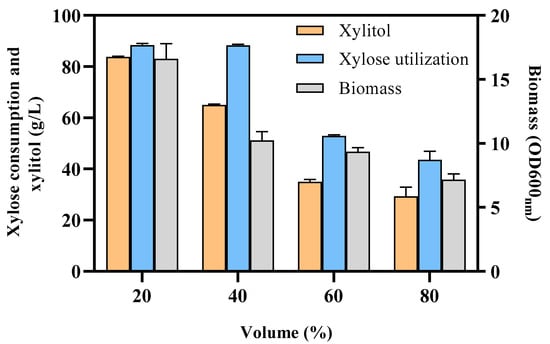
Figure 5.
Effect of oxygen supply of flask working volume on xylitol production using the recombinant strain BX12. Data are presented as mean of three replicates, and error bars represent the standard deviations.
4. Conclusions
Significant achievements have been made in constructing microbial cell factories for biochemicals production using either model or non-model microbial chassis combining systems biology, synthetic biology, and metabolic engineering strategies. However, only a few biochemical products were produced commercially, and challenges to obtain effective and compatible biological parts and chassis cells are still limiting the advancement of biomanufacturing.
Xylitol toxicity was investigated in this study indicating the suitability of E. coli strain with strong xylitol tolerance as the ideal host for xylitol production. Xylose reductases from different microorganisms were examined, and the one derived from Z. mobilis was the best in this study for xylitol production in E. coli. A recombinant E. coli strain BX12 was further engineered combining the better gene of sthA for cofactor NADPH balancing and efficient xylose transporter genes, significantly increasing xylitol production to 96.13 g/L, which provides efficient xylose reductase and recombinant strain for future improvement and industrial xylitol production.
The highest yield of xylitol has been achieved using pure xylose and the fermentation conditions were optimized in terms of oxygen supply level and organic nitrogen sources. However, xylitol production using cheap carbon sources such as lignocellulosic biomass and inexpensive nitrogen sources, such as urea, still needs to be investigated for industrial production.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/fermentation11030131/s1, Figure S1: Cell growth curve (A), xylitol production, xylose consumption, and final biomass (B) obtained with xylose reductase xyrA and Gra1 in E. coli, Figure S2: Effect of co-overexpression of advantageous genes for xylitol production. Table S1: Xylitol production with culture media of different xylose concentrations using the recombinant xylitol-producer strain BX1.
Author Contributions
Conceptualization, S.Y. and Z.H., methodology: S.Y., X.Y., L.Z. and J.L., investigation and validation, J.L., C.L. and X.Y., formal analysis and data curation, X.Y., J.L., Z.H. and S.Y., writing—original draft preparation, J.L., X.Y., writing—review and editing, X.Y., J.L., Z.H. and S.Y., supervision and project administration, S.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Key Research and Development Program of China (No. 2022YFA0911800 to SY), the International Science and Technology Cooperation base of Hubei Province (No. SH2318 to SY), the Innovation Base for Introducing Talents of Discipline of Hubei Province (No. 2019BJH021 to SY), the Technology Achievement Transformation Project of Wuhan Science and Technology Innovation Bureau (No. 2024030803010187 to SY). Funding was also supported by the State Key Laboratory of Biocatalysis and Enzyme Engineering.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Materials. Further inquiries can be directed to the corresponding authors.
Acknowledgments
We acknowledge the support from the State Key Laboratory of Biocatalysis and Enzyme Engineering.
Conflicts of Interest
The authors declare that they have a patent application associated with this study. S.Y. and L.Z. have filed a patent (CN202410887652.5) to protect strains for xylitol production, and S.Y. is the founder of Wuhan ZymoBiotech lnc and a Section Board Member of Fermentation.
References
- Wang, Y.B.; Chuang, C.Y.; Liao, J.F. Effects of xylitol in chewing gum on dental plaque and Streptococcus mutans. J. Food Drug Anal. 2020, 14, 9. [Google Scholar] [CrossRef]
- Parajó, J.C.; Domínguez, H.; Domínguez, J. Biotechnological production of xylitol. Part 1: Interest of xylitol and fundamentals of its biosynthesis. Bioresour. Technol. 1998, 65, 191–201. [Google Scholar] [CrossRef]
- Morais Junior, W.G.; Pacheco, T.F.; Trichez, D.; Almeida, J.R.M.; Gonçalves, S.B. Xylitol production on sugarcane biomass hydrolysate by newly identified Candida tropicalis JA2 strain. Yeast 2019, 36, 349–361. [Google Scholar] [CrossRef] [PubMed]
- Werpy, T.; Petersen, G. Top Value Added Chemicals from Biomass: Volume I–Results of Screening for Potential Candidates from Sugars and Synthesis Gas; National Renewable Energy Lab. (NREL): Golden, CO, USA, 2004; 76p. [Google Scholar]
- Saha, B.C.; Kennedy, G.J. Optimization of xylitol production from xylose by a novel arabitol limited co-producing Barnettozyma populi NRRL Y-12728. Prep. Biochem. Biotechnol. 2021, 51, 761–768. [Google Scholar] [CrossRef]
- Venkateswar Rao, L.; Goli, J.K.; Gentela, J.; Koti, S. Bioconversion of lignocellulosic biomass to xylitol: An overview. Bioresour. Technol. 2016, 213, 299–310. [Google Scholar] [CrossRef]
- Jain, H.; Mulay, S. A review on different modes and methods for yielding a pentose sugar: Xylitol. Int. J. Food Sci. Nutr. 2014, 65, 135–143. [Google Scholar] [CrossRef]
- Yokoyama, S.-I.; Suzuki, T.; Kawai, K.; Horitsu, H.; Takamizawa, K. Purification, characterization and structure analysis of NADPH-dependent d-xylose reductases from Candida tropicalis. J. Ferment. Bioeng. 1995, 79, 217–223. [Google Scholar] [CrossRef]
- Gallezot, P. Metal Catalysts for the Conversion of Biomass to Chemicals; Elsevier: Amsterdam, The Netherlands, 2013; pp. 1–27. [Google Scholar]
- Ahuja, V.; Bhatt, A.; Mehta, S.; Sharma, V.; Rathour, R.; Verdhan, S. Xylitol production by Pseudomonas gessardii VXlt-16 from sugarcane bagasse hydrolysate and cost analysis. Bioprocess. Biosyst. Eng. 2022, 45, 1019–1031. [Google Scholar] [CrossRef]
- Yuan, D.; Liu, B.; Jiang, L.; Chen, Y.; Xu, G.; Lin, J.; Yang, L.; Lian, J.; Jiang, Y.; Ye, L.; et al. XylR overexpression in Escherichia coli alleviated transcriptional repression by arabinose and enhanced xylitol bioproduction from xylose mother liquor. Appl. Biochem. Biotechnol. 2024, 196, 6624–6637. [Google Scholar] [CrossRef]
- Winkelhausen, E.; Kuzmanova, S. Microbial conversion of d-xylose to xylitol. J. Ferment. Bioeng. 1998, 86, 1–14. [Google Scholar] [CrossRef]
- Xu, P.; Bura, R.; Doty, S.L. Genetic analysis of D-xylose metabolism by endophytic yeast strains of Rhodotorula graminis and Rhodotorula mucilaginosa. Genet. Mol. Biol. 2011, 34, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Oh, E.J.; Ha, S.J.; Rin Kim, S.; Lee, W.H.; Galazka, J.M.; Cate, J.H.; Jin, Y.S. Enhanced xylitol production through simultaneous co-utilization of cellobiose and xylose by engineered Saccharomyces cerevisiae. Metab. Eng. 2013, 15, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Jeon, Y.J.; Shin, H.S.; Rogers, P.L. Xylitol production from a mutant strain of Candida tropicalis. Lett. Appl. Microbiol. 2011, 53, 106–113. [Google Scholar] [CrossRef]
- Yuan, X.; Tu, S.; Lin, J.; Yang, L.; Shen, H.; Wu, M. Combination of the CRP mutation and ptsG deletion in Escherichia coli to efficiently synthesize xylitol from corncob hydrolysates. Appl. Microbiol. Biotechnol. 2020, 104, 2039–2050. [Google Scholar] [CrossRef]
- Dhar, K.S.; Wendisch, V.F.; Nampoothiri, K.M. Engineering of Corynebacterium glutamicum for xylitol production from lignocellulosic pentose sugars. J. Biotechnol. 2016, 230, 63–71. [Google Scholar] [CrossRef]
- Povelainen, M.; Miasnikov, A.N. Production of xylitol by metabolically engineered strains of Bacillus subtilis. J. Biotechnol. 2007, 128, 24–31. [Google Scholar] [CrossRef]
- Bianchini, I.d.A.; Sene, L.; da Cunha, M.A.A.; Felipe, M.d.G.d.A. Short-term adaptation strategy improved xylitol production by Candida guilliermondii on sugarcane bagasse hemicellulosic hydrolysate. BioEnergy Res. 2022, 15, 1182–1194. [Google Scholar] [CrossRef]
- Guirimand, G.; Sasaki, K.; Inokuma, K.; Bamba, T.; Hasunuma, T.; Kondo, A. Cell surface engineering of Saccharomyces cerevisiae combined with membrane separation technology for xylitol production from rice straw hydrolysate. Appl. Biochem. Biotechnol. 2016, 100, 3477–3487. [Google Scholar] [CrossRef]
- Sarkar, P.; Mukherjee, M.; Goswami, G.; Das, D. Adaptive laboratory evolution induced novel mutations in Zymomonas mobilis ATCC ZW658: A potential platform for co-utilization of glucose and xylose. J. Ind. Microbiol. Biotechnol. 2020, 47, 329–341. [Google Scholar] [CrossRef]
- Ko, B.S.; Kim, D.M.; Yoon, B.H.; Bai, S.; Lee, H.Y.; Kim, J.H.; Kim, I.C. Enhancement of xylitol production by attenuation of intracellular xylitol dehydrogenase activity in Candida tropicalis. Biotechnol. Lett. 2011, 33, 1209–1213. [Google Scholar] [CrossRef]
- Hou, J.; Qiu, C.; Shen, Y.; Li, H.; Bao, X. Engineering of Saccharomyces cerevisiae for the efficient co-utilization of glucose and xylose. FEMS Yeast Res. 2017, 17, fox034. [Google Scholar] [CrossRef] [PubMed]
- Lou, J.; Wang, J.; Yang, Y.; Yang, Q.; Li, R.; Hu, M.; He, Q.; Du, J.; Wang, X.; Li, M.; et al. Development and characterization of efficient xylose utilization strains of Zymomonas mobilis. Biotechnol. Biofuels 2021, 14, 231. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Park, J.B.; Jang, S.W.; Ha, S.J. Enhanced xylitol production by mutant Kluyveromyces marxianus 36907-FMEL1 due to improved xylose reductase activity. Appl. Biochem. Biotechnol. 2015, 176, 1975–1984. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Chen, Z.; Wang, J.; Shen, W.; Li, Q.; Chen, X. Stepwise metabolic engineering of Candida tropicalis for efficient xylitol production from xylose mother liquor. Microb. Cell Fact. 2021, 20, 105. [Google Scholar] [CrossRef]
- Su, B.; Wu, M.; Zhang, Z.; Lin, J.; Yang, L. Efficient production of xylitol from hemicellulosic hydrolysate using engineered Escherichia coli. Metab. Eng. 2015, 31, 112–122. [Google Scholar] [CrossRef]
- Jin, L.-Q.; Xu, W.; Yang, B.; Liu, Z.-Q.; Zheng, Y.-G. Efficient biosynthesis of xylitol from xylose by coexpression of xylose reductase and glucose dehydrogenase in Escherichia coli. Appl. Biochem. Biotechnol. 2019, 187, 1143–1157. [Google Scholar] [CrossRef]
- Cunha, J.T.; Soares, P.O.; Romaní, A.; Thevelein, J.M.; Domingues, L. Xylose fermentation efficiency of industrial Saccharomyces cerevisiae yeast with separate or combined xylose reductase/xylitol dehydrogenase and xylose isomerase pathways. Biotechnol. Biofuels 2019, 12, 20. [Google Scholar] [CrossRef]
- de Freitas Branco, R.; Chandel, A.K.; Silva, S.S.d. Enzymatic production of xylitol: Current status and future perspectives. In D-Xylitol: Fermentative Production, Application and Commercialization; da Silva, S.S., Chandel, A.K., Eds.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 193–204. [Google Scholar]
- Liu, P.; Xu, H.; Zhang, X. Metabolic engineering of microorganisms for L-alanine production. J. Ind. Microbiol. Biotechnol. 2022, 49, kuab057. [Google Scholar] [CrossRef]
- Lee, J.H.; Lama, S.; Kim, J.R.; Park, S.H. Production of 1,3-propanediol from glucose by recombinant Escherichia coli BL21(DE3). Biotechnol. Bioprocess. Eng. 2018, 23, 250–258. [Google Scholar] [CrossRef]
- Zhou, S.; Causey, T.B.; Hasona, A.; Shanmugam, K.T.; Ingram, L.O. Production of optically pure D-lactic acid in mineral salts medium by metabolically engineered Escherichia coli W3110. Appl. Environ. Microbiol. 2003, 69, 399–407. [Google Scholar] [CrossRef]
- Zhu, X.; Tan, Z.; Xu, H.; Chen, J.; Tang, J.; Zhang, X. Metabolic evolution of two reducing equivalent-conserving pathways for high-yield succinate production in Escherichia coli. Metab. Eng. 2014, 24, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Yim, H.; Haselbeck, R.; Niu, W.; Pujol-Baxley, C.; Burgard, A.; Boldt, J.; Khandurina, J.; Trawick, J.D.; Osterhout, R.E.; Stephen, R.; et al. Metabolic engineering of Escherichia coli for direct production of 1,4-butanediol. Nat. Chem. Biol. 2011, 7, 445–452. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; He, Q.; Geng, B.; Yang, S. Microbial cell factories in the bioeconomy era: From discovery to creation. Biodes. Res. 2024, 6, 0052. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Wang, X.; Yang, Y.; Wang, Z.; Zhang, H.; Li, Y.; He, Q.; Li, M.; Yang, S. Cysteine supplementation enhanced inhibitor tolerance of Zymomonas mobilis for economic lignocellulosic bioethanol production. Bioresour. Technol. 2022, 349, 126878. [Google Scholar] [CrossRef]
- Zheng, Y.; Han, J.; Wang, B.; Hu, X.; Li, R.; Shen, W.; Ma, X.; Ma, L.; Yi, L.; Yang, S.; et al. Characterization and repurposing of the endogenous type I-F CRISPR-Cas system of Zymomonas mobilis for genome engineering. Nucleic Acids Res. 2019, 47, 11461–11475. [Google Scholar] [CrossRef]
- Qiu, M.; Shen, W.; Yan, X.; He, Q.; Cai, D.; Chen, S.; Wei, H.; Knoshaug, E.P.; Zhang, M.; Himmel, M.E.; et al. Metabolic engineering of Zymomonas mobilis for anaerobic isobutanol production. Biotechnol. Biofuels 2020, 13, 15. [Google Scholar] [CrossRef]
- Yang, Q.; Yang, Y.; Tang, Y.; Wang, X.; Chen, Y.; Shen, W.; Zhan, Y.; Gao, J.; Wu, B.; He, M.; et al. Development and characterization of acidic-pH-tolerant mutants of Zymomonas mobilis through adaptation and next-generation sequencing-based genome resequencing and RNA-Seq. Biotechnol. Biofuels 2020, 13, 144. [Google Scholar] [CrossRef]
- Agrawal, M.; Chen, R.R. Discovery and characterization of a xylose reductase from Zymomonas mobilis ZM4. Biotechnol. Lett. 2011, 33, 2127–2133. [Google Scholar] [CrossRef]
- Bianchini, I.d.A.; Jofre, F.M.; Queiroz, S.d.S.; Lacerda, T.M.; Felipe, M.d.G.d.A. Relation of xylitol formation and lignocellulose degradation in yeast. Appl. Microbiol. Biotechnol. 2023, 107, 3143–3151. [Google Scholar] [CrossRef]
- Salunkhe, S.S.; Raiker, V.A.; Rewanwar, S.; Kotwal, P.; Kumar, A.; Padmanabhan, S. Enhanced fluorescent properties of an OmpT site deleted mutant of green fluorescent protein. Microb. Cell Fact. 2010, 9, 26. [Google Scholar] [CrossRef]
- Song, H.; Yang, Y.; Li, H.; Du, J.; Hu, Z.; Chen, Y.; Yang, N.; Mei, M.; Xiong, Z.; Tang, K.; et al. Determination of Nucleotide Sequences within Promoter Regions Affecting Promoter Compatibility between Zymomonas mobilis and Escherichia coli. ACS Synth. Biol. 2022, 11, 2811–2819. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, Y.; Wang, R.X.; Yan, X.Y.; Wang, J.W.; Wang, X.; Chen, S.W.; Bai, F.W.; He, Q.N.; Yang, S.H. Metabolic engineering of Zymomonas mobilis for continuous co-production of bioethanol and poly-3-hydroxybutyrate (PHB). Green Chem. 2022, 24, 2588–2601. [Google Scholar] [CrossRef]
- Wang, X.; Gao, Q.; Bao, J. Enhancement of furan aldehydes conversion in Zymomonas mobilis by elevating dehydrogenase activity and cofactor regeneration. Biotechnol. Biofuels 2017, 10, 24. [Google Scholar] [CrossRef] [PubMed]
- Dunn, K.L.; Rao, C.V. Expression of a xylose-specific transporter improves ethanol production by metabolically engineered Zymomonas mobilis. Appl. Microbiol. Biotechnol. 2014, 98, 6897–6905. [Google Scholar] [CrossRef]
- Lekshmi Sundar, M.S.; Madhavan Nampoothiri, K. An overview of the metabolically engineered strains and innovative processes used for the value addition of biomass derived xylose to xylitol and xylonic acid. Bioresour. Technol. 2022, 345, 126548. [Google Scholar] [CrossRef]
- Ranieri, R.; Candeliere, F.; Moreno-Garcia, J.; Mauricio, J.C.; Rossi, M.; Raimondi, S.; Amaretti, A. Fermentative processes for the upcycling of xylose to xylitol by immobilized cells of Pichia fermentans WC1507. Front. Bioeng. Biotechnol. 2024, 12, 1339093. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).