Production of Bio-Improved Butter with Lactic Acid Bacteria Isolated from Traditional Cheese Matrix and Eye Fluid
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
Strains
2.2. Methods
2.2.1. Preparation of Butter Samples
2.2.2. pH Measurement
2.2.3. Viability Proportion Index (VPI)
2.2.4. Aroma Compounds Analysis
2.2.5. Textural Analysis
2.2.6. Quantitative Descriptive Analysis (QDA)
2.2.7. Statistical Analysis
3. Results and Discussion
3.1. Acidification and Microorganism Viability
3.1.1. Textural Properties
3.1.2. Aroma Compounds
3.1.3. Sensory Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ozcan, T.; Kurdal, E. The Effects of Using a Starter Culture, Lipase, and Protease Enzymes on Ripening of Mihalic Cheese. Int. J. Dairy Technol. 2012, 65, 585–593. [Google Scholar] [CrossRef]
- Aday, S.; Karagul-Yuceer, Y. Physicochemical and Sensory Properties of Mihalic Cheese. Int. J. Food Prop. 2014, 17, 2207–2227. [Google Scholar] [CrossRef]
- Turk Patent. Manyas Kelle Cheese Registration (Registration Number: 628). Turk Patent and Trademark Office 2020. Available online: https://www.ci.gov.tr/ (accessed on 8 June 2025).
- Hayaloglu, A.A.; Ozer, B.H.; Fox, P.F. Cheeses of Turkey: 2. Varieties Ripened under Brine. Dairy Sci. Technol. 2008, 88, 225–244. [Google Scholar] [CrossRef]
- Keser, G.; Ciniviz, M.; Baskaya Aral, I.; Ozcan, T.; Ersan Yılmaz, L.; Copur, U.O.; Kaner, O. Effect of Salt Concentration on the Structure and Preference of Mihalic Cheese. In Proceedings of the ICENS 7th International Conference on Engineering and Natural Science, Villahermosa, Mexico, 23–27 June 2021; pp. 298–303. [Google Scholar]
- Tırpanci Sivri, G.; Oksuz, O. Identification of Propionibacterium spp. Isolated from Mihalic Cheeses by MALDI-TOF MS. J. Tekirdag Agric. Fac. 2019, 16, 244–250. [Google Scholar] [CrossRef]
- Ozer, E.; Kesenkas, H. The Effect of Using Different Starter Culture Combinations on Ripening Parameters, Microbiological and Sensory Properties of Mihalic Cheese. J. Food Sci. Technol. 2019, 56, 1202–1211. [Google Scholar] [CrossRef]
- Bulut, B. Changes in Chemical Composition and Microbial Flora of Mihalic Cheeses That Produced from Raw and Pasteurized Milk During Ripening Periods. Master’s Thesis, Selcuk University, Konya, Turkiye, 2006. [Google Scholar]
- Camara, S.P.D.A.; Dias, C.M.; Rocha, L.; Dapkevicius, A.; Rosa, H.J.D.; de Borba, A.E.S.; Silveira, M.D.G.; Malcata, F.X.; Dapkevicius, M.D.L.E. Assessment of Autochthonous Lactic Acid Bacteria as Starter Cultures for Improved Manufacture of Pico Cheese Using a Cheese Model. Int. Dairy J. 2022, 128, 105294. [Google Scholar] [CrossRef]
- Durango Zuleta, M.M.; Grisales Rojas, L.F.; Sepúlveda Valencia, J.U.; Valdés Duque, B.E.; Moreno-Herrera, C.X. Growth Kinetics of Autochthonous Lactic Acid Bacteria Isolated from Double Cream Cheese as Potential Starter Culture. TecnoLógicas 2023, 26, e206. [Google Scholar] [CrossRef]
- Rampanti, G.; Cantarini, A.; Cardinali, F.; Milanović, V.; Garofalo, C.; Aquilanti, L.; Osimani, A. Technological and Enzymatic Characterization of Autochthonous Lactic Acid Bacteria Isolated from Viili Natural Starters. Foods 2024, 13, 1115. [Google Scholar] [CrossRef]
- Keser, G.; Ozcan, T. Cross-Over Fermentation Dynamics and Proteomic Properties of Acid Gels with Indigenous Lactobacillus spp. Isolated from Cheeses. Food Microbiol. 2025, 128, 104700. [Google Scholar]
- Liu, A.; Liu, Q.; Bu, Y.; Hao, H.; Liu, T.; Gong, P.; Zhang, L.; Tian, H.; Yi, H. Aroma Classification and Characterization of Lactobacillus delbrueckii subsp. bulgaricus Fermented Milk. Food Chem. X 2022, 15, 100385. [Google Scholar] [CrossRef]
- Guo, S.; Chen, M.; Wu, T.; Liu, K.; Zhang, H.; Wang, J. Probiotic Bifidobacterium animalis ssp. lactis Probio-M8 Improves the Properties and Organic Acid Metabolism of Fermented Goat Milk. J. Dairy Sci. 2022, 105, 9426–9438. [Google Scholar] [CrossRef] [PubMed]
- Ceylan, O.; Ozcan, T. Effect of the Cream Cooling Temperature and Acidification Method on the Crystallization and Textural Properties of Butter. LWT–Food Sci. Technol. 2020, 132, 109806. [Google Scholar] [CrossRef]
- Akgun, A. Improving the Acidification Kinetics, Structural and Sensory Properties of Low-Fat Yoghurt Using High-Pressure Homogenization Treatment. Mljekarstvo 2024, 74, 33–44. [Google Scholar] [CrossRef]
- Hussin, F.S.; Chay, S.Y.; Hussin, A.S.M.; Wan Ibadullah, W.Z.; Muhialdin, B.J.; Abd Ghani, M.S.; Saari, N. GABA Enhancement by Simple Carbohydrates in Yoghurt Fermented Using Novel, Self-Cloned Lactobacillus plantarum Taj-Apis362 and Metabolomics Profiling. Sci. Rep. 2021, 11, 9417. [Google Scholar] [CrossRef]
- Kurtuldu, O.; Ozcan, T. Effect of β-Glucan on the Properties of Probiotic Set Yoghurt with Bifidobacterium animalis subsp. lactis Strain Bb-12. Int. J. Dairy Technol. 2018, 71, 157–166. [Google Scholar] [CrossRef]
- Zianni, R.; Mentana, A.; Tomaiuolo, M.; Campaniello, M.; Iammarino, M.; Centonze, D.; Palermo, C. Volatolomic Approach by HS-SPME/GC–MS and Chemometric Evaluations for the Discrimination of X-Ray Irradiated Mozzarella Cheese. Food Chem. 2023, 423, 136239. [Google Scholar] [CrossRef]
- Lucan, M.; Ranilović, J.; Slačanac, V.; Cvetković, T.; Primorac, L.; Gajari, D.; Obrdalj, H.T.; Jukic, M.; Čačić, J.L. Physico-Chemical Properties, Spreadability and Consumer Acceptance of Low-Sodium Cream Cheese. Mljekarstvo/Dairy 2020, 70, 13–27. [Google Scholar] [CrossRef]
- ISO 8586:2012; Sensory Analysis—General Guidelines for the Selection, Training and Monitoring of Selected Assessors and Expert Sensory Assessors. International Organization for Standardization: Geneva, Switzerland, 2012.
- Nasr, N.M.; Abd-Alhalim, L.R. Characterization and Identification of Lactobacillus rhamnosus and Enterococcus durans as Probiotic Potential Isolated from Selected Dairy Products in Egypt. J. Umm Al-Qura Univ. Appl. Sci. 2024, 10, 168–177. [Google Scholar] [CrossRef]
- Fuso, A.; Bancalari, E.; Castellone, V.; Caligiani, A.; Gatti, M.; Bottari, B. Feeding Lactic Acid Bacteria with Different Sugars: Effect on Exopolysaccharides (EPS) Production and Their Molecular Characteristics. Foods 2023, 12, 215. [Google Scholar] [CrossRef]
- Han, N.R.; Byun, J.A.; Yu, S.; Yun, E.J.; Cheon, S.; Song, S.; Shim, S.; Choi, I.-G.; Lee, S.-H.; Kim, K.H. Evolution-Aided Improvement of the Acid Tolerance of Levilactobacillus brevis and Its Application in Sourdough Fermentation. Food Res. Int. 2024, 190, 114584. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Kim, J.H.; Kim, J.A.; Park, S.Y. Application of Lactobacillus brevis B7 Isolated from Kimchi to Ginseng-Supplemented Yogurt: Physicochemical and Sensory Properties. Korean J. Food Sci. Anim. Resour. 2022, 42, 252–265. [Google Scholar] [CrossRef]
- Sebastián Nicolás, J.L.; Contreras López, E.; Ramírez Godínez, J.; Cruz Guerrero, A.E.; Rodríguez Serrano, G.M.; Añorve Morga, J.; Jaimez Ordaz, J.; Castañeda Olid, A.; Pérez Escalante, E.; Ayala Niño, A.; et al. Milk Fermentation by Lacticaseibacillus rhamnosus GG and Streptococcus thermophilus SY 102: Proteolytic Profile and ACE-Inhibitory Activity. Fermentation 2021, 7, 215. [Google Scholar] [CrossRef]
- Fan, X.; Du, L.; Xu, J.; Shi, Z.; Zhang, T.; Jiang, X.; Zeng, X.; Wu, Z.; Pan, D. Effect of Single Probiotics Lacticaseibacillus casei CGMCC1.5956 and Levilactobacillus brevis CGMCC1.5954 and Their Combination on the Quality of Yogurt as Fermented Milk. LWT—Food Sci. Technol. 2022, 163, 113530. [Google Scholar] [CrossRef]
- Sezer, E.; Ayar, A.; Yilmaz, S.O. Fermentation of Dietary Fibre-Added Milk with Yoghurt Bacteria and L. rhamnosus and Use in Ice Cream Production. Fermentation 2022, 9, 3. [Google Scholar] [CrossRef]
- de Souza Oliveira, R.P.; Perego, P.; de Oliveira, M.N.; Converti, A. Effect of Inulin on the Growth and Metabolism of a Probiotic Strain of Lactobacillus rhamnosus in Co-Culture with Streptococcus thermophilus. LWT 2012, 47, 358–363. [Google Scholar] [CrossRef]
- Costa, T.J.N.; Costa, I.M.; Magalhães, L.M.M.; de Souza, M.R.; Rossi, G.A.M.; Salotti-Souza, B.M.; Fante, C.A. Technological Assessment and Predictive Modeling of Probiotic Lactose-Free Fermented Milk with Lacticaseibacillus paracasei GV17. Foods 2025, 14, 1176. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz-Ersan, L.; Ozcan, T.; Akpinar-Bayizit, A.; Turan, M.A.; Taskin, M.B. Probiotic Cream: Viability of Probiotic Bacteria and Chemical Characterization. J. Food Process. Preserv. 2017, 41, e12797. [Google Scholar] [CrossRef]
- Hurtado-Romero, A.; Zepeda-Hernández, A.; Cárdenas-Rangel, J.; Aguilar-Márquez, R.; Garcia-Amezquita, L.E.; Carrillo-Nieves, D.; García-Cayuela, T. Frozen Fermented Dairy Snacks with Probiotics and Blueberry Bagasse: Stability, Bioactivity, and Digestive Viability. Microorganisms 2025, 13, 86. [Google Scholar] [CrossRef] [PubMed]
- Lecuyer, S.; Rusconi, R.; Shen, Y.; Forsyth, A.; Vlamakis, H.; Kolter, R.; Stone, H.A. Shear Stress Increases the Residence Time of Adhesion of Pseudomonas aeruginosa. Biophys. J. 2011, 100, 341–350. [Google Scholar] [CrossRef]
- Ferreira, L.; Borges, A.; Gomes, D.; Dias, S.; Pereira, C.; Henriques, M. Adding Value and Innovation in Dairy SMEs: From Butter to Probiotic Butter and Buttermilk. J. Food Process. Preserv. 2020, 46, 14867. [Google Scholar] [CrossRef]
- Settachaimongkon, S.; van Valenberg, H.J.F.; Winata, V.; Wang, X.; Nout, M.R.; van Hooijdonk, T.C.M.; Smid, E.J.; Zwietering, M.H. Effect of Sublethal Preculturing on the Survival of Probiotics and Metabolite Formation in Set-Yoghurt. Food Microbiology 2015, 49, 104–115. [Google Scholar] [CrossRef]
- Chen, S.; Niu, H.; Wu, Y.; Sun, J.; Han, X.; Zhang, L. Influence of Lactic Acid on Cell Cycle Progressions in Lactobacillus bulgaricus during Batch Culture. Appl. Biochem. Biotechnol. 2021, 193, 912–924. [Google Scholar] [CrossRef]
- Mortensen, B.K. Butter and Other Milk Fat Products. In Encyclopedia of Dairy Sciences, 2nd ed.; Fuquay, J.W., Fox, P.F., McSweeney, P.L.H., Eds.; Academic Press: Cambridge, MA, USA, 2011; pp. 492–499. [Google Scholar]
- Chávez de la Vega, M.I.; Alatorre-Santamaría, S.; Gómez-Ruiz, L.; García-Garibay, M.; Guzmán-Rodríguez, F.; González-Olivares, L.G.; Cruz-Guerrero, A.E.; Rodríguez-Serrano, G.M. Influence of Oat β-Glucan on the Survival and Proteolytic Activity of Lacticaseibacillus rhamnosus GG in Milk Fermentation: Optimization by Response Surface. Fermentation 2021, 7, 210. [Google Scholar] [CrossRef]
- Oleksy-Sobczak, M.; Górska, S.; Piekarska-Radzik, L.; Ścieszka, S.; Klewicka, E. Exopolysaccharides Synthesized by Lacticaseibacillus rhamnosus ŁOCK 0943: Structural Characteristics and Evaluation of Biological and Technological Properties. Processes 2024, 12, 1192. [Google Scholar] [CrossRef]
- Silva, T.; Pires, A.; Gomes, D.; Viegas, J.; Pereira-Dias, S.; Pintado, M.; Henriques, M.; Pereira, C. Sheep’s Butter and Correspondent Buttermilk Produced with Sweet Cream and Cream Fermented by Aromatic Starter, Kefir and Probiotic Culture. Foods 2023, 12, 331. [Google Scholar] [CrossRef]
- Figueiroa, F.J.F.; De Marchi, F.E.; Santos, G.T.D.; Santos, W.B.R.D.; Kazama, D.C.D.S.; Leite, L.C.; Branco, A.F.; Damasceno, J.C. Production, Composition and Fatty Acid Profile of Milk and Butter Texture of Dairy Cows Fed Ground or Pelleted Concentrate with Sunflower and/or Lignosulfonate. Rev. Bras. Zootec. 2013, 42, 743–750. [Google Scholar] [CrossRef]
- Ozcan, T.; Akpinar-Bayizit, A.; Yilmaz-Ersan, L.; Cetin, K.; Delikanli, B. Evaluation of Fatty Acid Profile of Trabzon Butter. Int. J. Chem. Eng. Appl. 2016, 7, 190–194. [Google Scholar] [CrossRef]
- Ronholt, S.; Kirkensgaard, J.J.K.; Pedersen, T.B.; Mortensen, K.; Knudsen, J.C. Polymorphism, Microstructure, and Rheology of Butter: Effects of Cream Heat Treatment. Food Chem. 2012, 135, 1730–1739. [Google Scholar] [CrossRef]
- Tondhoosh, A.; Nayebzadeh, K.; Mohamadifar, A.; Homayouni-Rad, A.; Hosseinoghli, H. Industrial Application of Different Heat Treatments and Cream Fat Contents for Improving the Spreadability of Butter. Recent Pat. Food Nutr. Agric. 2016, 8, 107–115. [Google Scholar] [CrossRef]
- Chudy, S.M.; Cais-Sokolińska, D.; Tomaszewska-Gras, J. Physicochemical Characteristics and Consumers’ Preferences for Milk Fat Products. Appl. Sci. 2022, 12, 11986. [Google Scholar] [CrossRef]
- Kashaninejad, M.; Razavi, S.M.; Mazaheri Tehrani, M.; Kashaninejad, M. Effect of Extrusion Conditions and Storage Temperature on Texture, Colour and Acidity of Butter. Int. J. Dairy Technol. 2017, 70, 102–109. [Google Scholar] [CrossRef]
- Karakus, M.S.; Akgul, F.Y.; Korkmaz, A.; Atasoy, A.F. Evaluation of Fatty Acids, Free Fatty Acids and Textural Properties of Butter and Sadeyag (Anhydrous Butterfat) Produced from Ovine and Bovine Cream and Yoghurt. Int. Dairy J. 2022, 126, 105229. [Google Scholar] [CrossRef]
- Glibowski, P.; Zarzycki, P.; Krzepkowska, M. The Rheological and Instrumental Textural Properties of Selected Table Fats. Int. J. Food Prop. 2008, 11, 678–686. [Google Scholar] [CrossRef]
- Ewe, J.A.; Loo, S.Y. Effect of Cream Fermentation on Microbiological, Physicochemical and Rheological Properties of L. helveticus-Butter. Food Chem. 2016, 201, 29–36. [Google Scholar] [CrossRef]
- Ronholt, S.; Kirkensgaard, J.J.K.; Mortensen, K.; Knudsen, J.C. Effect of Cream Cooling Rate and Water Content on Butter Microstructure during Four Weeks of Storage. Food Hydrocoll. 2014, 34, 169–176. [Google Scholar] [CrossRef]
- Saleem, G.N.; Gu, R.; Qu, H.; Bahar Khaskheli, G.; Rashid Rajput, I.; Qasim, M.; Chen, X. Therapeutic Potential of Popular Fermented Dairy Products and its Benefits on Human Health. Front. Nutr. 2024, 11, 1328620. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Kristo, E.; LaPointe, G. Adding Apple Pomace as a Functional Ingredient in Stirred-Type Yogurt and Yogurt Drinks. Food Hydrocoll. 2020, 100, 105453. [Google Scholar] [CrossRef]
- Chen, X.; Gu, Z.; Peng, Y.; Quek, S.Y. What Happens to Commercial Camembert Cheese under Packaging? Unveiling Biochemical Changes by Untargeted and Targeted Metabolomic Approaches. Food Chem. 2022, 383, 132437. [Google Scholar] [CrossRef]
- Tian, H.; Yang, R.; Sun, X.; Yu, H.; Huang, J.; Yuan, H.; Lou, X.; Yuan, Z.; Chen, C. Screening of Goaty Flavor-Inhibiting Lactic Acid Bacteria and Their Effects on the Flavor Profiles of Goat Milk Cakes. Food Biosci. 2023, 53, 102504. [Google Scholar] [CrossRef]
- Gauglitz, J.M.; Aceves, C.M.; Aksenov, A.A.; Aleti, G.; Almaliti, J.; Bouslimani, A.; Brown, E.A.; Campeau, A.; Caraballo-Rodríguez, A.M.; Chaar, R.; et al. Untargeted Mass Spectrometry-Based Metabolomics Approach Unveils Molecular Changes in Raw and Processed Foods and Beverages. Food Chem. 2020, 302, 125290. [Google Scholar] [CrossRef]
- Sun, Y.; Peng, C.; Wang, J.; Guo, S.; Sun, Z.; Zhang, H. Mesopic Fermentation Contributes More to the Formation of Important Flavor Compounds and Increased Growth of Lactobacillus casei Zhang than Does High Temperature during Milk Fermentation and Storage. J. Dairy Sci. 2022, 105, 4857–4867. [Google Scholar] [CrossRef]
- Rajendran, S.; Silcock, P.; Bremer, P. Volatile Organic Compounds (VOCs) Produced by Levilactobacillus brevis WLP672 Fermentation in Defined Media Supplemented with Different Amino Acids. Molecules 2024, 29, 753. [Google Scholar] [CrossRef]
- Moiseenko, K.V.; Glazunova, O.A.; Savinova, O.S.; Shabaev, A.V.; Fedorova, T.V. Changes in Composition of Some Bioactive Molecules upon Inclusion of Lacticaseibacillus paracasei Probiotic Strains into a Standard Yogurt Starter Culture. Foods 2023, 12, 4238. [Google Scholar] [CrossRef]
- Liu, K.; Wang, D.; Yang, C.; Liu, Y.; Altankhuyag, O.E.; Yao, G. Comparative Genomics and Untargeted Metabolomics Analysis of Dairy-Derived Lacticaseibacillus rhamnosus Strains and Their Potential Applications in Dairy Fermentation. J. Dairy Sci. 2025, 7, 6814–6827. [Google Scholar] [CrossRef]
- Fox, P.F.; Guinee, T.P.; Cogan, T.M.; McSweeney, P.L.H. Fundamentals of Cheese Science, 2nd ed.; Springer: New York, NY, USA, 2017. [Google Scholar] [CrossRef]
- Clarke, H.J.; McCarthy, W.P.; O’Sullivan, M.G.; Kerry, J.P.; Kilcawley, K.N. Oxidative Quality of Dairy Powders: Influencing Factors and Analysis. Foods 2021, 10, 2315. [Google Scholar] [CrossRef]
- Panseri, S.; Soncin, S.; Chiesa, L.M.; Biondi, P.A. A Headspace Solid-Phase Microextraction Gas-Chromatographic Mass-Spectrometric Method (HS-SPME-GC/MS) to Quantify Hexanal in Butter during Storage as Marker of Lipid Oxidation. Food Chem. 2011, 127, 886–889. [Google Scholar] [CrossRef] [PubMed]
- Du, R.; Yu, L.; Yu, N.; Ping, W.; Song, G.; Ge, J. Characterization of Exopolysaccharide Produced by Levilactobacillus brevis HDE-9 and Evaluation of its Potential Use in Dairy Products. Int. J. Biol. Macromol. 2022, 217, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Bengoa, J.; da Silva, B.C.; Pagnoncelli, M.G.B.; Furlan, L.T.R.; da Silva Duarte, V.; da Silva Duarte, A.J.; Saad, S.M.I. Exopolysaccharide-Producing Lacticaseibacillus paracasei Strains Isolated from Kefir as Starter for Functional Dairy Products. Front. Microbiol. 2023, 14, 1110177. [Google Scholar] [CrossRef]
- Stefanovic, E.; Kilcawley, K.N.; Roces, C.; Rea, M.C.; O’Sullivan, M.; Sheehan, J.J.; McAuliffe, O. Evaluation of the Potential of Lactobacillus paracasei Adjuncts for Flavor Compounds Development and Diversification in Short-Aged Cheddar Cheese. Front. Microbiol. 2018, 9, 1506. [Google Scholar] [CrossRef]
- Champagne, C.P.; da Cruz, A.G.; Daga, M. Strategies to Improve the Functionality of Probiotics in Supplements and Foods. Curr. Opin. Food Sci. 2018, 22, 160–166. [Google Scholar] [CrossRef]

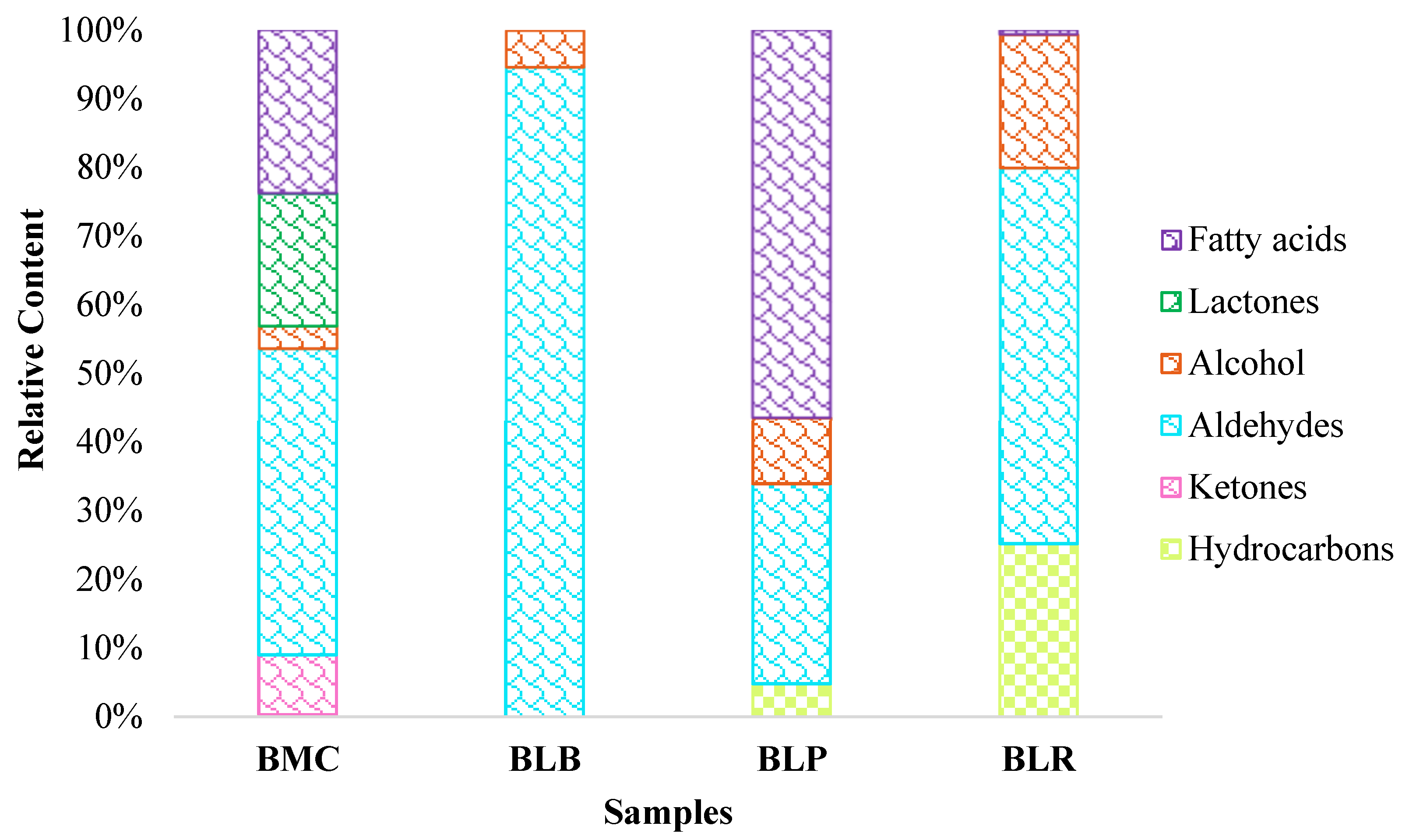
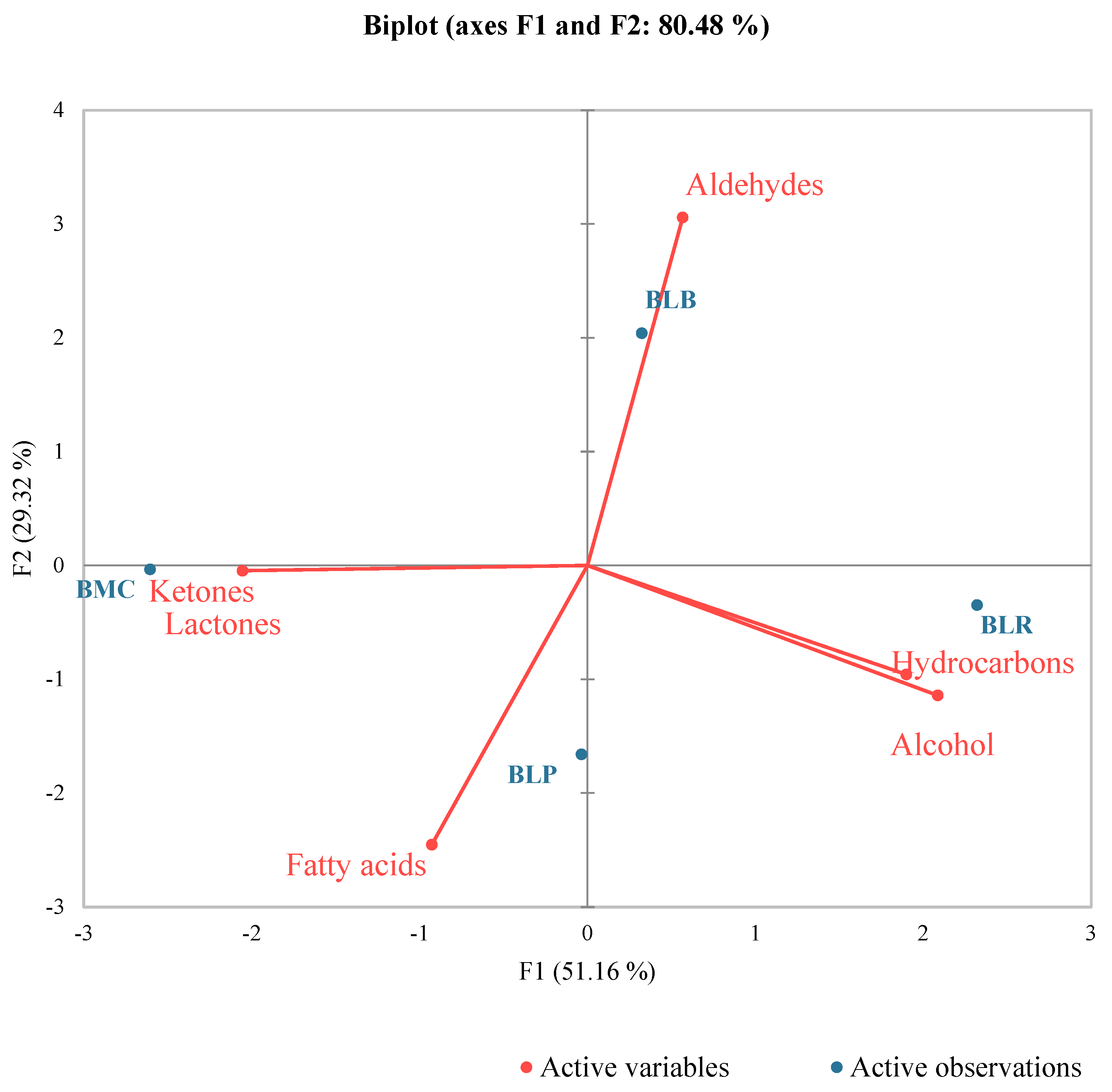
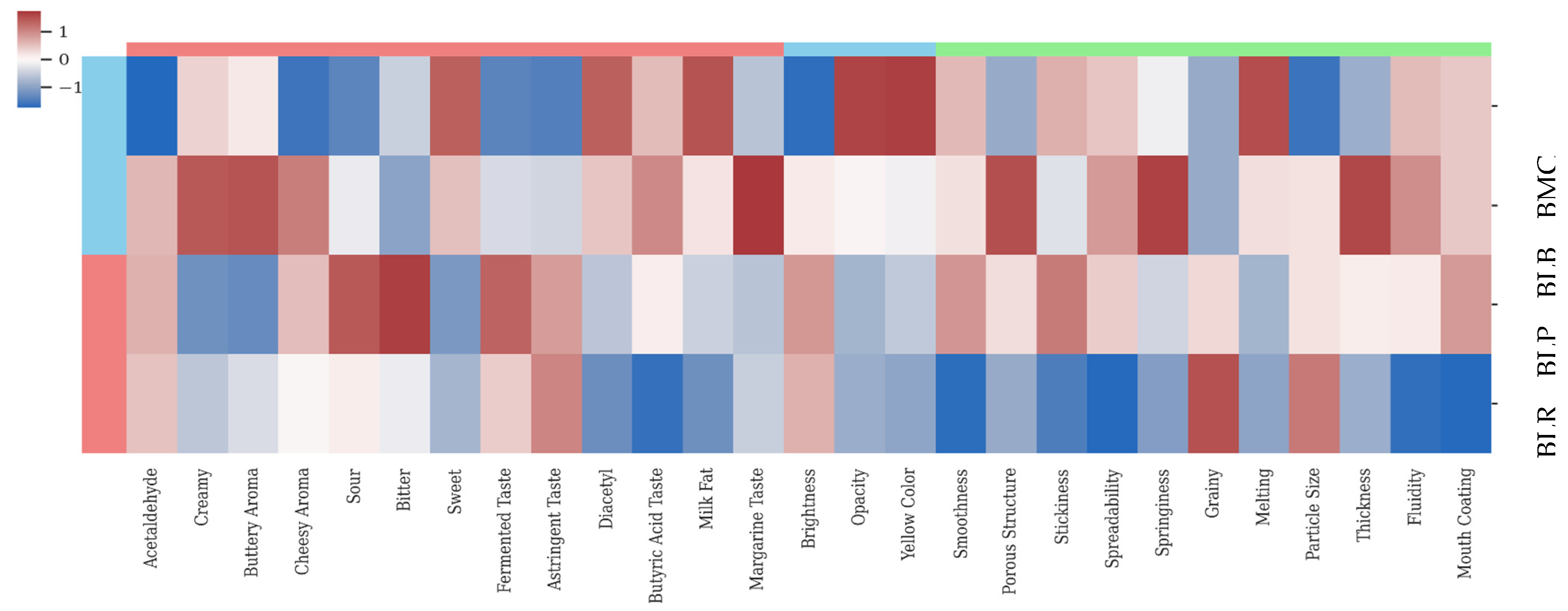
| Samples | Mixed Culture | Isolated Cultures | ||
|---|---|---|---|---|
| Lv. brevis | Ls. paracasei | Ls. rhamnosus | ||
| BMC | X | |||
| BLB | X | X | ||
| BLP | X | X | ||
| BLR | X | X | ||
| Descriptors | Definitions |
|---|---|
| Appearance Properties | |
| Opacity | The degree to which a product prevents light from passing through |
| Yellow Color | Visual perception of yellowness |
| Brightness | The surface of the sample appears bright to matte |
| Taste, Aroma, and Flavor Properties | |
| Creamy Aroma | Sweet and fatty cream |
| Cheesy Aroma | The aroma of fresh cheese |
| Buttery Aroma | Fresh or sweet cream |
| Fermented Flavor | Typical fermented yogurt aroma and taste |
| Acetaldehyde Aroma | Sharp, green, pungent, or fruity aroma, often described as green apple or unripe fruit. |
| Sweet Taste | The basic taste sensation of sugars or sweeteners |
| Sour Taste | A basic taste associated with acids such as lactic or citric acid |
| Bitter Taste | A basic taste perceived from substances like caffeine or certain peptides |
| Astringency | A drying, puckering mouthfeel |
| Diacetyl Aroma | A buttery or creamy aroma |
| Butyric Acid Aroma | The fatty acid with a strong, rancid, or cheesy odor |
| Milk Fat Aroma | The perception of natural milk fat |
| Margarine Flavor | A flavor often associated with vegetable oils |
| Textural Properties | |
| Smoothness | A texture without roughness; smooth to the touch |
| Stickiness | Sticks to any of the teeth, gums, or palate |
| Springiness | The elasticity |
| Grainy | The presence of small particles |
| Spreadability | The ease with which a product can be spread over a piece of bread |
| Porous Structure | Non-homogeny |
| Melting | Transition from solid to liquid at mouth temperature |
| Particle Size | The perceived or measured size of solid components |
| Thickness | The perceived or actual body or viscosity of the product |
| Fluidity | The ease with which a product flows, refers to the ability of a substance, particularly lipids, to flow and change shape without breaking apart. |
| Mouth Coating | The extent to which the product leaves a coating on the tongue or palate after swallowing |
| Samples | Vmax | Tvmax (min.) | pHvmax | Tend of pH 5.20 (min.) | ΔpH (Beginning of pH-Vmax pH) |
|---|---|---|---|---|---|
| BMC | 0.00483 c | 425 d | 5.33 a | 515 a | 0.98 c |
| BLB | 0.00700 b | 435 b | 5.22 b | 465 c | 1.12 a |
| BLP | 0.00500 c | 420 c | 5.31 a | 450 d | 0.92 d |
| BLR | 0.00840 a | 475 a | 5.20 b | 475 b | 1.07 b |
| p | ** | ** | ** | ** | ** |
| Samples | LAB (M17 Agar) | LAB (MRS Agar) |
|---|---|---|
| BMC | 1.084 a | 1.043 a |
| BLB | 1.077 b | 1.020 c |
| BLP | 1.081 a | 1.035 b |
| BLR | 1.008 c | 0.928 d |
| p | ** | ** |
| Texture Parameters | Samples | Storage Time (Day) | |||
|---|---|---|---|---|---|
| 1 | 30 | 60 | 90 | ||
| Firmness | BMC | 2925.48 ± 119.710 aA | 1894.64 ± 273.185 abB | 3621.18 ± 287.785 aA | 3687.59 ± 382.608 aA |
| BLB | 2887.16 ± 332.743 aA | 1659.77 ± 182.829 bcB | 1869.32 ± 148.499 bB | 2960.71 ± 198.962 aA | |
| BLP | 2290.16 ± 229.227 aA | 2198.76 ± 131.972 aA | 1589.87 ± 93.134 bB | 2450.47 ± 267.039 aA | |
| BLR | 2005.12 ± 252.595 aB | 1223.31 ± 17.240 cC | 1437.63 ± 127.938 bBC | 3760.27 ± 220.730 aA | |
| Work of Shear | BMC | 5229.89 ± 269.522 aA | 2665.12 ± 298.425 aB | 3976.62 ± 236.437 aAB | 4572.59 ± 295.459 aA |
| BLB | 4353.81 ± 271.059 abA | 2147.27 ± 198.731 abB | 2619.41 ± 220.866 bB | 3937.68 ± 145.371 aA | |
| BLP | 3143.74 ± 236.104 bcA | 2568.90 ± 140.179 aA | 2231.35 ± 151.135 bA | 3137.51 ± 295.475 aA | |
| BLR | 2916.76 ± 202.475 cB | 1402.18 ± 120.145 bD | 1957.24 ± 94.035 bC | 4189.68 ± 111.147 aA | |
| Stickiness | BMC | −2269.92 ± 49.245 aA | −1444.26 ± 122.698 aA | −2506.24 ± 146.285 aA | −2252.14 ± 196.717 aA |
| BLB | −2312.16 ± 96.589 aA | −1251.50 ± 54.667 abA | −1290.46 ± 65.189 bA | −1831.86 ± 87.644 aA | |
| BLP | −1594.58 ± 93.049 bA | −1679.03 ± 113.816 aA | −1174.73 ± 79.847 bA | −1734.93 ± 228.461 aA | |
| BLR | −1646.90 ± 129.556 bB | −884.13 ± 107.554 bC | −1069.15 ± 65.304 bBC | −2621.79 ± 126.832 aA | |
| Work of Adhesion | BMC | −424.58 ± 41.302 aA | −424.06 ± 25.990 aA | −505.96 ± 28.680 aA | −652.71 ± 54.730 aA |
| BLB | −494.48 ± 71.132 aA | −365.31 ± 33.216 aB | −416.96 ± 49.382 abAB | −475.45 ± 29.226 abA | |
| BLP | −356.00 ± 30.163 aA | −405.64 ± 32.139 aA | −348.31 ± 37.472 bcA | −300.92 ± 15.508 bA | |
| BLR | −460.17 ± 46.328 aA | −303.11 ± 38.863 aB | −258.38 ± 21.886 cB | −427.43 ± 38.656 bA | |
| BMC | BLB | BLP | BLR | |||||
|---|---|---|---|---|---|---|---|---|
| Storage Time (Day) | 1 | 90 | 1 | 90 | 1 | 90 | 1 | 90 |
| Appearance Properties | ||||||||
| Opacity | 6.40 aA | 7.80 aA | 5.20 abB | 7.20 aA | 5.20 abB | 6.40 abA | 5.80 abA | 6.00 abA |
| Yellow Color | 6.20 aB | 9.20 aA | 6.60 aB | 8.60 aA | 7.20 aA | 7.40 abA | 6.80 aA | 6.80 abA |
| Brightness | 9.40 abA | 9.00 abA | 10.00 aA | 10.00 aA | 10.40 aA | 10.60 aA | 9.60 abA | 10.60 aA |
| Taste, Aroma, and Flavor Properties | ||||||||
| Creamy Aroma | 7.80 bB | 8.40 aA | 8.40 aB | 9.20 aA | 7.60 bA | 7.80 bA | 7.46 bB | 8.80 aA |
| Cheesy Aroma | 0.00 aA | 0.00 aA | 0.00 aA | 0.00 aA | 0.00 aA | 0.20 aA | 0.00 aA | 0.00 aA |
| Buttery Aroma | 10.60 aA | 10.00 bA | 11.20 aA | 10.80 aA | 11.00 aA | 9.60 bB | 10.76 aA | 11.00 aA |
| Fermented Flavor | 5.80 abA | 5.80 bA | 6.40 aA | 7.00 aA | 7.10 aA | 8.40 aA | 6.10 aB | 8.40 aA |
| Acetaldehyde Aroma | 4.00 bB | 6.00 bA | 4.80 aB | 6.80 aA | 5.00 aB | 6.60 aA | 4.56 aB | 6.60 aA |
| Sweet Taste | 8.40 aA | 6.00 aB | 9.60 aA | 6.20 aB | 6.80 bA | 5.40 bB | 8.80 aA | 5.30 bB |
| Sour Taste | 4.30 bB | 5.80 bA | 5.40 aB | 7.00 aA | 6.40 aB | 7.80 aA | 4.32 bB | 7.00 aA |
| Bitter Taste | 0.00 bB | 2.80 aA | 0.00 bA | 0.00 bA | 0.00 bB | 3.20 aA | 0.00 bA | 0.00 bA |
| Astringency Taste | 0.67 abA | 0.52 abA | 0.91 aA | 0.82 aA | 1.19 aA | 1.10 aA | 1.24 aA | 1.26 aA |
| Diacetyl Aroma | 5.80 abA | 4.00 aB | 6.20 aA | 4.00 aB | 6.20 aA | 4.40 aB | 6.04 aA | 4.20 aB |
| Butyric Acid Taste | 0.20 aB | 2.00 aA | 0.40 aB | 2.00 aA | 0.60 aB | 1.60 aA | 0.20 aB | 1.80 aA |
| Milk Fat Aroma | 7.40 aA | 7.40 aA | 8.20 aA | 5.80 bB | 8.00 aA | 5.00 bB | 7.66 aA | 5.40 bB |
| Margarine Flavor | 0.00 bB | 1.00 aA | 1.80 aA | 0.00 bB | 0.00 bA | 0.00 bA | 0.00 bA | 0.00 bA |
| Textural Properties | ||||||||
| Smoothness | 7.40 aB | 12.60 aA | 7.80 aB | 11.60 aA | 7.80 aB | 12.40 aA | 7.80 aB | 9.20 abA |
| Porous Structure | 0.00 bA | 0.00 bA | 0.00 bB | 3.00 aA | 1.44 aA | 0.00 bB | 0.00 bA | 0.00 bA |
| Stickiness | 7.80 aB | 9.40 aA | 8.20 aA | 8.40 aA | 8.00 aB | 9.40 aA | 7.50 aB | 9.00 aA |
| Spreadability | 6.40 aB | 9.80 aA | 7.00 aB | 10.00 aA | 6.50 aB | 9.60 aA | 4.58 bB | 8.80 abA |
| Springiness | 7.60 abA | 6.60 bA | 8.40 aA | 8.20 aA | 8.40 aA | 6.00 bAB | 7.80 abA | 6.00 bAB |
| Grainy | 0.40 bB | 1.40 aA | 0.00 bB | 1.38 aA | 1.62 aA | 0.24 bB | 0.19 bB | 1.80 aA |
| Melting | 7.60 aA | 3.40 aB | 7.00 aA | 3.40 aB | 6.00 abA | 3.00 aB | 5.90 abA | 2.60 aB |
| Particle Size | 4.00 aA | 3.10 aA | 4.20 aA | 3.10 aA | 4.30 aA | 3.22 aA | 4.40 aA | 3.03 aA |
| Thickness | 4.00 aA | 3.20 aA | 4.40 aA | 3.00 aA | 4.30 aA | 3.20 aA | 4.20 aA | 3.00 aA |
| Fluidity | 4.80 aA | 3.40 aA | 5.40 aA | 3.40 aA | 5.00 aA | 3.00 aA | 4.50 aA | 2.80 aA |
| Mouth Coating | 7.80 aA | 6.40 abA | 7.40 aA | 7.20 aA | 7.30 aA | 8.25 aA | 7.10 aA | 7.20 aA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Keser, G.; Ozcan, T. Production of Bio-Improved Butter with Lactic Acid Bacteria Isolated from Traditional Cheese Matrix and Eye Fluid. Fermentation 2025, 11, 620. https://doi.org/10.3390/fermentation11110620
Keser G, Ozcan T. Production of Bio-Improved Butter with Lactic Acid Bacteria Isolated from Traditional Cheese Matrix and Eye Fluid. Fermentation. 2025; 11(11):620. https://doi.org/10.3390/fermentation11110620
Chicago/Turabian StyleKeser, Gokce, and Tulay Ozcan. 2025. "Production of Bio-Improved Butter with Lactic Acid Bacteria Isolated from Traditional Cheese Matrix and Eye Fluid" Fermentation 11, no. 11: 620. https://doi.org/10.3390/fermentation11110620
APA StyleKeser, G., & Ozcan, T. (2025). Production of Bio-Improved Butter with Lactic Acid Bacteria Isolated from Traditional Cheese Matrix and Eye Fluid. Fermentation, 11(11), 620. https://doi.org/10.3390/fermentation11110620







