The Effect of Carbon Sources on Carotenoid Synthesis by the Novel Rhodococcus corynebacterioides TAO1
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strain Isolation and Identification
2.2. Carotenoid Production
2.3. Determination of EPS Production
2.4. UV-Vis and FT-IR Spectroscopy Analyses
2.5. Statistical Analysis
3. Results and Discussion
3.1. Identification of the Bacterial Strain TAO1
3.2. The Effect of Different Carbon Sources on Carotenoid Production
3.3. The Effect of Molasses Concentration on Carotenoid Production
3.4. UV-Vis and FTIR Spectroscopy Analyses
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| EPS | Exopolysaccharide |
| FTIR | Fourier Transform Infrared Spectroscopy |
| UV-Vis | Ultra violet-visible spectrophotometer |
References
- Khaneja, R.; Perez-Fons, L.; Fakhry, S.; Baccigalupi, L.; Steiger, S.; To, E.; Sandmann, G.; Dong, T.C.; Ricca, E.; Fraser, P.D.; et al. Carotenoids found in Bacillus. J. Appl. Microbiol. 2010, 108, 1889–1902. [Google Scholar] [CrossRef]
- Cohen, A.C.; Dichiara, E.; Jofré, V.; Antoniolli, A.; Bottini, R.; Piccoli, P. Carotenoid profile produced by Bacillus licheniformis Rt4M10 isolated from grapevines grown in high altitude and their antioxidant activity. Int. J. Food Sci. Technol. 2018, 53, 2697–2705. [Google Scholar] [CrossRef]
- Das, A.; Yoon, S.H.; Lee, S.H.; Kim, J.Y.; Oh, D.K.; Kim, S.W. An update on microbial carotenoid production: Application of recent metabolic engineering tools. Appl. Microbiol. Biotechnol. 2007, 77, 505–512. [Google Scholar] [CrossRef]
- Saubenova, M.; Rapoport, A.; Venkatachalam, M.; Dufossé, L.; Yermekbay, Z.; Oleinikova, Y. Production of carotenoids by microorganisms. Fermentation 2024, 10, 502. [Google Scholar] [CrossRef]
- Gu, Z.; Deming, C.; Yongbin, H.; Zhigang, C.; Feirong, G. Optimization of carotenoids extraction from Rhodobacter sphaeroides. LWT-Food Sci. Technol. 2008, 41, 1082–1088. [Google Scholar] [CrossRef]
- Thanapimmetha, A.; Suwaleerat, T.; Saisriyoot, M.; Chisti, Y.; Srinophakun, P. Production of carotenoids and lipids by Rhodococcus opacus PD630 in batch and fed-batch culture. Bioprocess Biosyst. Eng. 2017, 40, 133–143. [Google Scholar] [CrossRef]
- Venil, C.K.; Dufossé, L.; Renuka Devi, P. Bacterial pigments: Sustainable compounds with market potential for pharma and food industry. Front. Sustain. Food Syst. 2020, 4, 100. [Google Scholar] [CrossRef]
- Crespo, T.J.; Montero, Z.; Fuentes, J.L.; Reig Garcia-Galbis, M.; Garbayo, I.; Vílchez, C.; Martínez-Espinosa, R.M. Exploring the valuable carotenoids for the large-scale production by marine microorganisms. Mar. Drugs 2018, 16, 203. [Google Scholar] [CrossRef] [PubMed]
- Samanta, A.K.; Chaudhuri, S.; Dutta, D. Antioxidant efficacy of carotenoid extract from bacterial strain Kocuria marina DAGII. Mater. Today Proc. 2016, 3, 3427–3433. [Google Scholar] [CrossRef]
- Naik, R.; Gupte, S. Characterization of pigment produced by high carotenoid yielding bacteria Paracoccus marcusii RSPO1 and evaluation of its anti-diabetic, anti-microbial and antioxidant properties. Nat. Prod. Res. 2024, 38, 968–977. [Google Scholar] [CrossRef]
- Mata-Gómez, L.C.; Montañez, J.C.; Méndez-Zavala, A.; Aguilar, C.N. Biotechnological production of carotenoids by yeasts: An overview. Microb. Cell Factories 2014, 13, 12. [Google Scholar] [CrossRef]
- Han, M.; He, Q.; Zhang, W.G. Carotenoids production in different culture conditions by Sporidiobolus pararoseus. Prep. Biochem. Biotechnol. 2012, 42, 293–303. [Google Scholar] [CrossRef]
- Maeda, I.; Inaba, A.; Koike, H.; Yoneyama, K.; Ueda, S.; Yoshida, K. Acyclic carotenoid and cyclic apocarotenoid cleavage by an orthologue of lignostilbene-α, β-dioxygenase in Rhodopseudomonas palustris. J. Biochem. 2013, 154, 449–454. [Google Scholar] [CrossRef]
- Saejung, C.; Apaiwong, P. Enhancement of carotenoid production in the new carotenoid-producing photosynthetic bacterium Rhodopseudomonas faecalis PA2. Biotechnol. Bioprocess Eng. 2015, 20, 701–707. [Google Scholar] [CrossRef]
- Ravi, G.; Venkata Dasu, V.; Pakshirajan, K. Exploring the impact of sodium acetate on lipid and carotenoid production in Rhodotorula mucilaginosa. Prep. Biochem. Biotechnol. 2025, 55, 590–605. [Google Scholar] [CrossRef] [PubMed]
- Cappelletti, M.; Presentato, A.; Piacenza, E.; Firrincieli, A.; Turner, R.J.; Zannoni, D. Biotechnology of Rhodococcus for the production of valuable compounds. Appl. Microbiol. Biotechnol. 2020, 104, 8567–8594. [Google Scholar] [CrossRef]
- Jiang, W.; Sun, J.; Gao, H.; Tang, Y.; Wang, C.; Jiang, Y.; Zhang, W.; Xin, F.; Jiang, M. Carotenoids production and genome analysis of a novel carotenoid producing Rhodococcus aetherivorans N1. Enzym. Microb. Technol. 2023, 164, 110190. [Google Scholar] [CrossRef]
- Negrete, P.S.; Ghilardi, C.; Pineda, L.R.; Pérez, E.; Herrera, M.L.; Borroni, V. Biosurfactant production by Rhodococcus ALDO1 isolated from olive mill wastes. Biocatal. Agric. Biotechnol. 2024, 57, 103106. [Google Scholar] [CrossRef]
- Liang, Y.; Yu, H. Genetic toolkits for engineering Rhodococcus species with versatile applications. Biotechnol. Adv. 2021, 49, 107748. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.K.; Lu, M.Y.; Cui, M.D.; Wang, X.; Wang, H.; Yang, Z.G.; Zhu, S.-J.; Zhou, Y.D.; Hu, G.; Hong, Q. Terrimonas soli sp. nov., isolated from farmland soil. Int. J. Syst. Evol. Microbiol. 2018, 68, 819–823. [Google Scholar] [CrossRef]
- Kabadayı, H.K.; Demiray, E.; Ertuğrul Karatay, S.; Dönmez, G. Enhanced cu (II) bioremoval by newly isolated Kluyveromyces marxianus with sodium dodecyl sulphate. Bioremediation J. 2025, 29, 204–210. [Google Scholar] [CrossRef]
- Danış, K.; Bingöl, B.N.; Kars, G. Production of biological hydrogen and bacterial carotenoids with Rhodobacter sphaeroides OU 001 in a biorefinery concept. Eurasian J. Biol. Chem. Sci. 2022, 5, 56–61. [Google Scholar] [CrossRef]
- Frengova, G.I.; Simova, E.D.; Beshkova, D.M.; Simov, Z.I. Production and monomer composition of exopolysaccharides by yogurt starter cultures. Can. J. Microbiol. 2000, 46, 1123–1127. [Google Scholar] [CrossRef]
- Fayez, D.; Youssif, A.; Sabry, S.; Ghozlan, H.; Eltarahony, M. Carotegenic Virgibacillus halodenitrificans from Wadi El-Natrun Salt Lakes: Isolation, optimization, characterization and biological activities of carotenoids. Biology 2022, 11, 1407. [Google Scholar] [CrossRef]
- Rodriguez-Concepcion, M.; Avalos, J.; Bonet, M.L.; Boronat, A.; Gomez-Gomez, L.; Hornero-Mendez, D.; Limon, M.C.; Meléndez-Martínez, A.J.; Olmedilla-Alonso, B.; Palou, A.; et al. A global perspective on carotenoids: Metabolism, biotechnology, and benefits for nutrition and health. Prog. Lipid Res. 2018, 70, 62–93. [Google Scholar] [CrossRef]
- Kars, G.; Demirel Kars, M.; Obalı, İ.; Emsen, A.; Gündüz, U. Investigation of antioxidant and cytotoxic effects of biotechnologically produced carotenoids from Rhodobacter sphaeroides OU 001. Gümüşhane Univ. J. Sci. Technol. 2020, 10, 559–568. [Google Scholar] [CrossRef]
- Çobanoğlu, Ş.; Yazıcı, A. Isolation, characterization, and antibiofilm activity of pigments synthesized by Rhodococcus sp. SC1. Curr. Microbiol. 2022, 79, 15. [Google Scholar] [CrossRef]
- Bhosale, P.; Gadre, R. Production of β-carotene by a mutant of Rhodotorula glutinis. Appl. Microbiol. Biotechnol. 2001, 55, 423–427. [Google Scholar] [CrossRef]
- Zhou, Q.; Zhang, P.; Zhang, G. Biomass and carotenoid production in photosynthetic bacteria wastewater treatment: Effects of light intensity. Bioresour. Technol. 2014, 171, 330–335. [Google Scholar] [CrossRef] [PubMed]
- Marzieh, M.N.; Sadat, M.N.S.; Elaheh, A.; Hamed, A. Carotenoid production by Rhodotorula glutinis in a substrate containing date syrup. In Proceedings of the 6th National Biotechnology Congress, Tehran, Iran, 13–15 August 2009. [Google Scholar]
- Khalil, M.A.; Sonbol, F.I.; Al-Madboly, L.A.; Aboshady, T.A.; Alqurashi, A.S.; Ali, S.S. Exploring the therapeutic potentials of exopolysaccharides derived from lactic acid bacteria and Bifidobacteria: Antioxidant, antitumor, and periodontal regeneration. Front. Microbiol. 2022, 13, 803688. [Google Scholar] [CrossRef]
- Chen, S.; Cheng, R.; Xu, X.; Kong, C.; Wang, L.; Fu, R.; Li, J.; Wang, S.; Zhang, J. The structure and flocculation characteristics of a novel exopolysaccharide from a Paenibacillus isolate. Carbohydr. Polym. 2022, 291, 119561. [Google Scholar] [CrossRef]
- Urai, M.; Anzai, H.; Ogihara, J.; Iwabuchi, N.; Harayama, S.; Sunairi, M.; Nakajima, M. Structural analysis of an extracellular polysaccharide produced by Rhodococcus rhodochrous strain S-2. Carbohydr. Res. 2006, 341, 766–775. [Google Scholar] [CrossRef]
- Urai, M.; Aizawa, T.; Anzai, H.; Ogihara, J.; Iwabuchi, N.; Neilan, B.; Couperwhite, I.; Nakajima, M.; Sunairi, M. Structural analysis of an extracellular polysaccharide produced by a benzene tolerant bacterium, Rhodococcus sp. 33. Carbohydr. Res. 2006, 341, 616–623. [Google Scholar] [CrossRef]
- Perry, M.B.; MacLean, L.L.; Patrauchan, M.A.; Vinogradov, E. The structure of the exocellular polysaccharide produced by Rhodococcus sp. RHA1. Carbohydr. Res. 2007, 342, 2223–2229. [Google Scholar] [CrossRef]
- Urai, M.; Yoshizaki, H.; Anzai, H.; Ogihara, J.; Iwabuchi, N.; Harayama, S.; Sunairi, M.; Nakajima, M. Structural analysis of mucoidan, an acidic extracellular polysaccharide produced by a pristaneassimilating marine bacterium, Rhodococcus erythropolis PR4. Carbohydr. Res. 2007, 342, 927–932. [Google Scholar] [CrossRef]
- Li, F.; Hu, X.; Li, J.; Sun, X.; Luo, C.; Zhang, X.; Li, H.; Lu, J.; Li, Y.; Bao, M. Purification, structural characterization, antioxidant and emulsifying capabilities of exopolysaccharide produced by Rhodococcus qingshengii QDR4-2. J. Polym. Environ. 2023, 31, 64–80. [Google Scholar] [CrossRef]
- Gharibzahedi, S.M.T.; Razavi, S.H.; Mousavi, M. Kinetic analysis and mathematical modeling of cell growth and canthaxanthin biosynthesis by Dietzia natronolimnaea HS-1 on waste molasses hydrolysate. RSC Adv. 2013, 3, 23495–23502. [Google Scholar] [CrossRef]
- Voaides, C.; Dima, R.J.A.Z. Effect of carbon source on carotenoid production by Rhodotorula sp. Arch. Zootech. 2011, 14, 75. [Google Scholar]
- Ram, S.; Mitra, M.; Shah, F.; Tirkey, S.R.; Mishra, S. Bacteria as an alternate biofactory for carotenoid production: A review of its applications, opportunities and challenges. J. Funct. Foods 2020, 67, 103867. [Google Scholar] [CrossRef]
- Jiang, Y.; Lv, Y.; Wu, R.; Lu, J.; Dong, W.; Zhou, J.; Zhang, W.; Xin, F.; Jiang, M. Consolidated bioprocessing performance of a two-species microbial consortium for butanol production from lignocellulosic biomass. Biotechnol. Bioeng. 2020, 117, 2985–2995. [Google Scholar] [CrossRef]
- Jiang, W.; Gao, H.; Sun, J.; Yang, X.; Jiang, Y.; Zhang, W.; Jiang, M.; Xin, F. Current status, challenges and prospects for lignin valorization by using Rhodococcus sp. Biotechnol. Adv. 2022, 60, 108004. [Google Scholar] [CrossRef]
- Styczynski, M.; Rogowska, A.; Gieczewska, K.; Garstka, M.; Szakiel, A.; Dziewit, L. Genome-based insights into the production of carotenoids by Antarctic bacteria, Planococcus sp. ANT_H30 and Rhodococcus sp. ANT_H53B. Molecules 2020, 25, 4357. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, C.; Prasastha, V.R.; Venkatachalam, M.; Dufossé, L. Natural substrates and culture conditions to produce pigments from potential microbes in submerged fermentation. Fermentation 2022, 8, 460. [Google Scholar] [CrossRef]
- Wibowo, S.; Prakoso, B.D.; Najihah, S.; Fajar, Z.H.; Widyarti, S.; Sabarudin, A.; Soeatmadji, D.W.; Sumitro, S.B. Synthesis and characterization of astaxanthin-metal ions (Cu2+ and Zn2+) complex. Rasayan J. Chem. 2021, 14, 877–886. [Google Scholar] [CrossRef]
- Naziri, D.; Hamidi, M.; Hassanzadeh, S.; Tarhriz, V.; Zanjani, B.M.; Nazemyieh, H.; Hejazi, M.A.; Hejazi, M.S. Analysis of carotenoid production by Halorubrum sp. TBZ126; an extremely halophilic archeon from Urmia Lake. Adv. Pharm. Bull. 2013, 4, 61. [Google Scholar] [CrossRef]
- Cvetković, D.; Marković, D. Stability of carotenoids toward UV-irradiation in hexane solution. J. Serbian Chem. Soc. 2008, 73, 15–27. [Google Scholar] [CrossRef]
- Henry, L.K.; Puspitasari-Nienaber, N.L.; Jarén-Galán, M.; van Breemen, R.B.; Catignani, G.L.; Schwartz, S.J. Effects of ozone and oxygen on the degradation of carotenoids in an aqueous model system. J. Agric. Food Chem. 2000, 48, 5008–5013. [Google Scholar] [CrossRef]
- Lóránd, T.; Deli, J.; Molnár, P.; Tóth, G. FT-IR study of some carotenoids. Helv. Chim. Acta 2002, 85, 1691–1697. [Google Scholar] [CrossRef]
- Meléndez-Martínez, A.J.; Stinco, C.M.; Mapelli-Brahm, P. Skin carotenoids in public health and nutricosmetics: The emerging roles and applications of the UV radiation-absorbing colourless carotenoids phytoene and phytofluene. Nutrients 2019, 11, 1093. [Google Scholar] [CrossRef]
- Sandmann, G. Carotenoid biosynthesis and biotechnological application. Arch. Biochem. Biophys. 2001, 385, 4–12. [Google Scholar] [CrossRef]

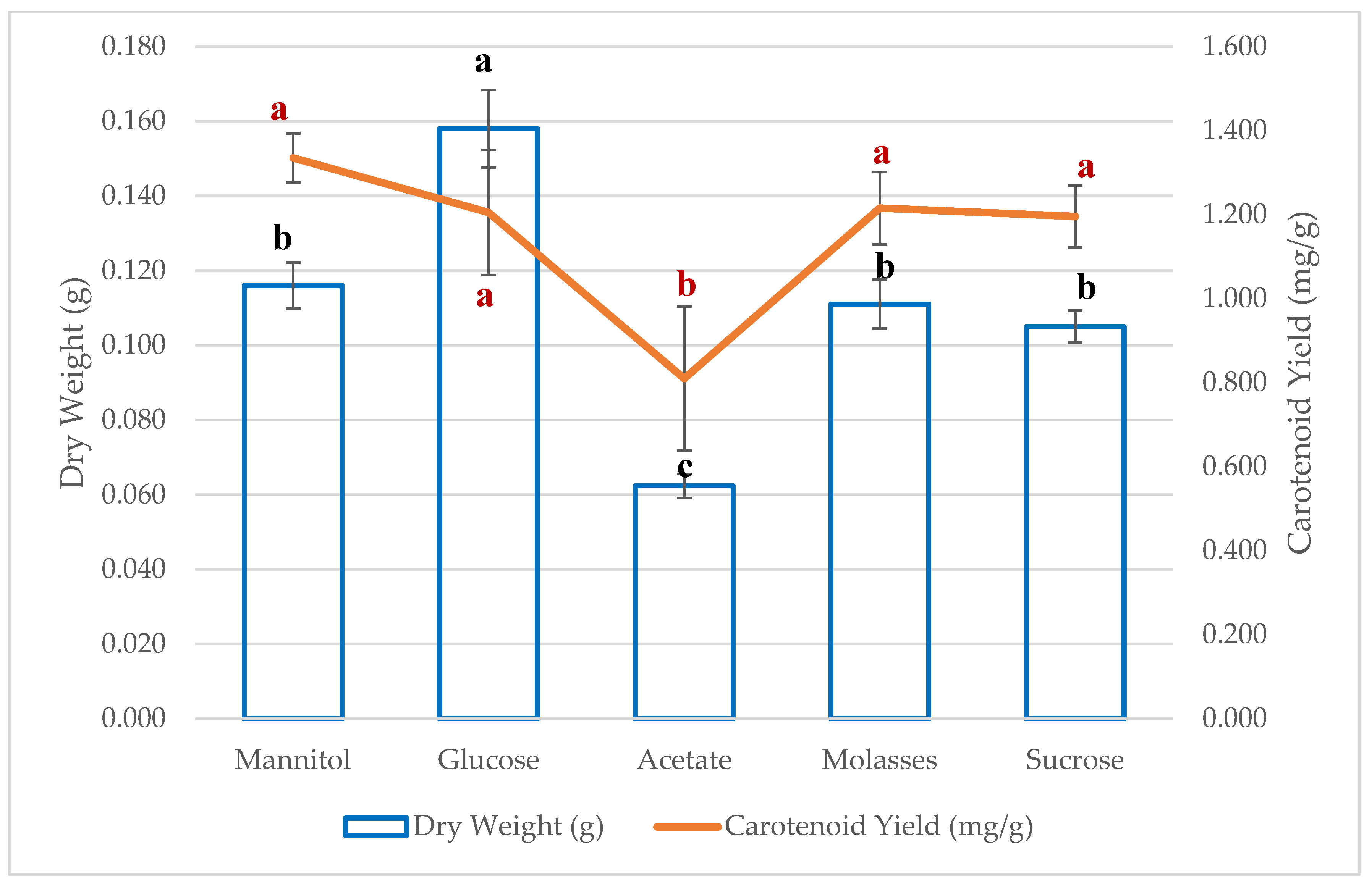
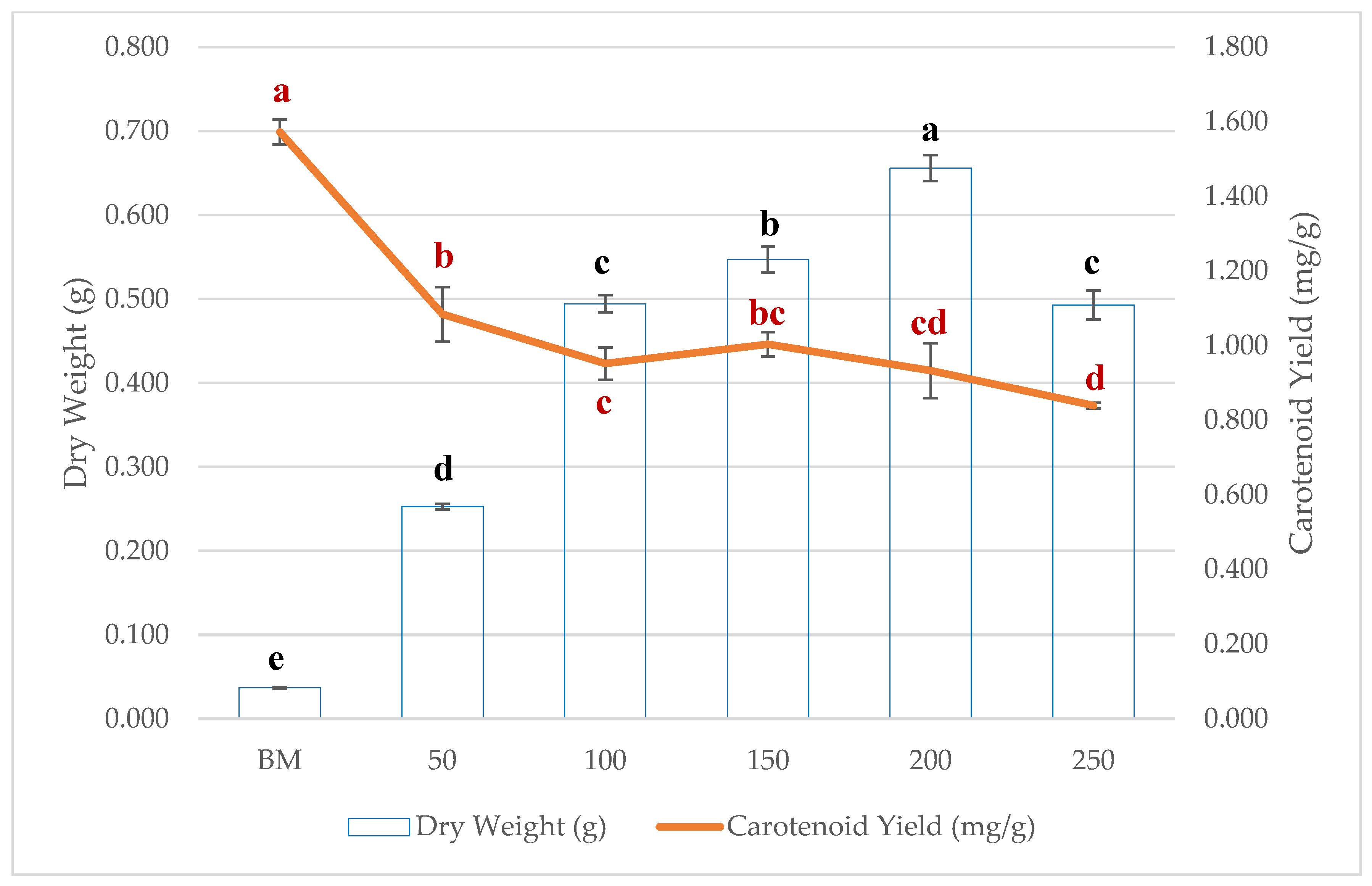
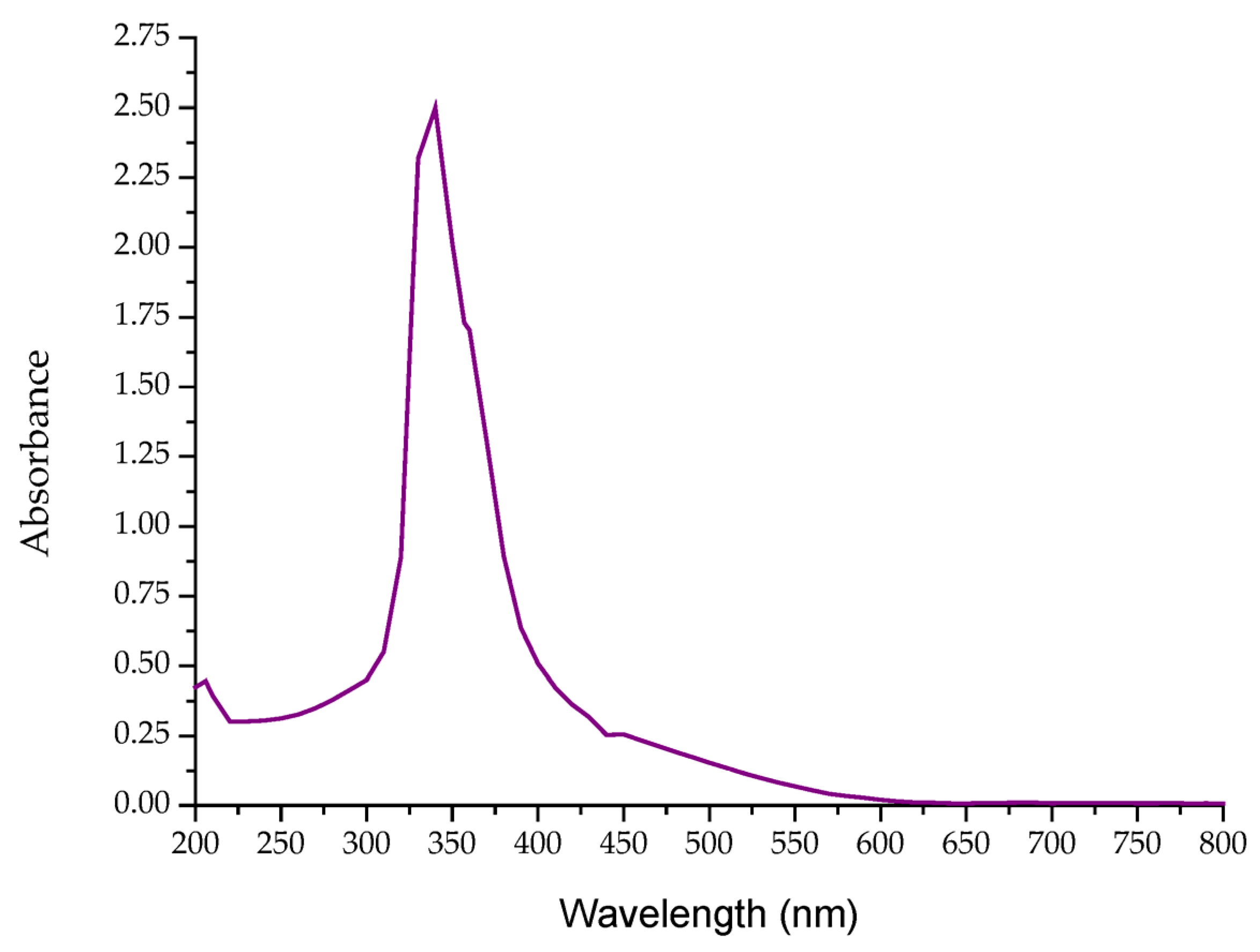
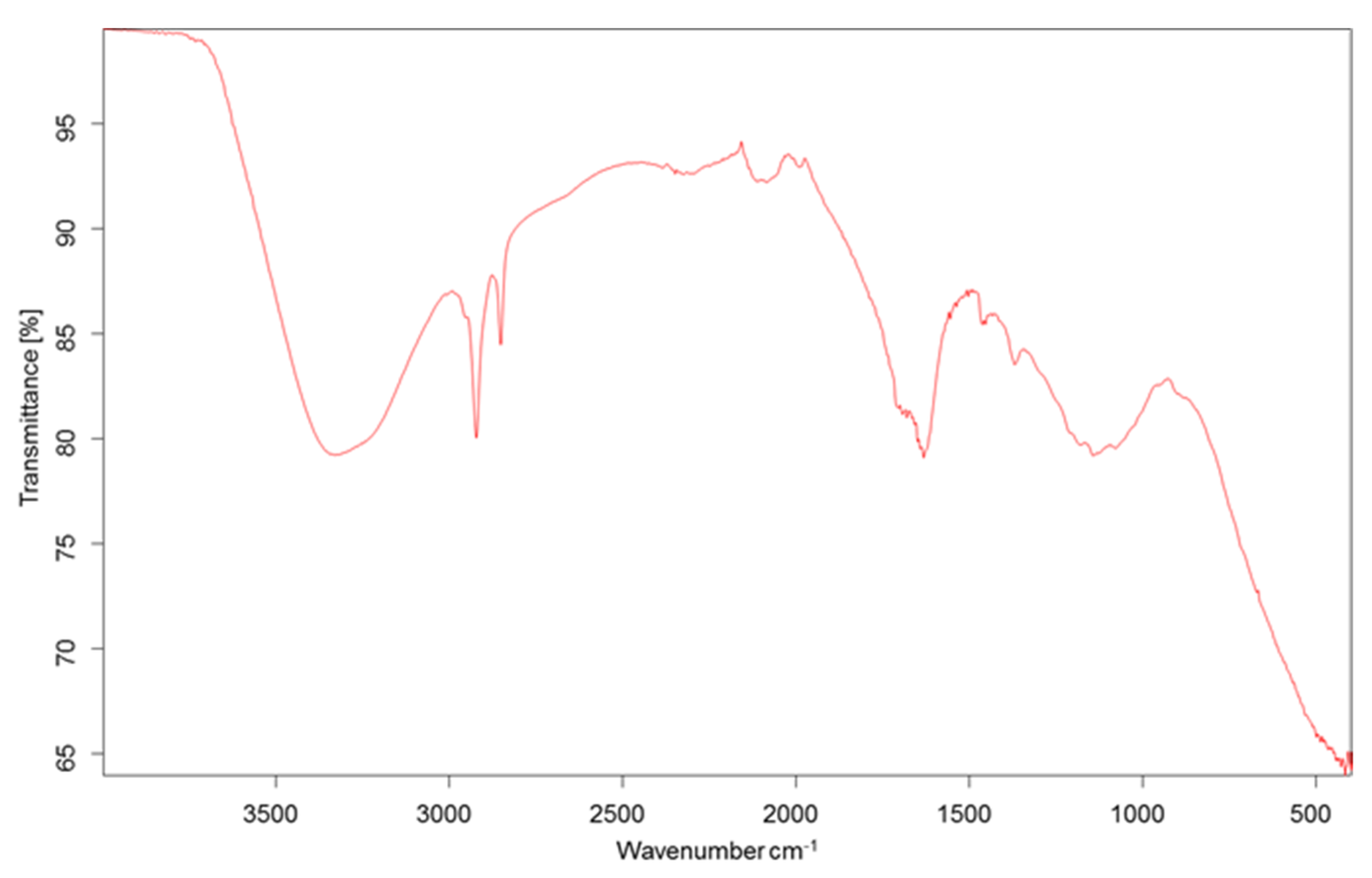
| Carbon Source | Dry Weight (g) | Carotenoid Yield (mg/g) | EPS (mg/L) |
|---|---|---|---|
| Mannitol | 0.116 ± 0.006 b | 1.335 ± 0.058 a | 10.354 ± 1.16 c |
| Glucose | 0.158 ± 0.01 a | 1.205 ± 0.149 a | 18.774 ± 1.857 b |
| Acetate | 0.062 ± 0.003 c | 0.81 ± 0.172 b | 10.664 ± 0.198 c |
| Molasses | 0.111 ± 0.022 b | 1.216 ± 0.348 a | 18.407 ± 0.962 b |
| Sucrose | 0.105 ± 0.004 b | 1.196 ± 0.074 a | 28.0 ± 0.167 a |
| Medium | Dry Weight (g) | Carotenoid Yield (mg/g) | EPS (mg/L) |
|---|---|---|---|
| BM | 0.037 ± 0.001 e | 1.572 ± 0.108 a | 17.449 ± 0.835 e |
| 50 | 0.253 ± 0.003 d | 1.084 ± 0.073 b | 19.496 ± 0.056 e |
| 100 | 0.494 ± 0.01 c | 0.952 ± 0.044 c | 23.105 ± 1.192 d |
| 150 | 0.547 ± 0.035 b | 1.004 ± 0.033 bc | 28.643 ± 1.486 c |
| 200 | 0.656 ± 0.049 a | 0.934 ± 0.074 cd | 41.346 ± 2.526 b |
| 250 | 0.493 ± 0.052 c | 0.84 ± 0.007 d | 71.583 ± 6.265 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yürümez Canpolat, E.; Artan Onat, T. The Effect of Carbon Sources on Carotenoid Synthesis by the Novel Rhodococcus corynebacterioides TAO1. Fermentation 2025, 11, 599. https://doi.org/10.3390/fermentation11100599
Yürümez Canpolat E, Artan Onat T. The Effect of Carbon Sources on Carotenoid Synthesis by the Novel Rhodococcus corynebacterioides TAO1. Fermentation. 2025; 11(10):599. https://doi.org/10.3390/fermentation11100599
Chicago/Turabian StyleYürümez Canpolat, Elif, and Tuba Artan Onat. 2025. "The Effect of Carbon Sources on Carotenoid Synthesis by the Novel Rhodococcus corynebacterioides TAO1" Fermentation 11, no. 10: 599. https://doi.org/10.3390/fermentation11100599
APA StyleYürümez Canpolat, E., & Artan Onat, T. (2025). The Effect of Carbon Sources on Carotenoid Synthesis by the Novel Rhodococcus corynebacterioides TAO1. Fermentation, 11(10), 599. https://doi.org/10.3390/fermentation11100599





