Evaluation of Nutritional Value and Rumen Degradation Rate of Six Unconventional Feeds Using In Vitro and In Situ Methods
Abstract
1. Introduction
2. Materials and Methods
2.1. Material
2.1.1. Ethics Committee Approval
2.1.2. Raw Materials for Experiments
2.1.3. Experimental Animal and Feeding Management
2.2. Experimental Design
2.2.1. In Situ Nutrient Digestibility
2.2.2. In Vitro Fermentation
2.3. Measurement of Treatments and Methods
2.3.1. Determination Method of Nutrient Content
2.3.2. Bioactive Ingredient Content
2.3.3. Rumen Fermentation Parameters
2.3.4. Analysis of Selected Data on In Situ Rumen Degradation Rates
2.4. Statistical Analyses
3. Results
3.1. Nutritional Composition of Experimental Raw Materials
3.1.1. Nutrient Levels
3.1.2. Amino Acid Content
3.1.3. Polyunsaturated Fatty Acids Content
3.1.4. Bioactive Ingredient Content
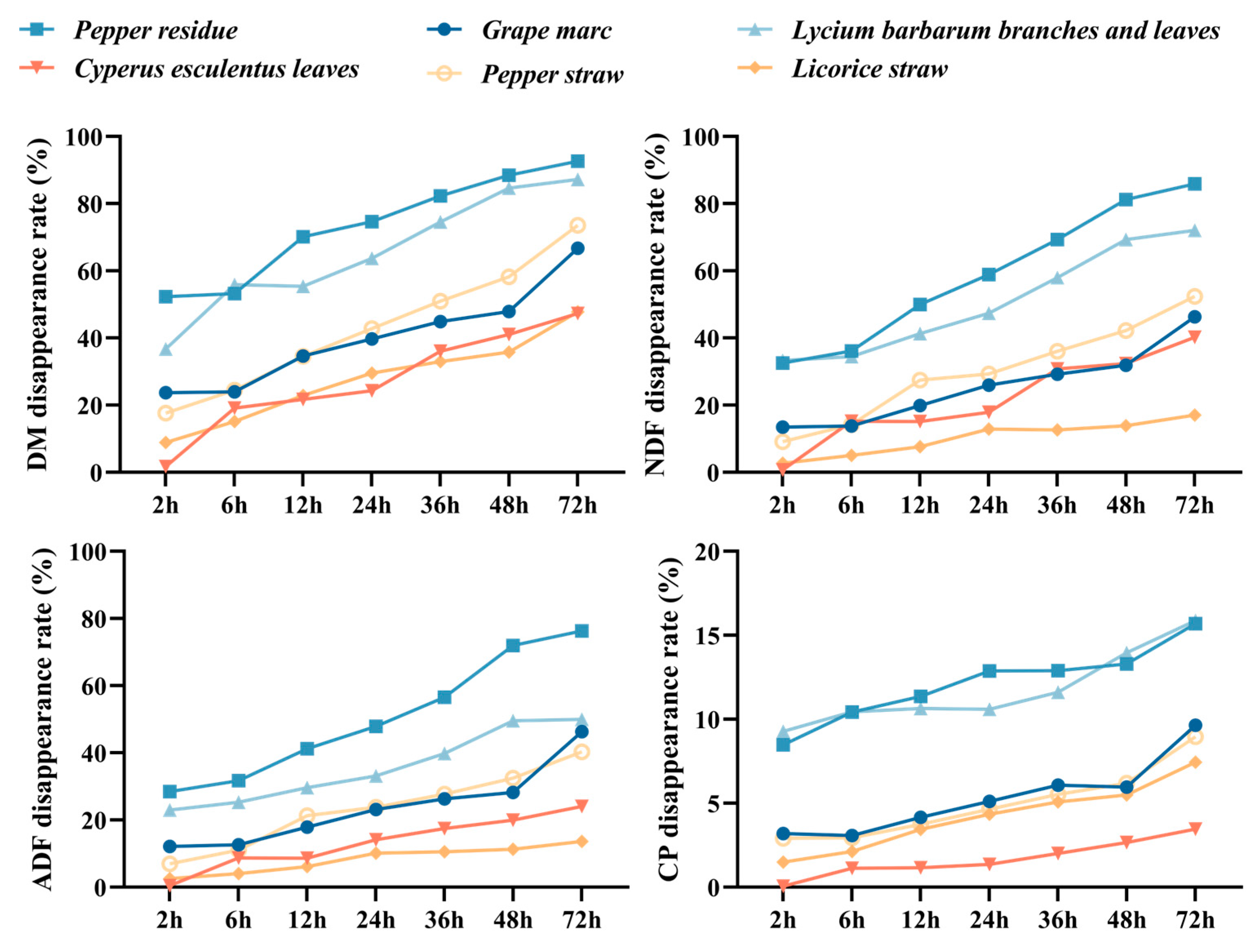
3.2. In Situ Nutrient Digestibility and Degradation Parameters
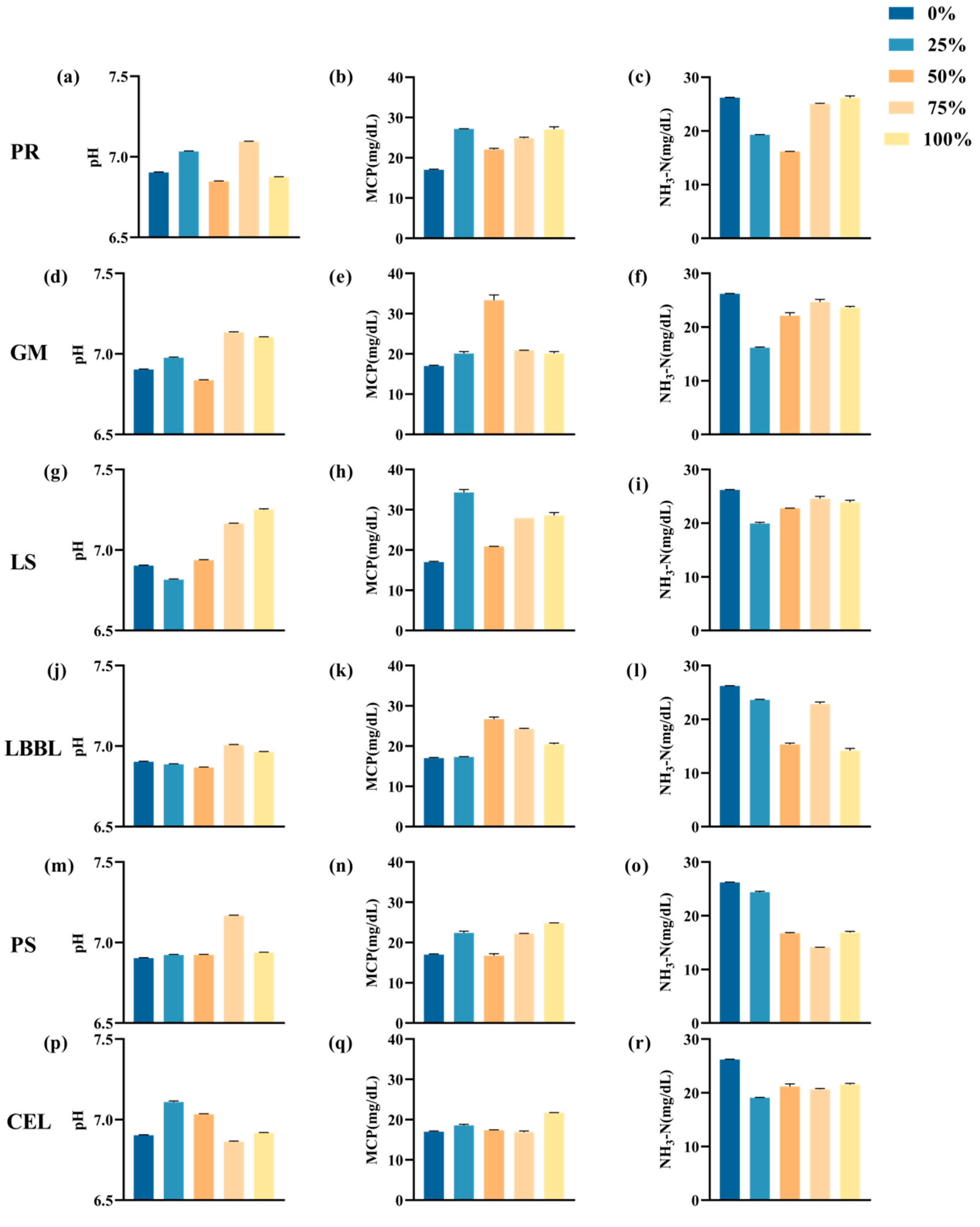
3.3. In Vitro Fermentation
3.3.1. In Vitro Gas Production
3.3.2. In Vitro Fermentation Parameters
4. Discussion
4.1. The Nutritional Components of Six Unconventional Feedstuffs
4.1.1. Nutritional Characteristics
4.1.2. Amino Acid Profile and Protein Quality
4.1.3. Polyunsaturated Fatty Acid Composition
4.1.4. Functional Bioactives and Potential Effects
4.2. Rumen Degradation and In Vitro Fermentation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mottet, A.; Teillard, F.; Boettcher, P.; De’ Besi, G.; Besbes, B. Review: Domestic Herbivores and Food Security: Current Contribution, Trends and Challenges for a Sustainable Development. Animal 2018, 12, s188–s198. [Google Scholar] [CrossRef]
- Oh, J.; Giallongo, F.; Frederick, T.; Pate, J.; Walusimbi, S.; Elias, R.J.; Wall, E.H.; Bravo, D.; Hristov, A.N. Effects of Dietary Capsicum Oleoresin on Productivity and Immune Responses in Lactating Dairy Cows. J. Dairy Sci. 2015, 98, 6327–6339. [Google Scholar] [CrossRef]
- Long, S.; Liu, S.; Wang, J.; Mahfuz, S.; Piao, X. Natural Capsicum Extract Replacing Chlortetracycline Enhances Performance via Improving Digestive Enzyme Activities, Antioxidant Capacity, Anti-Inflammatory Function, and Gut Health in Weaned Pigs. Anim. Nutr. 2021, 7, 305–314. [Google Scholar] [CrossRef]
- Kafantaris, I.; Kotsampasi, B.; Christodoulou, V.; Makri, S.; Stagos, D.; Gerasopoulos, K.; Petrotos, K.; Goulas, P.; Kouretas, D. Effects of Dietary Grape Pomace Supplementation on Performance, Carcass Traits and Meat Quality of Lambs. In Vivo 2018, 32, 807–812. [Google Scholar] [CrossRef]
- Long, L.N.; Kang, B.J.; Jiang, Q.; Chen, J.S. Effects of Dietary Lycium barbarum Polysaccharides on Growth Performance, Digestive Enzyme Activities, Antioxidant Status, and Immunity of Broiler Chickens. Poult. Sci. 2020, 99, 744–751. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Long, L.; Jiang, Q.; Kang, B.; Li, Y.; Yin, J. Effects of Dietary Supplementation of Lycium barbarum Polysaccharides on Growth Performance, Immune Status, Antioxidant Capacity and Selected Microbial Populations of Weaned Piglets. J. Anim. Physiol. Anim. Nutr. 2020, 104, 1106–1115. [Google Scholar] [CrossRef]
- Chen, H.; Guo, B.; Yang, M.; Luo, J.; Hu, Y.; Qu, M.; Song, X. Response of Growth Performance, Blood Biochemistry Indices, and Rumen Bacterial Diversity in Lambs to Diets Containing Supplemental Probiotics and Chinese Medicine Polysaccharides. Front. Vet. Sci. 2021, 8, 681389. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Luo, H.; Liu, K.; Jia, H.; Chen, Y.; Wang, Z. Antioxidant Effects of Liquorice (Glycyrrhiza uralensis) Extract during Aging of Longissimus Thoracis Muscle in Tan Sheep. Meat Sci. 2015, 105, 38–45. [Google Scholar] [CrossRef]
- Sajjadi, R.; Solati, A.A.; Motlagh, M.K.; Bonchenari, M.K. Immune Responses and Some Blood Metabolite Responses of femaleHolstein Calves to Dietary Supplementationwith Licorice Root (Glycyrrhiza glabra). Iran. J. Appl. Anim. Sci. 2014, 4, 505. [Google Scholar]
- Moral-Anter, D.; Campo-Sabariz, J.; Ferrer, R.; Martín-Venegas, R. Cyperus esculentus L. Tubers (Tiger nuts) Protect Epithelial Barrier Function in Caco-2 Cells Infected by Salmonella enteritidis and Promote Lactobacillus plantarum Growth. Nutrients 2020, 13, 71. [Google Scholar] [CrossRef]
- Bamgbose, A.M.; Eruvbetine, D.; Dada, W. Utilization of Tigernut (Cyperus rotundus, L.) Meal in Diets for Cockerel Starters. Bioresour. Technol. 2003, 89, 245–248. [Google Scholar] [CrossRef]
- Asaolu, V.O.; Akinlade, J.A.; Aderinola, O.A.; Okewoye, A.T.; Alalade, J.A. Performance of Grazing West African Dwarf Goats on Moringa Multinutrient Block Supplementation. Asian J. Anim. Sci. 2012, 6, 263–277. [Google Scholar] [CrossRef]
- ISO 6497:2002; Animal Feeding Stuffs—Sampling. International Organization for Standardization (ISO): Geneva, Switzerland, 2002.
- NY/T 816-2021; Feed Standard of Meat-Producing Sheep and Goats. The Ministry of Agriculture of the People’s Republic of China: Beijing, China; China Agriculture Press: Beijing, China, 2021.
- AOAC. Official Methods of Analysis, 20th ed.; AOAC International: Gaithersburg, MD, USA, 2016. [Google Scholar]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for Dietary Fiber, Neutral Detergent Fiber, and Nonstarch Polysaccharides in Relation to Animal Nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Menke, K.H.; Raab, L.; Salewski, A.; Steingass, H.; Fritz, D.; Schneider, W. The Estimation of the Digestibility and Metabolizable Energy Content of Ruminant Feedingstuffs from the Gas Production When They Are Incubated with Rumen Liquor in Vitro. J. Agric. Sci. 1979, 93, 217–222. [Google Scholar] [CrossRef]
- DB12/T 885-2019; Determination of Proanthocyanidins in Plant Extracts by UV/Vis Spectrophotometry. Tianjin Local Standard: Tianjin, China, 2019.
- Chaney, A.L.; Marbach, E.P. Modified Reagents for Determination of Urea and Ammonia. Clin. Chem. 1962, 8, 130–132. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Erwin, E.S.; Marco, G.J.; Emery, E.M. Volatile Fatty Acid Analyses of Blood and Rumen Fluid by Gas Chromatography. J. Dairy Sci. 1961, 44, 1768–1771. [Google Scholar] [CrossRef]
- Ørskov, E.R.; McDonald, I. The Estimation of Protein Degradability in the Rumen from Incubation Measurements Weighted According to Rate of Passage. J. Agric. Sci. 1979, 92, 499–503. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, F.; Li, F.; Wang, Z.; Guo, L.; Weng, X.; Sun, X.; He, Z.; Meng, X.; Liang, Z.; et al. Influence of Dietary Forage Neutral Detergent Fiber on Ruminal Fermentation, Chewing Activity, Nutrient Digestion, and Ruminal Microbiota of Hu Sheep. Animals 2025, 15, 314. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Wang, C.; Zhang, H.; Yu, L.; Dong, L.; Gong, D.; Yao, J.; Wang, H. Illumina Sequencing and Metabolomics Analysis Reveal Thiamine Modulation of Ruminal Microbiota and Metabolome Characteristics in Goats Fed a High-Concentrate Diet. Front. Microbiol. 2021, 12, 653283. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Xiong, Z.; Zhang, S.; Li, K.; Bu, Y.; Zheng, N.; Zhao, S.; Wang, J. Enrichment of Milk Antioxidant Activity by Dietary Supplementation of Red Clover Isoflavone in Cows and Its Improvement on Mice Intestinal Health. Food Chem. 2024, 446, 138764. [Google Scholar] [CrossRef]
- Abuelo, A.; Hernández, J.; Benedito, J.L.; Castillo, C. The Importance of the Oxidative Status of Dairy Cattle in the Periparturient Period: Revisiting Antioxidant Supplementation. J. Anim. Physiol. Anim. Nutr. 2015, 99, 1003–1016. [Google Scholar] [CrossRef] [PubMed]
- Seleem, M.S.; Wu, Z.H.; Xing, C.Q.; Zhang, Y.; Hanigan, M.D.; Bu, D.P. Effects of Rumen-Encapsulated Methionine and Lysine Supplementation and Low Dietary Protein on Nitrogen Efficiency and Lactation Performance of Dairy Cows. J. Dairy Sci. 2024, 107, 2087–2098. [Google Scholar] [CrossRef] [PubMed]
- Räisänen, S.E.; Lapierre, H.; Price, W.J.; Hristov, A.N. Lactational Performance Effects of Supplemental Histidine in Dairy Cows: A Meta-Analysis. J. Dairy Sci. 2023, 106, 6216–6231. [Google Scholar] [CrossRef]
- Luo, Z.; Ou, H.; Tan, Z.; Jiao, J. Rumen-Protected Methionine and Lysine Supplementation to the Low Protein Diet Improves Animal Growth through Modulating Colonic Microbiome in Lambs. J. Anim. Sci. Biotechnol. 2025, 16, 46. [Google Scholar] [CrossRef]
- Titze, N.; Chi, Y.-P.; Haese, E.; Hartung, J.; Rodehutscord, M. Linkage of in Situ Ruminal Degradation of Crude Protein with Ruminal Degradation of Amino Acids and Phytate from Different Soybean Meals in Dairy Cows. J. Dairy Sci. 2024, 107, 2011–2025. [Google Scholar] [CrossRef]
- Shepardson, R.P.; Harvatine, K.J. Effects of Fat Supplements Containing Different Levels of Palmitic and Stearic Acid on Milk Production and Fatty Acid Digestibility in Lactating Dairy Cows. J. Dairy Sci. 2021, 104, 7682–7695. [Google Scholar] [CrossRef]
- Ferlay, A.; Doreau, M.; Martin, C.; Chilliard, Y. Effects of Incremental Amounts of Extruded Linseed on the Milk Fatty Acid Composition of Dairy Cows Receiving Hay or Corn Silage. J. Dairy Sci. 2013, 96, 6577–6595. [Google Scholar] [CrossRef] [PubMed]
- Yi, X.; Wu, B.; Ma, J.; Cui, X.; Deng, Z.; Hu, S.; Li, W.; A, R.; Li, X.; Meng, Q.; et al. Effects of Dietary Capsaicin and Yucca schidigera Extracts as Feed Additives on Rumen Fermentation and Microflora of Beef Cattle Fed with a Moderate-Energy Diet. Fermentation 2022, 9, 30. [Google Scholar] [CrossRef]
- Singh, S.; Koli, P.; Bhadoria, B.K.; Agarwal, M.; Lata, S.; Ren, Y.; Du, X. Proanthocyanidins Modulate Rumen Enzyme Activities and Protein Utilization in Vitro. Molecules 2022, 27, 5870. [Google Scholar] [CrossRef]
- Duan, P.; Rehemujiang, H.; Zhang, L.; Lu, M.; Li, C.; Hu, L.; Wang, Y.; Diao, Q.; Xu, G. Lycium barbarum (Wolfberry) Branches and Leaves Enhance the Growth Performance and Improve the Rumen Microbiota in Hu Sheep. Animals 2024, 14, 1610. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Cheng, L.; Liu, J.; Zhang, S.; Sun, X.; Al-Marashdeh, O. Effects of Licorice Extract Supplementation on Feed Intake, Digestion, Rumen Function, Blood Indices and Live Weight Gain of Karakul Sheep. Animals 2019, 9, 279. [Google Scholar] [CrossRef]
- Zhao, W.; Shen, T.; Zhao, B.; Li, M.; Deng, Z.; Huo, Y.; Aernouts, B.; Loor, J.J.; Psifidi, A.; Xu, C. Epigallocatechin-3-Gallate Protects Bovine Ruminal Epithelial Cells against Lipopolysaccharide-Induced Inflammatory Damage by Activating Autophagy. J. Anim. Sci. Biotechnol. 2024, 15, 109. [Google Scholar] [CrossRef]
- Grabber, J.H. How Do Lignin Composition, Structure, and Cross-Linking Affect Degradability? A Review of Cell Wall Model Studies. Crop Sci. 2005, 45, 820–831. [Google Scholar] [CrossRef]
- Zhong, H.; Zhou, J.; Abdelrahman, M.; Xu, H.; Wu, Z.; Cui, L.; Ma, Z.; Yang, L.; Li, X. The Effect of Lignin Composition on Ruminal Fiber Fractions Degradation from Different Roughage Sources in Water Buffalo (Bubalus bubalis). Agriculture 2021, 11, 1015. [Google Scholar] [CrossRef]
- Du, S.; Xu, M.; Yao, J. Relationship between Fibre Degradation Kinetics and Chemical Composition of Forages and By-Products in Ruminants. J. Appl. Anim. Res. 2016, 44, 189–193. [Google Scholar] [CrossRef]
- Oskoueian, E.; Abdullah, N.; Oskoueian, A. Effects of Flavonoids on Rumen Fermentation Activity, Methane Production, and Microbial Population. Biomed. Res. Int. 2013, 2013, 349129. [Google Scholar] [CrossRef]
- An, Z.; Luo, G.; Abdelrahman, M.; Riaz, U.; Gao, S.; Yao, Z.; Ye, T.; Lv, H.; Zhao, J.; Chen, C.; et al. Effects of Capsicum Oleoresin Supplementation on Rumen Fermentation and Microbial Abundance under Different Temperature and Dietary Conditions in Vitro. Front. Microbiol. 2022, 13, 1005818. [Google Scholar] [CrossRef]
- Khiaosa-ard, R.; Mahmood, M.; Mickdam, E.; Pacífico, C.; Meixner, J.; Traintinger, L.-S. Winery By-Products as a Feed Source with Functional Properties: Dose–Response Effect of Grape Pomace, Grape Seed Meal, and Grape Seed Extract on Rumen Microbial Community and Their Fermentation Activity in RUSITEC. J. Anim. Sci. Biotechnol. 2023, 14, 92. [Google Scholar] [CrossRef]
- Patra, A.K.; Saxena, J. Exploitation of Dietary Tannins to Improve Rumen Metabolism and Ruminant Nutrition. J. Sci. Food Agric. 2011, 91, 24–37. [Google Scholar] [CrossRef]

| Ingredients | Content | Nutrients Levels † | Content |
|---|---|---|---|
| Alfalfa | 9.00 | Metabolic energy ‡ (MJ/kg) | 9.99 |
| Whole plant corn silage | 5.50 | CP | 15.72 |
| Corn straw | 45.50 | NDF | 38.47 |
| Corn | 12.00 | ADF | 21.26 |
| Wheat bran | 7.00 | EE | 6.26 |
| Soybean meal | 4.00 | NFC | 22.41 |
| Cottonseed meal | 12.70 | NFC/NDF | 0.58 |
| CaHPO4·2H2O | 1.30 | Ca | 0.98 |
| NaCl | 1.00 | TP | 0.66 |
| Premix * | 2.00 | Ash | 10.21 |
| Ingredient Composition | Amount (g/L DH2O) |
|---|---|
| NaHCO3 (Sodium carbonate, Solution B) | 39.00 |
| Na2HPO4 (Sodium phosphate, Solution C) | 5.70 |
| KH2PO4 (Potassium dihydrogen phosphate, Solution C) | 6.20 |
| MgSO4·7H2O (Magnesium sulfate heptahydrate, Solution C) | 0.60 |
| CaCl2·2H2O (Calcium chloride dihydrate, Solution A) | 0.13 |
| MnCl2·4H2O (Manganese(II) chloride tetrahydrate, Solution A) | 0.10 |
| CoCl2·6H2O (Cobalt(II) chloride hexahydrate, Solution A) | 0.01 |
| FeCl3·6H2O (Ferric chloride hexahydrate, Solution A) | 0.08 |
| Resazurine (0.1%, w/v) | 1.00 mL |
| Reduction solution (95 mL H2O, 4 mL 1 N NaOH, 625 mg Na2S·9H2O) | 40.00 mL |
| Items | Nutrient Levels | |||||
|---|---|---|---|---|---|---|
| PR | GM | PS | LBBL | LS | CEL | |
| DM, % | 88.11 ± 0.07 | 89.64 ± 0.01 | 92.80 ± 0.10 | 93.29 ± 0.04 | 90.30 ± 0.05 | 89.76 ± 0.02 |
| Ash, % | 12.42 ± 0.18 | 7.40 ± 0.17 | 11.67 ± 0.14 | 9.17 ± 0.22 | 7.63 ± 0.15 | 16.42 ± 0.97 |
| CP, % | 15.76 ± 0.10 | 10.84 ± 0.19 | 14.61 ± 0.29 | 14.93 ± 0.30 | 14.00 ± 0.11 | 4.45 ± 0.03 |
| EE, % | 1.91 ± 0.20 | 6.53 ± 0.07 | 1.33 ± 0.31 | 5.88 ± 1.09 | 4.53 ± 0.14 | 6.47 ± 0.06 |
| NDF, % | 35.71 ± 0.62 | 55.36 ± 1.69 | 48.96 ± 2.58 | 41.17 ± 0.39 | 58.01 ± 1.28 | 69.50 ± 0.56 |
| ADF, % | 30.35 ± 0.52 | 50.42 ± 1.69 | 31.56 ± 2.25 | 31.52 ± 0.16 | 46.78 ± 1.27 | 48.73 ± 1.43 |
| Ca, % | 0.66 ± 0.02 | 0.67 ± 0.01 | 0.97 ± 0.22 | 1.26 ± 0.14 | 1.73 ± 0.33 | 1.13 ± 0.27 |
| P, % | 0.48 ± 0.02 | 0.31 ± 0.13 | 0.14 ± 0.01 | 0.22 ± 0.01 | 0.16 ± 0.01 | 0.18 ± 0.02 |
| VA, ng/g | 0.06 ± 0.01 | 0.70 ± 0.01 | 0.74 ± 0.01 | 0.32 ± 0.01 | 0.70 ± 0.01 | 0.37 ± 0.01 |
| VC, μg/g | 1262.33 ± 22.40 | 342.33 ± 7.54 | 1373.00 ± 19.97 | 1529.67 ± 6.49 | 1121.67 ± 13.78 | 760.00 ± 10.79 |
| VE, μg/g | 30.93 ± 0.47 | 113.67 ± 2.33 | 210.00 ± 5.69 | 55.97 ± 1.54 | 143.33 ± 2.19 | 186.33 ± 4.33 |
| VB1, mg/g | 16.97 ± 0.13 | 2.62 ± 0.01 | 6.82 ± 0.08 | 10.02 ± 0.04 | 9.03 ± 0.10 | 4.24 ± 0.07 |
| VB2, ng/g | 0.51 ± 0.01 | 1.02 ± 0.01 | 0.52 ± 0.01 | 0.83 ± 0.01 | 0.64 ± 0.01 | 0.96 ± 0.01 |
| Items | Amino Acid Content | ||||||
|---|---|---|---|---|---|---|---|
| PR | GM | PS | LBBL | LS | CEL | ||
| Essential amino acids (EAAs) | Threonine | 0.43 | 0.44 | 0.52 | 0.49 | 0.54 | 0.13 |
| Lysine | 0.37 | 0.52 | 0.76 | 0.89 | 0.73 | 0.20 | |
| Leucine | 0.67 | 0.66 | 0.83 | 0.80 | 0.99 | 0.26 | |
| Isoleucine | 0.36 | 0.40 | 0.44 | 0.45 | 0.52 | 0.14 | |
| Methionine | 0.05 | 0.04 | 0.04 | 0.01 | 0.03 | 0.01 | |
| Phenylalanine | 0.40 | 0.38 | 0.55 | 0.47 | 0.57 | 0.14 | |
| Valine | 0.43 | 0.46 | 0.55 | 0.55 | 0.61 | 0.17 | |
| Total EAAs | 2.69 | 2.89 | 3.68 | 3.64 | 3.99 | 1.04 | |
| Non-essential amino acids (NEAAs) | Alanine | 0.47 | 0.44 | 0.58 | 0.54 | 0.60 | 0.25 |
| Proline | 1.32 | 0.70 | 1.17 | 1.42 | 1.13 | 0.18 | |
| Cystine | 0.09 | 0.05 | 0.03 | 0.03 | 0.04 | <0.01 | |
| Tyrosine | 0.24 | 0.23 | 0.31 | 0.28 | 0.44 | 0.08 | |
| Aspartic Acid | 1.58 | 0.50 | 0.56 | 0.72 | 0.58 | 0.13 | |
| Glutamic Acid | 1.19 | 1.13 | 1.00 | 1.30 | 0.71 | 0.21 | |
| Serine | 0.45 | 0.43 | 0.48 | 0.52 | 0.49 | 0.13 | |
| Glycine | 0.56 | 0.69 | 0.60 | 0.56 | 0.66 | 0.17 | |
| Histidine | 0.16 | 0.32 | 0.25 | 0.25 | 0.26 | 0.05 | |
| Arginine | 0.51 | 0.58 | 0.56 | 0.51 | 0.66 | 0.12 | |
| Total NEAAs | 6.58 | 5.07 | 5.54 | 6.12 | 5.57 | 1.33 | |
| Total AAs | 9.27 | 7.96 | 9.23 | 9.77 | 9.56 | 2.37 | |
| Items | Polyunsaturated Fatty Acids Content | |||||
|---|---|---|---|---|---|---|
| PR | GM | PS | LBBL | LS | CEL | |
| cis,cis-9,12-octadecadienoic acid(C18:2n6c) | 0.05 | 1.64 | 0.22 | 0.14 | 0.07 | 0.04 |
| cis,cis,cis-9,12,15-Octadecatrienoic acid(C18:3n3) | 0.05 | 0.09 | 0.36 | 0.11 | 0.43 | 0.05 |
| cis-9-octadecenoic acid(C18:1n9c) | 0.01 | 0.34 | 0.03 | 0.01 | 0.02 | 0.04 |
| Dodecanoic acid(C12:0) | <0.01 | <0.01 | 0.00 | <0.01 | 0.01 | <0.01 |
| Tetradecanoic acid(C14:0) | 0.00 | <0.01 | 0.01 | <0.01 | 0.01 | <0.01 |
| Hexadecanoic acid(C16:0) | 0.02 | <0.01 | 0.14 | 0.06 | 0.09 | 0.02 |
| Heptadecanoic acid(C17:0) | <0.01 | <0.01 | 0.00 | <0.01 | <0.01 | <0.01 |
| Octadecanoic acid(C18:0) | <0.01 | 0.07 | 0.03 | 0.01 | 0.00 | <0.01 |
| Eicosanoic acid(C20:0) | <0.01 | 0.01 | 0.01 | 0.01 | 0.00 | <0.01 |
| Docosanoic acid(C22:0) | <0.01 | 0.01 | 0.01 | 0.00 | 0.03 | <0.01 |
| Tetracosanoic acid(C24:0) | <0.01 | 0.01 | 0.01 | <0.01 | 0.02 | <0.01 |
| Items | Capsaicin (PR) | Proanthocyanidins (GM) | Capsaicin (PS) | Lycium Barbarum Polysaccharides (LBBL) | Liquiritin (LS) | Epigallocatechin (CEL) |
|---|---|---|---|---|---|---|
| Content | 0.02 | 8.48 | 0.08 | 9.24 | 4.24 | 1.77 |
| Items | Group | SEM | p-Value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| PR | GM | PS | LBBL | LS | CEL | ||||
| DM | a/% | 46.29 a | 22.81 c | 15.67 d | 9.15 e | 36.27 b | 2.45 f | 3.74 | <0.001 |
| b/% | 52.32 bc | 73.16 a | 78.05 a | 34.69 d | 57.91 b | 46.57 c | 3.69 | <0.001 | |
| c/(%/h) | 0.04 ab | 0.01 c | 0.02 bc | 0.04 ab | 0.04 ab | 0.04 a | 0.00 | 0.022 | |
| ED/% | 74.77 a | 45.14 c | 45.23 c | 27.56 e | 66.51 b | 29.82 d | 4.23 | <0.001 | |
| CP | a/% | 3.90 b | 2.94 bc | 2.40 cd | 1.28 de | 6.41 a | 0.38 e | 0.49 | <0.001 |
| b/% | 11.46 b | 11.81 b | 17.915 a | 5.93 b | 13.30 b | 4.36 b | 5.31 | <0.001 | |
| c/(% /h) | 0.10 a | 0.01 bc | 0.00 c | 0.03 b | 0.03 b | 0.02 bc | 0.01 | <0.001 | |
| ED/% | 12.47 a | 6.00 b | 4.95 c | 4.15 d | 12.12 a | 1.86 e | 0.97 | <0.001 | |
| NDF | a/% | 24.88 a | 12.22 c | 9.45 d | 2.96 e | 21.77 b | 2.22 e | 2.10 | <0.001 |
| b/% | 68.43 a | 55.05 b | 69.69 a | 23.71 d | 54.61 b | 38.770 c | 4.09 | <0.001 | |
| c/(% /h) | 0.03 a | 0.02 b | 0.02 b | 0.02 b | 0.04 a | 0.04 a | 0.00 | <0.001 | |
| ED/% | 59.99 a | 29.45 d | 32.17 c | 10.89 f | 50.58 b | 23.46 e | 3.56 | <0.001 | |
| ADF | a/% | 23.13 a | 11.07 c | 7.66 d | 2.41 e | 15.77 b | 1.53 e | 1.84 | <0.001 |
| b/% | 69.73 a | 45.99 b | 65.72 a | 17.91 c | 37.28 b | 24.19 c | 4.82 | <0.001 | |
| c/(%/h) | 0.02 b | 0.02 bc | 0.01 c | 0.02 bc | 0.04 a | 0.03 a | 0.00 | <0.001 | |
| ED/% | 51.27 a | 26.17 c | 25.74 c | 8.74 e | 35.78 b | 13.83 d | 3.39 | <0.001 | |
| Items | Group (%) | SEM | p-Value | |||||
|---|---|---|---|---|---|---|---|---|
| 0 | 25 | 50 | 75 | 100 | ||||
| PR | HAc | 16.34 e | 21.53 d | 22.49 c | 24.40 b | 30.82 a | 1.21 | <0.001 |
| PA | 11.60 d | 11.16 e | 11.99 c | 12.44 b | 13.83 a | 0.23 | <0.001 | |
| BA | 9.10 b | 8.74 d | 8.02 e | 8.99 c | 9.76 a | 0.15 | <0.001 | |
| A/P ratio, % | 1.41 e | 1.93 c | 1.88 d | 1.96 b | 2.23 a | 2.83 | <0.001 | |
| TVFAs | 37.04 e | 41.43 d | 42.49 c | 45.83 b | 54.41 a | 1.45 | <0.001 | |
| GM | HAc | 16.94 e | 30.60 a | 28.41 b | 24.21 c | 23.59 d | 1.25 | <0.001 |
| PA | 12.60 ab | 13.63 a | 11.66 bc | 10.84 cd | 10.21 e | 0.36 | 0.001 | |
| BA | 9.17 b | 10.26 a | 9.16 b | 8.99 b | 7.98 c | 0.20 | <0.001 | |
| A/P ratio, % | 1.34 c | 2.26 ab | 2.44 a | 2.23 b | 2.31 ab | 2.80 | <0.001 | |
| TVFAs | 38.71 e | 54.48 a | 49.22 b | 44.05 c | 41.77 d | 1.51 | <0.001 | |
| PS | HAc | 16.94 d | 34.99 c | 43.97 a | 38.04 b | 38.11 b | 2.46 | <0.001 |
| PA | 12.60 e | 14.89 d | 15.44 c | 16.54 a | 16.25 b | 0.37 | <0.001 | |
| BA | 9.17 a | 8.01 e | 8.10 d | 8.94 b | 8.80 c | 0.12 | <0.001 | |
| A/P ratio, % | 1.34 c | 2.35 b | 2.85 a | 2.30 b | 2.35 b | 2.79 | <0.001 | |
| TVFAs | 38.71 d | 57.90 c | 67.51 a | 63.52 b | 63.15 b | 2.73 | <0.001 | |
| LBBL | HAc | 16.94 e | 30.91 c | 26.65 d | 36.29 a | 31.78 b | 1.75 | <0.001 |
| PA | 12.60 e | 14.32 c | 13.05 d | 16.96 a | 15.71 b | 0.44 | <0.001 | |
| BA | 9.17 e | 9.94 c | 9.63 d | 11.65 a | 10.41 b | 0.23 | <0.001 | |
| A/P ratio, % | 1.34 e | 2.16 a | 2.04 c | 2.14 b | 2.02 d | 2.82 | <0.001 | |
| TVFAs | 38.71 e | 55.17 c | 49.34 d | 64.90 a | 57.91 b | 2.35 | <0.001 | |
| LS | HAc | 16.94 e | 22.52 d | 25.28 c | 30.49 b | 35.13 a | 1.68 | <0.001 |
| PA | 12.60 e | 13.84 d | 13.98 c | 15.72 b | 17.76 a | 0.48 | <0.001 | |
| BA | 9.17 d | 6.40 e | 9.59 c | 9.92 b | 11.13 a | 0.42 | <0.001 | |
| A/P ratio, % | 1.34 e | 1.63 d | 1.81 c | 1.94 b | 1.98 a | 2.84 | <0.001 | |
| TVFAs | 38.71 e | 42.76 d | 48.85 c | 56.13 b | 64.02 a | 2.44 | <0.001 | |
| CEL | HAc | 16.94 c | 33.59 a | 30.72 b | 31.22 b | 30.60 b | 1.59 | <0.001 |
| PA | 12.60 d | 18.95 a | 16.73 b | 17.12 b | 15.43 c | 0.57 | <0.001 | |
| BA | 9.17 d | 11.87 a | 11.24 b | 11.28 b | 10.93 c | 0.25 | <0.001 | |
| A/P ratio, % | 1.34 d | 1.77 c | 1.84 b | 1.82 b | 1.98 a | 2.84 | <0.001 | |
| TVFAs | 38.71 d | 64.41 a | 58.69 bc | 59.62 b | 56.95 c | 2.37 | <0.001 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, C.-Y.; Yang, S.-H.; Ma, Y.; Chen, D.; Yan, Z.-S.; Yuan, G.-H.; Lu, M.-L.; Diao, Q.-y.; Xu, G.-S.; Rehemujiang, H. Evaluation of Nutritional Value and Rumen Degradation Rate of Six Unconventional Feeds Using In Vitro and In Situ Methods. Fermentation 2025, 11, 594. https://doi.org/10.3390/fermentation11100594
Shi C-Y, Yang S-H, Ma Y, Chen D, Yan Z-S, Yuan G-H, Lu M-L, Diao Q-y, Xu G-S, Rehemujiang H. Evaluation of Nutritional Value and Rumen Degradation Rate of Six Unconventional Feeds Using In Vitro and In Situ Methods. Fermentation. 2025; 11(10):594. https://doi.org/10.3390/fermentation11100594
Chicago/Turabian StyleShi, Chen-Yang, Shi-Hong Yang, Yin Ma, Dong Chen, Ze-Sheng Yan, Guo-Hong Yuan, Mu-Long Lu, Qi-yu Diao, Gui-Shan Xu, and Halidai Rehemujiang. 2025. "Evaluation of Nutritional Value and Rumen Degradation Rate of Six Unconventional Feeds Using In Vitro and In Situ Methods" Fermentation 11, no. 10: 594. https://doi.org/10.3390/fermentation11100594
APA StyleShi, C.-Y., Yang, S.-H., Ma, Y., Chen, D., Yan, Z.-S., Yuan, G.-H., Lu, M.-L., Diao, Q.-y., Xu, G.-S., & Rehemujiang, H. (2025). Evaluation of Nutritional Value and Rumen Degradation Rate of Six Unconventional Feeds Using In Vitro and In Situ Methods. Fermentation, 11(10), 594. https://doi.org/10.3390/fermentation11100594







