1. Introduction
Pork is one of the main sources of protein in many regions around the world, providing essential amino acids, vitamins, and minerals, and is crucial for human nutrition and health [
1]. In piglet production, infections from pathogen and various stress factors (cold exposure, weaning, dietary changes, and sudden environmental shifts) often trigger intestinal inflammation, which causes mucosal damage and microbial imbalance, resulting in poor growth and even sudden fatalities [
2,
3]. The weaning period represents a particularly critical window for the formation of the intestinal microbiota [
4]. The intestinal microbial community of weaned piglets is highly susceptible to the stress of weaning, and thus easily influenced by external stimuli [
4]. Feed additives can improve the nutritional components of animal feed, promote growth, enhance immunity, improve the physical properties of feed, or prevent diseases, among other purposes [
5]. It is crucial to maintain intestinal health and minimize post-weaning stress by incorporating a range of feed additives, including peptides, organic and inorganic acids, prebiotics, probiotics, plant extracts, essential oils, and yeast [
6]. Probiotics in feed additives refer to a class of microorganisms that are beneficial to the health of the animal’s gut [
7]. These microorganisms mainly include lactic acid bacteria, bifidobacteria, etc. They are added to the feed of animals with the aim of promoting the growth of beneficial bacteria in the gut, suppressing the proliferation of harmful bacteria, thereby maintaining the balance of the gut flora, enhancing the animal’s digestive and absorptive capacity, improving immune function, reducing the occurrence of intestinal diseases, and promoting the growth and health of animals [
8]. As feed additives, probiotics are widely used in livestock and poultry farming, helping to improve the production performance and health level of animals [
9]. Furthermore, utilizing high-quality feed ingredients can improve digestibility and promote immunological characteristics.
Fermented feed, a green, environmentally friendly, and innovative alternative to conventional feed, offers a means to replace the use of antibiotics, improve animal health, enhance the nutritional value and utilization of feed, and improve the ecological environment of animal husbandry [
10]. Fermented feed contains a large number of small molecules that are easily absorbed by the animal’s intestines, various unsaturated fatty acids, and aromatic substances, which can improve palatability and thereby boost feed consumption [
11]. In recent years, the development and application of fermented feed technology have become a hot topic in the feed industry. The most commonly used microorganisms in fermented feed are lactic acid bacteria, yeast, and
Bacillus subtilis [
12]. Studies indicate that feeding sows with fermented feed can significantly increase key elements such as the number of piglets born, the number of live piglets, the birth weight of piglets, the average daily gain, and the average weaning weight, thereby exerting a certain promoting effect on the reproductive performance of sows and the growth performance of piglets [
12]. Previous studies have indicated that fermented bamboo shoot residue feed has a positive impact on the growth performance, serum parameters, and intestinal microbial population of weaned piglets [
13].
Cauliflower is a nutritionally rich vegetable, packed with vitamins such as Vitamin C, Vitamin K, Vitamin B6, folate, and dietary fiber, as well as essential minerals like potassium, magnesium, and manganese [
14]. It also contains antioxidants, including glucosinolates, which help protect cells from oxidative damage [
15]. Moreover, cauliflower is high in dietary fiber, which aids in digestive health. These characteristics make cauliflower an important part of a healthy diet. However, a large amount of vegetable waste generated from the leaves during the processing of cauliflower has become one of the main sources of agricultural non-point source pollution [
16]. Due to their perishable nature, the decomposition of cauliflower residue produces foul-smelling gases and leachate, which not only pollute the environment and soil but also promote the growth and spread of pathogens, thereby posing significant environmental pollution and potential health risks to local residents. Therefore, the rational development and utilization of agricultural waste such as cauliflower residue are beneficial for safeguarding the rural ecological environment and are of great significance in promoting the development of sustainable agriculture. In the previous studies, we found that the fermented feed of cauliflower residues could increase the average daily gain and final weight of lambs, promote the development of the rumen and small intestine of lambs, optimize the rumen flora structure of lambs, and promote the overall growth and development of lambs [
17]. However, at present, there are few studies on the influence of the fermented feed of cauliflower tail vegetables on piglet diarrhea. Considering the widespread focus on the health of piglets and in light of public health concerns, numerous countries worldwide have prohibited the use of antibiotics as feed additives. Due to the advantages of fermentation feed, such as the minimal toxic side effects and low likelihood of antibiotic resistance, it holds great potential as a substitute for antibiotics to alleviate piglet weaning stress [
18]. Therefore, this study aims to explore the effects of cauliflower residue fermented feed on the diarrhea rate, intestinal morphology, immune indicators, and gut microbiota of weaned piglets, providing a reference for the rational use and application of cauliflower residue fermented feed. The research results indicate that the fermented feed from cauliflower residue can improve the growth performance, apparent digestibility, and immune function of piglets, as well as reduce the diarrhea rate in piglets.
2. Materials and Methods
2.1. Animal Ethics Statement
This study has been approved by the Animal Protection and Use Committee of Northwest Minzu University, Lanzhou, China (No: XBMU-SM-2020010).
2.2. Preparation of Cauliflower Residue Fermented Feed
Bacillus subtilis (CCTCC NO: 2021528),
Saccharomyces cerevisiae (CICC NO: 1769), and
Lactiplantibacillus plantarum (CCTCC NO: M2021527) were sourced from the College of Life Science and Engineering at Northwest Minzu University. Cauliflower residues are composed of cauliflower leaves (harvested in November), provided by Lanzhou Ruiyuan Agricultural Technology Co., Ltd. (Lanzhou, China). The preparation of the cauliflower residue fermentation feed concentrate involved uniformly mixing these microbial agents in a 1:1:1 mass ratio. The production process of fermented feed from cauliflower residue involves drying and grinding the cauliflower residue, then mixing it with other basic feed ingredients in proportion. Subsequently, 0.35% of a composite microbial agent is added, and the mixture is uniformly blended before being placed into fermentation bags [
17]. After anaerobic fermentation at room temperature for 25 days, the fermented product is then dried in an oven to obtain the fermented feed. The nutritional needs of the pigs were based on the NRC (2012) guidelines. The composition and nutritional levels of the basic feed and the fermented feed made from cauliflower residue are detailed in
Table 1.
2.3. Experimental Design
This study was conducted at the pig farm of Lanzhou Ruiyuan Agricultural Technology Co., Ltd. (Lanzhou, China). The experiment utilized a completely randomized block design, selecting 42 weaned piglets (Duroc × [Landrace × Yorkshire], castrated male), with good health and similar weights (11.40 ± 1.36 kg) at 40 days of age, and randomly dividing them into two groups of 21 piglets each. There were 7 pens per treatment, each housing 3 pigs. The area of each pen was 120 cm × 80 cm (length × width), surrounded by stainless steel tubes, and equipped with a nipple water dispenser and a feed trough. Both groups of weaned piglets were subjected to consistent rearing and management conditions. The experiment lasted for 30 days, including an 8-day preliminary trial period followed by a 22-day formal trial period. During the adaptation period and feeding trials, all weaned piglets can freely obtain feed and water.
At the end of the experiment, 6 pigs close to the median body weight were selected from each group and euthanized. Each pig was intramuscularly injected with a dose of 80 mg/kg body weight of 10% pentobarbital sodium for anesthesia, and slaughtered 15 min later. The small intestine was taken from the abdominal cavity and divided into three parts according to physiological characteristics: the duodenum, jejunum, and ileum. A 2 cm segment was taken from each of the duodenum, jejunum, and ileum, and the intestinal specimens were washed with physiological saline and stored in 4% paraformaldehyde for 24 h for morphological examination. The contents of the jejunum, ileum, cecum, and colon were collected in pre-chilled tubes and immediately frozen in liquid nitrogen and stored at −80 °C until microbial composition analysis. The pH value of the intestinal contents was determined using a pH meter (NO.S400-B, Mettler Toledo Technology Co., Ltd., Changzhou, China). The contents were placed into a paper cup, the metal probe of the meter was completely immersed in the contents for 3 s, and the value was recorded after stabilization.
The growth trait data of piglets were measured according to our previous method, and the body shape index was calculated based on the measurement results [
17]. Each body size index is calculated using the following formulas:
2.4. Apparent Digestibility Measurement
Before the end of the feeding trial, piglet feces were collected continuously for three days, twice a day, in the morning and afternoon, with each collection consisting of approximately 200 g. After collection, the fecal samples are immediately mixed with 10% H2SO4 (10 mL/100 g) for nitrogen fixation, labeled, and then stored at −20 °C for further use. All of the growing pigs’ excrement was defrosted, blended, and then dried at 65 °C for 72 h before the natural moisture was restored at room temperature for 24 h. The fecal samples were used to determine the content of dry matter (DM), crude protein (CP), crude fat, crude fiber (CF), total calcium, and total phosphorus. Acid-insoluble ash is used as an indigestible marker to measure the apparent total digestibility of dry matter (DM), crude protein (CP), crude fiber (CF), total calcium (Ca), and total phosphorus (P).
According to the method of J. Chen et al., the content of calcium and phosphorus mineral components in feces is detected [
19]. Add 10 mL concentrated nitric acid and 1 mL hydrogen peroxide to digest the fecal sample in sequence. After boiling, add another 0.5 mL of perchloric acid and remove the acid at 180 °C for 2 h. Finally, use an Inductively Coupled Plasma Optical Emission Spectrometer (Agilent 5110 ICP-OES, Shanghai Shanfu Technology Co., Ltd., Shanghai, China) with 1% nitric acid and a constant volume of 10 mL to determine the content of calcium and phosphorus in the sample.
The apparent digestibility of nutrients is evaluated using the AIA method, and the apparent nutrient digestibility is calculated according to the following formula [
20]. The calculation formula is shown below:
where: WFI = weight of feed intake; NF = concentration of nutrient in feed; WEV = weight of total excreta voided; NE = concentration of nutrient in total excreta.
2.5. The Determination of Digestive Enzyme Activity
According to the manufacturer (Nanjing Jiancheng Bioengineering Research Institute, Nanjing, China), the activity of enzymes in duodenal, jejunal, and ileal tissues was measured using α-amylase (NO. C016-1-1), lipase (NO. A054-1-1), trypsin (NO. A080-2-2), and chymotrypsin (NO. AO80-3-1) enzyme activity assay kits.
2.6. Diarrhea Scores
Evaluate the diarrhea rate of piglets according to the method outlined by Peng et al. [
21]. During the experiment, the fecal condition of the piglets was observed daily, and sensory scoring was conducted for each instance of feces excreted by each piglet. The scoring system was applied to determine the rate of diarrhea as follows: 0 = hard feces; 1 = slightly soft feces; 2 = soft, partially formed feces; 3 = loose, semiliquid feces; 4 = watery, mucous-like feces. When the average score was over 2 for 2 consecutive days, pigs were identified as having diarrhea. The diarrhea rate was calculated according to the following equation:
2.7. Growth Performance
The growth performance was assessed by weighing the fasted weaned piglets at the beginning and end of the formal trial period, specifically on the first and last days, before morning feeding. The initial and final weights of the piglets were recorded, ensuring no significant differences in initial weights among the replicates. The average daily weight gain (ADG) was then calculated based on these initial and final weights. Throughout the trial, the feed consumption for each group was recorded on a daily basis per replicate, allowing for the calculation of the average daily feed intake (ADFI). Subsequently, the feed conversion ratio (Feed/Gain, F/G) was calculated based on ADG and ADFI.
2.8. H & E Stain
Evaluate the morphological characteristics of the intestinal tissues according to the methods outlined by Deng et al. [
22]. The mid-duodenum, mid-jejunum, and mid-ileum sample tissue of the piglets was fixed with 4% paraformaldehyde for 48 h to prepare a slice of 5 μm. Each intestinal tissue is sliced into six sections for observation. For morphological analysis, measure the villus height (V) and crypt depth (C) using image processing connected to an optical microscope. In each section, randomly select 5 different areas for observation and measurement. Calculate the V/C ratio to assess the relationship between villus height and crypt depth. At least 10 complete villi and associated crypt depths were measured for each tissue section in this study.
2.9. Serum Biochemical Indicators
At the conclusion of the experiment, 12 piglets were selected from each group for blood collection. Blood (10 mL) was drawn from the anterior vena cava and placed into sterile tubes (5 mL) or vacuum tubes containing heparin (5 mL). Non-anticoagulated samples were left at room temperature for 1 h and then centrifuged at 1500× g for 15 min (NO. AXTD530, Yancheng Anxin Technology Co., Ltd., Yancheng, China).
Enzyme-linked immunosorbent assay (ELISA) was employed to determine the levels of immunoglobulins (Ig) in piglet serum, in accordance with the instructions provided with the respective reagent kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China). The detected immunological parameters encompassed IgA, IgG, and IgM.
We used an automated biochemical analyzer (Mindray BS-420, Shenzhen Mindray Bio Medical Electronics Co., Ltd., Shenzhen, China) to examine a range of serum parameters, including alanine aminotransferase (ALT), albumin (Alb), blood urea nitrogen (BUN), triglycerides (TG), aspartate aminotransferase (AST), total cholesterol (T-Chol), glucose (Glu), and total protein (TP). These were measured using the method detailed by Zhang et al., involving the centrifugation of serum samples at 2500×
g at 4 °C [
23].
The umbilical cord serum concentration of total protein (TP), albumin (ALB), urea, glucose (GLU), total cholesterol (TC), and total glyceride (TG) was examined using an automated biochemical analyzer (Mindray BS-420, Shenzhen Mindray Bio-Medical Electronics Co., Ltd., Shenzhen, China).
2.10. Microbiology of Cecal Contents
Microbial genomic DNA was extracted from cecal contents utilizing a QIAamp Fast DNA Stool Mini Kit (Qiagen Ltd., Hilden, Germany). The V3-V4 region of the 16SrRNA gene underwent amplification with the universal primers F (5′-ACT CCT ACG GGA GGC AGC AG-3′) and R (5′-GGA CTA CHVGGG TWT CTAAT-3′). Purified amplicons were pooled in equimolar amounts and subjected to paired-end sequencing on the Illumina MiSeq PE300 platform/NovaSeq PE250 platform (Illumina, San Diego, CA, USA) according to the standard protocol of Majorbio Biopharmaceutical Technology Co., Ltd. (Shanghai, China). The Uslust algorithm was used to cluster operational taxa (OTU) with a confidence threshold of 0.7. The Shannon index, Chao 1 index, and ace index were used to analyze the α diversity.
2.11. Statistical Analysis
All data were analyzed using the GraphPad Prism 8.0 program (GraphPad Software, San Diego, CA, USA). Differences between treatments were further assessed using Duncan’s range of comparison tests. The experimental data were expressed as the means ± SEM, and p < 0.05 between groups was considered as a statistically significant difference.
4. Discussion
Early weaning can lead to piglet weaning stress syndrome, clinically manifested as growth retardation, weight loss, and severe diarrhea, ultimately resulting in decreased piglet constitution and reduced immunity, and making them more susceptible to secondary diseases from external pathogens such as bacteria or viruses [
24]. Currently, this issue is mainly addressed by incorporating antibiotics to the feed. However, a large amount of data indicates that there is a phenomenon of excessive or prolonged addition of antibiotics during use, which is inevitably linked to the development of bacterial resistance and the presence of antibiotic residues in animal products [
25]. This has prompted people to seek reliable and effective alternatives [
26]. Fermented feed has many advantages, such as minimal toxic side effects and low antibiotic resistance, making it a promising alternative to antibiotics in alleviating weaning stress in piglets [
27]. Therefore, this study comprehensively evaluated the application effects of cauliflower residue fermented feed on diarrheal piglets from aspects of growth performance, serum biochemical parameters, intestinal development, and microbial flora structure.
Growth performance is a core indicator for assessing the success of pig farm management, as increased growth leads to higher yields and greater economic benefits [
28]. The results indicate that fermented feed can improve the growth performance of weaned piglets. In this experiment, the average daily weight gain (ADG) of the piglets in the experimental group was significantly higher than that of the control group, and the final weight also increased, indicating that fermented feed can significantly improve the growth performance of piglets. The apparent digestibility of nutrients directly impacts the growth performance of animals, which is positively correlated with their digestive performance, meaning that the higher the apparent digestibility of nutrients in the feed, the better the animals’ growth performance [
29]. The results demonstrate that the digestibility of dry matter, crude protein, and total phosphorus significantly increased in the experimental group. This result indicates that fermented feed can improve piglets’ utilization of nutrients, thereby stimulating piglet growth. The body size index serves as a measure reflecting the interrelationships and proportions of various parts of livestock body development, providing insights into changes in the external characteristics of livestock and judging the extent of development across various parts [
30]. In the results of this experiment, the experimental group exhibited notably higher body height and chest width than the control group, while other body size indicators and the body size index displayed no significant differences, indicating that feeding fermented feed did not adversely affect the development of various parts of the weaned piglets’ bodies, rather, it was conducive to the development of their body height and chest width.
The intestines are responsible for the digestion, absorption, and transportation of nutrients, and are the largest digestive organ in piglets [
31]. They also serve as a crucial defense barrier in the animal body. Post-weaning diarrhea in piglets can damage the intestinal structure, leading to problems such as villous atrophy and increased crypt depth, which affect digestion, absorption, secretion, and barrier function [
32]. In the results of this experiment, the villus height to crypt depth ratio (V/C) and the villus height in the jejunum of the post-weaning piglets in the experimental group were significantly higher than those in the control group, while the crypt depth in the jejunum was significantly lower than that in the control group. This indicates that cauliflower residue fermented feed can promote the growth and development of the small intestine in post-weaning piglets, maintain the integrity of the intestinal mucosa, and thus improve the intestinal function of post-weaning piglets. The acidity of the digestive tract in livestock and poultry is an important factor influencing the digestive environment [
32]. It is also the basis for regulating the acid-base balance and electrolyte balance within the intestines [
33]. An appropriate level of acidity in the animal intestine ensures normal digestive function, making the intestinal pH value an important indicator of digestive performance. In this experiment, feeding fermented cauliflower residue feed significantly reduced the pH values of the duodenum and jejunum in piglets. This may be due to the colonization and growth of probiotics from the fermented feed in the intestines, leading to the production of a large amount of lactic acid, thereby reducing the pH value of the piglet intestines. The three main digestive enzymes in the digestive tract of livestock and poultry are amylase, lipase, and protease [
34]. Increased activity of these digestive enzymes can improve the utilization of nutrients in the body. The activity of digestive enzymes in pig intestines directly affects feed utilization and piglet growth performance. The results of this study indicate that adding fermented feed to the diet significantly increases the pancreatic protease activity in the duodenum and jejunum, and significantly increases the activity of ileal lipase, pancreatic protease, and chymotrypsin.
Immunoglobulins play a crucial role in the protective barrier and are closely related to the animal’s immune system [
35]. The strength of the body’s disease resistance can be indirectly reflected by the level of immunity [
36]. The results of this experiment demonstrate that providing fermented feed leads to an increase in the serum IgG and IgM levels in weaned piglets, with IgM significantly higher than the control group, indicating that cauliflower residue fermented feed helps improve the immune capacity of weaned piglets. Serum albumin (ALB) and urea (UREA) are important indicators of the body’s metabolic function, especially nitrogen metabolism [
37]. In this experiment, the levels of serum ALB and UREA in the experimental group were significantly increased, indicating that fermented cauliflower residue feed can promote the breakdown and metabolism of protein in the body, potentially promoting the growth and development of nursery pigs. The concentrations of total cholesterol (TC) and triglycerides (TG) in the serum are important indicators of the strength of lipid metabolism in the body [
38]. The level of TG in the serum can determine the development of adipose tissue and the deposition of fat, with higher TG levels indicating more fat deposition [
39]. In this experiment, the serum TC content in the experimental group was significantly lower than that in the control group, indicating that fermented cauliflower residue feed has a regulatory effect on lipid metabolism. The health status of the liver and heart can be assessed by the activity of alanine aminotransferase (ALT), aspartate aminotransferase (AST), and gamma-glutamyl transferase (GGT) in the serum [
40]. The lower the activity in the serum, the healthier the organ development [
41]. In this experiment, the ALT in the experimental group was significantly reduced, indicating that feeding fermented cauliflower residue feed can protect the liver function of nursery pigs to a certain extent. The health status of the biliary system can be reflected by alkaline phosphatase (ALP), with higher ALP activity in the serum indicating better animal growth, and ALP activity increases with the increase in the daily weight gain of pigs [
42]. In this experiment, the ALP in the serum of the experimental group was significantly higher than that in the control group, indicating that fermented cauliflower residue feed can increase the ALP activity in the serum of nursery pigs, thereby improving their growth performance.
The intestinal microbiota serves as a crucial symbiotic partner for the host, playing a pivotal role in intestinal metabolism. The pig’s intestinal microorganisms continuously colonize and establish a stable microbial ecosystem throughout the pig’s growth and development, contributing to the maintenance of the host’s health and normal physiological activities [
43]. Alpha diversity refers to the diversity within a specific area or ecosystem, typically measured by metrics reflecting community richness [
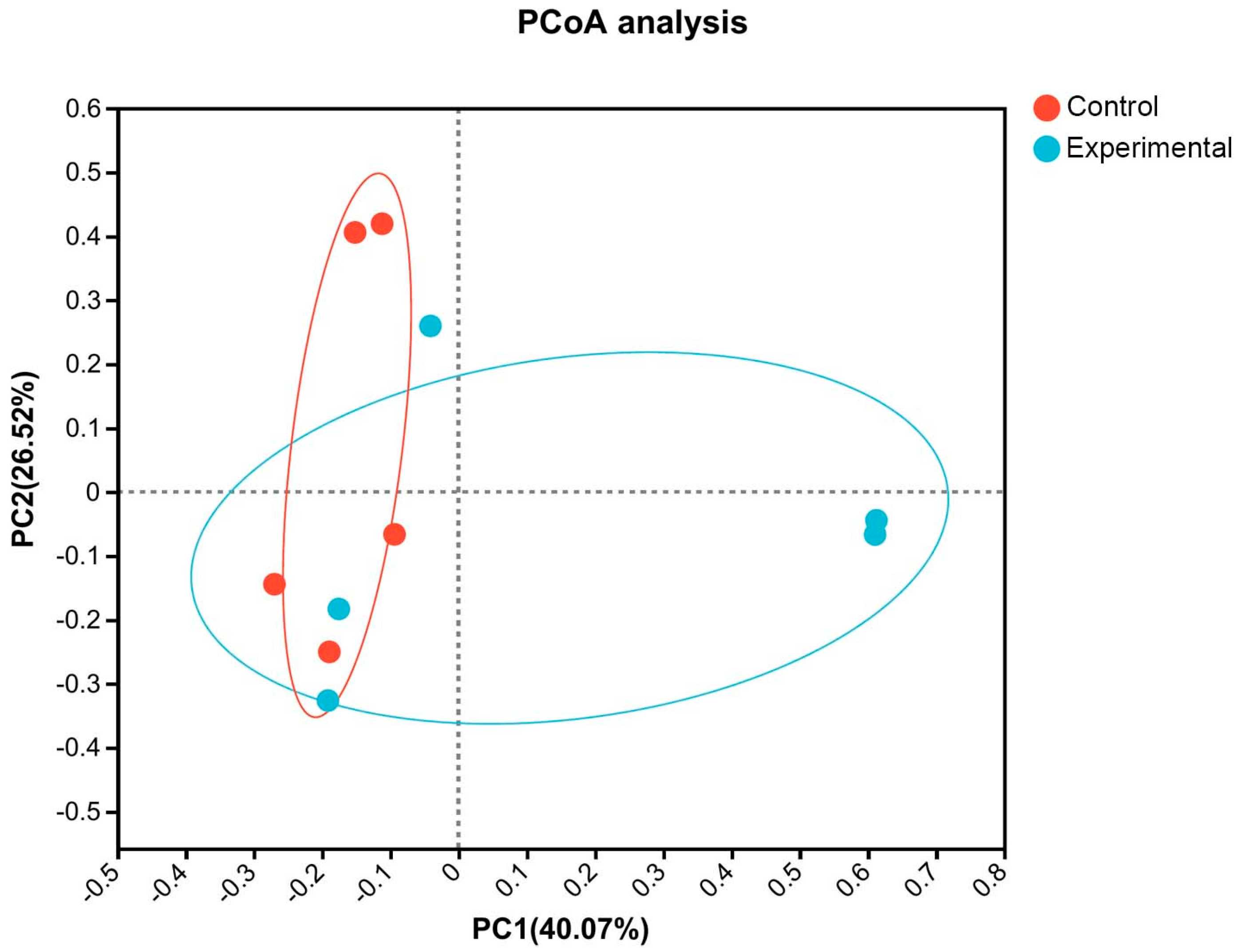
44]. This study indicates that adding fermented feed to the diet can increase the Chaol and ACE indices, reflecting enhanced microbial abundance in the gut of weaned piglets, and thus improving their resilience to unfavorable conditions. Therefore, fermented feed may favor the stability of the intestinal microbial community, aligning with its previously observed promotion of growth performance. Beta diversity reflects differences among different grouped samples and is often assessed using Principal Coordinates Analysis (PCoA) to observe differences among individuals or populations [
45]. The results of this study demonstrate that the microbial community structure of the six samples in the experimental group is notably similar and significantly different from that of the control group. This suggests that adding fermented feed to the diet of weaned piglets can significantly regulate their intestinal microbial community. In this study, the
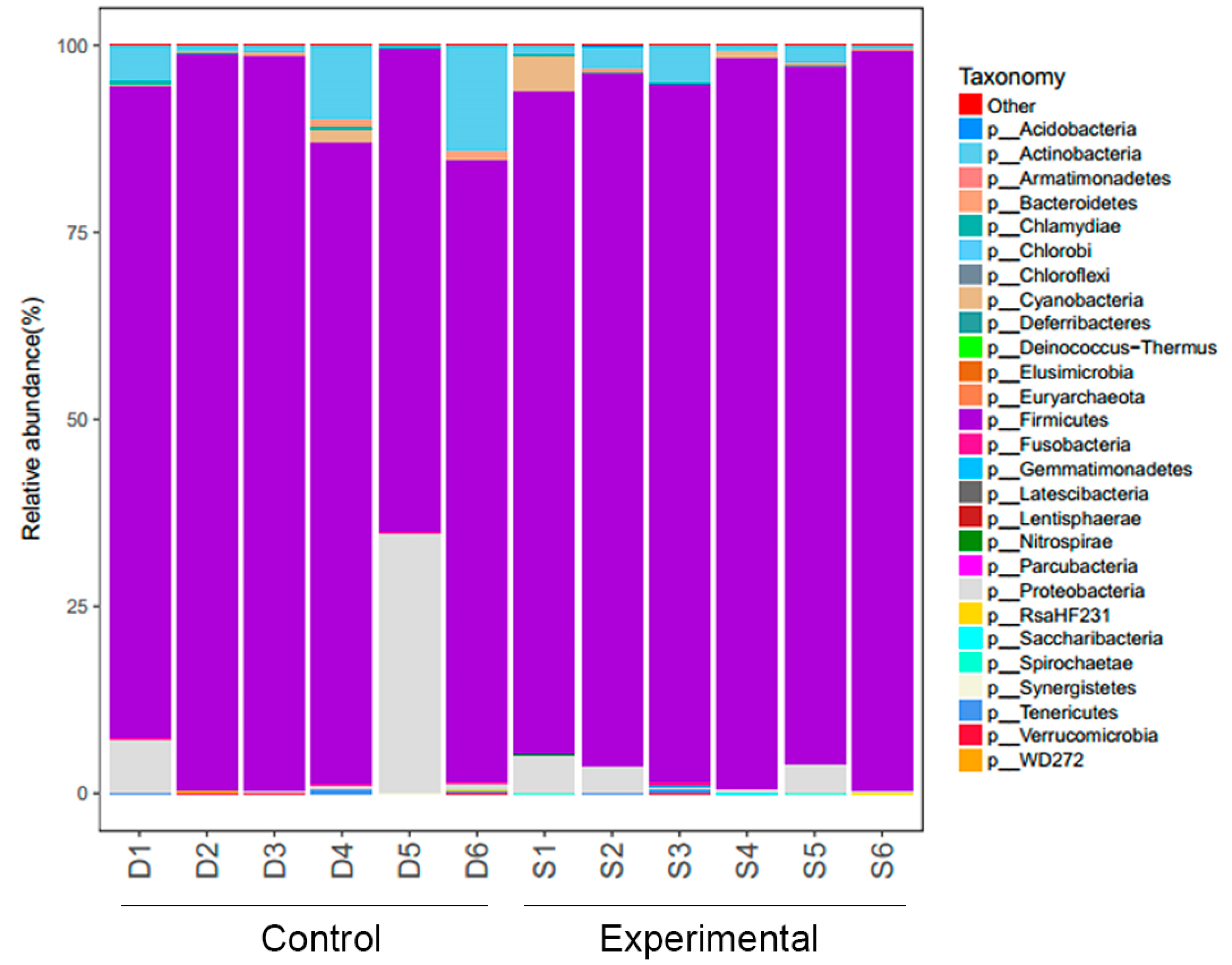
Firmicutes and
Proteobacteria were identified as dominant microbial communities in the intestines of weaned piglets. Changes in the relative proportion of
Firmicutes in the intestinal microbiota of piglets can lead to microbial imbalance, disrupted intestinal epithelial cell function, increased intestinal permeability, mucosal damage, immune response imbalance, and subsequently affect piglet growth performance [
29]. At the phylum level, the experimental group exhibited a higher abundance of
Firmicutes and
Cyanobacteria compared to the control group. Conversely, the abundance of
Proteobacteria,
Actinobacteria,
Tenericutes, and
Fusobacteria was lower in the experimental group, along with a significantly reduced abundance of
Bacteroidetes. Research indicates a close association between gut microbiota and obesity as well as host metabolic activities [
46]. Various studies have found that increasing the abundance of
Firmicutes, decreasing the abundance of
Bacteroidetes, and reducing the ratio of
Bacteroidetes to
Firmicutes all contribute to promoting fattening [
7,
28]. At the genus level, the relative abundance of 336 species was annotated across the samples. The genus-level analysis suggests that feeding fermented feed significantly boosts the number of
Lactobacillus, possibly related to the composite microbial agents added during the fermentation process. Additionally, it indirectly demonstrates that
Lactobacillus can inhibit the proliferation of
Streptococcus by significantly reducing its abundance. In conclusion, this research sheds light on the potential of fermented feed to modulate the gut microbiota of weaned piglets, highlighting its impact on their growth performance and intestinal microbial community structure.
In summary, the fermented feed of cauliflower residues significantly altered the diversity and community structure of the gut microbiota in diarrheal piglets, which may have profound effects on digestion and nutrient absorption. Further research is needed to explore the impact of these changes on the growth performance of piglets and to optimize the dosage and method of adding cauliflower residue fermented feed to achieve the best feeding effect. Although this study has certain limitations, the results still provide an effective method for preventing diarrhea in piglets. Future research should focus on the regulatory mechanisms of the metabolic products of cauliflower residue fermented feed on diarrheal piglets, identifying the specific molecules and cellular pathways involved. It is also important to explore the synergistic effects of cauliflower residue fermented feed with other intestinal health interventions to formulate more effective comprehensive prevention and treatment strategies.