Abstract
Green tea is a non-fermented tea with flavor and polyphenols. Aroma is one of the important quality indicators of tea. Fermented green tea wine can solve the problem of low-grade tea, which has more bitterness and less aroma. In this study, Camellia sinensis var. pubilimba Hung T. Chang (Kaishan white tea 2) was screened by orthogonal partial least squares-discriminant analysis (OPLS-DA) to benzyl alcohol and phenethyl alcohol presenting a fruity aroma, dimethyl sulfide presenting a green tea aroma, and rich tea polyphenols with contents of 2.08, 2.43, 12.26 and 3.72%, respectively. The optimal fermentation conditions for green tea wine were determined univariately as 1.5% yeast addition, 30 °Brix initial sugar, and fermentation temperature of 25 °C. The electronic sensory assessment showed that the saltiness, richness and umami were more prominent in green tea wine, while the response values of bitterness, astringency and aftertaste-A were lower. The order of aroma contribution can be seen as W1S > W5S > W2S > W2W > W1W > W3S > W6S. Kaisan white tea 2 gives green tea wine a clear tea aroma. This study provides better technical and theoretical strategies for the comprehensive quality assessment and control of fermented green tea wine quality.
1. Introduction
Tea (Camellia sinensis (L.) Kuntze) is widely consumed beverage worldwide, and China has emerged as a major global producer of tea owing to its abundant tea plant resources [1]. Chinese tea is conventionally categorized into different types, such as white, green, red, oolong, dark and yellow tea. Each tea has unique characteristics in appearance and color of leaves, taste and aroma of its infusion. Taste and aroma are the more significant quality indicators of tea. In China and other nations, the quality and grade of tea are normally assessed by tea appraisers according to an evaluation index system. Aroma usually accounts for 35% of the evaluation index of different teas, making it the more important factor in tea sensory quality [2,3]. The quality of aroma directly affects the consumer interpretation of tea quality. The tea aroma directly comes from the olfactory sense produced by volatile compounds, including the physical and mental stimulation brought about by the aroma compounds. Aroma is the result of the combination of various aroma compounds at a specific content and proportions, in conjunction with their comprehensive effects at the olfactory nerves [4,5]. The aroma compounds account for only about 0.01% of the dry tea mass. However, even though this proportion is very small, the sensory threshold of aroma and its value impart key qualities favored by consumers [3]. Nearly 600 aroma compounds have been assessed in teas, including over 280 compounds in green tea and more than 300 compounds in red and oolong tea [3,6].
Green tea belongs to the non-fermented tea category and has shown potential health benefits by having an optimum content of catechins and polyphenols, which are major components in tea leaves. The health benefits of green tea have been revealed significantly by manufacturers [7,8]. However, different types of green tea are also one of the significant factors. Their nutrients can regulate blood sugar, operate as antioxidants, and protect against cardiovascular diseases. A variety of research findings have proven that tea catechins assist in minimizing the risk of heart disease [9]. Although high-grade teas are widely marketed and profitable, seasonal influences can lead to stagnation or other problems with low-grade teas [10]. Consumers do not favor summer and autumn teas due to their heavy bitter taste and low aroma [11]. Current studies have shown that the intensity and characteristics of aroma cannot be assessed solely based on the quantity and content of aroma compounds. In fact, compounds of higher content do not necessarily produce a strong aroma. Different types of tea usually contain a variety of volatile organic compounds, yet not all volatile components play a potential role in the aroma quality of tea [3]. Therefore, deep-processed products using green tea as raw material constitute an advanced approach to solving the problem of wasted tea resources.
Tea wine is an alcoholic beverage with tea as the main raw material, which can be categorized into infused, prepared, and fermented types according to the processing methods [12]. Fermented tea wine has a rich flavor through yeast that converts various components in the tea leaves into special aroma substances [13]. Wang et al. [14] demonstrated that the aroma in fermented green tea juice was significantly higher than in unfermented, and the bitter and astringent substances were also reduced considerably. However, studies on the fermentation of tea wine using pure tea juice have mainly analyzed its chemical composition, and limited studies on the taste of tea wine have been reported.
Taste is an important indicator for evaluating tea wine quality. Currently, the taste of tea wine is based on the human senses, which can be easily disturbed by external factors [15]. E-sensory technology is an advanced analytical procedure based on the bionic senses to evaluate samples digitally. Hence, it provides a higher accuracy and reproducibility [16]. The common electronic sensory techniques mainly include the electronic nose, tongue, and eyes, which have been widely used in the analysis and evaluation of food flavor substances [15], such as fruits [17], wines [18] and tea beverages [19]. However, limited demonstrations have been reported on this technique for flavor analysis of green tea wine.
In order to more objectively and accurately determine the suitability of green tea varieties and the scientific evaluation of green tea wine quality, in this study green tea wine was fermented from green tea (Kaishan white tea) from Hezhou City, Guangxi, China, and the key aroma components of green tea were screened by multivariate statistical analysis. Finally, green tea wine quality was scientifically analyzed by an electronic sensory system. This provides technical and theoretical reference for the development and quality improvement of tea deep-processing products.
2. Materials and Methods
2.1. Green Tea Wine Preparation
The main tea-producing areas of Hezhou Bapu District and Zhaoping County, China, provided the green tea (Table 1). White granulated sugar was produced by Nanning Sugar Industry Co., Nanning, China, and RW Commercial Yeast purchased from the Anchor Yeast Co., Yichang, China. Green tea and water were extracted at 80 °C for 2 h at material to liquid ratio of 1:60. The tea broth was applied as the fermentation base and pasteurized for 30 min after adjusting the sugar content (solid soluble content, SSC) to 30 °Brix. When the tea broth was cooled to room temperature (RT), 0.1% activated yeast was added and fermented at 25 °C [20]. The dry yeast was activated for 30 min with liquid fermented (37 °C) [13]. Fermentation was completed when the SSC remained unchanged, and the tea wine was centrifuged to remove the yeast for determination (Figure 1).

Table 1.
Detailed description of different tea samples.
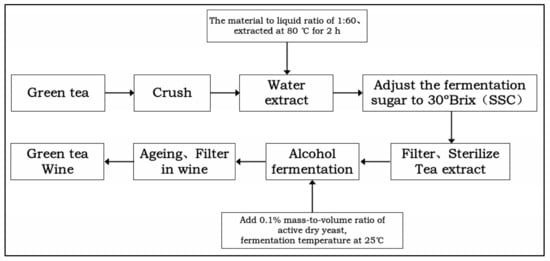
Figure 1.
Flow chart of tea wine fermentation.
2.2. Detection of Volatile Aromas
Green tea volatile aroma was detected by Headspace Solid-Phase Micro-extraction (HS-SPME, Agilent, Shanghai, China) combined with Gas Chromatography-Mass Spectrometry [GC–MS] (Agilent 8890-7000C, Shanghai, China), referring to the method of Chen et al. [21] with slight modifications. A total of 2 g of tea sample and 10 μL of 2-octanol (semi)-quantification) were placed in a 20 mL headspace bottle, and an aged 50/30 μm Carboxen/Polydimethylsiloxane/Divinylbenzene (CAR/PDMS/DVB) extraction head was inserted into the sample bottle and adsorbed at 60 °C for 30 min. The adsorbed head was removed and inserted into the injection port of the gas chromatography, and then desorbed at 250 °C for 3 min, and the instrument was activated to collect the data at the same time. GC–MS analysis employed a HP-INNOWAX column (60 mm × 0.25 mm × 0.25 µm, Agilent, CA, USA) with high purity helium carrier gas (≥99.999%) at constant flow rate of 1.0 mL/min. Injection mode was set to a split ratio of 5:1, with an inlet temperature of 250 °C. The oven temperature program started at 50 °C for 1.0 min, ramped to 220 °C at 3 °C/min, then 220 °C was held for 5 min. Electron bombardment (EI) ion source: 70 eV ionization energy, 230 °C ion source temperature, 250 °C transmission line temperature, 150 °C quadrupole temperature, mass range: 30 to 350 (m/z).
The volatile compounds were identified by their mass spectrometry fragment, which was matched with the National Institute of Standards and Technology (NIST) and Wiley mass spectral library, assisted by a mixture of n-alkane series to compare the experimental retention index (RI) with the values available in the NIST MS libraries. In addition, the relative content of volatile compounds was calculated by dividing the peak area of the isolated compound by the peak area of the internal standard. The quantification of alcohol, ester, aldehyde, oxygen heterocyclic and sulfo-compounds, hydrocarbons, etc., was carried out using semi-quantification. The standard curves were prepared by plotting the ratios of the response areas of the standard compounds and internal standard against their concentration ratios.
2.3. Single-Factor Experiment
A preliminary investigation of the factors affecting the alcoholic content and inclusions of fermented green tea wine was conducted using single-factor experiments, including the initial sugar, yeast addition and fermentation temperature.
2.4. Electronic Setups and Signals Acquiring
The E-nose (PEN3, Airsense Analytics, Schwerin, Germany) was used in this experiment, equipped with a metal oxide semiconductor (MOS) sensor array of 10 different MOS sensors. The sample was measured accurately in 25 mL of green tea wine in a sample bottle and equilibrated at room temperature for 20 min. The specific parameters for the electronic nose assay were 60 s of detection time, 60 s cleaning time, 98 s determination time, 200 mL/min carrier gas speed, and 200 mL/min injection flow rate [22].
An E-tongue (SA-402B, INSENT, Atsugi-shi, Japan) was applied to detect the taste of green tea wine. The E-tongue comprises an automatic sampler, a sensor array, and signal processing software [23]. A total of 50 mL of green tea wine was measured, and the sample was diluted with 50 mL of ddH2O and put in an electronic tongue detection cup for 3 min before detection. Each sample was cycled 4 times, and the last three sets of data were taken for analysis [24].
2.5. Statistical Analysis
All treatments were carried out in triplicate and experimental data were represented by mean value ± standard deviation (SD). Orthogonal Partial Least Squares-Discriminant Analysis (OPLS-DA)is a supervised discriminant analysis statistical method that employs partial least squares regression to model the relationship of sample data in order to predict results [25]. OPLS-DA was performed by SIMCA 14.1 software. Statistically significant differences (p < 0.05) were conducted by variance (ANOVA) analysis using SPSS 17.0 statistical software (SPSS Inc., Chicago, IL, USA) and Origin version 9.1 (OriginLab, Northampton, MA, USA).
3. Results and Discussion
3.1. Characteristic Aroma Analysis and Variety Selection of Green Tea Wine Fermentation Substrate
Tea aroma, the soul of tea, is one of the major factors contributing to tea quality and variety. This is mainly because the aroma can affect tea characteristics. The color of tea leaves, liquor color, and appearances of waste leaves were darker from green tea to black tea; meanwhile, the aroma became intensified due to the optimum oxidation of the leaf. Green tea tended to have a brisk taste, whereas oolong tea and black tea possessed asweeter taste, mainly due to glycoside degradation [26,27]. The volatile aroma of nine green tea samples are shown in Table 2 and Figure 2; the benzyl alcohol and phenethyl alcohol content in Kaishan white tea was higher than in other tea, with the benzyl alcohol content being up to two-fold higher, which indicated that this aroma substance could be used as a typical and unique aroma component of Kaishan white tea. Volatile compounds in tea produce the aroma. However, the aroma substances in tea are minimal, accounting for less than 0.1% of its total dry mass [11,27]. Moreover, a single sulfur-containing compound (dimethyl sulfide), which has a strong green tea aroma, has a high content of 12.94% in Kaisan white tea.

Table 2.
Volatile aroma compounds of different tea varieties.
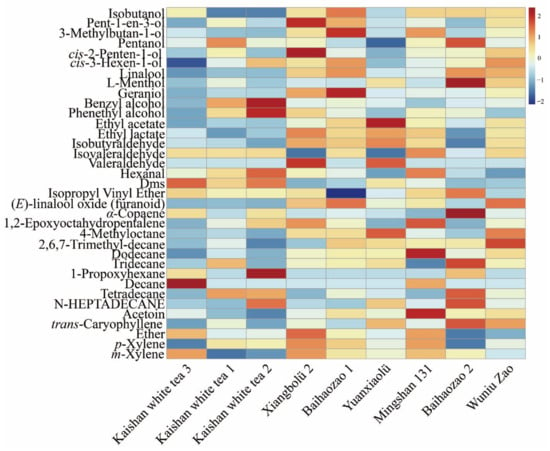
Figure 2.
Hotspot map of aroma differences between different varieties of green tea.
The results of the models constructed for all categories (Table 3) are based on the principle that R2and Q2 > 0.5 indicate an acceptable model [28], and therefore only models for alcohols and alkanes could be used for the analysis of variance in this result, in which the independent variable fit indices (R2x) were 1 and 1, the dependent variable fit indices (R2y) were 0.997 and 0.998, and the model prediction indices (Q2) were 0.992 and 0.994, respectively. After 200 replacement tests, the intercept between the Q2 regression line and the vertical axis is less than zero, indicating that the model is not overfitted and showing the validated model (Figure 3). Therefore, the result of this research concluded that the modeling results could be significant for key aroma components in green tea.

Table 3.
OPLS-DA analysis of each species.
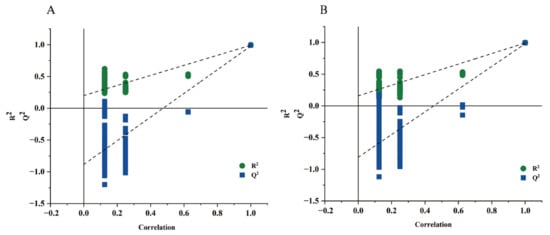
Figure 3.
Cross-validation of OPLS-DA model. Alcohol (A), and Alkane (B).
Variable Importance Projection (VIP) is usually used for the evaluation of the importance of variables in the OPLS-DA model, the larger VIP value indicating that the aroma substance are more important in the analysis of differences between green tea samples, and it is generally considered that variables with VIP > 1.0 make a higher contribution to the differentiation of the samples [29]. As shown in Figure 4(A1,B1), 10 key aroma substances were screened based on VIP > 1 and p < 0.05. Based on the distribution map, the key aroma substances can be analyzed for differences between different green teas, where compounds clustered together indicate that they are present in higher amounts in one tea than others, or present only in a certain tea [30]. Different aromas of a variety of teas are attributed to the various types of tea processing. However, various processing strategies determine the extent to which different precursor substances associate in the reactions of degradation process [27]. As shown in Figure 4 (A2,A3), Kaishan white tea 1 and 2 were located in the fourth quadrant with benzyl alcohol and phenylethanol, and Kaishan white tea 2 was located in the second quadrant with 1-Propoxyhexane, which indicated that benzyl alcohol, phenethyl alcohol, and 1-Propoxyhexane were the characteristic aromas of Kaishan white tea.
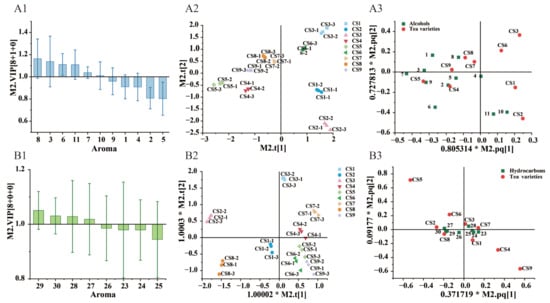
Figure 4.
VIP value, scores and loading plots of OPLS-DA modelof Alcohol (A1–A3), VIP value plot, scores plot and loading plot of Alkane (B1–B3).
Green tea is a non-fermented tea, rich in tea polyphenols and other functional components. Tea polyphenol content significantly affects the taste of green tea wine, while alcohol content also affects the extraction of tea polyphenols [31,32]. Therefore, this research investigated the effect of alcoholic fermentation on tea polyphenols. As shown in Figure 5, tea polyphenol content increased in green tea wine compared to green tea juice, with the highest in Kaisan white tea 3 at 3.72%, an increase of 32.38%. Kaishan white tea 1 had the highest alcohol content and the corresponding lowest residual sugar content of 14.50% vol and 12.45 °Brix, respectively. Fermentation of fruit wines are a biochemical process in which yeast converts sugars into alcohol and secondary metabolites. Sugar and alcohol content can reflect the fermentation level of fruit wines, while tea polyphenol is an important flavor-presenting substance in tea wine [32]. Kaishan white tea 2 did not have the highest tea polyphenols and alcohol content (3.48 and 14.2% vol), but its indexes were in line with the requirements for fruit wines, and it had the highest aroma content. Therefore, Kaishan white tea 2 was chosen as the best fermented tea base in this study.
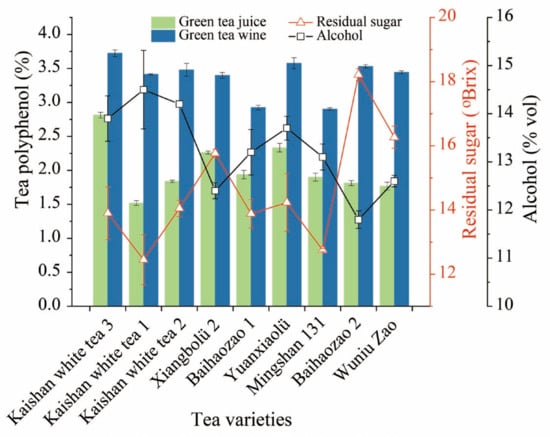
Figure 5.
Comparison of quality differences between different varieties of green tea juice and wine. Columnar, Tea polyphenols; Triangles, Residual sugar; Square-shaped, Alcohol.
3.2. Optimization of Fermentation Process of Green Tea Wine
Fermented tea wine taste is mostly catalyzed by yeast cells, thus factors affecting yeast fermentation activity can cause significant differences in tea wine taste. Therefore, this research investigated the effect of fermentation conditions on tea wine taste in different factors, such as yeast inoculum, initial sugar level, and fermentation temperature, and then determined the optimal fermentation process. As shown in Figure 6, the optimal fermentation conditions were determined by the single-factor experiments as 1.5% inoculum, 30 °Brix initial sugar level, and 25 °C fermentation temperature. The green tea wine quality obtained from fermentation under optimal conditions was 14.4% volume alcohol, 26.23% polyphenols and 8.7 °Brix sugar, respectively.
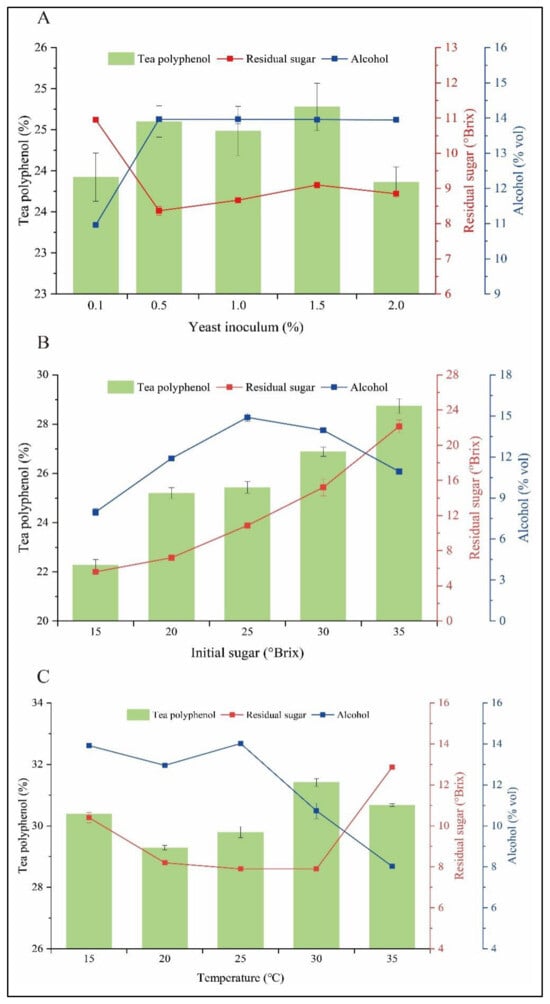
Figure 6.
Optimization of fermentation conditions for green tea wine. Yeast inoculums (A), initial sugar (B), temperature (C). Columnar, Tea polyphenols; Red square-shaped, Residual sugar; Blue square-shaped, Alcohol.
Temperature is one of the most important factors affecting the growth and metabolic efficiency of yeast. Within the optimal temperature range, the growth rate and metabolic capacity of yeast are positively correlated with temperature. When the temperature is higher than optimal temperature, yeast cells find it difficult to resist the extreme environment and irreversible damage occurs, leading to the decline of cell function or cell death [33,34]. Inoculum is a factor that affects the growth rate of microorganisms, and low inoculum will prolong the lag period. Within a certain range, an appropriate increase in the inoculum amount can promote the growth and metabolism of microorganisms. However, higher inoculum is not better, and too high inoculum will lead to an increase in secondary metabolites and premature death of microorganisms [35,36]. Yeast needs nutrients to grow, and tea juice is extremely low in carbon and nitrogen, failing to match the growth needs of the yeast growth. Sugar source acts as precursor for alcohol synthesis and determine the alcoholic yield, while providing a carbon source for yeast growth and facilitating the fermentation process [37].
3.3. Comprehensive Analysis of Green Tea Wine Taste by Electronic Sensory System
E-sensory technology is an advanced analysis system based on bionic senses to evaluate samples digitally. Hence, it provides a higher accuracy and reproducibility [16]. Taste is the most intuitive indicator to evaluate the quality of tea wine. As shown in Figure 7A, saltiness and richness were more prominent in green tea wine, while the response values for bitterness, astringency and aftertaste-A were lower. Compared with the black tea wine developed by Chen et al. [20], the green tea wine in this research had higher intensity of saltiness, while the sourness and bitterness of the black tea wine were more prominent. The reason for the difference may be the accumulation of organic acids and oxidation of amino acids in black tea after fermentation, resulting in an increase in sourness and decrease in saltiness compared to green tea wine [38].
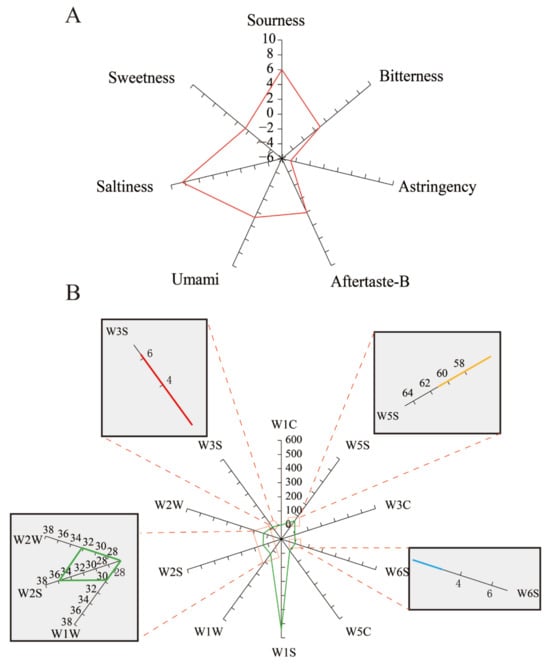
Figure 7.
Evaluation of electronic sensory of green tea wine quality.Radar chart of taste determination by the electronic tongue (A), and the aroma of tea wine by electronic nose analysis (B), red: W3S, yellow: W5S, blue: W6S, green: W2W, W2S and W1W.
For the sensitivity of each sensor, G/G0 indicates the response value. The electronic nose requires that the maximum G/G0 of the sensor for the samples to be tested is >0.5, so the response values of W1C, W3C and W5C in green tea wine was <0.5, indicating that the aromatic compounds in the volatile gases of green tea wine are low (Table 4). It has been demonstrated that the aromatic compound content decreases with fermentation time [37].

Table 4.
Electronic nose sensor performance and response value.
As shown in Figure 7B, the aroma components are mainly alkanes (W1S, W3S), nitrogen oxides (W5S), alcohols aldehydes and ketones (W2S) and sulfur-containing compounds (W1W, W2W). Based on G/G0, the order of aroma contribution can be seen as W1S > W5S > W2S > W2W > W1W > W3S > W6S. PLSR analysis of the aroma components along with the e-nose results showed that all the aroma components were positively correlated with the W5S, W1S and W1W sensors (Figure 8). This includes the hydrocarbon (W1S), which has the highest contribution value, with correlated aroma components 23–30.The dimethyl sulfur of the green tea aroma belongs to sulfide (W1W), which was consistent with the results of the e-nose. Aroma and taste together determine the flavor quality of tea and are dependent on volatile and non-volatile compounds. The contribution of a particular volatile compound to the tea aroma profile depends not only on its content but also on its odor activity value (OAV) and/or flavor dilution (FD) factor [39,40,41,42]. Technologies such as E-nose and E-tongue have been synthesized to discriminate sensory attributes of green tea during fermentation. The VOCs, not only serve as a homeostatic marker for plant homeopathic and environmental microorganisms, but also find utility in quality assessment and origin identification [43,44].
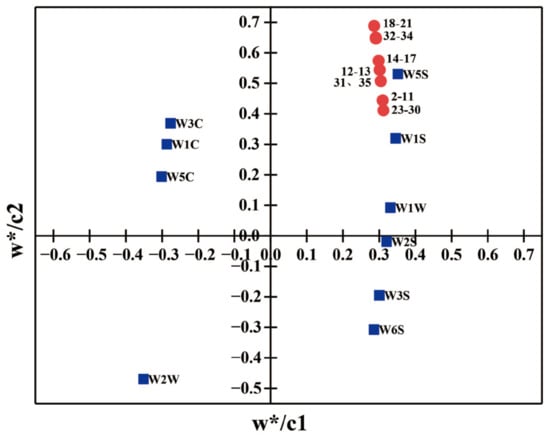
Figure 8.
PLSR analysis of aroma and electronic nose. Solid red circles represent aroma components, and the blue squares represent the e-nose sensors, “w*” indicates the projection coefficient of the independent variable on the X axis (w*/c1) and the projection coefficient of the dependent variable on the Y axis (w*/c2) in PLSR analysis.
4. Conclusions
Green tea, which is non-fermented, has the longest production history in China and around the globe. Due to its unique processing techniques, green tea has the lowest concentration of volatile compounds, and the aroma quality of green tea is endowed with pure, pleasant and long-lasting characteristics. The green tea volatile characteristic aroma was screened by OPLS-DA for the preparation of green tea wine with rich tea aroma. A variety of important factors were screened based on the VIP values, and the characteristic aromas were finally identified as benzyl alcohol, phenethyl alcohol and 1-Propoxyhexane by combining the aroma categories, among which the highest contents were found in Kaishan white tea 1 and 2. The reason for the different types of tea was mainly attributed in the variety of aroma compounds. However, the same type of tea can also have different flavors, mainly due to the different content of each odorant. Based on the comparison of tea polyphenol differences before and after fermentation, Kaishan white tea 2 did not have the highest tea polyphenols and alcohol content (3.48 and 14.2% vol), but its indexes were in line with the requirements for fruit wines, and it had the highest aroma content. It was finally determined that Kaishan white tea 2 was the subsequent fermentation substrate. The optimal fermentation conditions were determined univariately as 1.5% yeast addition, 30 °Brix initial sugar, and 25 °C fermentation temperature. The electronic sensory evaluation showed the saltiness and richness were more prominent in green tea wine, while the response values of bitterness, astringency and aftertaste-A were lower. The order of aroma contribution can be seen as W1S > W5S > W2S > W2W > W1W > W3S > W6S. This can be related to the presence of benzyl alcohol and phenethyl alcohol for the fruit aroma and dimethyl sulfide for the clear aroma of green tea in Kaishan white tea 2. Our results provide technical and theoretical information for developing and comprehensively evaluating tea wine quality for sustainable socioeconomic development.
Author Contributions
Conceptualization, F.W., B.L., J.C., F.Z. and G.C.; methodology, F.W., B.L., J.C., X.F., L.L. and H.C.; writing—original draft preparation, F.W., B.L., J.C. and K.K.V.; writing—review and editing, F.Z. and G.C.; electrochemical part, F.W., B.L. and J.C.; analytical part, X.F. and L.L. All authors have read and agreed to the published version of the manuscript.
Funding
This study was financially supported by the Project of Guangxi Agricultural Science and Technology Innovation Alliance (GNKM202415), and Guangxi Academy of Agricultural Sciences Basic Research Business Project (GNK2021YT117, GNK2024YP117).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available within the article.
Acknowledgments
The authors would like to thank the Guangxi Subtropical Crops Research Institute and the Guangxi Academy of Agricultural Sciences, Nanning, Guangxi, China, for providing the necessary facilities for this study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Liao, Y.; Zhou, X.; Zeng, L. How does tea (Camellia sinensis) produce specialized metabolites which determine its unique quality and function: A review. Crit. Rev. Food Sci. Nutr. 2021, 62, 3751–3767. [Google Scholar] [CrossRef]
- Zheng, X.Q.; Li, Q.S.; Xiang, L.P.; Liang, Y.R. Recent advances in volatiles of teas. Molecules 2016, 21, 338. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Wang, G.; Lin, S.; Liu, Z.; Wang, P.; Li, J.; Zhang, Q.; He, H. Digital evaluation of aroma intensity and odor characteristics of tea with different types—Based on OAV-splitting method. Foods 2022, 11, 2204. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.H.; Li, Y.F.; Li, M.; Wang, Y.J.; Zhang, L.; Wan, X.C.; Yang, X.G. Tea aroma formation from six model manufacturing processes. Food Chem. 2019, 285, 347–354. [Google Scholar] [CrossRef]
- Liu, C.; Wang, C.; Zheng, T.T.; Zhao, M.M.; Gong, W.Y.; Wang, Q.M.; Yan, L.; Zhang, W.J. Characterization of key odor-active compounds in Sun-Dried black tea by sensory and instrumental-directed flavor analysis. Foods 2022, 22, 1740. [Google Scholar] [CrossRef]
- Guo, X.Y.; Ho, C.T.; Schwab, W.; Wan, X.C. Aroma profiles of green tea made with fresh tea leaves plucked in summer. Food Chem. 2021, 363, 130328. [Google Scholar] [CrossRef] [PubMed]
- Gianfredi, V.; Nucci, D.; Abalsamo, A.; Acito, M.; Villarini, M.; Moretti, M.; Realdon, S. Green tea consumption and risk of breast cancer and recurrence—A systematic review and meta-analysis of observational studies. Nutrients 2018, 10, 1886. [Google Scholar] [CrossRef]
- Tan, H.-L.; Ojukwu, M.; Lee, L.-X.; Easa, A.M. Quality characteristics of green Tea’s infusion as influenced by brands and types of brewing water. Heliyon 2023, 9, e12638. [Google Scholar] [CrossRef] [PubMed]
- Zou, C.; Chen, D.-Q.; He, H.-F.; Huang, Y.-B.; Feng, Z.-H.; Chen, J.-X.; Wang, F.; Xu, Y.-Q.; Yin, J.-F. Impact of tea leaves categories on physicochemical, antioxidant, and sensorial profiles of tea wine. Front. Nutr. 2023, 10, 1110803. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, M.; Zhang, Z.; Jiang, J.; Gao, X.; Yue, P. Multiple responses optimization of instant dark tea production by submerged fermentation using response surface methodology. J. Food Sci. Technol. 2018, 55, 2579–2586. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, Y.; Yan, F.; Tang, Y.; Yu, B.; Chen, B.; Lu, L.; Yuan, L.; Wu, Z.; Chen, H. Monitoring Changes in the Volatile Compounds of Tea Made from Summer Tea Leaves by GC-IMS and HS-SPME-GC-MS. Foods 2022, 12, 146. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, S.; Sun, Y. Measurement of catechin and gallic acid in tea wine with HPLC. Saudi J. Biol. Sci. 2020, 27, 214–221. [Google Scholar] [CrossRef]
- Xu, W.; Wang, X.; Jia, W.; Wen, B.; Liao, S.; Zhao, Y.; Tang, Q.; Li, K.; Hua, Y.; Yang, Y.; et al. Dynamic changes in the major chemical and volatile components during the “Ziyan” tea wine processing. LWT—Food Sci. Technol. 2023, 186, 115273. [Google Scholar] [CrossRef]
- Wang, R.; Sun, J.; Lassabliere, B.; Yu, B.; Liu, S.Q. Biotransformation of green tea (Camellia sinensis) by wine yeast Saccharomyces cerevisiae. J. Food Sci. 2020, 85, 306–315. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Guo, C.; Guo, N.; Zhang, T.; Wang, Y.; Wang, Y.; Lin, X.; Wu, F.; Feng, Y.; Xing, G. Similarity Evaluation on the Compound TCM Formulation “Huoling Shengji Granule” and Its Placebo by Intelligent Sensory Evaluation Technologies and the Human Sensory Evaluation Method Based on Critical Quality Attributes. Evid.-Based Complement. Altern. Med. 2021, 2021, 6637326. [Google Scholar] [CrossRef]
- Rosa, A.R.D.; Leone, F.; Cheli, F.; Chiofalo, V. Fusion of electronic nose, electronic tongue and computer vision for animal source food authentication and quality assessment—A review. J. Food Eng. 2017, 210, 62–75. [Google Scholar] [CrossRef]
- Xiao, Z.; Jiang, X.; Niu, Y. Study on the interaction of sweet protein (thaumatin) with key aroma compounds in passion fruit juice using electronic nose, ultraviolet spectrum, thermodynamics, and molecular docking. LWT—Food Sci. Technol. 2022, 162, 113463. [Google Scholar] [CrossRef]
- Xia, Y.; Zha, M.; Liu, H.; Shuang, Q.; Chen, Y.; Yang, X. Novel Insight into the Formation of Odour—Active Compounds in Sea Buckthorn Wine and Distilled Liquor Based on GC–MS and E–Nose Analysis. Foods 2022, 11, 3273. [Google Scholar] [CrossRef]
- Wang, J.; Chang, M.; He, W.; Lu, X.; Fei, S.; Lu, G. Optimization of Electronic Nose Sensor Array for Tea Aroma Detecting based on Correlation Coefficient and Cluster Analysis. J. Chemosens. 2021, 9, 266. [Google Scholar] [CrossRef]
- Chen, J.; Lin, B.; Zheng, F.J.; Fang, X.C.; Ren, E.F.; Wu, F.F.; Verma, K.K.; Chen, G.L. Characterization of the Pure Black Tea Wine Fermentation Process by Electronic Nose and Tongue-Based Techniques with Nutritional Characteristics. ACS Omega 2023, 8, 12538–12547. [Google Scholar] [CrossRef]
- Chen, W.; Chen, J.; Pan, H.; Ding, L.; Ni, Z.; Wang, Y.; Zhou, J. Dynamical changes of volatile metabolites and identification of core fungi associated with aroma formation in Fu Brick tea during the fungal fermentation. LWT—Food Sci. Technol. 2024, 202, 116298. [Google Scholar] [CrossRef]
- Ma, T.; Wang, J.; Wang, H.; Lan, T.; Liu, R.; Gao, T.; Yang, W.; Zhou, Y.; Ge, Q.; Fang, Y.; et al. Is overnight fresh juice drinkable? The shelf life prediction of non-industrial fresh watermelon juice based on the nutritional quality, microbial safety quality, and sensory quality. Food Nutr. Res. 2020, 64, 4237. [Google Scholar] [CrossRef] [PubMed]
- Qiu, S.; Wang, J. Application of Sensory Evaluation, HS-SPME GC-MS, E-Nose, and E-Tongue for Quality Detection in Citrus Fruits. J. Food Sci. 2015, 80, S2296–S2304. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Li, Z.; Zou, S.; Dong, L.; Lin, X.; Chen, Y.; Zhang, S.; Ji, C.; Liang, H. Chemical Composition and Flavor Characteristics of Cider Fermented with Saccharomyces cerevisiae and Non-Saccharomyces cerevisiae. Foods 2023, 12, 3565. [Google Scholar] [CrossRef]
- Huang, X.; Tang, Q.; Chen, C.; Li, Q.; Lin, H.; Bai, S.; Zhao, J.; Li, J.; Wang, K.; Zhu, M. Combined analysis of transcriptome and metabolome provides insights into nano-selenium foliar applications to improve summer tea quality (Camellia sinensis). LWT 2023, 175, 114496. [Google Scholar] [CrossRef]
- Zhang, L.; Cao, Q.-Q.; Granato, D.; Xu, Y.-Q.; Ho, C.-T. Association between chemistry and taste of tea: A review. Trends Food Sci. Technol. 2020, 101, 139–149. [Google Scholar] [CrossRef]
- Lin, Y.; Wang, Y.; Huang, Y.; Song, H.; Yang, P. Aroma identification and classification in 18 kinds of teas (Camellia sinensis) by sensory evaluation, HS-SPME-GC-IMS/GC × GC-MS, and chemometrics. Foods 2023, 12, 2433. [Google Scholar] [CrossRef]
- Phan, Q.; DuBois, A.; Osborne, J.; Tomasino, E. Effects of Yeast Product Addition and Fermentation Temperature on Lipid Composition, Taste and Mouthfeel Characteristics of Pinot Noir Wine. Horticulturae 2022, 8, 52. [Google Scholar] [CrossRef]
- Deed, R.C.; Fedrizzi, B.; Gardner, R.C. Influence of Fermentation Temperature, Yeast Strain, and Grape Juice on the Aroma Chemistry and Sensory Profile of Sauvignon Blanc Wines. J Agric. Food Chem. 2017, 65, 8902–8912. [Google Scholar] [CrossRef]
- Mu, Y.; Zeng, C.; Qiu, R.; Yang, J.; Zhang, H.; Song, J.; Yuan, J.; Sun, J.; Kang, S. Optimization of the Fermentation Conditions of Huaniu Apple Cider and Quantification of Volatile Compounds Using HS-SPME-GC/MS. Metabolites 2023, 13, 998. [Google Scholar] [CrossRef]
- Indarti, K.; Apriani, E.F.; Wibowo, A.E.; Simanjuntak, P. Antioxidant Activity of Ethanolic Extract and Various Fractions from Green Tea (Camellia sinensis L.) Leaves. Pharmacogn. J. 2019, 11, 771–776. [Google Scholar] [CrossRef]
- López-Aguilar, R.; Zuleta-Prada, H.; Hernández-Montes, A.; Herbert-Pucheta, J.E. Comparative NMR Metabolomics Profiling between Mexican Ancestral & Artisanal Mezcals and Industrialized Wines to Discriminate Geographical Origins, Agave Species or Grape Varieties and Manufacturing Processes as a Function of Their Quality Attributes. Foods 2021, 10, 157. [Google Scholar] [CrossRef] [PubMed]
- Yun, J.; Cui, C.; Zhang, S.; Zhu, J.; Peng, C.; Cai, H.; Yang, X.; Hou, R. Use of headspace GC/MS combined with chemometric analysis to identify the geographic origins of black tea. Food Chem. 2021, 360, 130033. [Google Scholar] [CrossRef]
- Yang, X.; Chen, Q.; Liu, S.; Hong, P.; Zhou, C.; Zhong, S. Characterization of the effect of different cooking methods on volatile compounds in fish cakes using a combination of GC-MS and GC-IMS. Food Chem. X 2024, 22, 101291. [Google Scholar] [CrossRef] [PubMed]
- Halouska, S.; Fenton, R.J.; Barletta, R.G.; Powers, R. Predicting the in vivo mechanism of action for drug leads using NMR metabolomics. ACS Chem. Biol. 2012, 7, 166–171. [Google Scholar] [CrossRef]
- Kontkanen, D.; Inglis, D.L.; Pickering, G.J.; Reynolds, A. Impact of yeast inoculation methods for Icewine fermentation on yeast growth, fermentation time, yeast metabolite production and sensory profiles of Vidal Icewine. Am. J. Enol. Viticult. 2004, 55, 363–370. [Google Scholar] [CrossRef]
- Santa-Bell, E.; Kovacs, N.K.; Alács, B.; Molnar, Z.; Hornyánszky, G. Immobilization of Phenylalanine Ammonia-lyase via EDTA Based Metal Chelate Complexes—Optimization and Prospects. Period. Polytech. Chem. Eng. 2021, 65, 308–319. [Google Scholar] [CrossRef]
- Tang, F.; Cai, W.; Shan, C.; Guo, Z.; Hou, Q.; Zhang, Z.; Dong, Y. Dynamic changes in quality of jujube wine during fermentation. J. Food Process. Preserv. 2020, 44, e14704. [Google Scholar] [CrossRef]
- Liu, H.; Xu, Y.; Wen, J.; An, K.; Wu, J.; Yu, Y.; Zou, B.; Guo, M. A comparative study of aromatic characterization of Yingde Black Tea infusions in different steeping temperatures. LWT 2021, 143, 110860. [Google Scholar] [CrossRef]
- Yin, P.; Kong, Y.; Liu, P.; Wang, J.; Zhu, Y.; Wang, G.; Sun, M.; Chen, Y.; Guo, G.; Liu, Z. A critical review of key odorants in green tea: Identification and biochemical formation pathway. Trends Food Sci. Technol. 2022, 129, 221–232. [Google Scholar] [CrossRef]
- Chen, G.L.; Zheng, F.J.; Dong, W.B.; Wu, C.Q.; Lin, B.; Xu, S.S. Aroma components of eight black teas from Hezhou. J. South. Agricult. 2018, 49, 2532–2538. [Google Scholar]
- Chen, G.L.; Zheng, F.J.; Lin, B.; Xu, S.S.; Wu, C.Q.; Dong, W.B. Comparison and Analysis of Characteristic Aroma Components of 6 Main Green Teas in Hezhou. Sci. Technol. Food Ind. 2019, 40, 246–255. [Google Scholar]
- Su, P.; Kang, H.; Peng, Q.; Wicaksono, W.A.; Berg, G.; Liu, Z.; Ma, J.; Zhang, D.; Cernava, T.; Liu, Y. Microbiome homeostasis on rice leaves is regulated by a precursor molecule of lignin biosynthesis. Nat. Commun. 2024, 15, 23. [Google Scholar] [CrossRef] [PubMed]
- Di Donato, F.; Biancolillo, A.; Mazzulli, D.; Rossi, L.; D’Archivio, A.A. HSSPME/GC–MS volatile fraction determination and chemometrics for the discrimination of typical Italian Pecorino cheeses. Microchem. J. 2021, 165, 106133. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).