The Impact of Lactobacillus delbrueckii Hepatic Metabolism in Post-Weaning Piglets
Abstract
1. Introduction
2. Materials and Methods
2.1. Testing Materials and Sources
2.2. Design of Experiments and Formulation of Dietary Composition
2.3. Management of Animal Experiments
2.4. Detection Indices and Methodologies
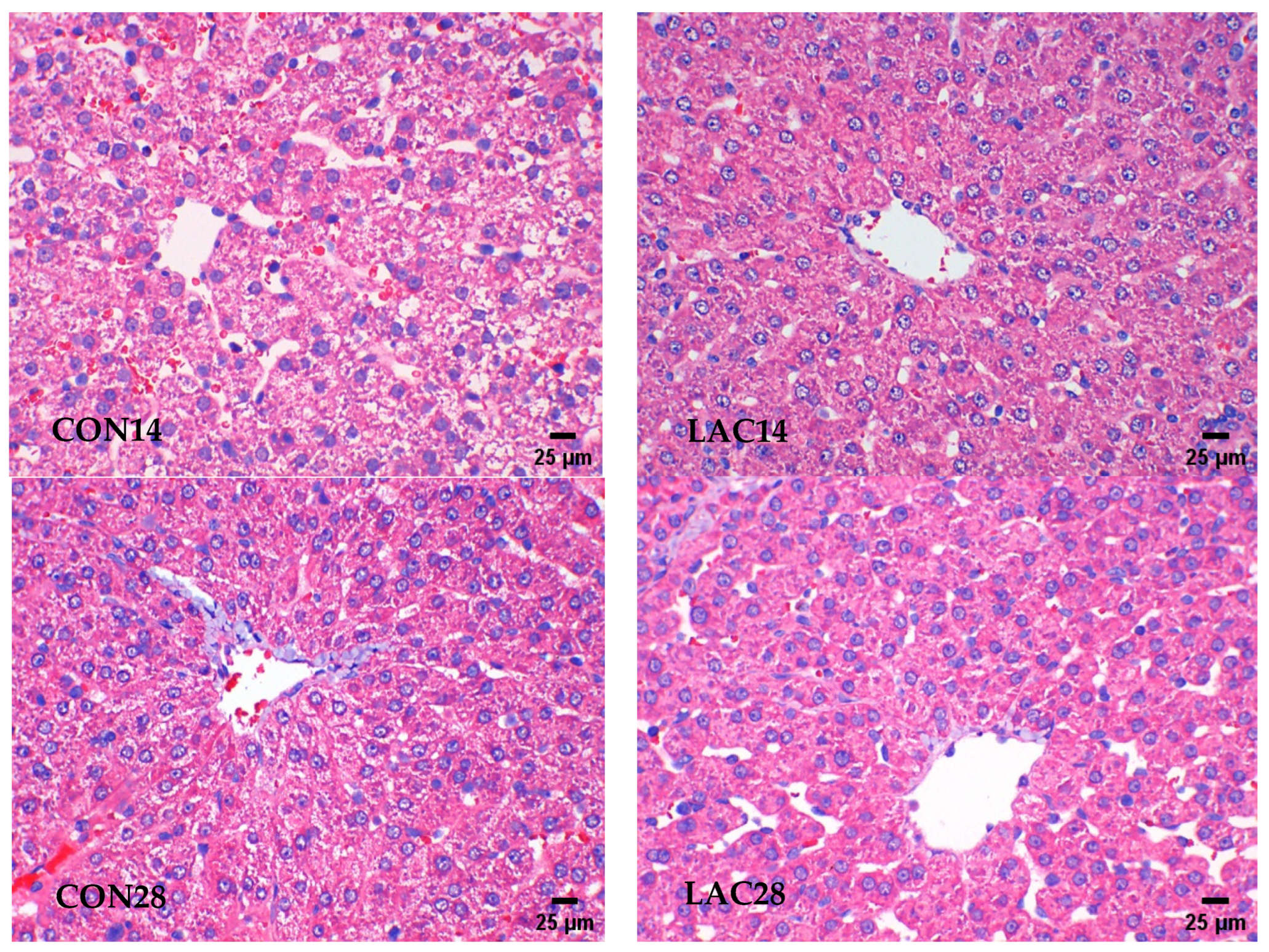
2.4.1. The Liver Tissue Was Examined Using Hematoxylin and Eosin (HE) Staining
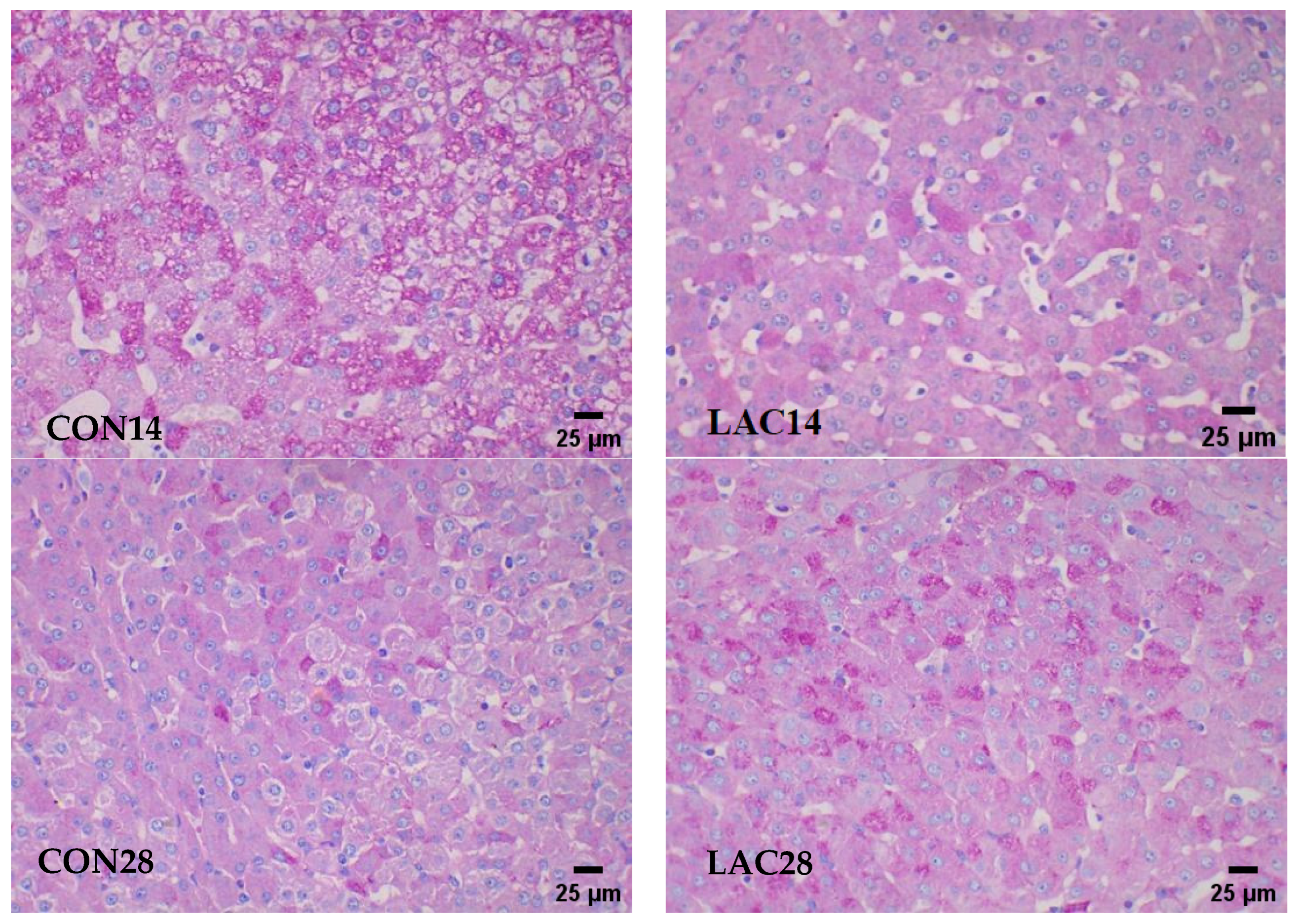
2.4.2. Revealing Hepatic Glycogen Accumulation through PAS Staining in Liver Tissue
2.4.3. Comprehensive Analysis of Hepatic Metabolites across the Entire Spectrum
2.5. Revealing the Identification of Metabolic Pathways
2.6. Data Processing and Analysis Procedures
3. Results and Analysis
3.1. The Impact of Lactobacillus delbrueckii on Hepatic Morphology in Post-Weaning Piglets
3.2. Impact of Lactobacillus delbrueckii on Hepatic Glycogen Content in Post-Weaning Piglets
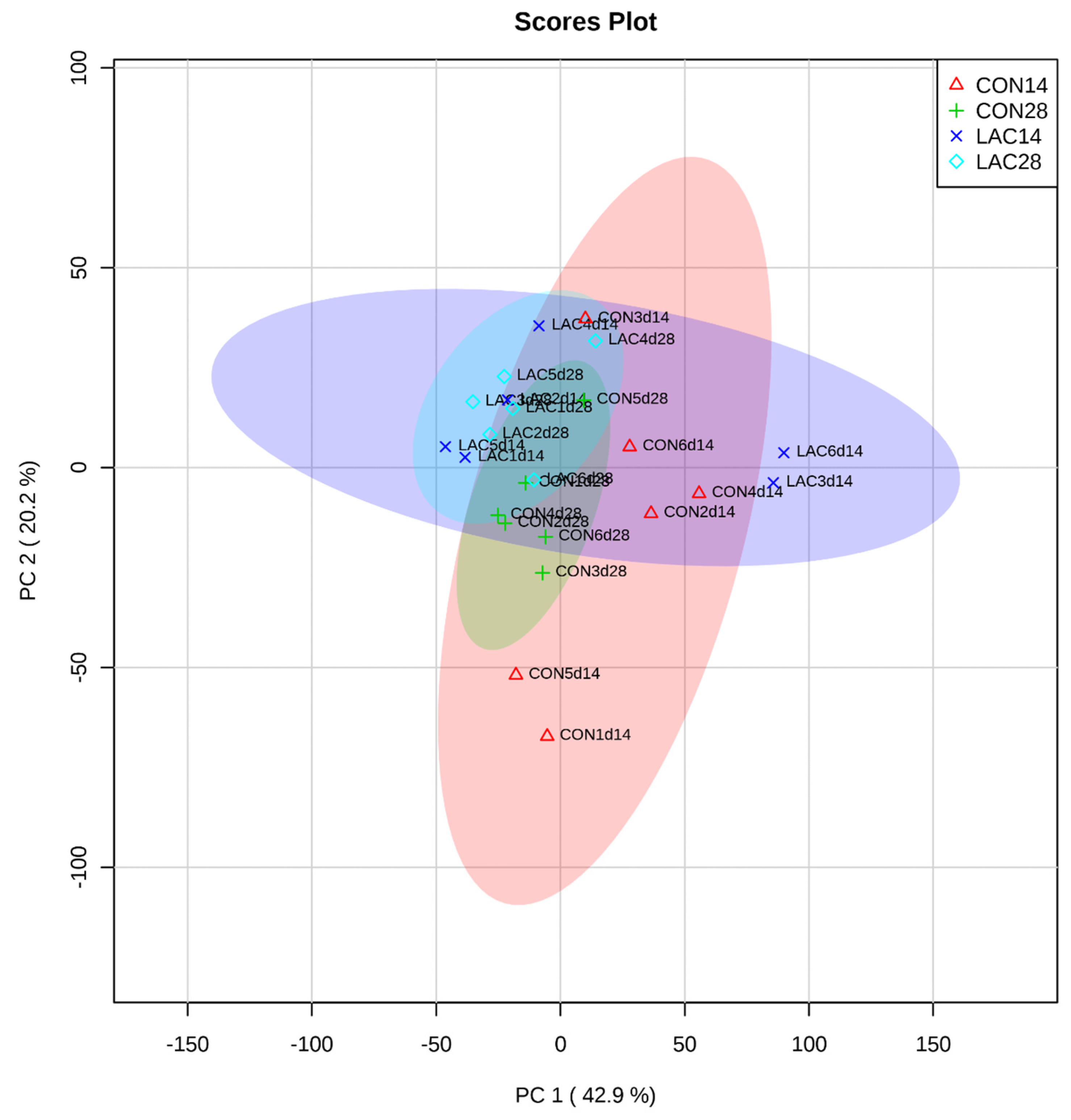
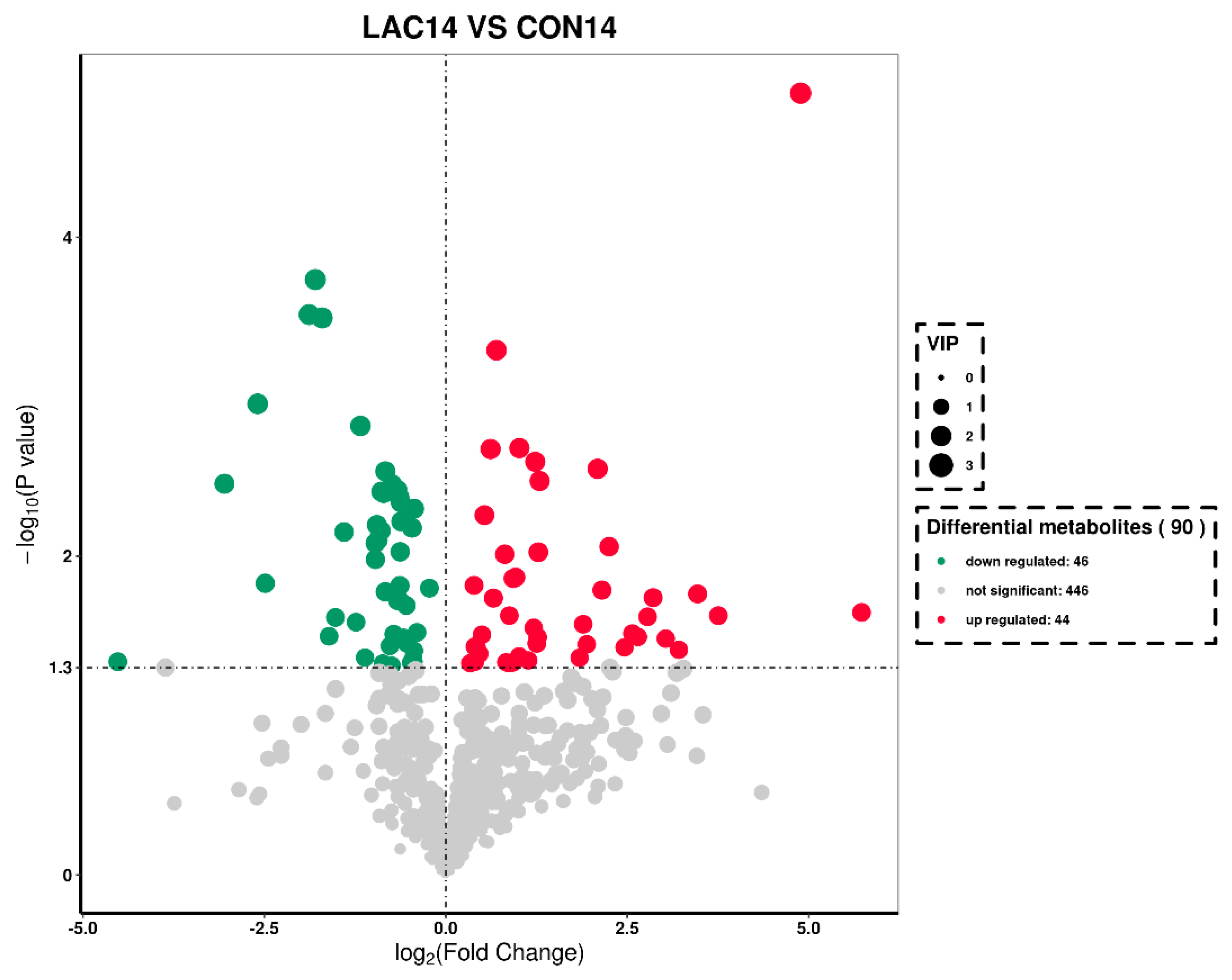
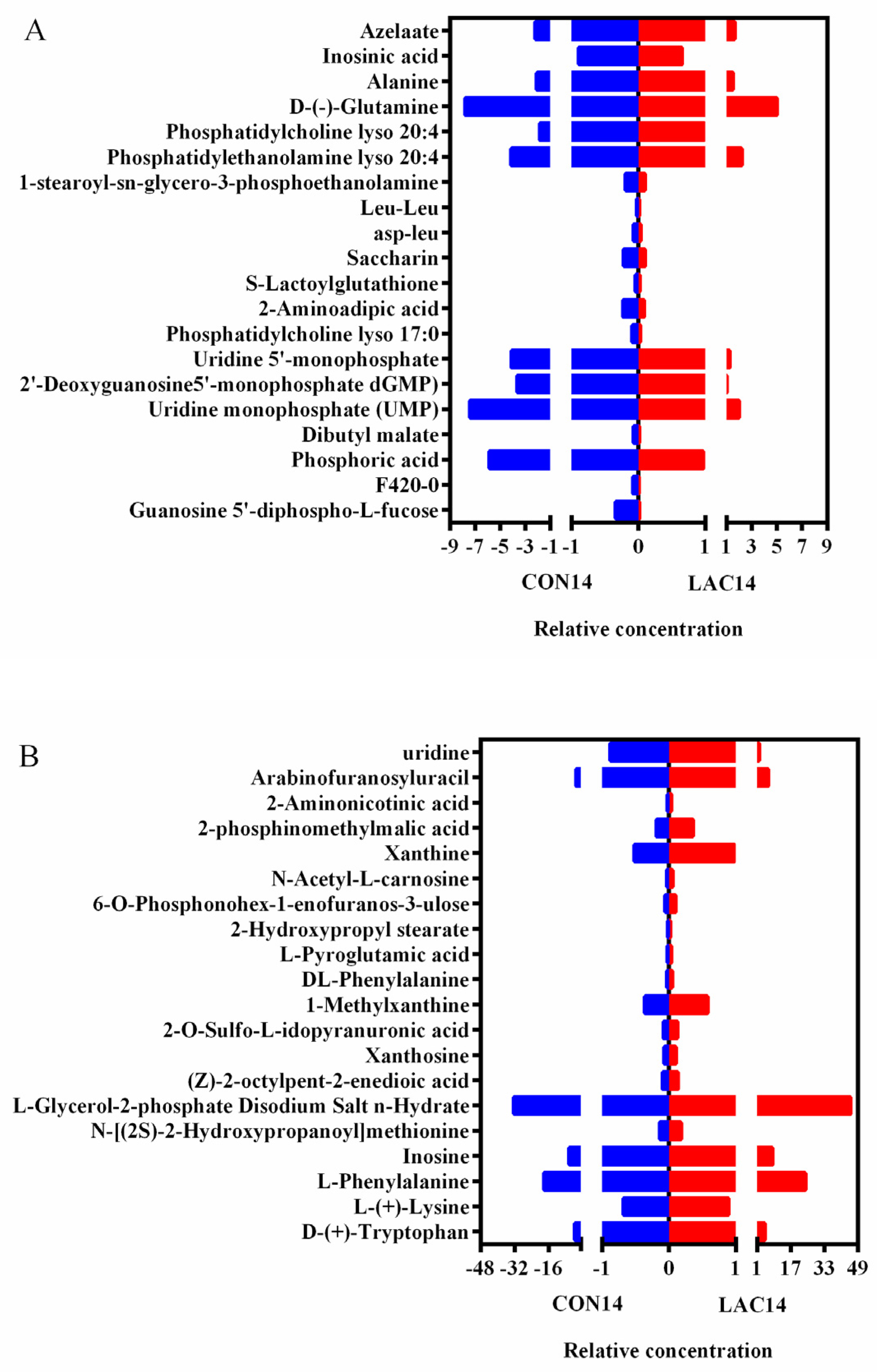
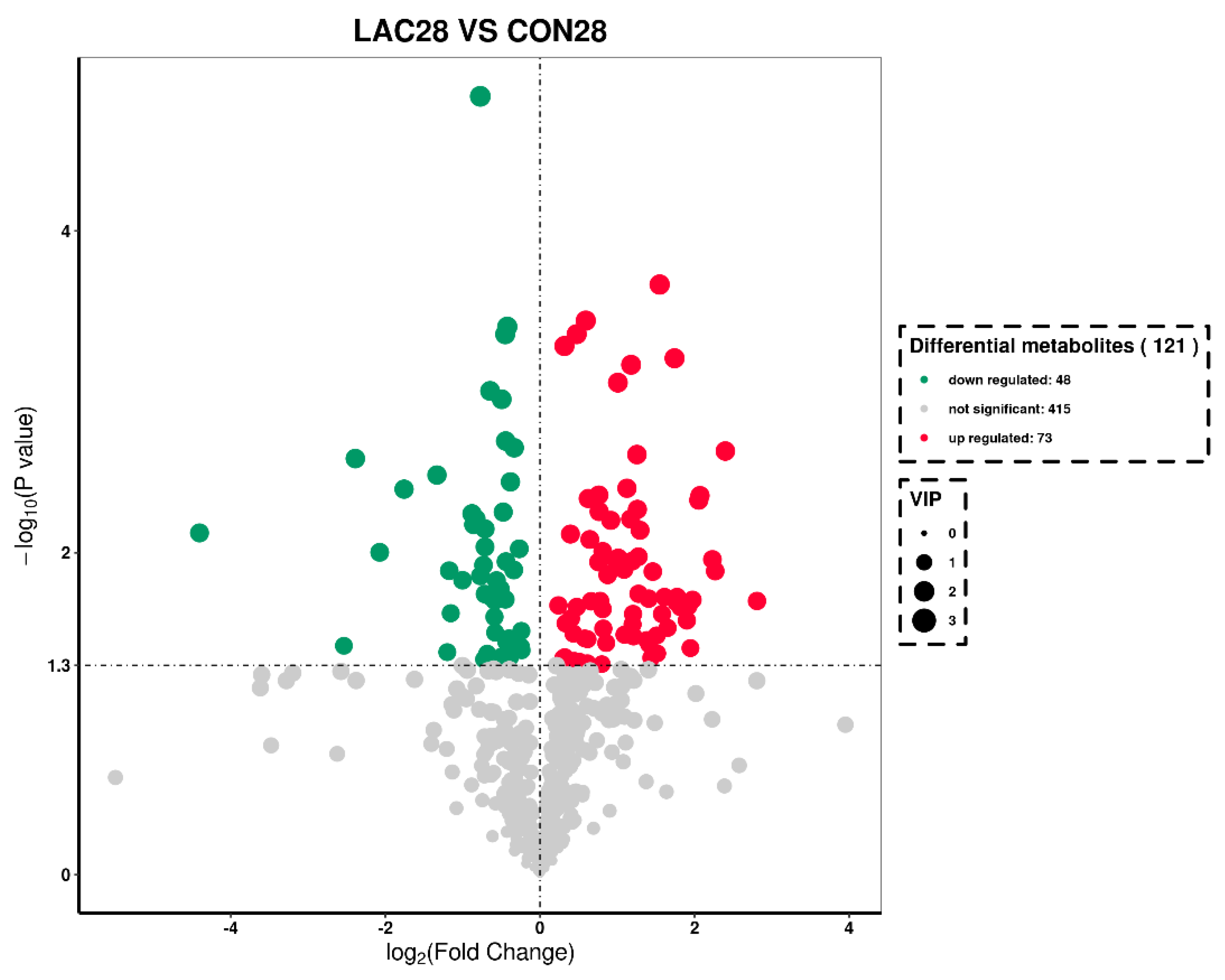
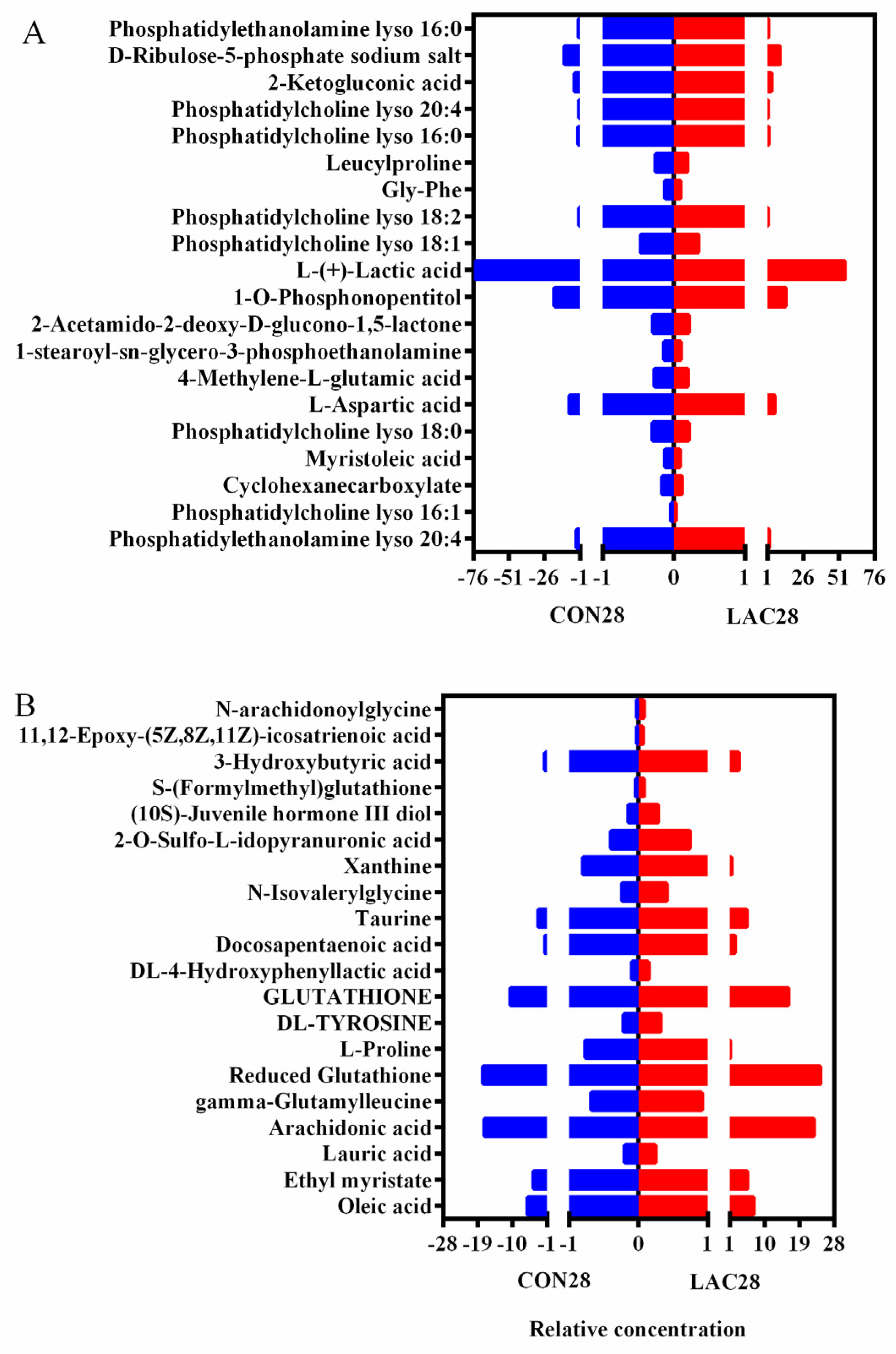
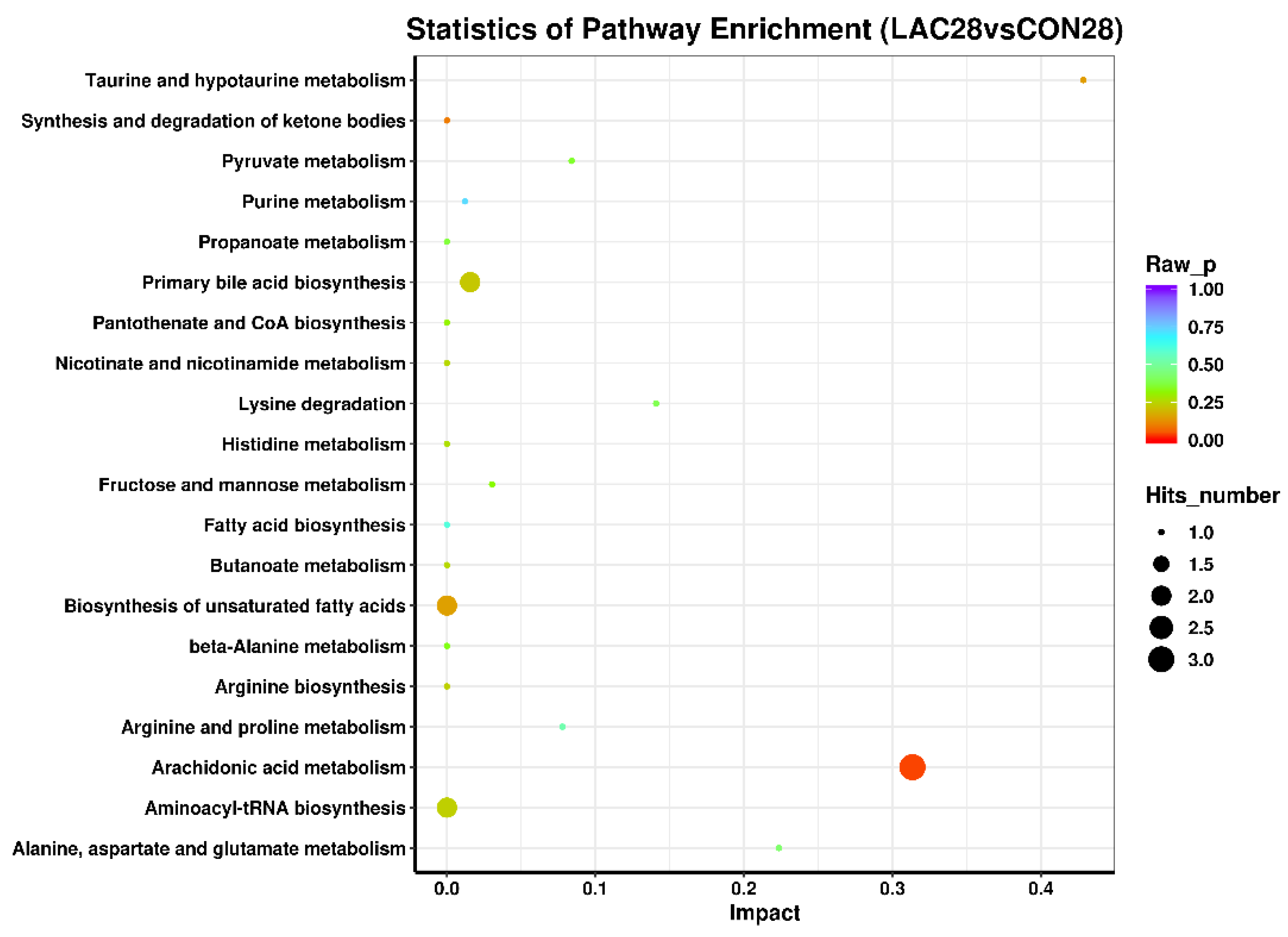
3.3. The Impact of Lactobacillus delbrueckii on Hepatic Metabolism in Post-Weaning Piglets
3.4. Impact of Lactobacillus delbrueckii on Hepatic Glycogen Content in Post-Weaning Piglets
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rogosa, M.; Hansen, P.A. Nomenclatural Considerations of Certain Species of Lactobacillus Beijerinck. Int. J. Syst. Evol. Microbiol. 1971, 21, 177–186. [Google Scholar] [CrossRef]
- Wen, W.; Yang, T.; Wei, L.; Huang, X. Biological Function of Lactobacillus delbrueckii and Its Application in Animal Production. Chin. J. Anim. Nutr. 2019, 31, 1533–1539. [Google Scholar] [CrossRef]
- Toba, T.; Yoshioka, E.; Itoh, T. Lacticin, a bacteri-ocin produced by Lactobacillus delbrueckii subsp. Lactis. Lett. Appl. Microbiol. 1991, 12, 43–45. [Google Scholar] [CrossRef]
- Dai, Y.; Tian, Z.; Nan, X. Progress of the Lactobacillus and Its Physiological Function. Agric. Prod. Process. (J. Agric. Sci.) 2009, 7, 24–26+29. [Google Scholar]
- Hansen, P.A. Type Strains of Lactobacillus Species. A Report by the Taxonomic Subcommittee on Lactobacilli and Closely Related Organisms. R. A Subcommittee of the International Committee on Nomenclature of Bacteria of the International Association of Microbiological Societies; American Culture Collection: Rockville, MD, USA, 1968; Corpus ID: 89670364. [Google Scholar]
- Li, Y.; Hou, S.; Peng, W.; Lin, Q.; Chen, F.; Yang, L.; Li, F.; Huang, X. Oral Administration of Lactobacillus delbrueckii during the Suckling Phase Improves Antioxidant Activities and Immune Responses after the Weaning Event in a Piglet Model. Oxidative Med. Cell Longev. 2019, 2019, 6919803. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Tian, S.; Bai, H. Lactic acid bacteria and lactic acid bacteria fermented food. J. Food Food Ind. 2005, 12, 3. [Google Scholar] [CrossRef]
- Giang, H.H.; Viet, T.Q.; Ogle, B.; Lindberg, J.E. Effects of Supplementation of Probiotics on the Performance, Nutrient Digestibility and Faecal Microflora in Growing-finishing Pigs. Asian-Australas. J. Anim. Sci. 2011, 24, 655–661. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, J.; Zhang, S.; Yang, F.; Thacker, P.A.; Zhang, G.; Qiao, S.; Ma, X. Oral Administration of Lactobacillus fermentum I5007 Favors Intestinal Development and Alters the Intestinal Microbiota in Formula-Fed Piglets. J. Agric. Food Chem. 2014, 62, 860–866. [Google Scholar] [CrossRef] [PubMed]
- Giang, H.H.; Viet, T.Q.; Ogle, B.; Lindberg, J.E. Growth performance, digestibility, gut environment and health status in weaned piglets fed a diet supplemented with potentially probiotic complexes of lactic acid bacteria. Livest. Sci. 2010, 129, 95–103. [Google Scholar] [CrossRef]
- He, T.; Zhu, Y.-H.; Yu, J.; Xia, B.; Liu, X.; Yang, G.-Y.; Su, J.-H.; Guo, L.; Wang, M.-L.; Wang, J.-F. Lactobacillus johnsonii L531 reduces pathogen load and helps maintain short-chain fatty acid levels in the intestines of pigs challenged with Salmonella enterica Infantis. Veter Microbiol. 2019, 230, 187–194. [Google Scholar] [CrossRef]
- Li, Y.; Hou, S.; Chen, J.; Peng, W.; Wen, W.; Chen, F.; Huang, X. Oral administration of Lactobacillus delbrueckii during the suckling period improves intestinal integrity after weaning in piglets. J. Funct. Foods 2019, 63, 103591. [Google Scholar] [CrossRef]
- Pluske, J.R.; Thompson, M.J.; Atwood, C.S.; Bird, P.H.; Williams, I.H.; Hartmann, P.E. Maintenance of villus height and crypt depth, and enhancement of disaccharide digestion and monosaccharide absorption, in piglets fed on cows’ whole milk after weaning. Br. J. Nutr. 1996, 76, 409–422. [Google Scholar] [CrossRef] [PubMed]
- Fuller, R. Probiotics in man and animals. J. Appl. Bacteriol. 1989, 66, 365–378. [Google Scholar] [PubMed]
- Meale, S.J.; Li, S.C.; Azevedo, P.; Derakhshani, H.; DeVries, T.J.; Plaizier, J.C.; Steele, M.A.; Khafipour, E. Weaning age influences the severity of gastrointestinal microbiome shifts in dairy calves. Sci. Rep. 2017, 7, 198. [Google Scholar] [CrossRef] [PubMed]
- Stokes, C.R. The development and role of microbial-host interactions in gut mucosal immune development. J. Anim. Sci. Biotechnol. 2017, 8, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Xiong, X.; Wang, X.; Tan, B.; Li, T.; Yin, Y. Effects of Weaning on Intestinal Upper Villus Epithelial Cells of Piglets. PLoS ONE 2016, 11, e0150216. [Google Scholar] [CrossRef] [PubMed]
- Gresse, R.; Chaucheyras-Durand, F.; Fleury, M.A.; Van de Wiele, T.; Forano, E.; Blanquet-Diot, S. Gut Microbiota Dysbiosis in Postweaning Piglets: Understanding the Keys to Health. Trends Microbiol. 2017, 25, 851–873. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Liu, P.; Guan, W.; Chen, F.; Deng, Y. Effects of Dietary Supplementation of Immuno-globulin of Yolk on Growth Performance, Serum Biochemical Parameters, Intestinal Morphology and Microbial Flora of Weaned Piglets. Chin. J. Anim. Nutr. 2017, 29, 271–279. [Google Scholar] [CrossRef]
- Nafikov, R.A.; Beitz, D.C. Carbohydrate and Lipid Metabolism in Farm Animals. J. Nutr. 2007, 137, 702–705. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Yi, S.; Xu, E.; Chen, X.; Dai, B.; Xiao, Y. Effects of Early Weaning on Glycogen Concent and Expression of Hepatic Genes Associated with Gluconeogenesis and Glycolysis in Piglets. Chin. J. Anim. Nutr. 2018, 30, 1019–1026. [Google Scholar]
- Li, R. Effects of Lactobacillus delbrueckii on Cholesterol Metabolism in Fattening Pigs; Hunan Agricultural University: Changsha, China, 2014. [Google Scholar]
- Nielsen, S.S.; Grøfte, T.; Tygstrup, N.; Vilstrup, H. Synthesis of acute phase proteins in rats with cirrhosis exposed to lipopolysaccharide. Comp. Hepatol. 2006, 5, 3. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.M.; Wang, X.Y.; Wu, H.T.; Wang, H.B.; Chen, S.K.; Zhu, H.L.; Liu, Y.L. Effects of glutamate on energy metabolism and mRNA expression of key enzymes and related regulatory factors in liver of piglets. Chin. J. Anim. Sci. 2016, 52, 38–43. [Google Scholar]
- Li, Q.; Liu, Y.; Che, Z.; Zhu, H.; Meng, G.; Hou, Y.; Ding, B.; Yin, Y.; Chen, F. Dietary L-arginine supplementation alleviates liver injury caused by Escherichia coli LPS in weaned pigs. J. Endotoxin Res. 2012, 18, 804–814. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.B. Regulative Effect of Flaxseed Oil on Intestinal and Liver Injury of Piglets after Lipopolysaccharide Challenge; Wuhan University of Light Industry: Wuhan, China, 2016. [Google Scholar]
- Baiceanu, A.; Mesdom, P.; Lagouge, M.; Foufelle, F. Endoplasmic reticulum proteostasis in hepatic steatosis. Nat. Rev. Endocrinol. 2016, 12, 710–722. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Shao, Y.; Yao, W.; Wang, T.; Xia, J.; Huang, F. Effects of Garcinol Supplementation on Growth Performance, Antioxidant Function and Liver Lipid Synthesis of Piglets under Oxidative Stress. Chin. J. Anim. Nutr. 2019, 31, 5834–5845. [Google Scholar]
- Sokol, H.; Seksik, P.; Furet, J.; Firmesse, O.; Mariat, D.; Beaugerie, L.; Dore, J.M. T1238 fecal microbiota alterations in inflammatory bowel disease (IBD): Decrease of Faecali-bacterium Prausnitzii but no quantitative variation of Escherichia coli. Gastroenterology 2008, 134, A-513. [Google Scholar] [CrossRef]
- Kobatake, E.; Nakagawa, H.; Seki, T.; Miyazaki, T. Protective effects and functional mechanisms of Lactobacillus gasseri SBT2055 against oxidative stress. PLoS ONE 2017, 12, e0177106. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Xia, Y.; Wang, G.; Xiong, Z.; Zhang, H.; Lai, F.; Ai, L. Lactobacillus plantarum AR501 alleviates the oxidative stress of D-Galactose-induced aging mice liver by up regulation of Nrf2-Mediated antioxidant enzyme expression. J. Food Sci. 2018, 83, 1990–1998. [Google Scholar] [CrossRef]
- Liu, M. Study on the Alleviating Effect of Lactobacillus desseri on Inflammatory Bowel Disease of Weaned Piglets; Hunan Agricultural University: Changsha, China, 2016. [Google Scholar]
- Chen, X.L.; Gong, J.P.; Xu, F.L. Effects and mechanisms of glycogen synthase kinase-3 inhibition induced by lipopolysaccharide pretreatment on liver glycogen. J. South. Med. Univ. 2014, 2, 201–205. [Google Scholar]
- Huang, L.H. Effects of Chitooligosaccharides on Alleviating Hepatic Oxidative Damage in Weaned Piglets; Sichuan Agricultural University: Chengdu, China, 2016. [Google Scholar]
- Li, L.Z.; Li, L.Y. Key Technologies of Modern Biochemical Drug Production; Chemical Industry Press: Beijing, China, 2006; pp. 145–150. [Google Scholar]
- Song, Y.H.; Feng, T. Clinical application of determination of free L-fucose in urine. Prog. Biochem. Biophys. 1999, 26, 513–516. [Google Scholar]
- Dong, Z.N.; Shen, W.M. Clinical significance of urinary L-fucose determination. Proc. Acad. Mil. Med. Sci. 1997, 4, 257. [Google Scholar]
- Fu, S.E.; Han, W.; Bian, J. Research progress of arachidonic acid. Yunnan Chem. Ind. 2004, 31, 31–32. [Google Scholar]
- Zhong, J. Effects of Arachidonic Acid (ARA) on the Growth and Hepatoprotective of Monopterus albus; Jiangxi Agricultural University: Nanchang, China, 2018. [Google Scholar]
- Winterbour, N.; Christine, C. Regulation of intracellular glutathione. Redox Biol. 2018, 22, 101086. [Google Scholar]

| Diet Ingredient | Content (%) | Nutrition Levels 2 | Content |
|---|---|---|---|
| Corn | 62.00 | DE (MJ/Kg) | 14.11 |
| Extruded soybean | 10.00 | CP (%) | 18.47 |
| Soybean meal | 14.00 | EE (%) | 4.40 |
| Whey powder | 5.00 | Lys (%) | 1.30 |
| Fermented soybean meal | 3.00 | Met (%) | 0.39 |
| Fish meal | 2.50 | Thr (%) | 0.80 |
| Calcium hydrophosphate | 1.40 | Met + Cys (%) | 0.70 |
| Calcium carbonate | 0.40 | Ca (%) | 0.70 |
| Choline | 0.10 | P (%) | 0.63 |
| Salt | 0.20 | AP (%) | 0.44 |
| Lysine | 0.42 | ||
| Methionine | 0.08 | ||
| Threonine | 0.10 | ||
| Premix 1 | 0.80 | ||
| Total | 100.00 |
| Time (min) | Flow Rate (mL/min) | A (%) | B (%) |
|---|---|---|---|
| 0 | 0.3 | 100 | 0 |
| 0 | 0.3 | 100 | 0 |
| 1.5 | 0.3 | 80 | 20 |
| 9.5 | 0.3 | 0 | 100 |
| 14.5 | 0.3 | 0 | 100 |
| 14.6 | 0.3 | 100 | 0 |
| 18 | 0.3 | 100 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Ma, L.; Liu, Z.; Huang, X. The Impact of Lactobacillus delbrueckii Hepatic Metabolism in Post-Weaning Piglets. Fermentation 2024, 10, 286. https://doi.org/10.3390/fermentation10060286
Wang X, Ma L, Liu Z, Huang X. The Impact of Lactobacillus delbrueckii Hepatic Metabolism in Post-Weaning Piglets. Fermentation. 2024; 10(6):286. https://doi.org/10.3390/fermentation10060286
Chicago/Turabian StyleWang, Xiaolong, Longteng Ma, Zhuying Liu, and Xinguo Huang. 2024. "The Impact of Lactobacillus delbrueckii Hepatic Metabolism in Post-Weaning Piglets" Fermentation 10, no. 6: 286. https://doi.org/10.3390/fermentation10060286
APA StyleWang, X., Ma, L., Liu, Z., & Huang, X. (2024). The Impact of Lactobacillus delbrueckii Hepatic Metabolism in Post-Weaning Piglets. Fermentation, 10(6), 286. https://doi.org/10.3390/fermentation10060286






