Nutritive and Fermentative Traits of African Stargrass (Cynodon nlemfuensis Vanderyst) Forage Preserved for Silage and Haylage
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Sites
2.2. Forage Management and Treatments Evaluated
2.3. Forage Sampling and Analysis
2.4. Experimental Design and Statistical Analysis
3. Results
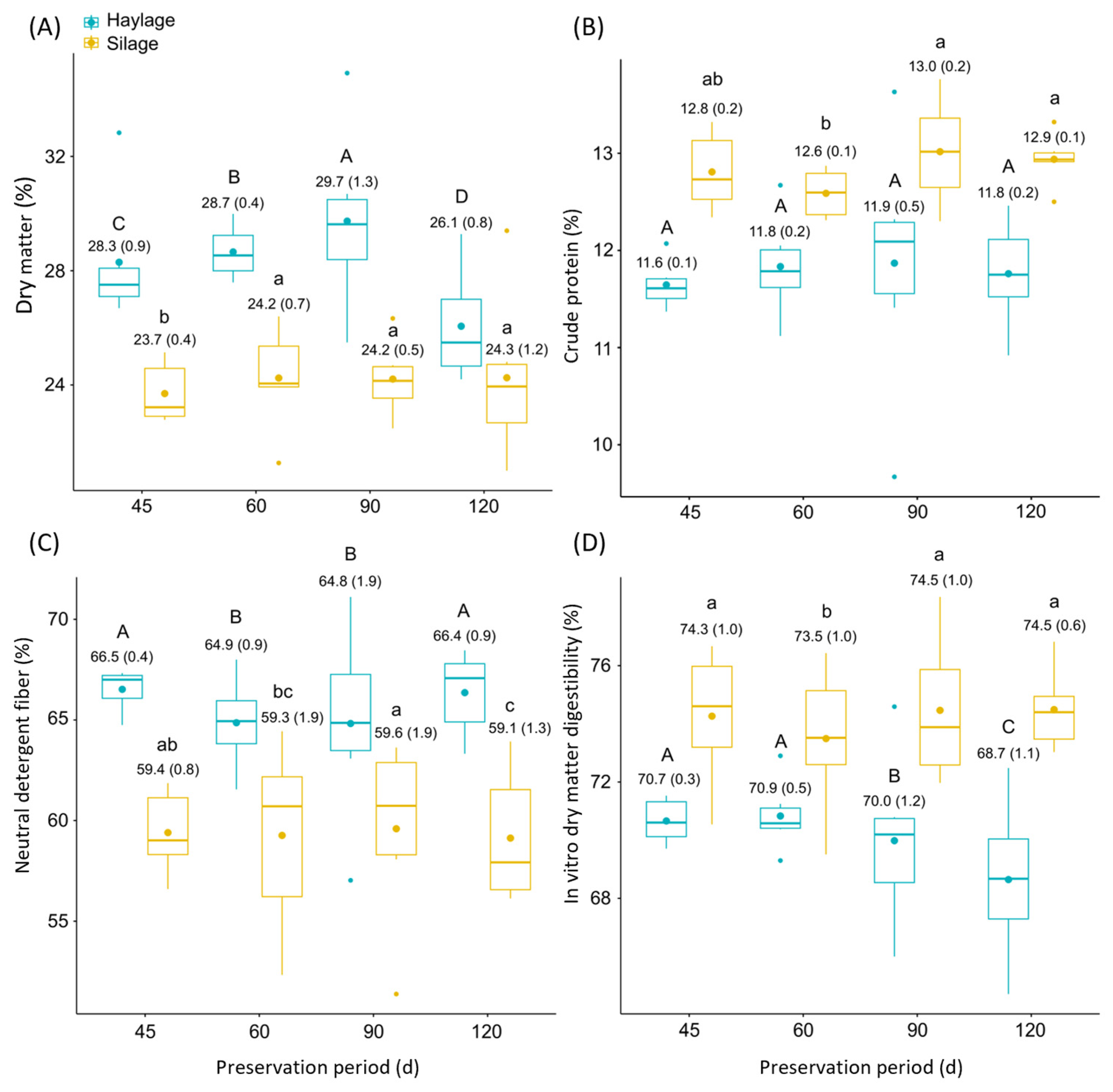
3.1. Nutritive Value of African Stargrass Silage and Haylage
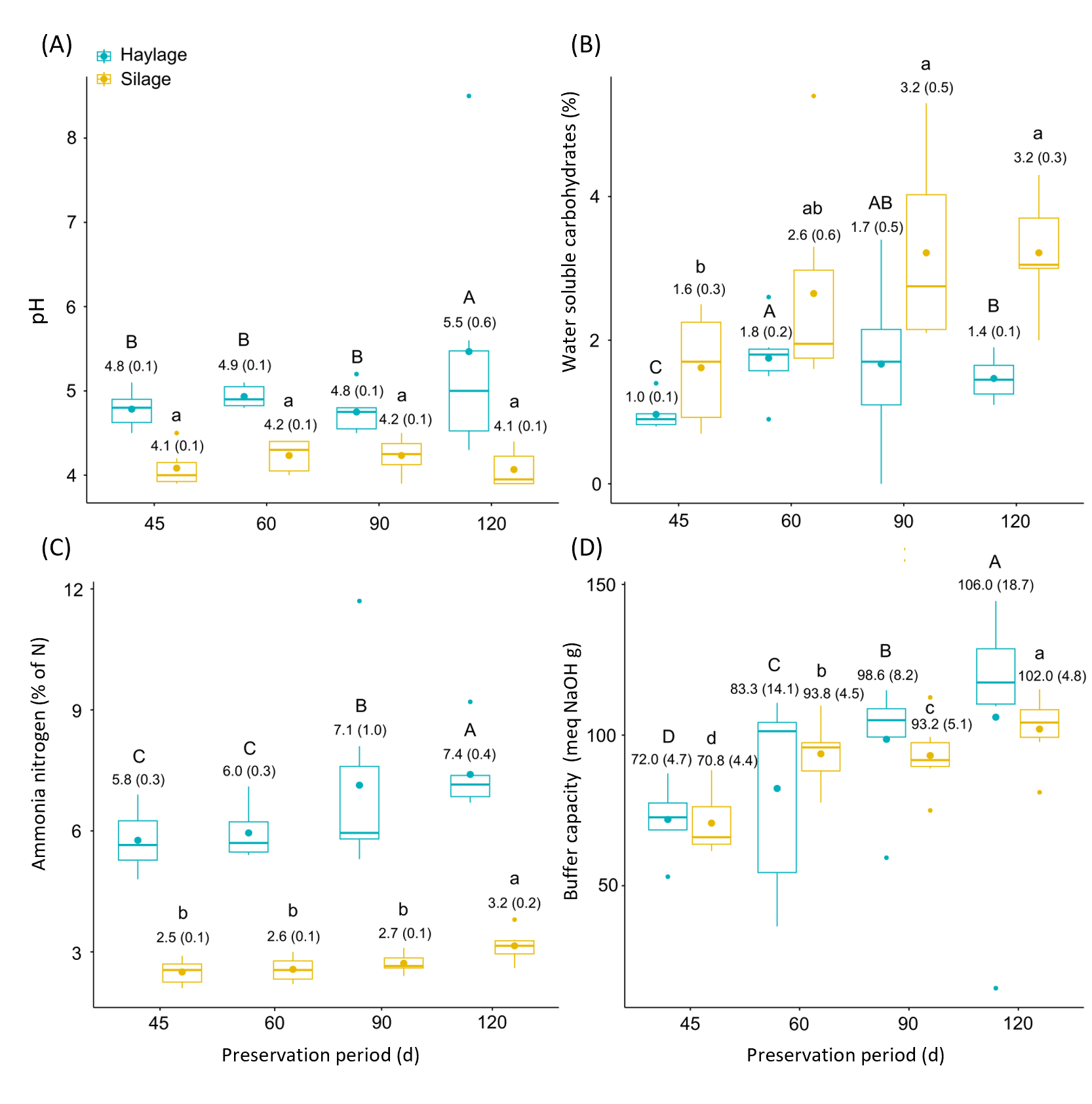
3.2. Fermentative Characteristics of African Stargrass Silage and Haylage
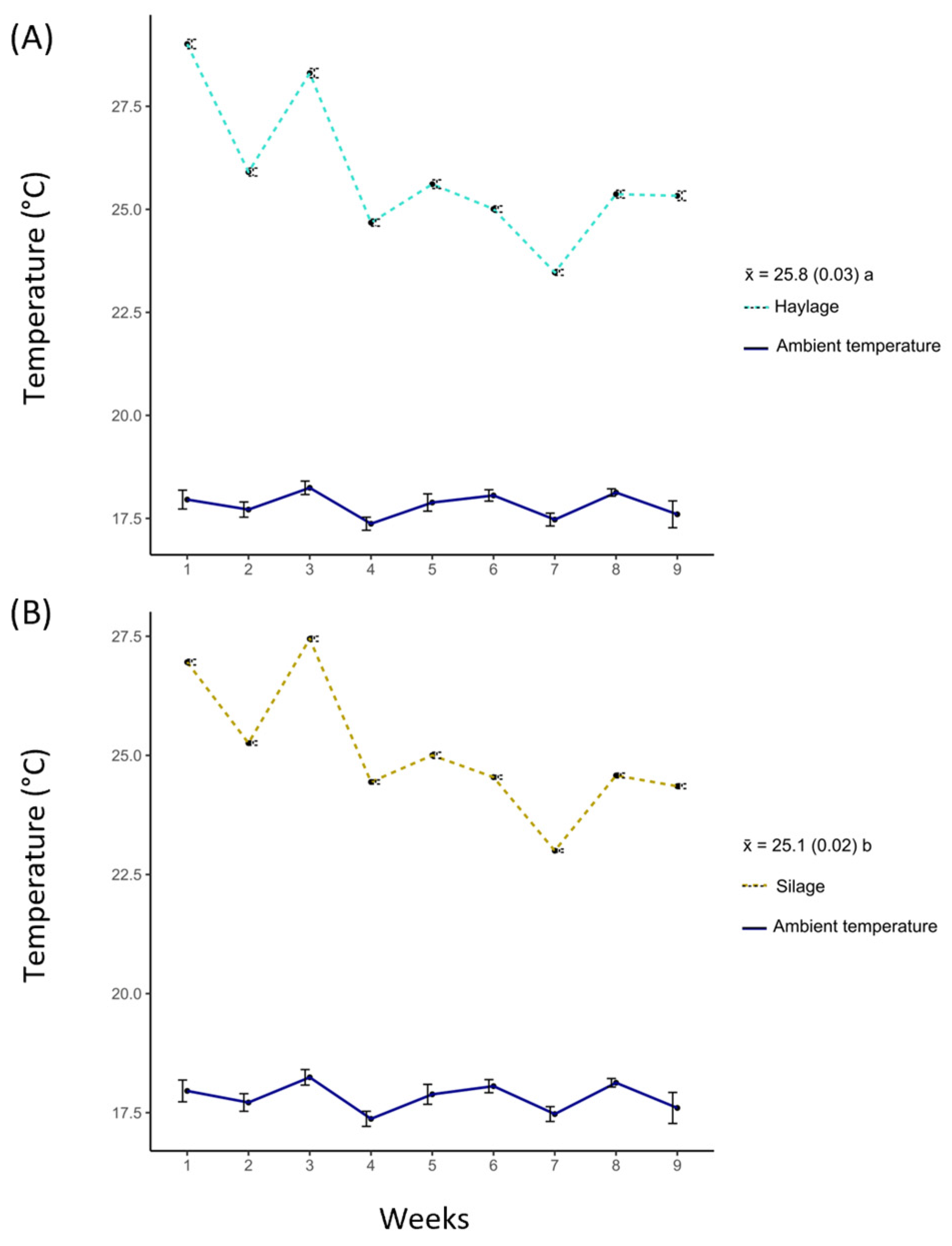
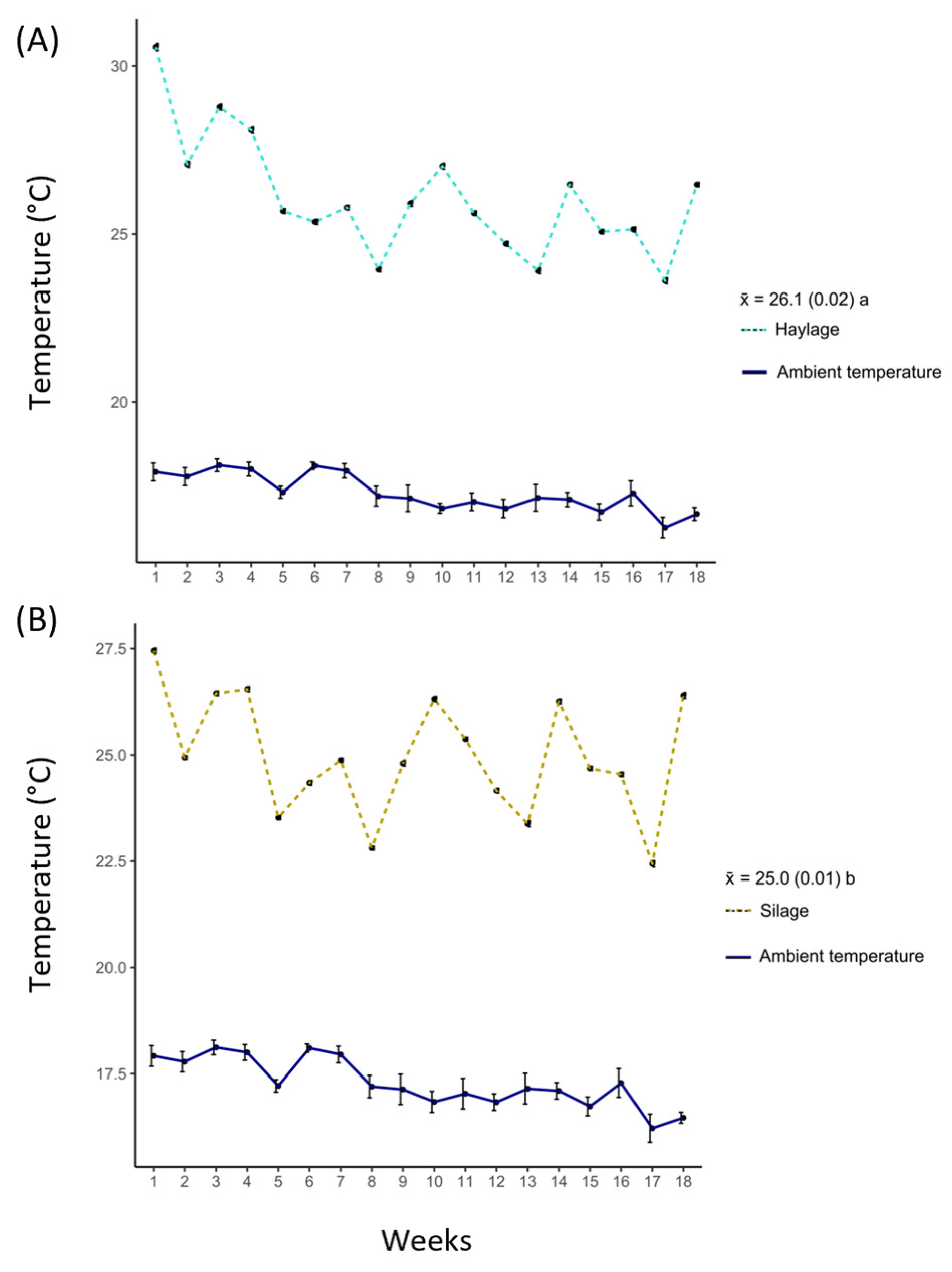
3.3. Temperature
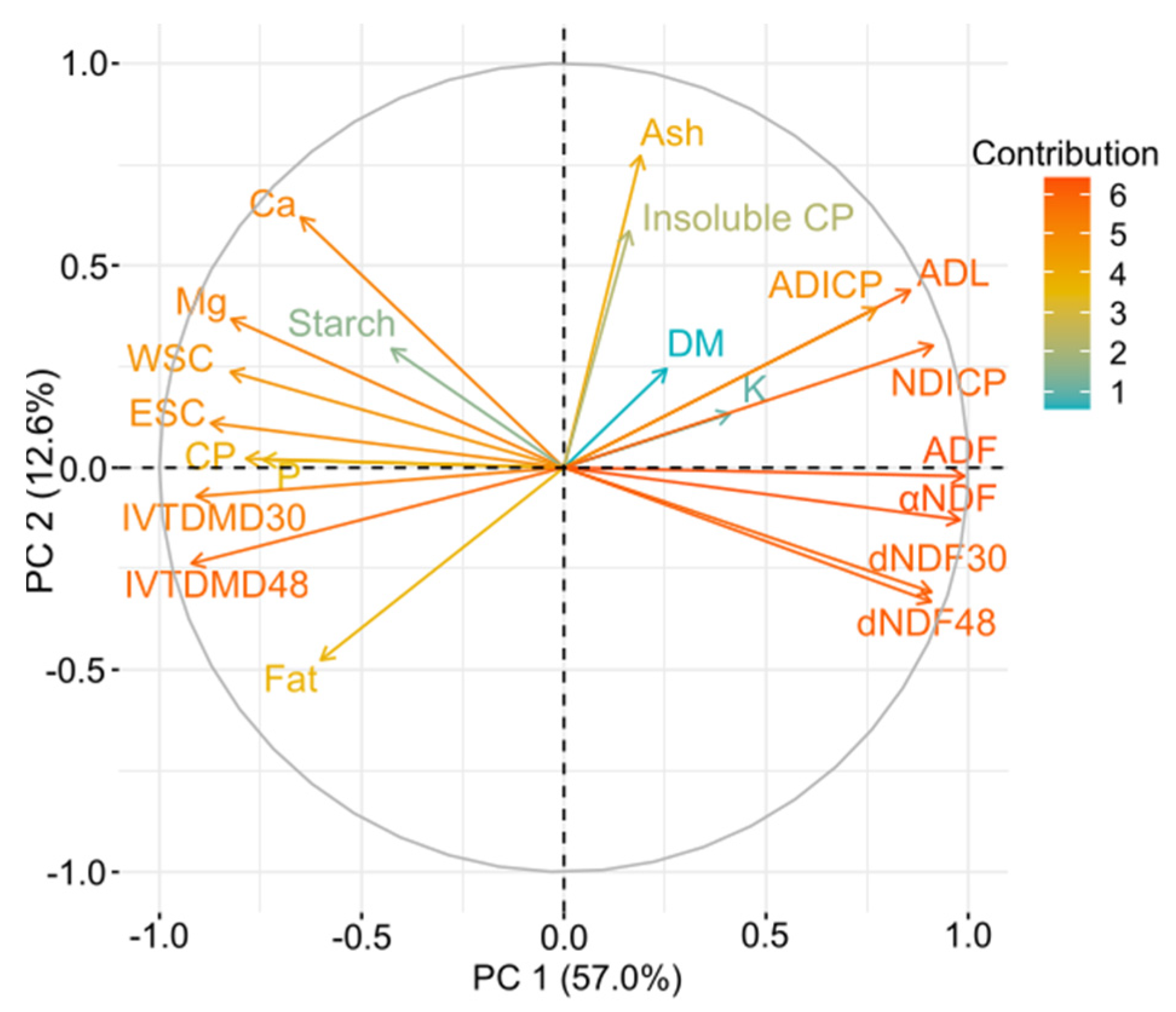
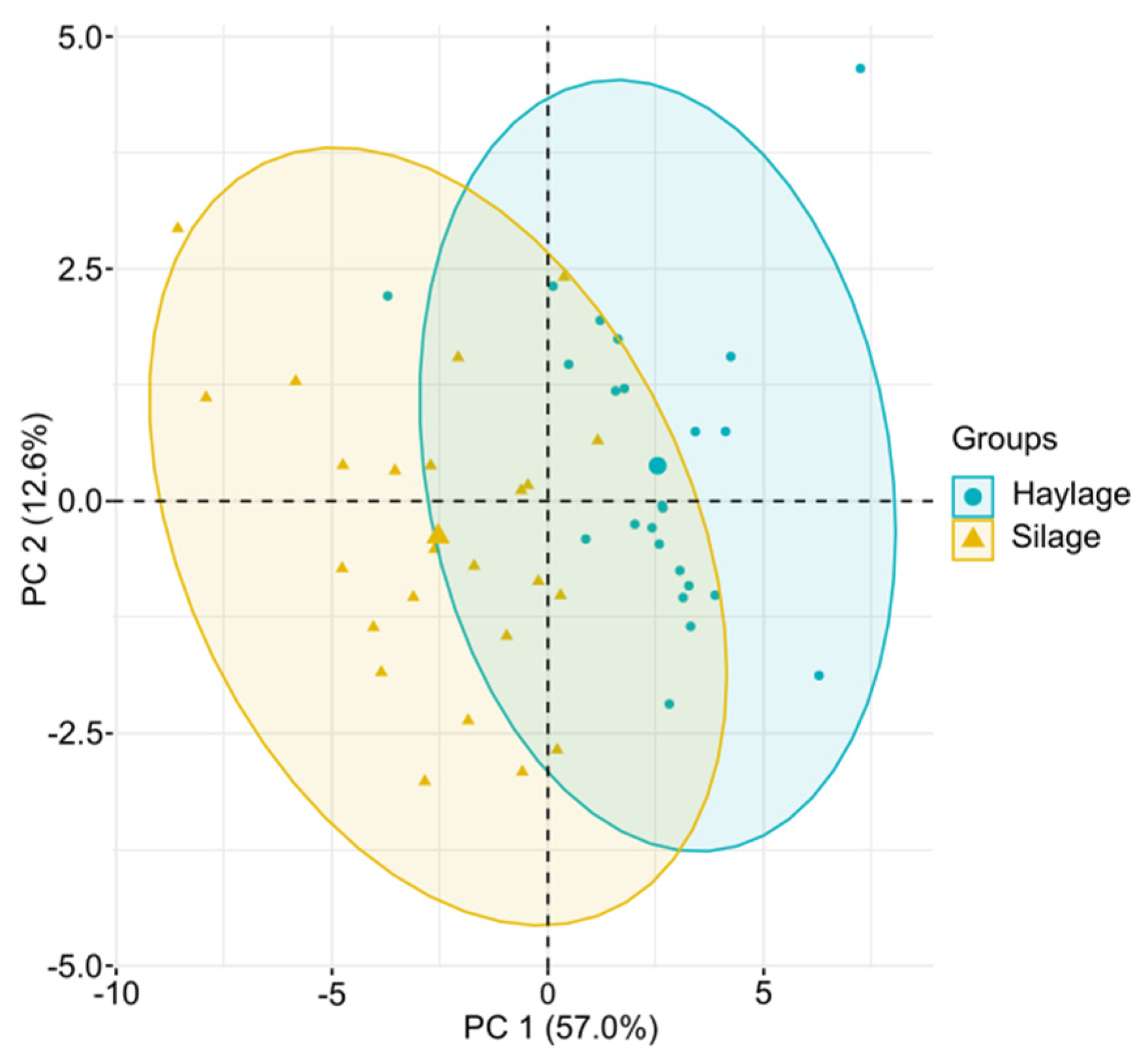
3.4. Principal Component Analysis (PCA)
4. Discussion
4.1. Nutritive Value of African Stargrass Silage and Haylage
4.2. Fermentative Characteristics of African Stargrass Silage and Haylage
4.3. Temperature
4.4. Principal Component Analysis (PCA)
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- López-González, F.; Estrada-Flores, J.G.; Avilés-Nova, F.; Yong-Ángel, G.; Hernández-Morales, P.; Martínez-Loperena, R.; Pedraza-Beltrán, P.E.; Castelán-Ortega, O.A. Agronomic evaluation and chemical composition of African star grass (Cynodon plectostachyus) in the southern region of the state of Mexico. Trop. Subtrop. Agroecosystems 2010, 12, 1–9. Available online: https://www.researchgate.net/publication/237042657_Agonomic_evaluation_and_chemical_composition_of_star_grass_Cynodon_plectostachyus_in_the_southern_region_of_the_State_of_Mexico (accessed on 25 June 2023).
- Ramírez-Rivera, E.J.; Rodríguez-Miranda, J.; Huerta-Mora, I.R.; Cárdenas-Cágal, A.; Juárez-Barrientos, J.M. Tropical milk production systems and milk quality: A review. Trop. Anim. Health Prod. 2019, 51, 1295–1305. [Google Scholar] [CrossRef] [PubMed]
- Sloat, L.L.; Gerber, J.S.; Samberg, L.H.; Smith, W.K.; Herrero, M.; Ferreira, L.G.; Godde, C.M.; West, P.C. Increasing importance of precipitation variability on global livestock grazing lands. Nat. Clim. Chang. 2018, 8, 214–218. [Google Scholar] [CrossRef]
- Gallup, J.L.; Sachs, J.D. Agriculture, Climate, and Technology: Why are the Tropics Falling Behind? Agric. Appl. Econ. Assoc. 2000, 82, 731–737. [Google Scholar] [CrossRef]
- Anjos, A.J.; Coutinho, D.N.; Freitas, C.A.S.; Macêdo, A.J.S.; Sena, H.P.; Mata e Silva, B.C.; Oliveira, G.M.; Raimundi, T.A.J. Potentials and challenges in making silages using tropical forages. Sci. Electron. Arch. 2020, 13, 129–136. [Google Scholar] [CrossRef]
- Panditharatne, S.; Allen, V.G.; Fontenot, J.P.; Jayasuriya, M.C.N. Ensiling Characteristics of Tropical Grasses as Influenced by Stage of Growth, Additives and Chopping Length. J. Anim. Sci. 1986, 63, 197–207. [Google Scholar] [CrossRef]
- Hughes, M.P.; Jennings, P.G.A.; Mlambo, V.; Lallo, C.H.O. Mitigating the Nutritional Limitations to Animal Production from Tropical Pastures: A Review. In Proceedings of the Conference: Caribbean Food Crop Society 347 forty Ninth Annual Meeting, Virgin Islands, VI, USA, 30 June–6 July 2013; Volume XLIX, pp. 1–30. [Google Scholar]
- Molina, A.M.G.; Roa, L.B.; Alzate, S.R. Ensilaje como fuente de alimentación para el ganado. Rev. Lasallista Investig. 2004, 1, 66–71. [Google Scholar]
- Khan, S.H.; Shahzad, M.A.; Nisa, M.; Sarwar, M. Nutrients intake, digestibility, nitrogen balance and growth performance of sheep fed different silages with or without concentrate. Trop. Anim. Health Prod. 2011, 43, 795–801. [Google Scholar] [CrossRef] [PubMed]
- Del Pozo, P.P. Del pozo. 2002. Bases ecofisiologicas para el manejo de los pastos tropicales.pdf. Pastos 2002, XXXII, 109–137. [Google Scholar]
- Sollenberger, L.E. Sustainable production systems for Cynodon species in the subtropics and tropics. Rev. Bras. Zootec. 2008, 37, 85–100. [Google Scholar] [CrossRef]
- Taliaferro, C.M.; Rouquette, F.M.; Mislevy, P. Bermudagrass and Stargrass. In Agronomy Monographs; Moser, L.E., Burson, B.L., Sollenberger, L.E., Eds.; American Society of Agronomy, Crop Science Society of America, Soil Science Society of America: Madison, WI, USA, 2016; pp. 417–475. [Google Scholar] [CrossRef]
- Daniel, J.L.P.; Bernardes, T.F.; Jobim, C.C.; Schmidt, P.; Nussio, L.G. Production and utilization of silages in tropical areas with focus on Brazil. Grass Forage Sci. 2019, 74, 188–200. [Google Scholar] [CrossRef]
- Villalobos, L.; Arce, J.; WingChing-Jones, R. Producción de biomasa y costos de producción de pastos estrella africana (Cynodon nlemfuensis), kikuyo (Kikuyuocloa clandestina) y ryegrass perenne (Lolium perenne) en lecherías de costa rica. RAC 2013, 37, 91–103. [Google Scholar] [CrossRef]
- Elizondo-Salazar, J.A. Producción de biomasa y calidad nutricional de tres forrajes cosechados a dos alturas. Mesoam. Agron. 2017, 28, 329. [Google Scholar] [CrossRef]
- Quaresma, J.P.S.; Abreu, J.G.D.; Almeida, R.G.D.; Cabral, L.D.S.; Oliveira, M.A.D.; Rodrigues, R.C. Recuperação de matéria seca e composição química de silagens de gramíneas do gênero Cynodon submetidas a períodos de pré-emurchecimento. Ciência Agrotecnologia 2010, 34, 1232–1237. [Google Scholar] [CrossRef]
- Vendramini, J.; Mislevy, P. Agronomy Department, UF/IFAS Extension; The Institute of Food and Agricultural Sciences, University of Florida: Gainesville, FL, USA, 2021; p. 4. [Google Scholar]
- Caldwell, G. Holistic Goat Care. A Comprehensive Guide to Raising Healthy Animal, Preventing Common Ailments, and Troubleshooting Problems; Chelsea Green Publishing: Hartford, VT, USA, 2017. [Google Scholar]
- Granados-Marín, C.; WingChing-Jones, R.; Rojas-Bourrillón, A. Ensilaje de pasto Estrella Africana (Cynodon nlemfuensis) con la adición de melaza, suero de leche e inóculos microbiales. URJ 2014, 6, 47–56. [Google Scholar] [CrossRef]
- Pineda, L.; Chacón, P.; Boschini, C. Evaluación de la calidad del ensilado de pasto Estrella Africana (Cynodon nlemfluensis) mezclado con tres diferentes aditivos. Agron. Costarric. 2016, 40, 11–27. [Google Scholar]
- Chacón-Hernández, P.A.; Vargas-Rodríguez, C.F. Consumo de Pennisetum purpureum cv. King Grass a tres edades de cosecha en caprinos. Agron. Mesoam. 2009, 21, 267. [Google Scholar] [CrossRef]
- Vásquez, A. Estudio Detallado de los Suelos de la Estación Experimental de Ganado Lechero El Alto; Facultad de Ciencias Agroalimentarias, Escuela de Fitotecnia, Universidad de Costa Rica: San José, Costa Rica, 1982. [Google Scholar]
- Cubero, J.F.; Rojas, A.; WingChing-Jones, R. Uso del inóculo microbial elaborado en finca en ensilaje de maíz (Zea mays). Valor nutricional y fermentativo. RAC 2010, 34, 237–250. [Google Scholar] [CrossRef]
- Villalobos, L.; Arce, J. Efecto del picado sobre las características nutricionales y fermentativas de ensilajes de pastos Kikuyo, Ryegrass perenne y Alpiste forrajero. RAC 2016, 40, 65–74. [Google Scholar] [CrossRef]
- Villalobos-Villalobos, L.A.; Arce-Cordero, J.A. Effects of commercial and farm-made inoculants on the chemical composition and fermentation of kikuyu grass silages. Crop Forage Turfgrass Manag. 2022, 8, e20159. [Google Scholar] [CrossRef]
- Cherney, J.H.; Digman, M.F.; Cherney, D.J. Handheld NIRS for forage evaluation. Comput. Electron. Agric. 2021, 190, 106469. [Google Scholar] [CrossRef]
- Berzaghi, P.; Riovanto, R. Near infrared spectroscopy in animal science production: Principles and applications. Ital. J. Anim. Sci. 2009, 8, 39–62. [Google Scholar] [CrossRef]
- McDonald Henderson, P.A.R. Buffering capacity of herbage samples as a factor in ensilage. J. Sci. Food Agric. 1962, 13, 395–400. [Google Scholar] [CrossRef]
- AOAC International. Official Methods of Analysis, 15th ed.; AOAC International: Rockville, MD, USA, 1990. [Google Scholar]
- R Core Team. A Language and Environment for Statistical Computing. Available online: https://www.R-project.org/ (accessed on 6 March 2022).
- McDonald, P.; Henderson, A.R.; Heron, S.J. The Biochemistry of Silage, 2nd ed.; Chalcombe Publications: Welton, UK, 1991; ISBN 0-948617-22-5. [Google Scholar]
- Santos, E.M.; Zanine, A.D.M. Silagem de gramíneas tropicais. Colloq. Agrar. 2006, 2, 32–45. [Google Scholar] [CrossRef]
- Borreani, G.; Giaccone, D.; Mimosi, A.; Tabacco, E. Comparison of Hay and Haylage from Permanent Alpine Meadows in Winter Dairy Cow Diets. J. Dairy Sci. 2007, 90, 5643–5650. [Google Scholar] [CrossRef] [PubMed]
- McCormick, M.E.; Han, K.J.; Moreira, V.R.; Blouin, D.C.; Forbes, S. Forage conservation efficiency and lactation response to bahiagrass conserved as barn-stored hay, outdoor-stored hay, or baleage. J. Dairy Sci. 2011, 94, 2500–2507. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.V.F.; Gómez Castro, A.G.; Perea, J.M.; García, A.; Guim, A.; Pérez Hernández, M. Fatores que afetam o valor nutritivo da silagens de forrageiras tropicais. Arch. Zootec. 2010, 59, 25–43. [Google Scholar] [CrossRef]
- Nath, C.D.; Neres, M.A.; Scheidt, K.C.; Bersot, L.D.S.; Monteiro Sunahara, S.M.; Sarto, J.R.W.; Stangarlin, J.R.; Gomes, S.D.; Sereno, M.J.; Perin, A.P. Characterization of Tifton 85 bermudagrass haylage with different layers of polyethylene film and storage time. Asian-Australas. J. Anim. Sci. 2018, 31, 1197–1204. [Google Scholar] [CrossRef]
- Rooke, J.A.; Hatfield, R.D. Biochemistry of Ensiling. In Agronomy Monographs; Buxton, D.R., Muck, R.E., Harrison, J.H., Eds.; American Society of Agronomy, Crop Science Society of America, Soil Science Society of America: Madison, WI, USA, 2003; pp. 95–139. [Google Scholar] [CrossRef]
- Costa, M.L.L.D.; Rezende, A.S.C.D.; Fonseca, M.G.; Lage, J.; Pimentel, P.G.; Mizubuti, I.Y.; Freitas, G.P.D.; Moreira, G.R.; Lana, Â.M.Q.; Saliba, E.D.O.S. Fermentation pattern of tropical grass haylage and digestibility compared to hay in equine diet. Semin. Ciências Agrárias 2018, 39, 2125. [Google Scholar] [CrossRef]
- Weinberg, Z.G.; Szakacs, G.; Ashbell, G.; Hen, Y. The effect of temperature on the ensiling process of corn and wheat. J. Appl. Microbiol. 2001, 90, 561–566. [Google Scholar] [CrossRef]
- Vendramini, J.M.B.; Desogan, A.A.; Silveira, M.L.; Sollenberger, L.E.; Queiroz, O.C.M.; Anderson, W.F. Nutritive Value and Fermentation Parameters of Warm-Season Grass Silage. Prof. Anim. Sci. 2010, 26, 193–200. [Google Scholar] [CrossRef]
- Dehghani, M.R.; Weisbjerg, M.R.; Hvelplund, T.; Kristensen, N.B. Effect of enzyme addition to forage at ensiling on silage chemical composition and NDF degradation characteristics. Livest. Sci. 2012, 150, 51–58. [Google Scholar] [CrossRef]
- Evangelista, A.R.; Lima, J.A.D.; Bernardes, T.F. Avaliação de algumas características da silagem de gramínea estrela roxa (Cynodon nlemfuensis Vanderyst). R. Bras. Zootec. 2000, 29, 941–946. [Google Scholar] [CrossRef]
- Castro, F.G.F.; Nussio, L.G.; Haddad, C.M.; Campos, F.P.D.; Coelho, R.M.; Mari, L.J.; Toledo, P.D.A. Características de fermentação e composição químico-bromatológica de silagens de capim-tifton 85 confeccionadas com cinco teores de matéria seca. R. Bras. Zootec. 2006, 35, 7–20. [Google Scholar] [CrossRef]
- Kagan, I.A.; Kirch, B.H.; Thatcher, C.D.; Teutsch, C.D.; Pleasant, R.S. Chromatographic profiles of nonstructural carbohydrates contributing to the colorimetrically determined fructan, ethanol-soluble, and water-soluble carbohydrate contents of five grasses. Anim. Feed. Sci. Technol. 2014, 188, 53–63. [Google Scholar] [CrossRef]
- Rambau, M.D.; Fushai, F.; Callaway, T.R.; Baloyi, J.J. Dry matter and crude protein degradability of Napier grass (Pennisetum purpureum) silage is affected by fertilization with cow-dung bio-digester slurry and fermentable carbohydrate additives at ensiling. Transl. Anim. Sci. 2022, 6, txac075. [Google Scholar] [CrossRef] [PubMed]
- Wahyudi, A.; Hendraningsih, L.; Sutawi; Setyobudi, R.H.; Mel, M. Fermentation quality of Pennisetum purpureum cv. Mott ensiled with Lactobacillus plantarum and sugarcane molasses in tropic. IOP Conf. Ser. Earth Environ. Sci. 2019, 293, 012007. [Google Scholar] [CrossRef]
- Zhao, J.; Yin, X.-J.; Dong, Z.-H.; Wang, S.-R.; Li, J.-F.; Shao, T. Dynamics in fermentation quality, bacterial community, and metabolic profile during silage fermentation of late-harvested elephant grass. Lett. Appl. Microbiol. 2023, 76, ovac036. [Google Scholar]
- Jones, D.I.H. The effect of cereal incorporation on the fermentation of spring-and autumn-cut silages in laboratory silos. Grass Forage Sci. 1988, 43, 167–172. [Google Scholar] [CrossRef]
- Zhao, G.; Wu, H.; Li, L.; He, J.; Hu, Z.; Yang, X.; Xie, X. Effects of applying cellulase and starch on the fermentation characteristics and microbial communities of Napier grass (Pennisetum purpureum Schum.) silage. J. Anim. Sci. Technol. 2021, 63, 1301–1313. [Google Scholar] [CrossRef]
- Kung, L.; Shaver, R.D.; Grant, R.J.; Schmidt, R.J. Silage review: Interpretation of chemical, microbial, and organoleptic components of silages. J. Dairy Sci. 2018, 101, 4020–4033. [Google Scholar] [CrossRef] [PubMed]
- Gouvêa, V.N.; Vendramini, J.M.B.; Sollenberger, L.E.; De Oliveira, F.C.L.; Dubeux, J.C.B.; Moriel, P.; Cecato, U.; Soares Filho, C.V.; Sanchez, J.M.D.; Yarborough, J.K.; et al. Inoculant effects on fermentation characteristics, nutritive value, and mycotoxin concentrations of bermudagrass silage. Crop Forage Turfgrass Manag. 2020, 6, e20054. [Google Scholar] [CrossRef]
- Playne, M.J.; McDonald, P. The buffering constituents of herbage and of silage. J. Sci. Food Agric. 1966, 17, 264–268. [Google Scholar] [CrossRef]
- Li, D.; Ni, K.; Zhang, Y.; Lin, Y.; Yang, F. Fermentation characteristics, chemical composition and microbial community of tropical forage silage under different temperatures: Asian-Australas. J. Anim. Sci. 2019, 32, 665–674. [Google Scholar] [CrossRef] [PubMed]
- Ashbell, G.; Weinberg, Z.G.; Hen, Y.; Filya, I. The effects of temperature on the aerobic stability of wheat and corn silages. J. Ind. Microbiol. Biotechnol. 2002, 28, 261–263. [Google Scholar] [CrossRef]
- Kim, K.H. Uchida Comparative studies of ensiling characteristics between temperate and tropical species. 2. Mechanical pr-treatments and changes in water soluble carbohydrate fractions during ensilage. Jpn. J. Grassl. Sci. 1991, 36, 426–433. [Google Scholar]
| Parameter | Fresh (Silage) | Wilted 48 h (Haylage) |
|---|---|---|
| Dry matter 1 | 20.8 | 27.3 |
| Nutritive values 2 | ||
| CP | 13.9 | 12.7 |
| EE | 3.5 | 2.9 |
| αNDF | 68.6 | 71.3 |
| ADF | 36.4 | 38.5 |
| ADL | 2.8 | 3.8 |
| DNDF | 56.5 | 53.2 |
| IVDMD | 70.2 | 66.6 |
| NEL | 1.1 | 1.3 |
| Preservation Period (d) | ADF | ADL | EE | Starch | DNDF | NEL |
|---|---|---|---|---|---|---|
| Haylage | ||||||
| 45 | 43.46 B | 5.87 B | 2.26 | 0.32 | 38.57 A | 1.18 |
| 60 | 42.52 D | 5.94 B | 2.19 | 0.35 | 37.02 B | 1.17 |
| 90 | 42.86 C | 6.11 B | 2.18 | 0.22 | 36.60 C | 1.17 |
| 120 | 43.86 A | 6.47 A | 2.13 | 0.16 | 37.07 B | 1.12 |
| Silage | ||||||
| 45 | 37.76 ab | 4.92 | 2.83 | 0.86 b | 34.94 a | 1.29 |
| 60 | 37.41 c | 5.18 | 2.65 | 1.26 a | 33.91 c | 1.25 |
| 90 | 37.94 a | 4.95 | 2.78 | 0.16 c | 34.59 b | 1.28 |
| 120 | 37.66 bc | 5.02 | 2.76 | 0.15 c | 34.12 c | 1.28 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Picado-Pérez, T.; Lemus, R.; Rivera, D.; Villalobos-Villalobos, L.A. Nutritive and Fermentative Traits of African Stargrass (Cynodon nlemfuensis Vanderyst) Forage Preserved for Silage and Haylage. Fermentation 2024, 10, 268. https://doi.org/10.3390/fermentation10060268
Picado-Pérez T, Lemus R, Rivera D, Villalobos-Villalobos LA. Nutritive and Fermentative Traits of African Stargrass (Cynodon nlemfuensis Vanderyst) Forage Preserved for Silage and Haylage. Fermentation. 2024; 10(6):268. https://doi.org/10.3390/fermentation10060268
Chicago/Turabian StylePicado-Pérez, Tania, Rocky Lemus, Daniel Rivera, and Luis A. Villalobos-Villalobos. 2024. "Nutritive and Fermentative Traits of African Stargrass (Cynodon nlemfuensis Vanderyst) Forage Preserved for Silage and Haylage" Fermentation 10, no. 6: 268. https://doi.org/10.3390/fermentation10060268
APA StylePicado-Pérez, T., Lemus, R., Rivera, D., & Villalobos-Villalobos, L. A. (2024). Nutritive and Fermentative Traits of African Stargrass (Cynodon nlemfuensis Vanderyst) Forage Preserved for Silage and Haylage. Fermentation, 10(6), 268. https://doi.org/10.3390/fermentation10060268






