Abstract
Effective sulfur dioxide (SO2) management is crucial in winemaking to minimize oxidative changes in wine flavor during storage. This study explored the impact of various SO2 management techniques on Solaris white wine’s flavor components and sensory properties. Five treatments were administered: ‘SO2 in juice’ (50 mg/L SO2 added to juice pre-fermentation), ‘Control’ (60 mg/L SO2 added post-fermentation), ‘Low SO2’ (50 mg/L SO2 post-fermentation), ‘High SO2’ (100 mg/L SO2 post-fermentation), and ‘No SO2’ (no SO2 added). The ‘Control’ followed a standard procedure, in which the achieved level of free sulfite is measured and extra SO2 added to reach the recommended level of free sulfite for the pH of the wine. Here, 50 + 10 mg/L was added. Volatile compounds were analyzed using dynamic headspace sampling coupled with gas chromatography–mass spectrometry after 0, 3, 6, and 12 months of storage. Sensory evaluation by a trained panel after 12 months revealed stronger perceptions of ‘overall impression’, ‘chemical’, ‘bitter’, ‘overripe fruit’, and ‘honey’ notes in the ‘No SO2’ and ‘SO2 in juice’ wines. The data underscore the significant influence of SO2 management on the flavor stability of Solaris white wines, emphasizing the need for strategic SO2 interventions during winemaking to enhance sensory quality over time.
1. Introduction
During the storage of white wines, there is a potential for the loss of initial freshness and fruity properties. Setting aside issues caused by various microorganisms, the primary concern for the degradation of young wine quality lies in chemical changes, especially alterations in the aroma compound profile due to diverse types of reactions. Notably, oxidation (of ethanol), Strecker, and early-stage Maillard reactions are considered significant contributors to flavor changes during the storage of white wine [1].
Free SO2 stands out as the most commonly used preservative agent in wine. The addition of SO2 is often conducted already to the must, where it serves to prevent must oxidation and inhibit the growth of undesirable microorganisms during fermentation [2]. In bottled wine, it not only limits acetaldehyde formation but also binds acetaldehyde, thereby protecting or enhancing the wine’s aroma. Extensive research has been conducted on the preservative function of SO2 on wine volatile compounds. For instance, Jackowetz et al. [3] found that the level of SO2 significantly affects acetaldehyde production and degradation during alcoholic fermentation. Similarly, Garde-Cerdán and Ancín-Azpilicueta [4] explored wine stored with SO2 in bottles, revealing higher concentrations of volatile compounds, particularly esters and alcohols, compared to wine aged in bottles without SO2. SO2 also influences carbonyl aging-related compounds in red wines [5], where the increase in oxidative compounds may be a consequence of aldehydes forming bisulfites once SO2 undergoes oxidation. However, few studies have specifically addressed the effects of SO2 levels on acetaldehyde and other oxidation-related compounds during the aging of white wine [3,4]. Specifically, wines with a high content of acetaldehyde may require more SO2 to attain adequate levels of free or active SO2. In this context, the timing of SO2 addition is crucial in winemaking. The amount added is also pivotal and depends especially on pH, as well as on wine style and cultivar [2]. White wines are often intended for consumption within a year after release. Adding low levels of SO2 may fail to protect the wine from early oxidation, while high levels may pose a risk of inducing health problems in sensitive consumers and have adverse effects on sensory quality. Therefore, there is an evident need for a more profound understanding of the role SO2 plays in relation to wine aging, particularly concerning the development of volatile compounds.
In order to evaluate if changes in volatile compounds are large enough to actually impact the sensory quality of wine, sensory evaluation is needed. Descriptive sensory methods are frequently employed in characterizing wine flavor [6,7]. These methods rely on samples that represent a relatively extensive sensory space to obtain clear discrimination. However, when sample variations are subtle, perceiving minor differences with these methods can be challenging. Instead, the difference from the control test emerges as a practical and sensitive method for use in food quality control programs, such as those for wine [8] and cheese [9].
Solaris is the primary cultivar grown in Denmark for white wine production. This interspecific hybrid cultivar holds high value for organic wine production in cool and cold climate regions due to its excellent disease tolerance and early ripening properties. As the wine industry expands in Nordic countries, there is a growing need to understand how to control wine quality from these cold climate cultivars. In a previous study, we assessed the quality of a selection of Danish Solaris wines and observed that differences in vintage seemed less characteristic than differences arising from sulfur management by producers [10]. The wines were segregated into two main clusters: half were associated with fruity and floral descriptors, while the other half was characterized by less pleasant flavors. Combining data on free and total SO2 and vinification methods by producers, oxidation appeared to result from poor sulfite management. The present work aims to investigate the effect of storage time and SO2 addition practice (timing and amount) on the profiles of volatile compounds and their relationship to the sensory properties of Solaris white wines.
2. Materials and Methods
2.1. Chemical Standards
Chemical standards for volatile compounds were sourced from reputable suppliers: Sigma-Aldrich (St. Louis, MO, USA), Fluka (Madrid, Spain), and Aldrich (Madrid, Spain). Ethanol (HPLC grade, 99.9%) and L (+)-tartaric acid (>99.5%) were procured from Sigma-Aldrich (Kiev, Ukraine).
2.2. Winemaking
Grapes of the Solaris cultivar were hand harvested in Pometet, Copenhagen University, Denmark, at a sugar content of 22.6 °Brix, with total acidity (as tartaric acid) at 10.2 g/L and pH at 3.03. The grapes were destemmed, crushed, and directly pressed in a 40 L hydropress (Speidel). As depicted in Figure 1, five treatments involving various SO2 management strategies were conducted in duplicate. In the first treatment, 50 mg/L of SO2 (sulfur dioxide water solution at 5% m/v) was added to the juice for SO2 stabilization two days before fermentation (SO2 in juice). During this period, all batches were placed in a cold room (3 °C) for clearing. The clear juice was racked into 5 L fermenters filled with CO2 prior to racking to minimize oxidation. Each treatment is in duplicate. In the remaining four treatments, SO2 was administered post-fermentation. The treatment receiving an addition of 60 mg/L SO2 was labeled as the ‘Control.’ It followed a standard procedure involving pH-dependent SO2 addition, a common practice in wine production [11]. Initially, 50 mg of SO2 was added (similar to the ‘Low SO2’ treatment); however, after assessing the free sulfite level and pH, an extra 10 mg of SO2/l was added to achieve the recommended SO2 level at the wine’s current pH, equating to a molecular SO2 level of 0.8 ppm. Samples with added SO2 concentrations of 0, 50, and 100 mg/L were designated as ‘No SO2,’ ‘Low SO2,’ and ‘High SO2’ wines, respectively. All fermentations were conducted in a 5 L bioreactor maintained at 18 °C in a temperature-controlled environment. To initiate fermentation, 0.2 g/L of commercial yeast Saccharomyces cerevisiae, bayanus (Lalvin DV10TM from Lallemand, Fredericia, Denmark) was added to all samples. Throughout fermentation, samples were collected from the musts on a daily basis for density and acetaldehyde analysis until all wines reached dryness. Density was measured with a DMA35 Density meter from Anton Paar (Tokyo, Japan). Following fermentation, the wines were carefully racked, and varying amounts of SO2 were added (see Figure 1). SO2 addition occurred shortly after fermentation, when acetaldehyde concentrations were at their lowest, aiming to minimize SO2 binding and optimize free SO2 levels. Free and total SO2 levels were measured immediately post-bottling. All wines were bottled in 375 mL bottles with screw caps and stored in a wine cellar under dark conditions at a temperature range of 15–18 °C. Chemical analyses were conducted periodically during storage (at 0, 3, 6, and 12 months post-bottling), with sensory evaluations performed on the wines after 12 months of storage.
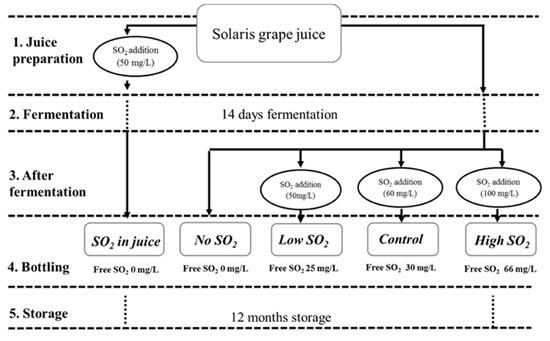
Figure 1.
The experimental design and sample names were used for each SO2 management.
2.3. SO2 Measurement
The measurement of free and total sulfur dioxide (SO2) utilized a modified Ripper iodine redox titration method, following the protocol outlined by Tanner and Sandoz [12]. Total and free SO2 levels in the wine were assessed at 3, 6, and 12 months post-bottling.
2.4. Analysis of Volatile Compounds
Volatile compound analysis followed a previously published method [6]. Dynamic headspace sampling (DHS) was employed to extract aroma compounds. Each wine sample (20 mL) was transferred to a 100 mL flask, to which 1 mL of 4-methyl-1-pentanol solution in water (5 mg/L, Aldrich, Steinheim, Germany) was added as an internal standard. Volatile compounds were trapped on a Tenax-TA trap (200 mg, mesh size 60/80, Buchem BV, Apeldoorn, The Netherlands) using a purge volume of 2 L (100 mL/min for 20 min). The trapped volatile compounds were then analyzed using a thermal desorption gas chromatography mass spectrometry system (Perkin Elmer TurboMatrix 350, Shelton, CT, USA, coupled to a 7890A GC/5975C VL MSD from Agilent Technologies, Palo Alto, CA, USA), equipped with a DB-Wax column (Agilent J&W, Palo Alto, CA, USA, 30 m × 0.25 mm × 0.25 mm). Data were analyzed using MSD Chemstation G1701EA software (Version E.01.00.237, Agilent Technologies Inc., Palo Alto, CA, USA), with compound identification based on mass spectra comparison with a standard library (Wiley275.l, HP product no. G1035A). Linear retention indices (RI) were calculated using a homologous series of alkanes (C5–C22) for further verification of compound identification. The RI values were compared to the RI of authentic standards or reported literature RI. Quantification of volatile compounds relied on calibration curves established using synthetic wine, as detailed in previous work [6]. Duplicate analyses were performed on each biological duplicate. Acetaldehyde quantification employed a modified DHS method described by Zhang and Petersen [13], taking acetaldehyde’s high volatility and low breakthrough volume in Tenax-TA traps into account. The method included conditions similar to those described above, except that headspace purge volume was decreased to 80 mL (40 mL/min for 2 min) and a lower cryo-focusing temperature (−20 °C) was used in the second step of the thermal desorption.
2.5. Sensory Analysis
A sensory evaluation of the finished wines after 12 months of storage was conducted using the difference from the control method [14,15] in standard sensory booths at the University of Copenhagen. An external panel with nine trained panelists (seven female and two male) participated and conducted the testing with informed consent.
2.5.1. Panel Training
Three training sessions, each lasting 1 ½ h, were conducted. During the initial session, five pairs of samples (sample names as indicated in Figure 1) and a list of wine fault attributes were presented to the panelists. Each pair contained two samples: a blind sample of the four treatments and the control sample (SO2 addition of 60 mg/L), which was used as a reference. The fifth pair was control vs. control. In each pair, the panelists were asked to evaluate differences between the control and test wines’ overall impressions using an open evaluation sheet and checking wine fault attributes from the list provided. This led to a total of 13 descriptive terms. Subsequent sessions introduced reference standards for each term, and then the wines were tasted again, allowing panelists to mark the degree of difference between paired samples. In discussion with the panel, the eight most relevant attributes were selected. In the final training session, panelists evaluated four pairs of samples to familiarize themselves with the evaluation procedure and the difference from control scaling.
2.5.2. Panel Wine Evaluation
Approximately 20 mL of wine, served at 9 °C, was presented in ISO standard wine glasses with watch-glass lids. Cold water and crackers were provided for palate cleansing. Samples were presented in pairs, with the control wine and an experimental wine coded with three-digit numbers. Panelists were instructed to taste the wine pairs and evaluate the difference in pre-determined sensory attributes between each sample and the control using a 15 cm unstructured line scale with anchors labeled “no difference” and “extreme difference.” Sample pairs were presented randomly to each assessor and evaluated in one session, with sessions repeated three times on different days.
2.6. Data Analysis
A one-way analysis of variance (ANOVA) was conducted on the concentrations of volatile compounds using SPSS (IBM SPSS Statistics v.22, Chicago, IL, USA), with ‘sample’ treated as a fixed effect. For sensory data analysis, a two-way ANOVA was employed, considering ‘product’ as a fixed factor and ‘assessor’ as a random factor. Tukey’s post hoc test was subsequently utilized to ascertain the extent of differences between samples, with a significance level set at 5%.
3. Results and Discussion
3.1. Acetaldehyde Production and Degradation during Wine Fermentation
Acetaldehyde stands out as the most significant carbonyl compound quantitatively produced by yeast during alcoholic fermentation. Figure 2A illustrates that, excluding the ‘SO2 in juice’ sample, acetaldehyde production peaked (400–500 mg/L) around fermentation days 2–3 for all samples, followed by a rapid decline over the subsequent three days, in line with density changes (Figure 2B). While no significant differences in peak acetaldehyde values were observed between the control and other treatments, the ‘SO2 in juice’ sample displayed a lower peak acetaldehyde level than the control. The delayed onset of fermentation, documented by the delayed decrease in density of the ‘SO2 in juice’ treatment, illustrates the antimicrobial impact of sulfite addition even on a strong cultural yeast [2]. The impact of SO2 addition on acetaldehyde production can be due to its influence on various pathways. These are, for example, the alcohol dehydrogenase-catalyzed formation of acetaldehyde from ethanol by mitochondria in yeast [16] and the pyruvate decarboxylase-catalyzed decarboxylation of pyruvate, which has acetaldehyde as the end product [17]. The bonding of acetaldehyde with SO2 could also provide an explanation. However, the final acetaldehyde concentrations were comparable across all samples. The patterns shown in Figure 2 are very similar to those found by other authors [3].
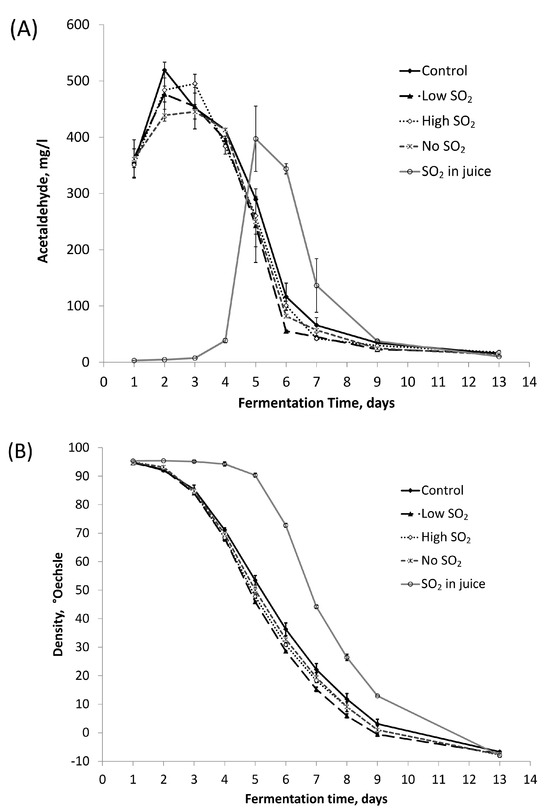
Figure 2.
The production and degradation of (A) acetaldehyde and (B) change in density during fermentation. The values shown are averages of fermentation replicates with a standard deviation.
3.2. Free SO2 Levels and Acetaldehyde Concentration during Wine Storage
The consumption of SO2 serves as a reliable indicator of wine oxidation. In our study, significant decreases in SO2 levels were observed during bottle aging, particularly within the first 6 months (Figure 3B). The ‘Low SO2’ sample experienced the most significant relative loss of free SO2 (87.1% after 12 months). Although the wine with high SO2 addition retained a level of approximately 24.5 mg/L after 12 months, expected to safeguard the wines at a pH of approximately 3.1 [18], the remaining samples fell below 10 mg/L after 12 months, a level potentially concerning for continued wine aging.
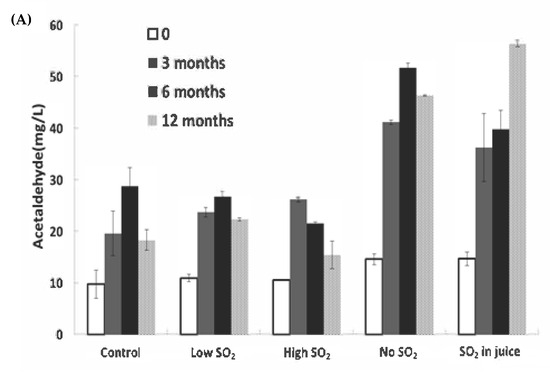
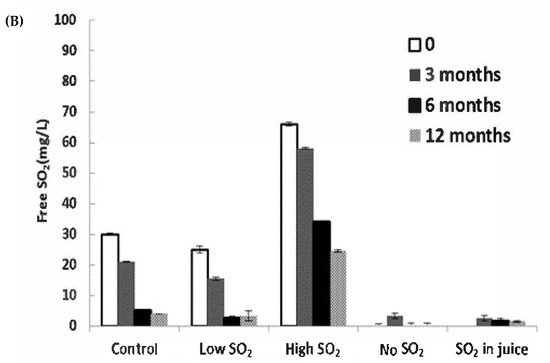
Figure 3.
Concentrations of (A) acetaldehyde and (B) free SO2 in wines during storage. The values shown are averages of fermentation replicates with a standard deviation.
Acetaldehyde can also arise post-alcoholic fermentation through ethanol oxidation when exposed to air [19]. Acetaldehyde concentrations did not significantly differ among wines immediately after fermentation. However, post-bottling, acetaldehyde markedly increased until 6 months of aging in all wines, particularly in the ‘No SO2’ and ‘SO2 in juice’ samples, which contained the lowest free SO2 levels (Figure 3A,B). After 12 months of aging, significant differences were observed between the control wine and the wine without SO2 addition. The lowest acetaldehyde level was found in the ‘High SO2’ sample, although not significantly different from the control wine. SO2 can inhibit aldehyde formation by competing with hydrogen peroxide, which induces ethanol oxidation [3]. The decline in acetaldehyde over 12 months of storage could be attributed to SO2 depletion, aligning with the findings of Bueno et al. [20]. Alternatively, it could result from rapid polymerization reactions of wine phenolics mediated by acetaldehyde [21,22], or acetaldehyde may decrease through reactions with alcohols to form dioxolanes [23].
3.3. Changes in Volatile Compounds during 3, 6, and 12 Months of Storage
Volatile compound variations were monitored during storage at 3, 6, and 12 months post-bottling (Table 1). Several acetate esters, such as propyl acetate, 2-methylpropyl acetate, hexyl acetate, and phenethyl acetate, declined during storage across all wine samples. These esters often create fruity aromas like banana and pears and floral aromas like rose. Conversely, several ethyl esters, including ethyl 2-methylbutyrate, ethyl 3-methylbutyrate, ethyl pyruvate, and ethyl 9-decenoate, increased over time. This is in accordance with the general observation that many acetate esters decrease during bottle storage while ethanol directly reacts with organic acid to generate a range of ethyl esters [24].

Table 1.
Changes in volatile compounds during storage at 3, 6, and 12 months (μg/L). Averages of all treatments. Only compounds with a p-value < 0.001 are shown.
Additionally, certain compounds known as markers for oxidation, including β-damascenone and vitispirane isomers, demonstrated substantial changes during storage. β-Damascenone, usually imparting fruity and floral notes, exhibited a slight decrease after 12 months of bottle aging, in line with the findings of Chisholm et al. [25] on aged Vidal blanc wine. Conversely, mono-terpene alcohols like linalool and hotrienol increased in wines without SO2 protection during storage, consistent with the observations of Zoecklein et al. [26] in aged Riesling white wines.
Monoterpenes can undergo considerable fluctuations due to isomerization and/or breakdown, potentially influenced by biochemical rearrangement in addition to hydrolysis [27]. During storage, significant increases were observed in the levels of 2,4,5-trimethyl-1,3-dioxolane (associated with green and phenolic notes), furfural (bread, almond, and sweet aromas), diethyl succinate, and ethyl pyruvate (with fruity, sweet, vegetable, and caramel nuances) in the ‘No SO2’ wine. Previous studies by others have also noted similar rises in these compounds in oxidized or aged wines [28,29,30]. Escudero et al. [28] identified 2,4,5-trimethyl-1,3-dioxolane as a key odorant in oxidized wine. Therefore, these compounds may serve as valuable indicators of wine aging.
The increase in 2,4,5-trimethyl-1,3-dioxolane levels is attributed to oxygen exposure, as it undergoes a condensation reaction with 2,3-butanediol and acetaldehyde [23]. Consequently, the rise in acetaldehyde concentration during wine aging coincides with the formation of 2,4,5-trimethyl-1,3-dioxolane. Similar findings were reported in beer by Vanderhaegen et al. [31], supporting this observation.
Among the compounds mentioned in this section, however, only ethyl 2-methylbutyrate, ethyl 3-methylbutyrate, linalool, and 2,4,5-trimethyl-1,3-dioxolane are present in concentrations above the threshold (Table 2). These compounds are further discussed in the next section.

Table 2.
Volatile compounds were quantified in the wines with different SO2 managements. Values are presented as averaged concentrations over replicates (μg/L) in finished wines after 12 months of storage. Levels not connected by the same letter are significantly different, and significance levels are presented as ‘ns’ (p > 0.05), ‘*’ (p ≤ 0.05), ‘**’ (p ≤ 0.01), or ‘***’ (p ≤ 0.001).
3.4. Effect of SO2 Management on Volatile Compounds in Final Wines (12 Months of Storage)
Table 2 presents the identified and quantified volatile compounds in the finished wine (12 months post-bottling). A total of 68 volatile compounds, including 31 esters, 17 alcohols, 8 aldehydes, 7 terpenes, 3 ketones, 1 sulfur compound, and a dioxolane, were identified. Given that the contribution of volatile compounds to wine aroma depends on their concentration surpassing the perception threshold, the odor activity value (OAV) was introduced to identify potent odorants. OAV was calculated as the ratio between the compound concentration and its odor threshold. Results revealed that 15 out of 68 quantified volatile compounds exceeded the odor threshold (log OAV > 0) in the wine, suggesting their potential as aroma contributors. Among them, eight esters, notably ethyl hexanoate (apple peel, fruit) and ethyl octanoate (fruit, fat), exhibited high OAV values, contributing fruity and fatty nuances to the wine. The robust presence of esters in Solaris white wines has been previously reported by Liu et al. [10]. While concentrations of these esters did not significantly differ between wines or displayed minor variations, indicating minimal influence from SO2 treatments, seven volatile compounds with OAV log values above 0 in at least one sample exhibited significant differences between treatments. These included acetaldehyde (pungent, overripe fruit odor), 3-methylbutanal (malty), and 2,4,5-trimethyl-1,3-dioxolane (green, phenolic), with notably higher concentrations in wines with ‘No SO2’ and ‘SO2 in juice.’ These findings align with previous research demonstrating elevated levels of acetaldehyde and 2,4,5-trimethyl-1,3-dioxolane in oxidized Solaris wines [10]. When sulfur dioxide is added to wine containing free acetaldehyde, it forms a sulfonated adduct [20,46], potentially explaining the low acetaldehyde content in wines with post-fermentation SO2 addition.
The presence of the acetal 2,4,5-trimethyl-1,3-dioxolane significantly increased in wines exhibiting the highest acetaldehyde levels. Vanderhaegen et al. [47] proposed that an equilibrium between 2,4,5-trimethyl-1,3-dioxolane, acetaldehyde, and 2,3-butanediol could rapidly establish due to the escalating acetaldehyde concentration. This molecule has also been suggested as a marker for detecting oxidation in bottled beer during aging [47].
β-Damascenone, known for its profound impact on wine aroma owing to its low odor threshold value [48], contributes delicate notes of apple, rose, and honey to white wines. In this study, β-Damascenone exhibited the highest concentration in the ‘No SO2’ wine, followed by ‘SO2 in juice’, and the lowest concentration in the ‘High SO2’ wine. This finding is consistent with the model wine study conducted by Daniel et al. [49], which demonstrated that the reaction between sulfur dioxide and β-damascenone yields 4-oxo-4-(2,6,6-trimethylcyclohexa-1,3-dien-1-yl) butane-2-sulfonate as the major non-volatile adduct. Linalool, contributing flowery notes, exhibited variations similar to those of β-damascenone. It is, however, unclear whether similar reactions with sulfur dioxide take place.
3.5. Effect of SO2 Management on Sensory Properties in Final Wines (12 Months of Storage)
Half of the attributes used in the sensory vocabulary had previously been selected to describe oxidized wines [10,50]. The ‘Control’ wine served as the sensory reference in the difference from control test, which enables the detection of subtle sensory differences by directly comparing attributes with the control sample in paired evaluations.
ANOVA of the panel difference ratings revealed that all attributes significantly discriminated among wine samples with different SO2 management (see Table 3). Specifically, the ‘No SO2’ and ‘SO2 in juice’ wines exhibited significantly stronger flavors compared to the ‘Control’ and ‘High SO2’ wines, except for ‘citrus.’ It is reasonable to attribute the greater ‘chemical’, ‘honey’, and ‘overripe fruit’ flavors in the ‘No SO2’ and ‘SO2 in juice’ wines to oxidation. The presence of elevated levels of acetaldehyde in these Solaris wines may contribute to the ‘chemical’ and ‘overripe fruit’ flavors. The ‘honey’ flavor could be attributed to the high levels of phenylethyl acetate and 2-pheneylethanol. Sensory analyses supported the significant impact of the absence of SO2 during storage on flavor attributes associated with oxidation.

Table 3.
Results from the difference from control sensory profiling, rating the intensity differences from the four experimental Solaris wines to the control (addition of 60 mg/L SO2). All wines were stored for 12 months.
No significant sensory differences were perceived between the ‘Control’ and ‘High SO2’ wines, nor between the ‘No SO2’ and ‘SO2 in juice’ wines. Despite the ‘High SO2’ wine containing significantly higher levels of volatile compounds such as nonanal and decanal, which contribute citrus notes, as well as compounds like linalool, β-damascenone, and hotrienol, which impart floral notes, these differences were not detected in the sensory analysis.
4. Conclusions
This study investigated the impact of sulfur dioxide (SO2) addition on the volatile and sensory characteristics of Solari’s white wine. The presence of free SO2 notably decreased the levels of free acetaldehyde during wine storage while also decreasing the concentrations of other aldehydes, particularly at higher dosage levels. Conversely, in the absence of free SO2, acetaldehyde levels increased significantly, accompanied by elevated levels of its associated compound, 2,4,5-trimethyl-1,3-dioxolane, carrying ‘green’ and ‘phenolic’ aroma notes, both surpassing well above their respective odor thresholds. Additionally, other volatile compounds such as 3-methylbutanal and β-damascenone increased in these wines, likely contributing to amplified sensory impressions of ‘chemical’, ‘overripe fruit’, and ‘honey’ notes.
In the finished wines after 12 months of storage, regardless of SO2 management, crucial esters defining Solari's wine aroma remained unaltered in concentration. However, even a low addition of sulfite improved the sensory quality significantly. This underscores the significance of employing moderate levels of SO2 post-Solaris wine fermentation to avoid oxidation of the final product, but higher SO2 additions conferred no significant benefits at the timespan studied. The progressive loss of free sulfite and, thus, oxidative protection was very evident during storage, and the expected lifespan of the wine needs to be taken into account when the sulfite level is defined pre-bottling.
Author Contributions
Conceptualization, T.B.T.-A. and M.A.P.; methodology, T.B.T.-A., M.A.P. and W.L.P.B.; formal analysis, S.Z., J.L. and M.A.P.; investigation, S.Z.; resources, W.L.P.B., M.A.P. and T.B.T.-A.; data curation, S.Z., M.A.P. and T.B.T.-A.; writing—original draft preparation, S.Z. and J.L.; writing—review and editing, S.Z., J.L., T.B.T.-A., M.A.P. and W.L.P.B.; visualization, S.Z.; supervision, M.A.P., W.L.P.B. and T.B.T.-A.; project administration, T.B.T.-A. and M.A.P.; funding acquisition, M.A.P., W.L.P.B. and T.B.T.-A. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by The Danish Ministry of Food, Agriculture and Fisheries (GUDP, No 34009-13-0725), Faculty of Science, the University of Copenhagen, and a grant from the Chinese Scholarship Council (No 201206300044).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Raw data can be obtained by contacting M.A.P.
Acknowledgments
We are truly thankful for the technical assistance of the staff at the Pometum and in the labs.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study, in the collection, analysis, or interpretation of data, in the writing of the manuscript, or in the decision to publish the results.
References
- Oliveira, C.M.; Ferreira, A.C.S.; De Freitas, V.; Silva, A.M.S. Oxidation mechanisms occurring in wines. Food Res. Int. 2011, 44, 1115–1126. [Google Scholar] [CrossRef]
- Jackson, R.S. Fermentation. In Wine Science, 5th ed.; Jackson, R.S., Ed.; Academic Press: San Diego, CA, USA, 2020; pp. 462–571. [Google Scholar] [CrossRef]
- Jackowetz, J.N.; Dierschke, S.; Mira de Orduña, R. Multifactorial analysis of acetaldehyde kinetics during alcoholic fermentation by Saccharomyces cerevisiae. Food Res. Int. 2011, 44, 310–316. [Google Scholar] [CrossRef]
- Garde-Cerdán, T.; Ancín-Azpilicueta, C. Effect of SO2 on the formation and evolution of volatile compounds in wines. Food Control 2007, 18, 1501–1506. [Google Scholar] [CrossRef]
- Ferreira, V.; Bueno, M.; Franco-Luesma, E.; Cullere, L.; Fernandez-Zurbano, P. Key changes in wine aroma active compounds during bottle storage of Spanish red wines under different oxygen levels. J. Agric. Food Chem. 2014, 62, 10015–10027. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Petersen, M.; Liu, J.; Toldam-Andersen, T. Influence of pre-fermentation treatments on wine volatile and sensory profile of the new disease tolerant cultivar Solaris. Molecules 2015, 20, 19791. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Bredie, W.L.P.; Sherman, E.; Harbertson, J.F.; Heymann, H. Comparison of rapid descriptive sensory methodologies: Free-choice profiling, flash profile and modified flash profile. Food Res. Int. 2018, 106, 892–900. [Google Scholar] [CrossRef] [PubMed]
- Brajkovich, M.; Tibbits, N.; Peron, G.; Lund, C.M.; Dykes, S.I.; Kilmartin, P.A.; Nicolau, L. Effect of screwcap and cork closures on SO2 levels and aromas in a Sauvignon Blanc wine. J. Agric. Food Chem. 2005, 53, 10006–10011. [Google Scholar] [CrossRef] [PubMed]
- Price, E.J.; Linforth, R.S.T.; Dodd, C.E.R.; Phillips, C.A.; Hewson, L.; Hort, J.; Gkatzionis, K. Study of the influence of yeast inoculum concentration (Yarrowia lipolytica and Kluyveromyces lactis) on blue cheese aroma development using microbiological models. Food Chem. 2014, 145, 464–472. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Toldam-Andersen, T.B.; Petersen, M.A.; Zhang, S.; Arneborg, N.; Bredie, W.L.P. Instrumental and sensory characterisation of Solaris white wines in Denmark. Food Chem. 2015, 166, 133–142. [Google Scholar] [CrossRef]
- Henderson, P. Sulphur dioxide. The science behind this anti-microbial, anti-oxidant wine additive. J. Pract. Winery Vineyard 2014, 1, 54–60. [Google Scholar]
- Tanner, H.; Sandoz, M. Bestimmung der freien SO2 neben Askorbinsäure und anderen Reduktonen unter Zuhilfenahme von Glyoxal. Schweiz. Z. Obs.-Weinbau 1972, 108, 331–337. [Google Scholar]
- Zhang, S.; Petersen, M.A. Comparison of headspace methods for detecting highly volatile compounds in wine. In 14th Weurman Symposium; Taylor, A., Mottram, D., Eds.; Context Products Ltd.: Cambridge, UK, 2015; pp. 579–582. [Google Scholar]
- Munoz, A.M.; Civille, G.V.; Carr, B.T. Sensory Evaluation in Quality Control; Springer: New York, NY, USA, 1992. [Google Scholar]
- Costell, E. A comparison of sensory methods in quality control. Food Qual. Prefer. 2002, 13, 341–353. [Google Scholar] [CrossRef]
- Bakker, B.M.; Bro, C.; Kotter, P.; Luttik, M.A.H.; van Dijken, J.P.; Pronk, J.T. The mitochondrial alcohol dehydrogenase adh3p is involved in a redox shuttle in Saccharomyces cerevisiae. J. Bacteriol. 2000, 182, 4730–4737. [Google Scholar] [CrossRef] [PubMed]
- Berlowska, J.; Kregiel, D.; Ambroziak, W. Pyruvate decarboxylase activity assay in situ of different industrial yeast strains. Food Technol. Biotechnol. 2009, 47, 96–100. [Google Scholar]
- Smith, C. Review of basics on sulfur dioxide—Part I. In Enology Briefs; University of California: Davis, CA, USA, 1982; pp. 1–4. [Google Scholar]
- Danilewicz, J.C. Review of reaction mechanisms of oxygen and proposed intermediate reduction products in wine: Central role of iron and copper. Am. J. Enol. Vitic. 2003, 54, 73–85. [Google Scholar] [CrossRef]
- Bueno, M.; Marrufo-Curtido, A.; Carrascón, V.; Fernández-Zurbano, P.; Escudero, A.; Ferreira, V. Formation and accumulation of acetaldehyde and strecker aldehydes during red wine oxidation. Front. Chem. 2018, 14, 6–20. [Google Scholar] [CrossRef]
- Lee, D.F.; Swinny, E.E.; Jones, G.P. NMR identification of ethyl-linked anthocyanin-flavanol pigments formed in model wine ferments. Tetrahedron Lett. 2004, 45, 1671–1674. [Google Scholar] [CrossRef]
- Rivasgonzalo, J.C.; Bravoharo, S.; Santosbuelga, C. Detection of compounds formed through the reaction of malvidin 3-Mmonoglucoside and catechin in the presence of acetaldehyde. J. Agric. Food Chem. 1995, 43, 1444–1449. [Google Scholar] [CrossRef]
- Peppard, T.L.; Halsey, S.A. The occurrence of 2 geometrical-Iisomers of 2,4,5-Trimethyl-1,3-Dioxolane in beer. J. Inst. Brew. 1982, 88, 309–312. [Google Scholar] [CrossRef]
- Zhang, D.; Wie, Z.; Han, Y.; Duan, Y.; Shi, B.; Ma, W. A Review on Wine Flavour Profiles Altered by Bottle Aging. Molecules 2023, 28, 6522. [Google Scholar] [CrossRef]
- Chisholm, M.G.; Guiher, L.A.; Zaczkiewicz, S.M. Aroma characteristics of aged Vidal Blanc wine. Am. J. Enol. Vitic. 1995, 46, 56–62. [Google Scholar] [CrossRef]
- Zoecklein, B.W.; Hackney, C.H.; Duncan, S.E.; Marcy, J.E. Effect of fermentation, aging and thermal storage on total glycosides, phenol-free glycosides and volatile compounds of White Riesling (Vitis vinifera L.) wines. J. Ind. Microb. Biotechnol. 1999, 22, 100–107. [Google Scholar] [CrossRef]
- Wilson, B.; Strauss, C.R.; Williams, P.J. Changes in free and glycosidically bound monoterpenes in developing Muscat grapes. J. Agric. Food Chem. 1984, 32, 919–924. [Google Scholar] [CrossRef]
- Escudero, A.; Cacho, J.; Ferreira, V. Isolation and identification of odorants generated in wine during its oxidation: A gas chromatography-olfactometric study. Eur. Food Res. Technol. 2000, 211, 105–110. [Google Scholar] [CrossRef]
- Escudero, A.; Asensio, E.; Cacho, J.; Ferreira, V. Sensory and chemical changes of young white wines stored under oxygen. An assessment of the role played by aldehydes and some other important odorants. Food Chem. 2002, 77, 325–331. [Google Scholar] [CrossRef]
- Zea, L.; Moyano, L.; Moreno, J.A.; Medina, M. Aroma series as fingerprints for biological ageing in fino sherry-type wines. J. Sci. Food Agric. 2007, 87, 2319–2326. [Google Scholar] [CrossRef]
- Vanderhaegen, B.; Neven, H.; Verachtert, H.; Derdelinckx, G. The chemistry of beer aging—A critical review. Food Chem. 2006, 95, 357–381. [Google Scholar] [CrossRef]
- Etiévant, P.X. Wine. In Volatile Compounds in Foods and Beverages; Maarse, H., Ed.; Marcel Dekker: New York, NY, USA, 1991; pp. 483–546. [Google Scholar] [CrossRef]
- Guth, H. Comparison of different white wine varieties in odor profiles by instrumental analysis and sensory studies. Abstr. Pap. Am. Chem. Soc. 1997, 213, 3027–3032. [Google Scholar] [CrossRef]
- Takeoka, G.R.; Buttery, R.G.; Turnbaugh, J.G.; Benson, M. Odor Thresholds of various branched esters. LWT-Food Sci. Technol. 1995, 28, 153–156. [Google Scholar] [CrossRef]
- Takeoka, G.R.; Buttery, R.G.; Teranishi, R.; Flath, R.A.; Guntert, M. Identification of additional pineapple volatiles. J. Agric. Food Chem. 1991, 39, 1848–1851. [Google Scholar] [CrossRef]
- Ahmed, E.M.; Dennison, R.A.; Dougherty, R.H.; Shaw, P.E. Flavor and odor thresholds in water of selected orange juice components. J. Agric. Food Chem. 1978, 26, 187–191. [Google Scholar] [CrossRef]
- Gómez-Míguez, M.J.; Cacho, J.F.; Ferreira, V.; Vicario, I.M.; Heredia, F.J. Volatile components of Zalema white wines. Food Chem. 2007, 100, 1464–1473. [Google Scholar] [CrossRef]
- Tao, Y.S.; Zhang, L. Intensity prediction of typical aroma characters of cabernet sauvignon wine in Changli County (China). LWT-Food Sci. Technol. 2010, 43, 1550–1556. [Google Scholar] [CrossRef]
- Pino, J.A.; Queris, O. Analysis of volatile compounds of mango wine. Food Chem. 2011, 125, 1141–1146. [Google Scholar] [CrossRef]
- Ferreira, V.; Lopez, R.; Cacho, J.F. Quantitative determination of the odorants of young red wines from different grape varieties. J. Sci. Food Agric. 2000, 80, 1659–1667. [Google Scholar] [CrossRef]
- Peinado, R.A.; Moreno, J.; Bueno, J.E.; Moreno, J.A.; Mauricio, J.C. Comparative study of aromatic compounds in two young white wines subjected to pre-fermentative cryomaceration. Food Chem. 2004, 84, 585–590. [Google Scholar] [CrossRef]
- Franco, M.; Peinado, R.A.; Medina, M.; Moreno, J. Off-vine grape drying effect on volatile compounds and aromatic series in must from Pedro Ximenez grape variety. J. Agric. Food Chem. 2004, 52, 3905–3910. [Google Scholar] [CrossRef] [PubMed]
- Cullere, L.; Escudero, A.; Cacho, J.; Ferreira, V. Gas chromatography-olfactometry and chemical quantitative study of the aroma of six premium quality Spanish aged red wines. J. Agric. Food Chem. 2004, 52, 1653–1660. [Google Scholar] [CrossRef] [PubMed]
- Moyano, L.; Zea, L.; Moreno, J.; Medina, M. Analytical study of aromatic series in sherry wines subjected to biological aging. J. Agric. Food Chem. 2002, 50, 7356–7361. [Google Scholar] [CrossRef]
- Ferreira, A.C.S.; de Pinho, P.G.; Rodrigues, P.; Hogg, T. Kinetics of oxidative degradation of white wines and how they are affected by selected technological parameters. J. Agric. Food Chem. 2002, 50, 5919–5924. [Google Scholar] [CrossRef]
- Coetzee, C.; Buica, A.; du Toit, W.J. The use of SO2 to bind acetaldehyde in wine: Sensory Implications. Afr. J. Enol. Vitic. 2018, 39. [Google Scholar] [CrossRef]
- Vanderhaegen, B.; Neven, H.; Coghe, S.; Verstrepen, K.J.; Verachtert, H.; Derdelinckx, G. Evolution of chemical and sensory properties during aging of top-fermented beer. J. Agric. Food Chem. 2003, 51, 6782–6790. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, V.; Ortin, N.; Escudero, A.; Lopez, R.; Cacho, J. Chemical characterization of the aroma of Grenache rose wines: Aroma extract dilution analysis, quantitative determination, and sensory reconstitution studies. J. Agric. Food Chem. 2002, 50, 4048–4054. [Google Scholar] [CrossRef] [PubMed]
- Daniel, M.A.; Elsey, G.M.; Capone, D.L.; Perkins, M.V.; Sefton, M.A. Fate of damascenone in Wine: The role of SO2. J. Agric. Food Chem. 2004, 52, 8127–8131. [Google Scholar] [CrossRef]
- Ferreira, A.C.S.; Hogg, T.; de Pinho, P.G. Identification of key odorants related to the typical aroma of oxidation-spoiled white wines. J. Agric. Food Chem. 2003, 51, 1377–1381. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).